Abstract
Chronic thromboembolic pulmonary hypertension (CTEPH) is a potentially curable disease when treated with pulmonary thromboendarterectomy (PTE). However, even at experienced surgical centers, nearly one-third of patients with CTEPH will be deemed inoperable for reasons including distal disease, comorbidities, or out-of-proportion pulmonary hypertension. It is in these patients with inoperable CTEPH that pulmonary hypertension (PH)-targeted medical therapy and balloon pulmonary angioplasty have potential therapeutic value.
Previous unblinded cohort trials have assessed PH-targeted medical therapy in various subpopulations of CTEPH patients using epoprostenol, treprostinil, sildenafil, bosentan, and iloprost, each demonstrating measurable pulmonary hemodynamic effects. However, riociguat, a soluble guanylate cyclase stimulator, is the first FDA-approved therapy for inoperable CTEPH to demonstrate both an improvement in functional capabilities (6-minute walk time) as well as significant gains in secondary pulmonary hemodynamic end points in a large placebo-controlled trial.
Balloon pulmonary angioplasty is an interventional procedure using telescoping catheters placed in the pulmonary arteries, through which wires and balloons are used to mechanically disrupt chronic clot material and relieve pulmonary vascular obstruction. Contemporary case series from multiple centers worldwide have demonstrated pulmonary hemodynamic improvement with this approach.
As a result of these advances, patients with inoperable CTEPH who had few options as recently as 5 years ago now have alternatives with emerging evidence of therapeutic efficacy.
Keywords: pulmonary hypertension, chronic thromboemboli, pulmonary hypertension targeted medical therapy, balloon pulmonary angioplasty
Introduction
Since the 1980s, pulmonary thromboendarterectomy (PTE) has been recognized as an effective treatment for selected patients with chronic thromboembolic pulmonary hypertension (CTEPH). When the surgery was performed at an experienced center, there was a decline in perioperative mortality rates, and long-term pulmonary hemodynamic and functional status benefits were realized; as a result, the procedure was regarded as a potential cure for this form of pulmonary hypertension.1–3 However, it has also become evident that there are identifiable CTEPH patients for whom PTE surgery is not an option—notably, those with technically inoperable chronic thromboembolic disease and those whose comorbidities preclude a reasonable expectation that surgery will have short- or long-term benefits. This observation was underscored in the results of the International Prospective CTEPH registry, where 36.6% of enrolled patients were deemed inoperable. The most common reasons included surgical inaccessibility of disease (47.8%), comorbidities (13.4%), and an “imbalance” between the degree of pulmonary vascular resistance (PVR) and the amount of accessible chronic thromboembolic disease (10.1%).4 Additional patient groups that present unique problems include those with symptomatic, residual pulmonary hypertension (PH) following PTE surgery as well as CTEPH patients with operable disease awaiting surgery with severe pulmonary hypertension and right heart dysfunction. It is in these patients that a role for PH-targeted medications and possibly balloon pulmonary angioplasty exists. In published recommendations from both the 2013 World Symposium of Pulmonary Hypertension in Nice5 and the 2016 ERS/ESC Pulmonary Hypertension Guidelines,6 these therapies should be considered for CTEPH patients when a surgical option has been excluded.
Pulmonary Hypertension Targeted Medical Therapy
Inoperable Chronic Thromboembolic Pulmonary Hypertension
Several clinical trials have examined the efficacy of PH-targeted medical therapy in CTEPH patients with inoperable disease and in those with residual PH following endarterectomy surgery. Early investigations involved cohort, case-controlled trials that were typically small in size. A summary of seminal trials and cohort studies of PH-targeted medical therapy is presented in Table 1.
Table 1.
Selected trials of pulmonary hypertension-directed medical therapy in inoperable chronic thromboembolic pulmonary hypertension.
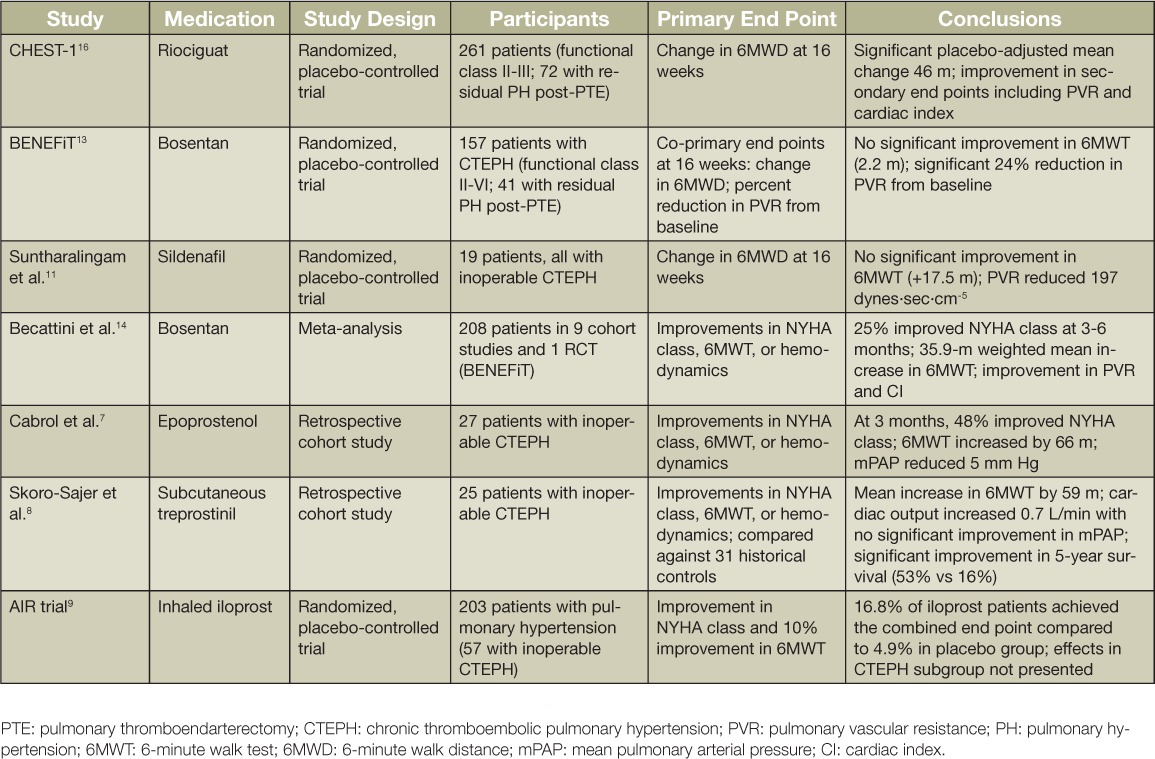
Intravenous epoprostenol and subcutaneous treprostinil have both been evaluated in retrospective cohort studies. Cabrol and colleagues retrospectively analyzed 27 patients with inoperable CTEPH who were treated with epoprostenol. After 3 months of therapy, there was a decrease in mean pulmonary artery (PA) pressure (mPAP) (56 + 9 mm Hg to 51 + 8 mm Hg), total pulmonary resistance (29.3 + 7.0 U/m2 to 23.0 + 5.0 U/m2), and an increase in the 6-minute walk test (6MWT) of 66 m. NYHA functional status improved by one class in 11 of 23 patients.7 In a single-center uncontrolled observational study, 28 patients with severe inoperable CTEPH were treated with subcutaneous treprostinil. Right heart catheterization was repeated in 19 patients after 19 + 6.3 months of treatment. Treprostinil therapy was associated not only with a significant reduction in PVR but also an improvement in the 6MWT (59 m), World Health Organization (WHO) functional class, brain natriuretic peptide (BNP) levels, and cardiac output. The 5-year survival rate was 53% compared with 16% in untreated historical controls.8 Early work in this patient group with inhaled iloprost, another prostacyclin analogue, demonstrated some efficacy, although less so than in idiopathic pulmonary arterial hypertension (PAH).9 Although the long-term benefit of inhaled iloprost in patients with inoperable CTEPH was not examined by Krug and colleagues, an acute pulmonary hemodynamic benefit was observed. With the administration of 5 ug of inhaled iloprost to 20 CTEPH patients (12 with distal chronic thromboembolic lesions), these investigators were able to show a decline in PVR from 1057 + 404.3 to 821.3 + 294.3 dynes·sec·cm−5 and a reduction in mean PA pressure from 50.55 + 8.43 to 45.75 + 8.09 mm Hg. This was accompanied by an increase in cardiac output from 3.66 + 1.05 to 4.05 + 0.91 L/min. Of note, 16 of the 20 patients were already receiving one or more PAH-specific medical therapies at the time.10
Other early studies examining the efficacy of other classes of PH-targeted medical therapy for patient with inoperable CTEPH were similarly limited. Sildenafil, a phosphodiesterase-5 inhibitor, was evaluated in a small, single-center, randomized placebo-controlled trial.11 In a 12-week pilot study, Suntharalingam and colleagues enrolled 19 patients with inoperable CTEPH to assess the benefit of sildenafil (9 patients receiving drug) in this group. Though there was no significant difference detected in the 6MWT (the primary end point), there was an improvement in WHO functional class and an average 197 dynes·sec·cm−5 difference in PVR between treatment groups. Control subjects were then transitioned to open-label sildenafil use and reassessed at 12 months. Significant improvement in 6-minute walk distance (6MWD), activity and symptom scores (CAMPHOR), cardiac index, PVR, and N-terminal (NT)-proBNP values (1000 to 811 pg/mL) were noted.11 In a larger patient group, Reichenberger and colleagues conducted an open-label study of sildenafil (50 mg 3×/d) in 104 patients with inoperable CTEPH. After 3 months of therapy, there was a modest decrease in pulmonary vascular resistance (863 + 38 dynes·sec·cm−5 to 759 + 62 dynes·sec·cm−5), with an increase in 6MWT from 310 + 11 m to 361 + 15 m; this distance further improved to 366 + 18 m after 12 months of sildenafil.12
An endothelin receptor antagonist (bosentan) was evaluated in the first randomized controlled trial in patients with inoperable CTEPH. In the Bosentan Effects in Inoperable Forms of Chronic Thromboembolic Pulmonary Hypertension (BENEFiT) trial, 157 patients (116 of whom were deemed inoperable) were randomized to either bosentan or placebo.13 There were two coprimary end points investigated: improvement in 6MWT and percent change from baseline in PVR. After 16 weeks of treatment, bosentan did not improve 6MWT (+ 2.2 m; 95% CI −22.5 to +26.8 m) but did reduce PVR 24% from baseline. A meta-analysis of the effects of bosentan on the 6MWT that included the BENEFiT study as well as eight uncontrolled cohort studies using bosentan demonstrated a significant weighted mean improvement of 35.9 m in the 6MWT.14 A new endothelin receptor antagonist, macitentan, is currently being evaluated in inoperable CTEPH in the ongoing MERIT-1 trial.15
Riociguat, a soluble guanylate cyclase stimulator and the first agent in this pharmaceutical class, was the first PH-targeted medical therapy to achieve a statistically significant improvement in 6MWD in a large, randomized, controlled trial. This resulted in FDA approval for use in CTEPH patients with inoperable disease and in those with residual PH following endarterectomy surgery. In the Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1 (CHEST-1) study, 261 patients with CTEPH (189 of whom were deemed inoperable) were randomized to 16 weeks of either riociguat (starting at 1 mg and titrated to 2.5 mg or the maximum tolerated dose) or placebo.16 In the riociguat-treated group, there was a placebo-adjusted mean change of 46 m (P < .001). Additionally, there were positive hemodynamic benefits in the treated group, with a mean reduction in PVR of 246 dynes·sec·cm−5 as well as a reduction in NT-proBNP with a placebo-adjusted least square mean difference of −444 pg/mL. Long-term follow-up of patients treated with riociguat was reported in 237 patients who continued on in the open-label CHEST-2 trial. At 2 years of riociguat use, overall survival was 93% (95% CI: 89%–96%) and clinical worsening-free survival was 82% (95% CI: 84%–92%) without additional safety concerns. After 12 weeks of open-label use of riociguat, the patients previously receiving placebo in CHEST-1 achieved a similar improvement in 6MWD to those in the extended riociguat treatment arm.17
The CHEST-2 mortality results can be contrasted with those of Scholzel et al. in an observational study of patients with inoperable CTEPH treated with PDE-5 inhibitors, endothelin receptor antagonists, prostacyclin analogs, or a combination of these.18 In this study, 32 patients were followed over a mean time period of 3.4 years. Even with advancement of non-riociguat medical therapies, the 1- and 3-year rates of freedom from clinical worsening (a composite that included death, need for IV prostacyclin, worsening functional class, or 15% drop in 6MWT) were 74% and 60%, respectively. Mortality during this time period was 34% (11 of 32 patients).18 Whether a mortality benefit will be seen in clinical practice with riociguat has not been determined.
Interpretation of survival benefit with PH-targeted therapy is difficult given the potential confounders affecting both survival and access to medical therapy. Therefore, clinical follow-up of patients with inoperable CTEPH should further focus on functional improvement as evidenced by 6MWD. Additionally, unlike numerous investigations in WHO group I PAH patients, there are no data on the use of combined multiple classes of PH-targeted medical therapy in inoperable CTEPH patients.
Right Heart Dysfunction and Residual Pulmonary Hypertension after Thromboendarterectomy Surgery
Following PTE, right ventricular dysfunction and residual pulmonary hypertension can be a significant cause of morbidity and mortality in the immediate postoperative period. A retrospective analysis from a single center revealed that in a group of 500 CTEPH patients undergoing PTE between 2006 and 2010, a postoperative mortality of 10.3% was observed in those patients with a postoperative PVR > 500 dynes·sec·cm−5; this compared to a 0.9% mortality rate in patients with a PVR below this level.1 Theoretically, initiation of PH-targeted medical therapies in these patients immediately after surgery may worsen hypoxemia due to nonspecific loss of hypoxemic vasoconstriction. Therefore, short-acting agents such as inhaled vasodilators and iloprost have been studied in this setting. In a small, single-center study, patients with residual PH at ICU admission following PTE who were treated with inhaled iloprost had significantly lower PVR for 60 minutes following treatment compared to placebo.19 Alternatively, inhaled nitric oxide (NO) may be considered in patients with postoperative right ventricular failure since inhaled NO has been demonstrated to lower PVR and helps improve oxygenation after PTE.20,21
After the immediate perioperative period, however, the presence of residual PH carries potential adverse consequences. Depending on definitions, up to 35% of patients undergoing PTE surgery will have residual PH.22–24 Freed et al. found that patients with a postoperative mean pulmonary arterial pressure over 30 mm Hg remained limited in exercise capacity and were more symptomatic compared to those with a mean pulmonary arterial pressure less than 30 mm Hg.23 Furthermore, an mPAP > 38 mm Hg and a PVR > 425 dynes·sec·cm−5 measured 3 to 6 months after PTE correlated with long-term mortality due to CTEPH.24
Patients with residual PH were included in both the BENEFiT and CHEST-1 studies. In the BENEFiT trial, bosentan-treated patients with residual PH actually saw an 11.9-m loss in the 6MWT compared to placebo-treated patients despite a 34% reduction in PVR compared to baseline.13 In CHEST-1, those with residual PH treated with riociguat experienced a 27-m improvement in the 6MWT compared to a 1.8-m improvement in the placebo-treated patients; they also experienced improvements in secondary end points including reduction in PVR (−154 ± 127 dyn.sec.cm−5 with riociguat compared to −11 ± 205 dyn.sec.cm−5 with placebo) and improvement in functional class (38% with riociguat vs 16% with placebo).16 For this reason, riociguat is considered a first-line oral therapy for patients with residual PH after PTE.
“Bridging Therapy” Prior to Pulmonary Thromboendarterectomy
A subgroup of CTEPH patients in whom PH-targeted medical therapy is increasingly used includes operable patients awaiting surgery. Whereas this approach might seem logical for hemodynamically unstable patients, the indication for “bridging therapy” in the majority of operable CTEPH patients remains controversial. Previous reports have suggested that a preoperative PVR > 1000 dynes·sec·cm−5 carries with it higher postoperative mortality and morbidity risks.1,25,26 The presumption, therefore, has been that if PH and right heart function can be positively affected in these high-risk patients, an improvement in postoperative outcomes might be realized. Nagaya and colleagues, one of the first groups to investigate this hypothesis, administered intravenous prostacyclin at a mean dose of 6 + 1 ng/kg/min for a duration of 46 + 12 days prior to PTE surgery in 12 patients with operable CTEPH, each with a PVR > 1200 dynes·sec·cm−5. This resulted in a significant preoperative reduction in PVR (1,510 + 53 to 1,088 + 58 dynes·sec·cm−5) and a decline in plasma BNP levels. While one patient in the treatment group died during the first 30 postoperative days, no one died in the group of 21 patients with a preoperative PVR < 1200 dynes·sec·cm−5. Pulmonary hemodynamic outcome in both patient groups was comparable.27
In another study, Reesink and colleagues conducted a 16-week, randomized, single-blind study using bosentan as a bridge to PTE surgery in 25 patients with operable CTEPH. Out of the 25 enrolled patients, 13 received bosentan. The mean differences in change from baseline between those receiving bosentan and those not receiving the drug were as follows: total pulmonary resistance decrease of 299 dynes/cm5 (P = .004), 6MWD increase of 33 m (P = .014), mean pulmonary artery pressure decrease of 11 mm Hg (P = .005), and increase in cardiac index 0.3 L/min/m2 (P = .08). Though postoperative mean PA pressure and total pulmonary resistance were lower in the bosentan group, this did not achieve statistical significance. Three patients died postoperatively in the no-bosentan group compared to no deaths in the bosentan-treated patients. Otherwise, for those who survived surgery, the short-term postoperative clinical course (ICU days, ventilator days, occurrence of lung injury) between groups was comparable.28
In a more recent study, Surie and colleagues conducted a pilot study of 15 patients with operable CTEPH to assess the effect of the preoperative use of bosentan, with a focus on right ventricular (RV) function.29 Eight of these 15 patients were randomly assigned to receive bosentan in addition to “best standard of care” for 16 weeks, with pre- and post-treatment evaluation of right heart function and remodeling using cardiac magnetic resonance imaging. Discernible improvement in RV stroke volume index (6 vs 1 mL/m−2, P = .037), RV ejection fraction (8% vs 4%, P = .028), RV mass (−3 vs 2 g/m−2, P = .001), RV isovolumic relaxation time (−0.03 vs 0.01 msec, 0.041), and LV ejection fraction (8% vs −2%, P = .037) were noted. This was accompanied by a change in mPAP (−11 vs 5 mm Hg in the non-bosentan group, P = .011) and an increase in 6MWD (20 vs −4 m in the non-bosentan group).29 Though the above studies may have demonstrated some pulmonary hemodynamic benefits with the use of selected PH-targeted therapies in patients with operable disease, the effects on postsurgical outcomes remain unproven.
Uncertainties regarding the use of PH-targeted medical therapy in this group are further fueled by two recent reports. In a retrospective analysis of patients referred for PTE surgery between 2005 and 2007, Jensen and colleagues revealed that the use of PAH-specific medical therapy in patients with surgical CTEPH increased from 19.9% of patients in 2005 to 31.9% in 2006 and to 37% in 2007. They also observed that this practice was associated with a significant delay in time to referral without having a discernible benefit on measured postoperative outcomes.30 The report of the long-term outcomes from the International Prospective CTEPH Registry reinforced these observations. Although patients receiving bridging therapy were more likely to have a more compromised hemodynamic profile preoperatively—with higher right atrial pressure, higher PVR, and lower cardiac index—the postoperative PVR was similar between groups; furthermore, postoperative complications (including the presence of postoperative PH and the incidence of reperfusion lung injury) were not significantly different whether or not PH-targeted medical therapy was prescribed before the endarterectomy. However, multivariate analysis in operated patients disclosed that bridging therapy with PH-targeted medications increased the risk of death. Possible explanations included a delay in surgical referral resulting from the use of medical therapies, a more difficult operation due to the effect of medications on the thrombus being endarterectomized, and a greater use of medications in the more hemodynamically compromised patients, or it may just be a marker of more extensive disease preoperatively.31
Balloon Pulmonary Angioplasty
Balloon pulmonary angioplasty (BPA), sometimes referred to as percutaneous transluminal pulmonary angioplasty, is a percutaneous approach for the treatment of CTEPH. It relies on the use of telescoping catheters placed in a central vein, through which wires and balloons are guided to mechanically disrupt chronic clot material and relieve pulmonary vascular obstruction. Despite first being described in 1988,32 it is only in recent years that BPA has emerged as a viable treatment option for CTEPH. In 2001, a case series described 18 patients who underwent BPA with unacceptably high (61%) complication rates.33 As a result, BPA was not a widely accepted approach in the treatment of CTEPH until 2012, when several publications from medical centers in Japan and Norway demonstrated the safety and efficacy of a refined approach.34–37 Since then, interest in BPA has rapidly expanded with an accompanying proliferation in the number of BPA-related publications. BPA now carries a class IIb recommendation for the treatment of inoperable CTEPH in the most recent European guidelines and may be considered in patients who are technically inoperable or carry an unfavorable risk:benefit ratio for PTE surgery (Figure 1).
Figure 1.
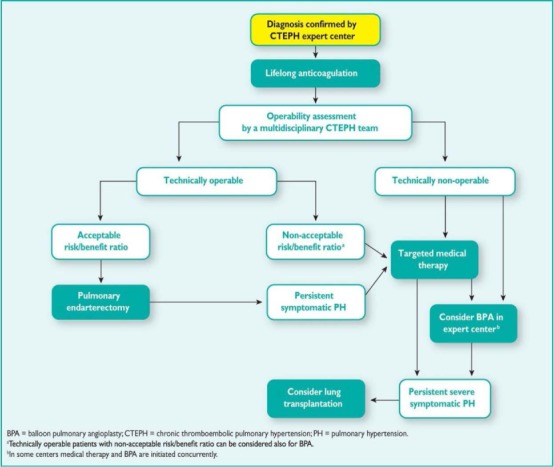
Treatment algorithm for chronic thromboembolic pulmonary hypertension.6
While there is considerable variation in the technical approach and patient selection among BPA centers, particularly as it relates to determination of operability, there are some common guiding principles of the procedure. First, to achieve hemodynamic and clinical end points, BPA requires more than one interventional session. During each BPA session, attempts are made to maximize the number of segments treated while minimizing the risks of BPA-associated lung injury. The improvement in mPAP does not occur immediately following BPA.35,38 Completely occluded vessels and pouch defects without angiographically visible runoff are less suited for BPA and are associated with higher complication and lower success rates. Patients with higher mPAP (> 35 mm Hg) prior to BPA and more severe PH may be at higher risk of BPA-associated lung injury.33,35 Finally, the use of intravascular imaging techniques and measurement of pressure gradients are useful tools to maximize efficacy, efficiency, and safety of BPA.39
Although histological examination of vessels after BPA is limited, two patterns have been described in post-mortem examination. The first demonstrated enlargement of the pulmonary arterial lumen by incision and compression of the thrombi without dissection of the vessel wall. In the second, the media of the PA was dissected by angioplasty and the organized thrombi were forced to one side, leaving a larger pseudo-lumen with formation of a new intimal layer.40
Outcomes
Despite differences in patient selection, differences in background use of PH-targeted therapies, and variations in the technical approach to BPA, all of the published series since 2012 have demonstrated substantial improvements in hemodynamics, functional class, and markers of heart failure such as BNP or NT-proBNP (Table 2).41 Many of the large BPA series have included patients who may have been surgical candidates had they been evaluated at an experienced PTE center. This observation does not necessarily detract from reported BPA results but rather highlights the ongoing subjectivity in determining which intervention might be best for any given patient. The mPAP across the series improved from a range of 40 to 47 mm Hg pre-BPA to a range of 24 to 34 mm Hg following BPA. The majority of patients also achieved NYHA/WHO functional class II status following this intervention. Some of the series also report decreased requirements for supplemental oxygen with BPA.
Table 2.
Balloon pulmonary angioplasty results.
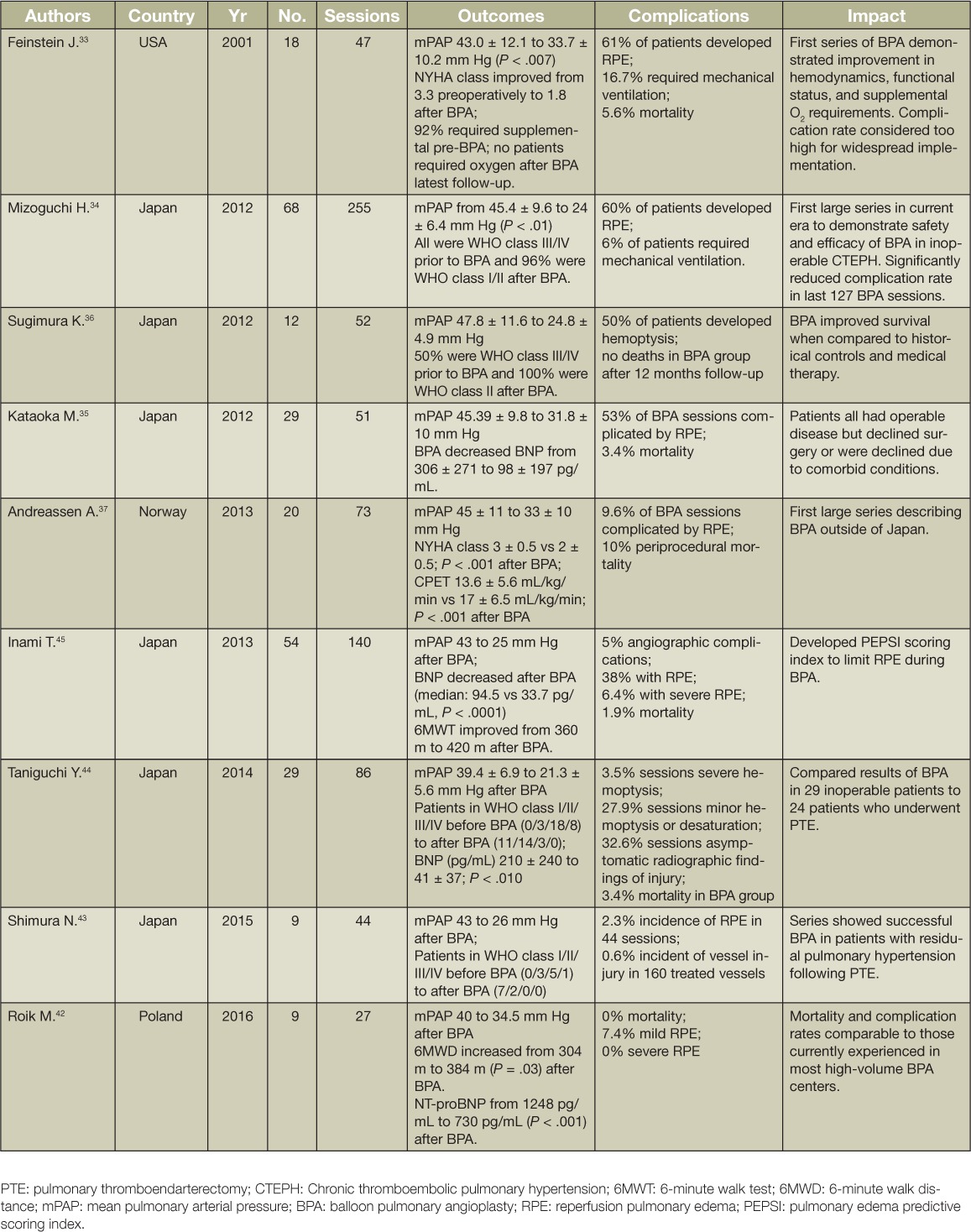
Perhaps more importantly, safety of BPA has improved dramatically from the first series reported in 2001. The rates of severe BPA-associated lung injury that required major interventions such as noninvasive ventilation and mechanical ventilation declined from 16.7% in the initial series by Feinstein et al. in 2001 to 6% in the large series by Matsubara et al. in 2012. In subsequent reports, the rate of severe BPA-associated lung injury has declined even further, in the range of 0% to 3.5%, while the incidence of mild BPA-associated lung injury has declined to less than 40%.42–45
The improved safety of BPA owes to a steep technical learning curve incorporating improved operator skill, the use of intravascular imaging modalities such as intravascular ultrasound and optical coherence tomography,46,47 the use of intravascular pressure gradient measurements,42,48 the characterization of specific lesion types,49 and the adaptation of interventional technique to the severity of PH. However, BPA requires significant quantities of iodinated contrast and radiation exposure under fluoroscopy. While there are currently no reports of complications related to either of these exposures, the length of each BPA session is often limited to the maximum safe levels of radiation and contrast.
Future Directions
With the rapid evolution of BPA, clinical practice is outpacing what is represented in the published literature. To date, no single technical approach has become dominant; instead, multiple high-volume centers are refining the technique in parallel.
The use of PH-targeted medical therapy is common in patients who undergo BPA. In some reports, this approach has been initiated to “stabilize” patients or lower mPAP prior to BPA. The benefit of this approach on outcomes is unknown, but an obvious detrimental effect has not been observed. How PH-targeted medical therapies compare to BPA for the treatment of inoperable CTEPH is also unknown and currently under investigation. In the small series by Sugimura, patients with inoperable CTEPH were treated aggressively with medical therapy prior to BPA, which resulted in improved cardiac output/index, PVR, and functional status but little change in mPAP.36 The patients then underwent BPA and had significant further reductions in mPAP and PVR and further improvement in WHO functional class and 6MWT. This suggests that BPA combined with medical therapy may have an advantage over medical therapy alone, although larger prospective studies are necessary to substantiate this finding.
The use of BPA in conjunction with PTE surgery is another approach currently being explored. A recent publication describes a combined approach in three patients with high PVR, each with operable CTEPH in one lung and inoperable disease in the other lung. The patients underwent PTE on the operable lung and BPA on the inoperable lung during the rewarming phase of surgery.50 Ultimately, the number of patients with clot distribution likely to benefit from this approach is small, and as already demonstrated in a previously published series,42 BPA may emerge as a reliable tool for the treatment of residual PH after PTE. As the collective BPA experience grows, there will assuredly be an interest in comparing BPA directly to PTE in select patient populations.
Conclusion
As has been the case for PTE surgery, proper patient selection for management with PH-targeted medical therapies or BPA is paramount in achieving favorable outcomes. Although these treatment options are currently indicated only for patients who are deemed inoperable, the diagnostician should appreciate that operability assessment is subjective and depends largely on the level of surgical experience at any given center.4 Though progress achieved in this area has been remarkable over the past several years, refinements of the treatment algorithm for CTEPH will likely evolve as operable CTEPH is better defined, and the optimal application of medical therapies and BPA will achieve greater clarity once appropriate clinical trials become available.
Key Points
Even at experienced surgical centers, nearly one-third of patients with CTEPH will be deemed inoperable for reasons including distal disease, comorbidities, or out-of-proportion pulmonary hypertension (PH). It is in these patients with inoperable CTEPH that PH-targeted medical therapy and balloon pulmonary angioplasty have potential therapeutic value.
Though many PAH-targeted medications (including sildenafil, bosentan, iloprost, and epoprostenol) have demonstrated hemodynamic improvement in inoperable CTEPH, riociguat is the only FDA-approved therapy for both inoperable CTEPH and residual PH after surgery based on the demonstrated improvements in both 6MWD and pulmonary hemodynamics in a placebo-controlled trial.
Balloon pulmonary angioplasty is an interventional procedure using telescoping catheters placed in the pulmonary arteries, through which wires and balloons are used to mechanically disrupt chronic clot material and relieve pulmonary vascular obstruction. Contemporary case series from multiple centers worldwide have demonstrated pulmonary hemodynamic improvement with this approach.
Conflict of Interest Disclosure
Dr. Fernandes conducts research funded by Actelion Pharmaceuticals and is on the speaker's bureau for Bayer Pharmaceuticals US, Inc.; Dr. Auger is a formal advisor to and conducts research on behalf of Bayer Corporation. Dr. Poch conducts research funded by Actelion Pharmaceuticals and is on the speaker's bureau for Bayer Pharmaceuticals US, Inc.
References
- 1. Madani MM, Auger WR, Pretorius V, . et al. Pulmonary endarterectomy: Recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012. July; 94( 1): 97– 103. [DOI] [PubMed] [Google Scholar]
- 2. Mayer E, Jenkins D, Lindner J, . et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. J Thorac Cardiovasc Surg. 2011. March; 141( 3): 702– 10. [DOI] [PubMed] [Google Scholar]
- 3. Delcroix M, Lang I, Pepke-Zaba J, . et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation. 2016. March 1; 133( 9): 859– 71. [DOI] [PubMed] [Google Scholar]
- 4. Pepke-Zaba J, Delcroix M, Lang I, . et al. Chronic thromboembolic pulmonary hypertension (CTEPH): Results from an international prospective registry. Circulation. 2011. November 1; 124( 18): 1973– 81. [DOI] [PubMed] [Google Scholar]
- 5. Kim NH, Delcroix M, Jenkins DP, . et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013. December 24; 62( 25 Suppl): D92– 9. [DOI] [PubMed] [Google Scholar]
- 6. Galiè N, Humbert M, Vachiery J-L, . et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2016. January 1; 37( 1): 67– 119. [DOI] [PubMed] [Google Scholar]
- 7. Cabrol S, Souza R, Jais X, . et al. Intravenous epoprostenol in inoperable chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2007. April; 26( 4): 357– 62. [DOI] [PubMed] [Google Scholar]
- 8. Skoro-Sajer N, Bonderman D, Wiesbauer F, . et al. Treprostinil for severe inoperable chronic thromboembolic pulmonary hypertension. J Thromb Haemost. 2007. March; 5( 3): 483– 9. [DOI] [PubMed] [Google Scholar]
- 9. Olschewski H, Simonneau G, Galiè N, . et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002. August 1; 347( 5): 322– 9. [DOI] [PubMed] [Google Scholar]
- 10. Krug S, Hammerschmidt S, Pankau H, Wirtz H, Seyfarth HJ.. Acute improved hemodynamics following inhaled iloprost in chronic thromboembolic pulmonary hypertension. Respiration. 2008. August; 76( 2): 154– 9. [DOI] [PubMed] [Google Scholar]
- 11. Suntharalingam J, Treacy CM, Doughty NJ, . et al. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008. August; 134( 2): 229– 36. [DOI] [PubMed] [Google Scholar]
- 12. Reichenberger F, Voswinckel R, Enke B, . et al. Long-term treatment with sildenafil in chronic thromboembolic pulmonary hypertension. Eur Resp J. 2007. November; 30: 922– 7. [DOI] [PubMed] [Google Scholar]
- 13. Jais X, D'Armini AM, Jansa P, . et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNoperable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008. December 16; 52( 25): 2127– 34. [DOI] [PubMed] [Google Scholar]
- 14. Becattini C, Manina G, Busti C, Gennarini S, Agnelli G.. Bosentan for chronic thromboembolic pulmonary hypertension: Findings from a systematic review and meta-analysis. Thromb Res. 2010. July; 126( 1): e51– 6. [DOI] [PubMed] [Google Scholar]
- 15. ClinicalTrials.gov [Internet]. Bethesda, MD: U.S. National Institutes of Health; Clinical Study to Assess the Efficacy, Safety and Tolerability of Macitentan in Subjects With Inoperable Chronic Thromboembolic Pulmonary Hypertension (MERIT-1); 2013 Dec 20 [cited 2016 Sep 29]. Available from: https://clinicaltrials.gov/ct2/show/nct02021292. [Google Scholar]
- 16. Ghofrani HA, D'Armini AM, Grimminger F, . et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013. July 25; 369( 4): 319– 29. [DOI] [PubMed] [Google Scholar]
- 17. Simonneau G, D'Armini AM, Ghofrani H-A, . et al. Predictors of long-term outcomes in patients treated with riociguat for chronic thromboembolic pulmonary hypertension: Data from the chest-2 open-label, randomised, long-term extension trial. Lancet Resp Med. 2016. May; 4( 5): 372– 80. [DOI] [PubMed] [Google Scholar]
- 18. Scholzel BE, Post MC, Thijs Plokker HW, Snijder RJ.. Clinical worsening during long-term follow-up in inoperable chronic thromboembolic pulmonary hypertension. Lung. 2012. April; 190: 161– 7. [DOI] [PubMed] [Google Scholar]
- 19. Kramm T, Eberle B, Guth S, Mayer E.. Inhaled iloprost to control residual pulmonary hypertension following pulmonary endarterectomy. Eur J Cardiothorac Surg. 2005. December; 28( 6): 882– 8. [DOI] [PubMed] [Google Scholar]
- 20. Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J.. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. 1991. November 9; 338( 8776): 1173– 4. [DOI] [PubMed] [Google Scholar]
- 21. Gårdebäck M, Larsen FF, Rådegran K.. Nitric oxide improves hypoxaemia following reperfusion oedema after pulmonary thromboendarterectomy. Br J Anaesth. 1995. December; 75( 6): 798– 800. [DOI] [PubMed] [Google Scholar]
- 22. Bonderman D, Skoro-Sajer N, Jakowitsch J, . et al. Predictors of outcome in chronic thromboembolic pulmonary hypertension. Circulation. 2007. April 24; 115( 16): 2153– 8. [DOI] [PubMed] [Google Scholar]
- 23. Freed DH, Thomson BM, Berman M, . et al. Survival after pulmonary thromboendarterectomy: Effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011. February; 141( 2): 383– 7. [DOI] [PubMed] [Google Scholar]
- 24. Cannon JE, Su L, Kiely DG, . et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: Results from the United Kingdom national cohort. Circulation. 2016. May 3; 133( 18): 1761– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Darteville P, Fadel E, Mussot S, . et al. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004. April; 23( 4): 637– 48. [DOI] [PubMed] [Google Scholar]
- 26. Fernandes TM, Poch DS, Papamatheakis DG, . et al. Pre-operative use of pulmonary arterial hypertension-targeted medication and the effects on post-pulmonary endarterectomy morbidity and mortality. J Heart Lung Transplant. 2015. April; 34( 4, Supplement): S159. [Google Scholar]
- 27. Nagaya N, Sasaki N, Ando M, . et al. Prostacyclin therapy before pulmonary thromboendarterectomy in patients with chronic thromboembolic pulmonary hypertension. Chest. 2003. February; 123( 2): 338– 43. [DOI] [PubMed] [Google Scholar]
- 28. Reesink HJ, Surie S, Kloek JJ, . et al. Bosentan as a bridge to pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2010. January; 139( 1): 85– 91. [DOI] [PubMed] [Google Scholar]
- 29. Surie S, Reesink HJ, Marcus T, . et al. Bosentan treatment is associated with improvement of right heart ventricular function and remodeling in chronic thromboembolic pulmonary hypertension. Clin Cardiol. 2013. November; 36( 11): 698– 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jensen KW, Kerr KM, Fedullo PF, . et al. Pulmonary hypertensive medical therapy in chronic thromboembolic pulmonary hypertension before pulmonary thromboendarterectomy. Circulation. 2009. September 29; 120( 13): 1248– 54. [DOI] [PubMed] [Google Scholar]
- 31. Delcroix M, Lang I, Pepke-Zaba J, . et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension. Results from an International Prospective Registry. Circulation. 2016. March 1; 133( 9): 859– 71. [DOI] [PubMed] [Google Scholar]
- 32. Voorburg JA, Cats VM, Buis B, Bruschke AV.. Balloon angioplasty in the treatment of pulmonary hypertension caused by pulmonary embolism. Chest. 1988. December; 94( 6): 1249– 53. [DOI] [PubMed] [Google Scholar]
- 33. Feinstein JA, Goldhaber SZ, Lock JE, . et al. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001. January 2; 103( 1): 10– 3. [DOI] [PubMed] [Google Scholar]
- 34. Mizoguchi H, Ogawa A, Munemasa M, . et. al Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012. December; 5( 6): 748– 55. [DOI] [PubMed] [Google Scholar]
- 35. Kataoka M, Inami T, Hayashida K, . et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012. December; 5( 6): 756– 62. [DOI] [PubMed] [Google Scholar]
- 36. Sugimura K, Fukumoto Y, Satoh K, . et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012; 76( 2): 485– 8. [DOI] [PubMed] [Google Scholar]
- 37. Andreassen AK, Ragnarsson A, Gude E, . et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart. 2013. October; 99( 19): 1415– 20. [DOI] [PubMed] [Google Scholar]
- 38. Hosokawa K, Abe K, Oi K, Mukai Y, Hirooka Y, Sunagawa K.. Negative acute hemodynamic response to balloon pulmonary angioplasty does not predicate the long-term outcome in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2015. June 1; 188: 81– 3. [DOI] [PubMed] [Google Scholar]
- 39. Ogawa A, Matsubara H.. Balloon pulmonary angioplasty: A treatment option for inoperable patients with chronic thromboembolic pulmonary hypertension. Front Cardiovasc Med. 2015. February 17; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kitani M, Ogawa A, Sarashina T, Yamadori I, Matsubara H.. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2014. December; 7( 6): 857– 9. [DOI] [PubMed] [Google Scholar]
- 41. Kimura M, Kohno T, Kawakami T, . et al. Balloon pulmonary angioplasty attenuates ongoing myocardial damage in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2016. March 15; 207: 387– 9. [DOI] [PubMed] [Google Scholar]
- 42. Roik M, Wretowski D, Łabyk A, . et al. Refined balloon pulmonary angioplasty driven by combined assessment of intra-arterial anatomy and physiology--Multimodal approach to treated lesions in patients with non-operable distal chronic thromboembolic pulmonary hypertension--Technique, safety and efficacy of 50 consecutive angioplasties. Int J Cardiol. 2016. January 15; 203: 228– 35. [DOI] [PubMed] [Google Scholar]
- 43. Shimura N, Kataoka M, Inami T, . et al. Additional percutaneous transluminal pulmonary angioplasty for residual or recurrent pulmonary hypertension after pulmonary endarterectomy. Int J Cardiol. 2015. March 15; 183: 138– 42. [DOI] [PubMed] [Google Scholar]
- 44. Taniguchi Y, Miyagawa K, Nakayama K, . et al. Balloon pulmonary angioplasty: An additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014. August; 10( 4): 518– 25. [DOI] [PubMed] [Google Scholar]
- 45. Inami T, Kataoka M, Shimura N, . et al. Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv. 2013. July; 6( 7): 725– 36. [DOI] [PubMed] [Google Scholar]
- 46. Ikeda N, Kubota S, Okazaki T, . et al. Comparison of intravascular optical frequency domain imaging versus intravascular ultrasound during balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Catheter Cardiovasc Interv. 2016. June; 87( 7): E268– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jorge E, Baptista R, Calisto J, . et al. Optical coherence tomography of the pulmonary arteries: A systematic review. J Cardiol. 2016. January; 67( 1): 6– 14. [DOI] [PubMed] [Google Scholar]
- 48. Inami T, Kataoka M, Shimura N, . et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: A breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv. 2014. November; 7( 11): 1297– 1306. [DOI] [PubMed] [Google Scholar]
- 49. Inohara T, Kawakami T, Kataoka M, . et al. Lesion morphological classification by OCT to predict therapeutic efficacy after balloon pulmonary angioplasty in CTEPH. Int J Cardiol. 2015. October 15; 197: 23– 5. [DOI] [PubMed] [Google Scholar]
- 50. Wiedenroth CB, Liebetrau C, Breithecker A, . et al. Combined pulmonary endarterectomy and balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. J Heart Lung Transplant. 2016. May; 35( 5): 591– 6. [DOI] [PubMed] [Google Scholar]


