Abstract
Pulmonary thromboendarterectomy (PTE), also referred to as pulmonary endarterectomy, is the definitive treatment for chronic thromboembolic pulmonary hypertension (CTEPH). The true incidence of CTEPH is unknown and difficult to ascertain; however, most experts agree that approximately 4% to 5% of all patients who have an acute episode of pulmonary embolism (PE) will continue to develop CTEPH. Based on an incidence rate of about 0.1% for acute PE, this translates into a CTEPH incidence of approximately 10,000 to 15,000 annually in the United States alone. Furthermore, there are patients with CTEPH who have no history of prior PE or deep vein thrombosis, adding to the estimated number. Despite these facts, the disease remains significantly underdiagnosed, and currently there are only about 300 PTEs performed nationwide, the majority of which are done at the University of California, San Diego (UCSD) Health System. The technical aspects of the procedure can be somewhat challenging and require meticulous and complete dissection of the entire pulmonary vascular tree, with the patient under profound hypothermic circulatory arrest. However, the determination of true CTEPH patients and those who would benefit from surgery can also be challenging and relies heavily on the experience of the CTEPH team. In this article, we will highlight some key points about the disease and describe the surgical techniques of PTE.
Keywords: chronic thromboembolic pulmonary embolism, CTEPH, pulmonary thromboendarterectomy, pulmonary hypertension
Introduction
Pulmonary thromboendarterectomy (PTE) remains the preferred and potentially curative option for patients with chronic thromboembolic pulmonary hypertension (CTEPH). The disease is characterized by intraluminal thrombus organization, fibrotic scar tissue-like stenosis, and the ensuing vascular remodeling of unaffected pulmonary vessels. Although the exact prevalence and annual incidence of CTEPH are unknown, studies suggest that this condition may occur in approximately 4% to 5% of patients after an acute pulmonary embolism, making CTEPH one of the most common causes of precapillary pulmonary hypertension (PH). Despite the fact that surgery can be potentially curative, there are only about 0.9 to 1 endarterectomies per million of the population performed in the United States annually. The numbers are slightly better in Europe, at about 1.7 per million of the population.1 In addition, even when the diagnosis is made, there is often a delay or lack of referral to expert centers for consideration of PTE surgery. Education of physicians to identify CTEPH, and training of PTE surgeons to master the techniques of this challenging operation, should remain a priority in the treatment of this disease.
Considerable progress has been made over the past decade in understanding the etiology, prevalence, natural history, and therapeutic approach to CTEPH. Acute pulmonary thromboembolism and its chronic sequelae are significant causes of morbidity and mortality in the United States and the world. However, the chronic disease process, even when established and symptomatic, is notoriously underdiagnosed because of the nonspecific nature of the two major symptoms—effort dyspnea and fatigue—and the fact that physical findings may be elusive until right heart failure occurs. Another factor is that patients affected by CTEPH may present with a variety of debilitating cardiopulmonary symptoms.
Once chronic PH develops, the prognosis is poor and even worse for those who do not have intracardiac shunts. Thus, patients with primary PH and those with PH due to pulmonary emboli fall into a higher risk category than those with Eisenmenger's syndrome and encounter a higher mortality rate. In fact, once the mean pulmonary pressure in patients with thromboembolic disease reaches 50 mm Hg or higher, the 3-year mortality approaches 90%.2 Therefore, despite an improved understanding of pathogenesis, diagnosis, and management, pulmonary emboli and the long-term sequelae of CTEPH remain frequent and often fatal disorders.
Pulmonary endarterectomy is a technically demanding operation currently performed in only a few select centers around the world. Proper patient selection, meticulous surgical technique, and careful postoperative management have contributed to the effectiveness of this procedure.3 A true endarterectomy (not an embolectomy) of all affected segments of the lung is essential to clear all affected areas of the pulmonary vasculature. When performed at an experienced center, the procedure ameliorates PH by improving lung ventilation–perfusion mismatch, significantly improving right ventricular (RV) dysfunction and tricuspid regurgitation, limiting retrograde expansion of thromboembolic material, and preventing arteriopathic changes in the remaining patent small pulmonary vessels.
Factors Involved in Patient Selection
The assessment of operability in patients with CTEPH is crucial since surgery can be potentially curative. Operability evaluation should be performed by an interdisciplinary expert team including pulmonologists, cardiologists, radiologists, and surgeons.4 Although the majority of patients with significant CTEPH will benefit from PTE surgery, the selection of appropriate patients remains quite subjective. It is typically based on multiple factors, including the severity of the patient's symptoms and the severity of PH and right heart dysfunction. Perhaps the most important factor in determining a suitable candidate relies on the correlation of severity of the PH versus the degree and extent of the obstruction. Like any other surgical procedure, individual patient factors including comorbidities, technical challenges, and level of expectations for long-term benefits all figure crucially in the decision-making process.
As a general rule, symptomatic patients should be offered surgery regardless of the severity of PH or RV dysfunction as long as there is a corresponding degree of obstructive disease. In such cases, neither the value of pulmonary vascular resistance (PVR) nor the degree of RV dysfunction will exclude a patient from surgical consideration. However, the endarterectomy and postoperative care are made more challenging by the presence of severe PH and right heart failure preoperatively and more distal presentation of the disease. Generally, if the disease is in the main, lobar, or even proximal segmental pulmonary artery branches, surgical clearing by endarterectomy is feasible. Distal segmental and subsegmental disease is much more difficult to remove and, at some centers, may render the patient inoperable. Since technical operability is ultimately determined by the surgeon's experience, a second surgical opinion is recommended in such cases.4,5 Ultimately, surgical candidate selection depends on the correlation of accessible surgical disease and the severity of PH and RV dysfunction. It should be emphasized that limited distal obstruction of pulmonary vasculature in combination with high PVR is a risk factor, but not an absolute contraindication, to surgery.
Other factors that will render the endarterectomy somewhat challenging due to potential increased risk include “distal” disease in patients with ventriculoatrial shunts, venous access catheters, or pacemaker leads and patients who have undergone splenectomy. However, none of these are absolute contraindications for surgery. Furthermore, age per se is not a contraindication as studies have indicated sustained benefits in patients older than 70 and 80 years of age.6,7
Patients with significant comorbid conditions such as end-stage lung disease or with a life-limiting malignancy are not only at considerable perioperative risk but are also unlikely to realize the functional status benefit that would be achieved with PTE. Although surgery may be technically feasible, such an aggressive intervention might be considered ill-advised. Fundamentally, the surgeon should anticipate that PTE will have a meaningful outcome for the patient undergoing this complex and technically challenging procedure. Otherwise, patients who are deemed truly inoperable should be evaluated for other treatment options, including PH-targeted medical therapy or balloon pulmonary angioplasty.
Surgical Techniques
Pulmonary thromboendarterectomy follows four basic principles: (1) The endarterectomy must be bilateral and therefore performed through a median sternotomy approach; (2) perfect visualization is essential and accomplished by using cardiopulmonary bypass and periods of circulatory arrest that are usually limited to 20 minutes at a time and supported by cooling to about 20°C; (3) identification of the correct dissection plane is crucial; and (4) a complete endarterectomy is essential. The techniques of this procedure are well established and have been described in detail elsewhere.8–10
After a median sternotomy is made, the pericardium is incised longitudinally and attached to the wound edges. Full cardiopulmonary bypass is instituted with high ascending aortic cannulation and two caval cannulae. The heart is emptied on bypass, and a temporary pulmonary artery vent is placed in the midline of the main pulmonary artery about 1 cm distal to the pulmonary valve. The patient is then cooled to 20°C with the pump oxygenator, and surface cooling with both the head jacket and the cooling blanket is begun. When ventricular fibrillation occurs, an additional vent is placed in the left atrium through the right superior pulmonary vein. Initially, it is most convenient for the primary surgeon to stand on the patient's left side and perform the endarterectomy on the right side (Figure 1).
Figure 1.
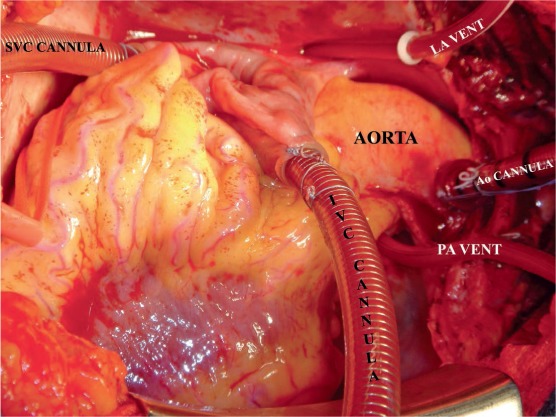
Intraoperative set-up for pulmonary endarterectomy. Please note that initially the surgeon stands on the left side of the patient. The left atrial vent is placed via the right superior pulmonary artery and directed into the left ventricle while the patient is cooling and the heart fibrillating. SVC: superior vena cava; IVC: inferior vena cava; PA: pulmonary artery; LA: left atrial.
The approach to the right pulmonary artery is made medial (not lateral) to the superior vena cava (Figure 2), and the superior vena cava is fully mobilized. Once the core temperature has reached 20°C, an aortic cross-clamp is applied and myocardial protection is provided through a single dose of antegrade cold blood cardioplegia. Additional myocardial protection is provided by using a cooling jacket surrounding the heart throughout the remainder of the procedure. Both tourniquets are now secured around the superior and inferior venae cavae. A modified cerebellar retractor is then used to expose the pulmonary artery between the aorta and the superior vena cava. A longitudinal incision is made in the center of the right pulmonary artery from beneath the ascending aorta out under the superior vena cava and entering the lower lobe branch of the pulmonary artery just after the takeoff of the middle lobe artery. Any loose thrombus, if present, is now removed. This is necessary to obtain good visualization. It is most important to recognize that (1) an embolectomy without subsequent endarterectomy is quite ineffective, and (2) in most patients with CTEPH, direct examination of the pulmonary vascular bed at operation generally shows no obvious embolic material. If the bronchial circulation is not excessive, the endarterectomy plane can be found during this early dissection. However, while a small amount of dissection can be performed before the initiation of circulatory arrest, it is unwise to proceed unless perfect visibility is obtained because the development of a correct plane is essential. Once circulatory arrest is initiated, the patient undergoes exsanguination. The correct plane appears pearly white, is smooth and silky in appearance, and lies between the intima and media (Figure 3). It is rare for this to exceed one 20-minute period for each side.
Figure 2.
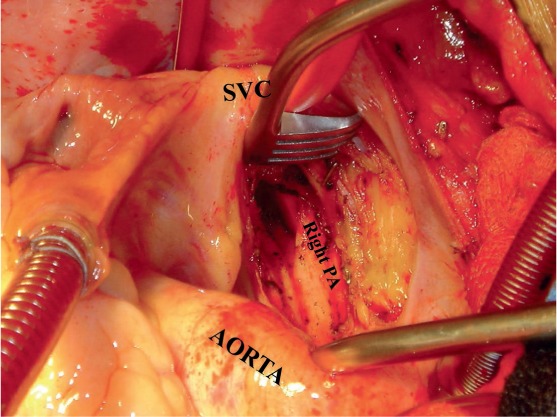
Surgical approach to the right pulmonary artery (PA) and endarterectomy. Please note that the right PA is approached between the aorta and superior vena cava (SVC) and not lateral to the SVC.
Figure 3.
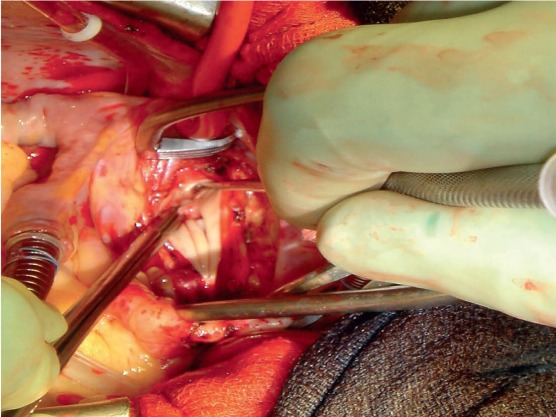
The plane of dissection is raised posteriorly initially and then a complete endarterectomy is performed.
The endarterectomy is then performed with an eversion technique. Because the vessel is everted and subsegmental branches are being worked on, a perforation here will become completely inaccessible and invisible later. It is important that each subsegmental branch is followed and freed individually until it ends in a “tail,” beyond which there is no further obstruction. Residual material should never be cut free; the entire specimen should “tail off” and come free spontaneously. Once the right-sided endarterectomy is completed, circulation is restarted and the arteriotomy is repaired with a continuous 6-0 polypropylene suture. The hemostatic nature of this closure is aided by the initial dissection; whenever possible, the full thickness of the pulmonary artery should be preserved immediately adjacent to the incision.
After closure of the right arteriotomy, the surgeon moves to the patient's right side. A left pulmonary arteriotomy is made slightly lateral to the pulmonary vent site and extended to the pericardial reflection, avoiding entry into the left pleural space. Care must be taken to avoid injury to the left phrenic nerve. Additional lateral dissection does not enhance intraluminal visibility, may endanger the left phrenic nerve, and makes subsequent repair of the left pulmonary artery more difficult. The heart is wrapped within a cooling jacket and retracted using a mesh-like basket retractor shown in Figure 4.
Figure 4.
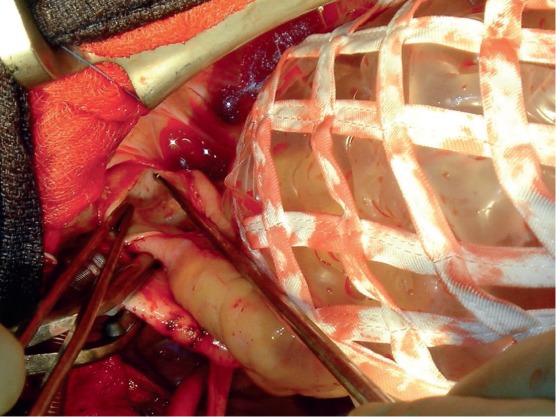
Surgical approach to the left pulmonary artery. The heart is retracted medially using a net-like retractor and the left pulmonary artery is exposed.
The left-sided dissection is virtually analogous in all respects to that accomplished on the right. The main obstructing material is a thickened scar-like tissue that obstructs each branch distally, and removal of only the gross thrombus visible on initial inspection will be ineffective. The duration of circulatory arrest intervals is subject to the same restriction as the right. The specimen is followed in each segmental and subsegmental branch to ensure complete removal of the endarterectomy material. Whenever possible, the left lower lobe should be dissected circumferentially and the specimen followed distally. The left main bronchus crosses anteriorly, and it is extremely important to have a firm grasp of the specimen at this location. If the specimen breaks, it is particularly difficult to regain exposure of these distal branches.
After completion of the endarterectomy, cardiopulmonary bypass is reinstituted and warming is commenced. During warming, a 10°C temperature gradient is maintained between the perfusate and body temperature. The rewarming period generally takes approximately 90 to 120 minutes but varies according to the patient's body mass. If other cardiac procedures are required, such as coronary artery or mitral or aortic valve surgery, these are conveniently performed during the systemic rewarming period. The most common concomitant procedure is closure of the foramen ovale followed by coronary bypass surgery.
Although tricuspid valve regurgitation is variable in these patients and often moderate to severe, tricuspid valve repair is not necessary unless there is an anatomic abnormality with the valve leaflets, chords, or overall structure. Tricuspid regurgitation secondary to annular dilation is left alone because RV remodeling occurs within a few days, with the return of tricuspid competence.11 Once all associated procedures are completed, myocardial cooling is discontinued. The left atrial vent is removed and the vent site is repaired. All air is removed from the heart, and the aortic cross-clamp is removed.
When the patient has fully rewarmed, cardiopulmonary bypass is discontinued. With a successful endarterectomy, cardiac output is generally high with low systemic vascular resistance. Temporary atrial and ventricular epicardial pacing wires are placed. Wound closure is routine and similar to other cardiac procedures.
Surgical Classification
According to a new surgical classification proposed by our group at the University of California, San Diego (UCSD), there are four levels of pulmonary occlusive disease related to organized thrombus. Level 0 (zero) denotes no surgical evidence for chronic thromboembolic disease. Level I disease (Figure 5 a) indicates that the obstructive material and the plane of dissection involve one of the main pulmonary arteries. In case of complete obstruction of one of the major arteries and complete nonperfusion of the entire lung, the letter “C” (i.e., Level IC) is added; this distinction is important given the technical differences in endarterectomy required in this setting. In Level II disease (Figure 5 b), the fibrous tissue starts at the level of the lobar branches or past the takeoff of the upper lobe artery. Level III disease (Figure 5 c) presents a more challenging surgical situation in which the disease is distal and starts at the segmental branches, where occlusive disease may not initially be evident. The endarterectomy plane must be carefully and painstakingly raised in each segmental branch. Level IV disease (Figure 5 d) symbolizes chronic thromboembolic disease starting at the subsegmental level only. This represents the most challenging of patients, as endarterectomy can be quite difficult at this level, and extensive surgical experience is required to achieve optimal results.
Figure 5.
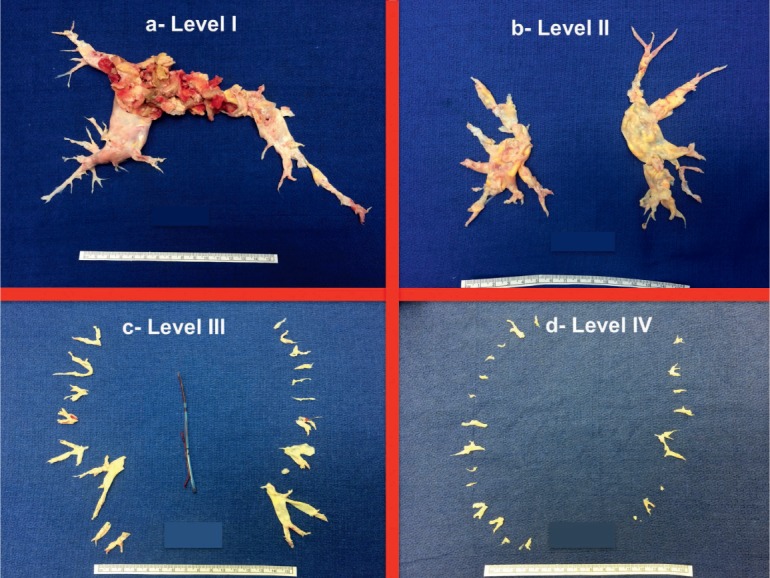
University of California, San Diego classification of pulmonary endarterectomy disease levels. This figure illustrates typical surgical specimens classified based on the most proximal level of obstruction, levels I to IV.
Outcomes
When performed by an experienced surgeon, PTE can be potentially curative. The benefits of PTE surgery are well established with multiple case series from a number of institutions with high-volume experience. Reports have confirmed improved hemodynamics, functional status, and exercise capacity following surgery.1 The in-hospital mortality at our center has decreased over time, from 4.4% for the last tertile of the first 1500 patients9 to 2.2% for the most recent 500-patient cohort reported.12 Currently at UCSD, the mortality is between 1% and 2% despite increased referrals of higher-risk and more distal disease patients.
In a majority of patients after successful PTE surgery, postoperative hemodynamic values become normal or near normal. This usually follows with significantly improved RV function and resolution of tricuspid regurgitation. From the UCSD and International CTEPH databases, improvements from PVR 700 to 800 dyn·s·cm−5 to 250 dyn·s·cm−5 have been experienced,1,12 a drop of around 65% following surgery. Other parameters that have been shown to improve markedly following PTE include mean pulmonary arterial pressure (mPAP) (down from 46 to 26 mm Hg)12 and median 6-minute walk distance (up from 362 to 459 m).1 Most patients also experience a shift towards improved NYHA functional class.
Although most patients enjoy a significantly improved hemodynamic state and improved functional class, residual PH (> 500 dyn.s.cm−5) after surgery remains the most important cause of early postoperative morbidity and mortality. At UCSD, mortality rates were 10.3% and 0.9% in those with and without residual PH, respectively.12 Similarly, in the International CTEPH Registry, residual PH affected 16.7% of patients and was associated with a higher early mortality.1 Data from the UK National Cohort showed that higher mPAP, right atrial pressure, and PVR as well as a lower cardiac index were negatively correlated with long-term survival in multivariate analyses.13
Conclusion
Surgical removal of thromboembolic material by means of a complete endarterectomy provides patients with CTEPH an opportunity for relief from this debilitating and ultimately fatal disease. Although PTE is technically demanding for the surgeon, requiring careful dissection of the pulmonary artery planes and use of circulatory arrest, excellent short- and long-term results can be achieved. As PTE has undergone successive improvements in operative technique, it can offer patients an acceptable mortality rate and anticipation of significant clinical improvement. With this growing experience, PTE can be an option for all patients with CTEPH regardless of the degree of PH or RV failure; it may even be offered to some very high-risk candidates when there is evidence of thromboembolic disease. Technical advances of the procedure over the last 4 decades, and particularly in the past decade, have significantly improved outcomes. Newly designed instruments allow better visualization and more complete endarterectomy in the distal segmental and subsegmental branches (Figure 6).
Figure 6.
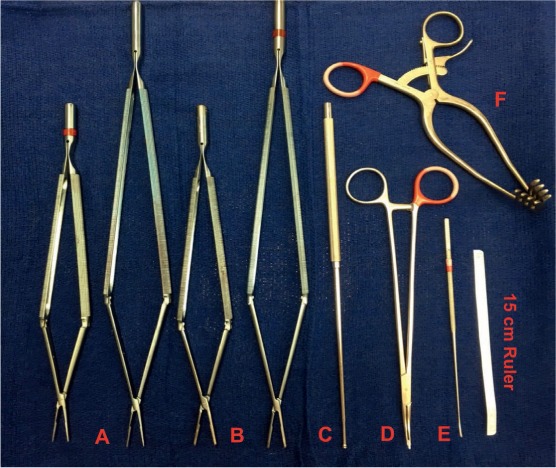
Instruments used during pulmonary endarterectomy (PEA). (A) 1-mm tip and (B) 2.5-mm tip double action PTE forceps; (C) PEA suction/dissector; (D) small pledget/mini-nut for fine dissection; (E) microendarterectomy spatula for identification of the correct plane; (F) modified cerebellar retractor for exposure of the right pulmonary artery. PTE: pulmonary thromboendarterectomy.
The primary problem remains that this is an under-recognized condition, and unfortunately there are still a large number of patients with CTEPH who carry other diagnoses and are mistreated as such. Increased understanding of CTEPH, its prevalence, and the availability of a surgical treatment should benefit more patients. Educating physicians to identify CTEPH, and training of PTE surgeons to master the techniques of the operation, should remain a priority in the treatment of this disease.
Key Points
Chronic thromboembolic pulmonary hypertension (CTEPH) is significantly underdiagnosed, but it is one of the most common causes of precapillary pulmonary hypertension, occurring in approximately 4% to 5% of patients after an acute pulmonary embolism.
Pulmonary thromboendarterectomy can be curative in many patients with chronic thromboembolic pulmonary hypertension regardless of the degree of pulmonary hypertension or right heart failure.
Education of physicians to identify CTEPH and training surgeons to master the techniques of pulmonary thromboendarterectomy should be a priority for treating this disease.
Conflict of Interest Disclosure
The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Mayer E, Jenkins DP, Lindner J, . et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an International Prospective Registry. J Thorac Cardiovasc Surg. 2011. March; 141( 3): 702– 10. [DOI] [PubMed] [Google Scholar]
- 2. Riedel M, Stanek V, Widimsky J, Prerovsky I.. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982. February; 81( 2): 151– 8. [DOI] [PubMed] [Google Scholar]
- 3. Taboada D, Pepke-Zaba J, Jenkins DP, . et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Resp J. 2014. December; 44( 6): 1635– 45. [DOI] [PubMed] [Google Scholar]
- 4. Kim NH, Delcroix M, Jenkins DP, . et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013. December 24; 62( 25 Suppl): D92– 9. [DOI] [PubMed] [Google Scholar]
- 5. Lang IM, Madani M.. Update on chronic thromboembolic pulmonary hypertension. Circulation. 2014. August 5; 130( 6): 508– 18. [DOI] [PubMed] [Google Scholar]
- 6. Berman M, Hardman G, Sharples L, . et al. Pulmonary endarterectomy: outcomes in patients > 70. Eur J Cardiothorac Surg. 2012. June; 41( 6): e154– 60. [DOI] [PubMed] [Google Scholar]
- 7. Pretorius V, Poch DS, Auger WR, . et al. Should we offer pulmonary endarterectomy to octogenarians? 5th World Symposium on Pulmonary Hypertension 2013: CTEPH; 2013 Feb 27; Nice, France p 54– 58. [Google Scholar]
- 8. Jamieson SW, Kapelanski DP.. Pulmonary endarterectomy. Curr Probl Surg. 2000. March; 37( 3): 165– 252. [DOI] [PubMed] [Google Scholar]
- 9. Jamieson SW, Kapelanski DP, Sakakibara N, . et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003. November; 76( 5): 1457– 62. [DOI] [PubMed] [Google Scholar]
- 10. Madani MM, Jamieson SW.. Pulmonary endarterectomy for chronic thromboembolic disease, operative techniques. Thorac Cardiovasc Surg. 2007; 11( 4): 264– 74. [Google Scholar]
- 11. Raisinghani A, Ben-Yehuda O.. Echocardiography in chronic thromboembolic pulmonary hypertension. Semin Thorac Cardiovasc Surg. 2006. Fall; 18( 3): 230– 5. [DOI] [PubMed] [Google Scholar]
- 12. Madani MM, Auger WR, Pretorius V, . et al. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012. July; 94( 1): 97– 103. [DOI] [PubMed] [Google Scholar]
- 13. Cannon JE, Su L, Kiely DG, . et al. Dynamic risk stratification of patient long-term outcome after pulmonary endarterectomy: results from the United Kingdom National Cohort. Circulation. 2016. May 3; 133( 18): 1761– 71. [DOI] [PMC free article] [PubMed] [Google Scholar]


