Abstract
Olmesartan-induced enteropathy mimics celiac disease clinically and pathologically. As in celiac disease, the pathologic findings are villous atrophy and increased intraepithelial lymphocytes. Clinical presentation of olmesartan-induced enteropathy includes diarrhea, weight loss, and nausea. In contrast to celiac disease, tissue transglutaminase is not elevated and there is no response to a gluten-free diet. Including this entity in the differential diagnosis of sprue-like enteropathy is critical for its early diagnosis since replacing olmesartan with an alternative antihypertensive drug can simplify the diagnostic workup and provide both clinical and histologic improvement.
Keywords: olmesartan-induced enteropathy, celiac disease, diarrhea
Case Presentations
Case 1
A 69-year-old woman presented with weight loss, postprandial emesis, volume depletion, and electrolyte abnormalities. Her medical history was remarkable for hypertension, with blood pressure controlled using metoprolol, amlodipine, and olmesartan. Esophagogastroduodenoscopy (EGD) was performed and revealed gross scalloping of the mucosa throughout the duodenum. Biopsies of the duodenum showed villous blunting and intraepithelial lymphocytic infiltrates (Figure 1). Labs revealed a normal IgA (204 mg/dL), negative tissue transglutaminase IgA (< 3 U/mL), positive HLA-DQ2, and negative DQ8. A strict gluten-free diet for 2 months did not help, and she was empirically treated with prednisone given severe inflammation observed in the small intestine. Symptoms improved rapidly but recurred when the prednisone was weaned. She was started on azathioprine as a steroid-sparing medication with unsuccessful cessation of steroids. Repeat EGD at the time of symptomatic remission on prednisone and azathioprine showed persistent villous blunting similar to the previous biopsy (Figure 2). Eventually, due to financial considerations, her antihypertensive regimen was adjusted, and olmesartan was switched to losartan. Following reports of sprue-like enteropathy linked to olmesartan, azathioprine was discontinued. Another EGD 6 months later showed normal endoscopic and histologic remission with normal villous architecture and no inflammatory infiltrates (Figure 3). She continues to feel well on losartan at 12 months follow-up.
Figure 1.
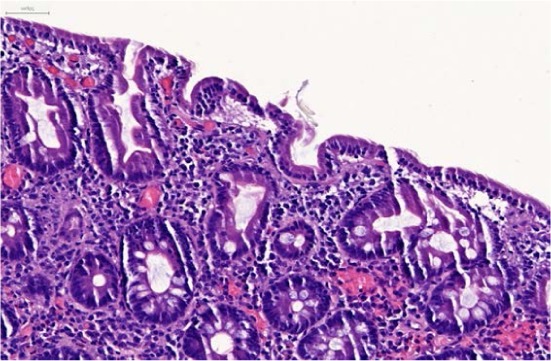
Marked villous blunting, mild inflammation in lamina propria, and slight increase of intraepithelial lymphocytes.
Figure 2.
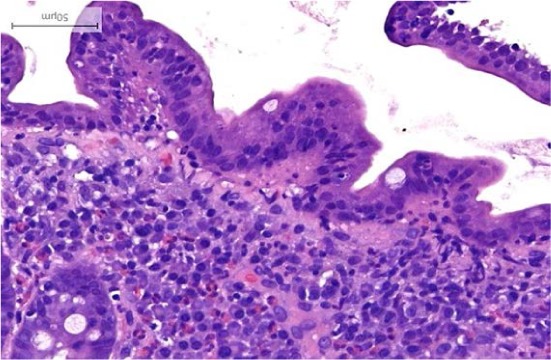
Persistent moderate villous blunting observed 12 months later while on prednisone and azathioprine.
Figure 3.
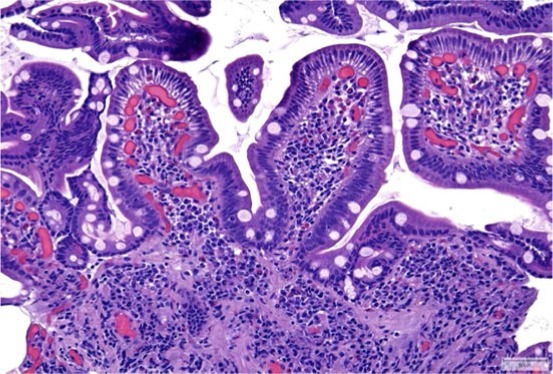
Preserved villous architecture and no increase in intraepithelial lymphocytes 6 months after stopping olmesartan.
Case 2
A 67-year-old woman with a history of asthma and hypertension presented with a 1-month history of severe, watery diarrhea accompanied by abdominal bloating and distention. Initial work-up was notable for mild hypokalemia with normal liver panel, lipase, and complete blood count. Stool studies including fecal leukocytes, Clostridium difficile toxin PCR, rotavirus ELISA, bacterial cultures, and Giardia and Cryptosporidium antigen testing all returned negative; however, fecal fat staining returned abnormal, which suggested malabsorption. Tissue transglutaminase IgA antibody testing was negative for celiac disease. Colonoscopy to the distal ileum was normal, with biopsies negative for microscopic colitis. Her diarrhea persisted, and on review of the patient's medications, she was found to have been taking olmesartan for 2 years. Olmesartan-associated sprue was suspected, and EGD revealed duodenal mucosal changes consistent with sprue-like enteropathy with moderate villous blunting, mild active inflammation, and increased intraepithelial lymphocytes (IELs) by CD3 immunohistochemical staining (Figures 4, 5). Improvement in diarrhea was seen 1 week after discontinuation of olmesartan, and complete resolution of symptoms was observed at 1 month.
Figure 4.
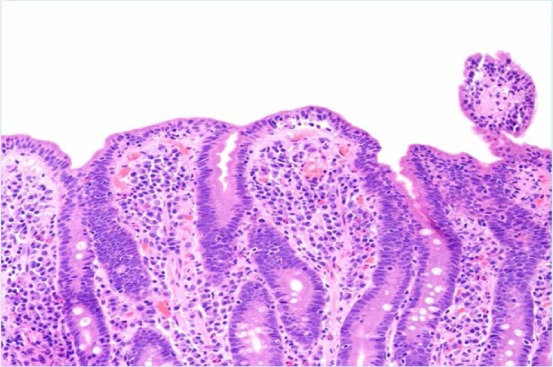
Duodenal biopsy demonstrating moderate villous blunting.
Figure 5.
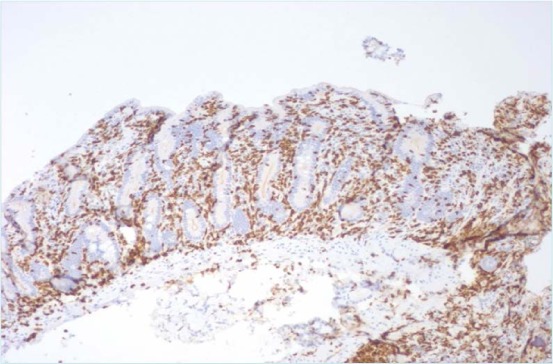
Increased duodenal intraepithelial lymphocytes on CD3 immunohistochemical staining.
Case 3
A 66-year-old woman with a past medical history of hypertension on olmesartan (40 mg daily) presented with a 6-month history of diarrhea, nausea, and 25-pound weight loss. Although her symptoms were initially relieved within a few days, they recurred 2 weeks later with multiple loose stools throughout the day. She had no abdominal pain. She was empirically treated with levofloxacin, metronidazole, and prednisone without improvement in her symptoms. In addition, she attempted to avoid several potential food triggers, with no alleviation of her symptoms. On admission, she was afebrile and hemodynamically stable. Stool culture including Clostridium difficile was unremarkable. EGD and colonoscopy were performed. Biopsy of the esophagus showed lymphocytic infiltration with basal hyperplasia. The small bowel had marked chronic inflammation, villous atrophy, and focal acute lymphocytosis (Figure 6). Random biopsies of the colon revealed chronic inflammation with reactive epithelial changes and prominent lymphocytosis (Figure 7). Serum transglutaminase IgA antibody was 9 units and IgG antibody was 2 units (normal 0–19). The patient was initially advised to adhere to a gluten-free diet and was started on budesonide for microscopic colitis. However, the negative celiac serologies on two occasions suggested an alternative diagnosis. Olmesartan was discontinued after about 10 years of therapy, and she was started on losartan. At follow-up 1 month later, she had gained about 14 pounds and reported that her diarrhea had resolved.
Figure 6.
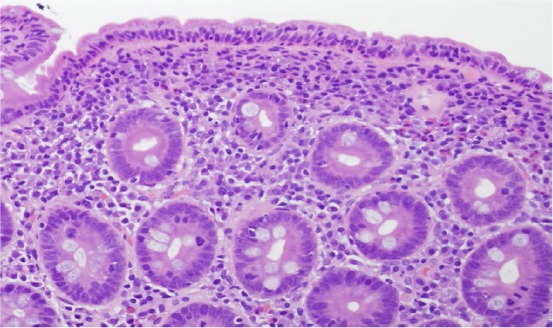
Small bowel biopsy showing villous atrophy with flat mucosa and increased intraepithelial lymphocytes in the surface epithelium (H and E, × 20).
Figure 7.
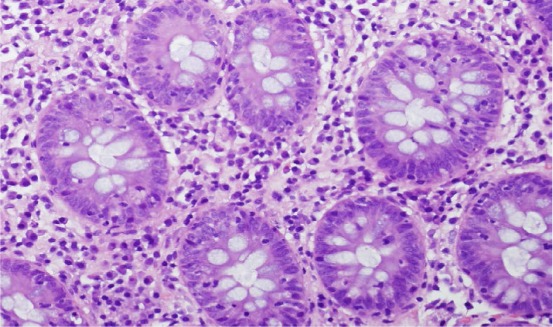
Colon biopsy showing increased intraepithelial lymphocytes in crypts with minimally increased chronic inflammatory cells in the lamina propria (H and E, × 20).
Discussion
Small intestinal villous atrophy and inflammatory infiltrates with negative celiac serologies (sprue-like enteropathy) presents a diagnostic challenge. The differential diagnosis is broad and includes autoimmune and drug-induced enteropathies, malignancies, infections, post-infectious enteropathy, and immunodeficient disorders.
Olmesartan is one of several angiotensin II receptor antagonists available for the treatment of hypertension. Patients with olmesartan-induced enteropathy typically present with diarrhea, weight loss, nausea, vomiting, and low albumin. Although biopsy findings mimic those of celiac disease, olmesartan-induced enteropathy can be distinguished from celiac disease by the presence of normal celiac serologies and, importantly, by the absence of a response to a gluten-free diet.1 Histological changes described in olmesartan-induced enteropathy can range from intraepithelial lymphocytosis and lymphocytic proliferation of the lamina propria to marked villous atrophy.2
In 2012, Rubio-Tapia et al. identified 22 patients on olmesartan who developed clinical features of chronic diarrhea, weight loss, and sprue-like enteropathy, evidenced by villous atrophy and mucosal inflammation on intestinal biopsy.1 All cases demonstrated negative serologic testing for celiac disease by IgA tissue transglutaminase, and a majority had failed prior therapy with a gluten-free diet. Notably, all 22 patients experienced resolution of symptoms upon withdrawal of olmesartan and discontinuation of a gluten-free diet.1 The mean duration of exposure to olmesartan was 3.1 years, suggesting a potential drug-induced, cell-mediated damage rather than a type I hypersensitivity reaction.
In our small case series, the time between olmesartan exposure to onset of symptoms ranged from 6 to 120 months. Thus, clinicians need to consider olmesartan-induced sprue regardless of how long a patient has been on olmesartan without side effects. Furthermore, one of three patients in our case series had not only small bowel involvement but also chronic inflammation and prominent lymphocytosis suggestive of lymphocytic colitis,1 demonstrating that olmesartan-associated enteropathy may affect any part of the gastrointestinal tract. Microscopic colitis can be found in this entity, with up to 22% of patients affected in one case series.1
The exact mechanisms through which olmesartan induces malabsorptive enteropathy remains unknown. Previous reports suggest cell-mediated immunity damage given the delay of enteropathy onset following olmesartan use. Two pathways have been proposed, including (1) the inhibitory effects of angiotensin II receptor blockers (ARBs) on transforming growth factor β, and (2) a disproportionate activation of angiotensin II receptor type 2 (AT2) receptors by angiotensin II after blocking AT1 receptors with olmesartan, which results in apoptosis of enterocytes.1,3
All three patients described in this series improved after withdrawal of olmesartan. Steroids may ameliorate symptoms in 95% of cases.2 In our first case presentation, azathioprine and prednisone offered clinical relief but did not restore histological changes (Figure 2). Replacing olmesartan with losartan, another ARB, provided both clinical and histologic improvement (Figure 3), indicating that this enteropathy is likely specific to olmesartan and not an ARB class effect. A recent epidemiological study from France showed a significant association between hospitalization for malabsorption and use of olmesartan compared with other ARBs and angiotensin-converting enzyme inhibitors.2 However, we note that while enteropathy with other ARBs seems to be very rare, it has also been reported in one patient with irbesartan.2
Conclusion
Clinicians and patients should be aware that olmesartan can cause an enteropathy clinically and histopathologically similar to celiac disease as well as a colopathy similar to microscopic colitis. Failure to recognize olmesartan-induced enteropathy may result in patients continuing on a medication that is injurious to the gastrointestinal tract or embarking on an unnecessary and expensive medical evaluation, frequently with steroid use, with both options disrupting quality of life. Time to recognition of this entity is often prolonged, suggesting the need for increased awareness in both patients and clinicians.
Conflict of Interest Disclosure
The authors have completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
References
- 1. Rubio-Tapia A, Herman ML, Ludvigsson JF, . et al. Severe sprue-like enteropathy associated with olmesartan. Mayo Clin Proc. 2012. August; 87( 8): 732– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marthey L, Cadiot G, Seksik P, . et al. Olmesartan-associated enteropathy: results of a national survey. Aliment Pharmacol Ther. 2014. November; 40( 9): 1103– 9. [DOI] [PubMed] [Google Scholar]
- 3. Ianiro G, Bibbo S, Montalto M, Ricci R, Gasbarrini A, Cammarota G.. Systematic review: sprue-like enteropathy associated with olmesartan. Aliment Pharmacol Ther. 2014. July; 40( 1): 16– 23. [DOI] [PubMed] [Google Scholar]


