Systemic infection in preterm infants has two categories with distinct aetiologies and outcomes.
Early onset infection—acquired in the intrapartum period and presenting in the first 48-72 hours after birth.
Late onset infection—usually acquired in hospital and clinically evident more than 72 hours after birth (usually after the first week of life).
Early onset infection
Incidence
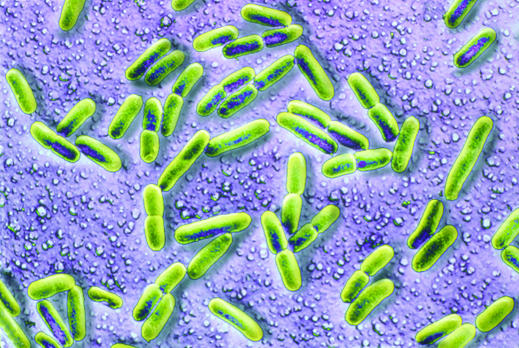
Early onset systemic infection includes bacteraemia, pneumonia, meningitis, and urinary tract infection. Although rare, early onset infection is a serious complication of preterm birth. In North America the incidence of bacteraemia proved by culture in very low birthweight (< 1500 g) infants is 1.5% of live births. The principal pathogens responsible are Group B streptococci and Escherichia coli. Congenital listeriosis (caused by spread of Listeria monocytogenes across the placenta after the mother has eaten infected food) is rare in North America and the United Kingdom but more common in some areas in Europe.
Figure 1.
Gram negative bacilli, particularly E coli, are an increasingly common cause of early onset infection in very low birthweight infants
In many developed countries the incidence of Group B streptococcal infection has fallen in the past decade, perhaps because of the greater use of intrapartum antibiotic treatment for women with specific risk factors. During this period, however, there has been a rise in the incidence of infection with Gram negative coliforms Microbiological surveillance should be continued to identify changes in the epidemiology of early onset infection, particularly antibiotic resistance.
Table 1.
Micro-organisms causing early onset infection in very low birthweight infants*
|
Rate per 1000 live born infants |
||
|---|---|---|
| 1991-93 | 1998-2000 | |
| Group B streptococcus |
5.9 |
1.7 |
| Other Gram positive organisms |
5.0 |
4.0 |
|
E coli |
3.2 |
6.8 |
| Other Gram negative organisms | 5.1 | 2.6 |
Adapted from Stoll BJ, et al. N Engl J Med 2002;347: 240-7
Presentation and treatment
Most infants with early onset systemic infection will present with signs of sepsis, such as respiratory distress or fever, in the first 12 hours after birth. Presentation can be delayed, and microbiological diagnosis may be difficult, especially if the mother has received intrapartum antibiotics. As it is difficult to rule out systemic infection in the symptomatic preterm neonate, empirical antibiotic treatment is given to all preterm infants with signs consistent with sepsis. Empirical antibiotic treatment is often indicated for preterm infants who seem well but who have specific risk factors for systemic infection, such as prolonged rupture of amniotic membranes.
Table 2.
Risk factors for early onset Group B streptococcal infection in preterm infants
| • Group B streptococcal infection in mother's previous baby |
| • Group B streptococcal infection in mother's vagina or urine during pregnancy |
| • Mother has fever during labour |
| • Prolonged rupture of amniotic membranes (> 18 hours) |
Intravenous penicillin plus an aminoglycoside, such as gentamicin, is the commonly used firstline antibiotic regimen for suspected early onset systemic infection. In an asymptomatic infant, if blood, urine, or cerebrospinal fluid examination and cultures do not confirm infection, antibiotics are often stopped after 48 hours. Parenteral antibiotics are continued for up to 14 days when bacteraemia is confirmed, and for up to three weeks in infants with meningitis. If listeriosis is suspected then ampicillin is often substituted for penicillin, although both are probably equally effective. L monocytogenes is, however, resistant to all cephalosporins.
Table 3.
Presenting features of early onset systemic infection in preterm infants
| • Respiratory distress |
| • Unstable temperature |
| • Floppiness |
| • Irritability |
| • Poor feeding |
| • Early onset jaundice |
| • Apnoea |
| • Poor perfusion |
| • Tachycardia |
| • Seizures |
Mortality in very low birthweight infants with early onset systemic infection is close to 40%, three times higher than in infants of the same gestation without infection. Congenital listeriosis is associated with a mortality rate of about 50%. Early onset systemic infection, particularly meningitis, is also associated with a high incidence of neurodevelopmental impairment, including cerebral palsy, and visual and hearing impairment in surviving children. The appropriate use of preventive strategies, such as intrapartum antibiotic prophylaxis, and the early empirical treatment of infected infants, may help reduce the incidence and severity of such adverse outcomes.
Table 4.
Main organisms causing late onset systemic infection in very low birthweight infants
| Gram positive organisms |
| • Coagulase negative staphylococci |
| • Staphylococcus aureus |
| • Enterococci |
| Gram negative organisms |
| • E coli |
| • Klebsiella spp |
| • Pseudomonas spp |
| Fungi |
| • Mainly Candida spp |
Late onset infection
Incidence
In contrast to early onset infection, late onset infection acquired in hospital is common, occurring in about 20% of very low birthweight infants. Most infections are caused by Gram positive organisms. Coagulase negative staphylococci account for half of all late onset infections. Risk of infection is inversely related to gestational age and birth weight, and directly related to the severity of illness at birth. These risk factors reflect the need for invasive interventions such as prolonged ventilation or vascular access. Late onset systemic infection with Gram negative organisms is often associated with specific complications of preterm birth, such as urinary tract infection.
Figure 2.
A preterm infant may need invasive interventions, such as ventilation and vascular access, which may increase the risk of infection
Nosocomial infection is the most common serious complication related to central venous catheters (“long lines”), which are often used to deliver parenteral nutrition to preterm infants. It is uncertain, however, whether using central venous catheters further increases the risk of infection in a population that is already at risk. The central venous catheter, or an associated thrombus, can act as a nidus for infection. The catheter may need to be removed to clear the infection.
Figure 3.
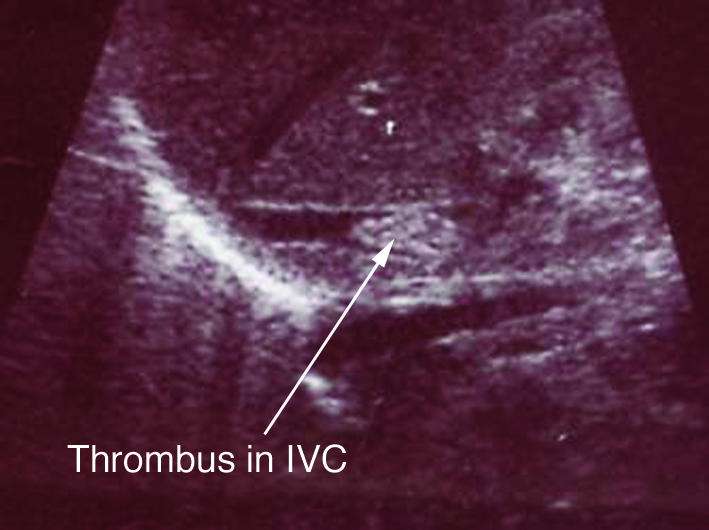
Central venous catheters, often used to deliver parenteral nutrition to preterm infants, can act as a nidus for infection. This ultrasonogram shows a long line associated (infected) thrombus in the inferior vena cava (IVC)
Presentation and treatment
The clinical presentation of systemic infection, particularly with coagulase negative staphylococci, can be insidious. Diagnosis depends on the early recognition of presenting clinical and laboratory signs.
Clinical signs include:
Increasing apnoea
Feeding intolerance or abdominal distension
Increased respiratory support
Lethargy and hypotonia.
Laboratory signs include:
Abnormal white blood cell count
Unexplained metabolic acidosis
Hyperglycaemia.
Good management includes the early investigation of suspected infection (a chest x ray is needed if pneumonia is suspected), and microbiological culture of blood, urine, and cerebrospinal fluid if meningitis is suspected.
Coagulase negative staphylococci are skin commensals and may contaminate samples that have been taken inappropriately. Conversely, blood samples that are of insufficient volume may give falsely reassuring negative results on culture. Blood samples for microbial culture (ideally, at least 1 ml) should be obtained from peripheral sites rather than indwelling cannulas. Urine should be obtained by suprapubic aspiration or “in out” aseptic catheterisation of the bladder.
Figure 4.
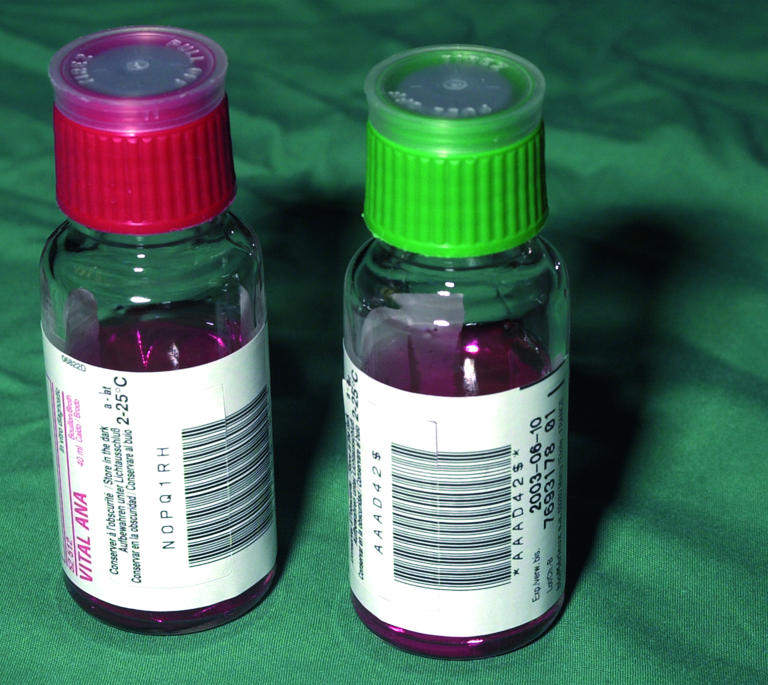
If a blood sample of insufficient volume is obtained the result may be false negative. Skin contaminants in samples that have been taken inappropriately may give false positive results
Antibiotic treatment is usually started as soon as these investigations have been done, and stopped when appropriate cultures are confirmed as negative—usually after 48 hours. As antibiotics are often prescribed empirically for infants with suspected sepsis, their rational use is essential to limit the emergence of antibiotic resistant bacteria. The firstline treatment for suspected nosocomial sepsis in preterm infants should include an antistaphylococcal antibiotic, such as flucloxacillin, and an aminoglycoside. Flucloxacillin, however, is not active (at least in vitro) against coagulase negative staphylococci. Vancomycin or teicoplanin is indicated for an infant with confirmed, or strongly suspected, coagulase negative staphylococcal infection, or in infants with a poor response to firstline antibiotics.
Table 5.
Risk factors for invasive fungal infection in very low birthweight infants
| • Fungal colonisation |
| • Severe illness at birth |
| • Use of multiple courses of antibiotics, especially third generation cephalosporins |
| • Use of parenteral nutrition |
| • Presence of a central venous catheter |
| • Use of H2 receptor antagonists |
The high mortality in preterm infants with late onset infection is mainly associated with Gram negative coliform infection, or with invasive fungal infection. Coagulase negative staphylococcal infection, although common, is associated with a more benign clinical course. Meningitis is rare and associated mortality is lower than with infection from other organisms. Inflammatory cascades associated with even “low grade” systemic infection may play a part in the pathogenesis of white matter and other brain damage that may result in neurodevelopmental impairment. Hospital acquired infection is associated with preterm infants who have a substantially prolonged hospital stay. This has important implications for resources and costs in health services
Table 6.
Antifungal treatments for preterm infants
| • Amphotericin B |
| • Lipid complex amphotericin B |
| • 5-Fluorocytosine (flucytosine) |
| • Triazoles (fluconazole, itraconazole) |
| • Imidazoles (miconazole, ketoconazole) |
Invasive fungal infection
The clinical presentation of invasive fungal and bacterial infection is similar, and this may cause a delay in diagnosis and treatment. The diagnosis may be further delayed if the organism cannot be recovered consistently from blood, cerebrospinal fluid, or urine. A high index of suspicion and the use of additional clinical tests, including retinal examination, echocardiography, and renal ultrasonography, may be needed to confirm the suspected diagnosis. Although systemic antifungal treatment is often given before the diagnosis is confirmed, about one third of very low birthweight infants with invasive fungal infection die. The role of prophylactic antifungal treatment for preterm infants at high risk of invasive fungal infection is still unclear. Topical prophylaxis (for example, with nystatin) can reduce fungal colonisation and infection. Some evidence shows that systemic prophylaxis with fluconazole reduces the incidence of invasive fungal infection, and possibly the mortality rate in very low birthweight infants. The effect of this intervention on longer term outcomes, including neurodevelopment and the emergence of antifungal resistance, is still to be determined.
Figure 5.
The layout and organisation of the neonatal unit may have an important effect on infection control practices
Adjunctive treatments for systemic infection
Because of the high mortality and morbidity associated with systemic infection in preterm infants, even with appropriate antibiotic treatment, adjunctive therapies that might improve outcomes have been sought. Recent attention has focused on the potential role of immunomodulatory drugs, and several large multicentre randomised controlled trials of these interventions are under way. Proposed adjuvant therapies for sepsis in preterm infants include:
Polyclonal immunoglobulin
Colony stimulating factors—for example, granulocyte-macrophage colony stimulating factor (GM-CSF)
Granulocyte transfusions
Anticytokine treatments.
Prevention of nosocomial infection
The impact of infection control practices may be affected by organisational issues (such as the layout of the neonatal intensive care unit, staffing levels, and throughput of patients) as well as training and educational factors. Continued research at all of these levels is needed if efforts to reduce the burden of infection in preterm infants are to be successful.
Figure 6.
Hand washing is a cornerstone of infection control
Some specific infection control practices, such as aseptic handling of central venous catheters and compliance with hand washing, are effective in reducing the incidence of hospital acquired infection in preterm infants.
The overall mortality rate in preterm infants with late onset systemic infection is substantially higher than in those without infection
Invasive fungal infection, mainly caused by Candida spp, accounts for about 10% of all cases of late onset sepsis in preterm infants
Recent research has indicated that the routine use of alcohol solutions as hand rubs after any patient contact may achieve a greater reduction in bacterial contamination of hands than conventional washing with medicated soap. Other measures, such as routine use of gowns, hats, and masks by staff or parents, are much less effective in preventing infection. The use of prophylactic antibiotics is not substantially beneficial for very low birthweight infants, and may contribute to the emergence of antibiotic resistant bacteria in the neonatal intensive care unit.
Conclusion
Systemic infection, particularly nosocomial infection, is an important cause of morbidity and mortality in preterm infants. Infants born after very short gestations and require intensive care and undergo invasive procedures are most at risk. Clinical and laboratory diagnosis can be difficult, potentially leading to delayed treatment. New prevention and treatment strategies are needed because morbidity is high despite antimicrobial treatment.
Linda Clerihew is specialist registrar in the Neonatal Intensive Care Unit at Ninewells Hospital and Medical School, Dundee.
The ABC of preterm birth is edited by William McGuire, senior lecturer in neonatal medicine, Tayside Institute of Child Health, Ninewells Hospital and Medical School, University of Dundee; and Peter W Fowlie, consultant paediatrician, Perth Royal Infirmary and Ninewells Hospital and Medical School, Dundee. The series will be published as a book in spring 2005.
Competing interests: WMcG received a grant from Pfizer UK for a national study of fungal infection in preterm infants.
The coloured scanning electron micrograph of Escherichia coli bacteria is with permission of BSIP/Science Photo Library. The ultrasonogram showing long line associated (infected) thrombus in inferior vena cava is courtesy of Dr Gavin Main.
References
- • Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110: 285-91 [DOI] [PubMed] [Google Scholar]
- • Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med 2002;347: 240-7 [DOI] [PubMed] [Google Scholar]
- • Fanaroff AA, Korones SB, Wright LL, Verter J, Poland RL, Bauer O, et al. Incidence, presenting features, risk factors and significance of late onset septicaemia in very low birth weight infants. The National Institute of Child Health and Human Development Neonatal Research Network. Pediatr Infect Dis J 1998;17: 593-8 [DOI] [PubMed] [Google Scholar]
- • Saiman L, Ludington E, Pfaller M, Rangel-Fraustro S, Wilbin TR, Dawson J, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey Study Group. Pediatr Infect Dis J 2000;19: 319-24 [DOI] [PubMed] [Google Scholar]
- • Adams-Chapman I, Stoll BJ. Prevention of nosocomial infections in the neonatal intensive care unit. Curr Opin Pediatr 2002;14: 157-64 [DOI] [PubMed] [Google Scholar]