Abstract
Vitamin D deficiency has been related to metabolic syndrome (MetS) in polycystic ovary syndrome (PCOS). The vitamin D-binding protein (DBP) is the main protein involved in vitamin D transport. Two single-nucleotide polymorphisms (SNPs) of the DBP gene, rs4588 and rs7041, have been associated with low circulating levels of 25-hydroxyvitamin D [25(OH)D] in various populations, but not in women with PCOS. Therefore, we determined the genotype and haplotype distribution of DBP gene polymorphisms and investigated the associations between these genetic variants and their haplotypes with PCOS, MetS, and 25(OH)D levels in women with PCOS and controls from the South of Brazil. The sample included 291 women (191 with PCOS and 100 controls). All participants were genotyped for polymorphisms rs2282679, rs4588, and rs7041. Serum 25(OH)D levels were determined in a subset of 102 participants. Women with PCOS were younger and had significantly higher body mass index, blood pressure, and insulin resistance than the control group (p<0.05). The prevalence of MetS in PCOS and controls was 26.5% and 4.8% respectively. Levels of 25(OH)D were lower in PCOS women with MetS, even after adjustment for age (p = 0.033). No associations were observed between PCOS and the polymorphisms or their haplotypes. A higher frequency of genotype TT of rs7041 was found in PCOS participants with MetS (OR: 2.21, 95%CI:1.08–4.52; p = 0.027). This same genotype was associated with lower 25(OH)D levels in both PCOS and control women (OR: 4.40, 95%CI:1.62–12.00; p = 0.002). In conclusion, these findings indicate that DBP gene polymorphisms and their haplotypes are not directly associated with PCOS. In contrast, the TT genotype of SNP rs7041 was associated with MetS in PCOS women, and with lower 25(OH)D levels in both PCOS and control groups.
Introduction
Polycystic ovary syndrome (PCOS), a heterogeneous disease characterized by chronic anovulation and manifestations of hyperandrogenism [1, 2], affects between 9% and 18% of women of reproductive age, depending on diagnostic criteria [1–3]. Women with PCOS suffer from metabolic abnormalities, including insulin resistance (IR), obesity, and metabolic syndrome (MetS) [4–6]. Accumulating evidence suggests that vitamin D deficiency is associated with IR and MetS in PCOS [7]. Vitamin D deficiency may also be linked to central obesity, lipid profile, and body mass index (BMI) in these women [7–13].
Both the vitamin D receptor (VDR) and the vitamin D-binding protein (DBP) play a key role in vitamin D metabolism. VDR is expressed in many tissues and organs, such as those involved in calcium homeostasis, glucose metabolism, and reproduction [14], whereas DBP is the main protein involved in vitamin D transport [15, 16]. Two well-studied single-nucleotide polymorphisms (SNPs) of the DBP gene, rs4588 and rs7041, have been previously shown to be strongly associated with low circulating 25-hydroxyvitamin D [25(OH)D] levels in genome-wide association studies [17, 18] and in various populations [19–24].
Although there have been reports about VDR polymorphisms in PCOS [25–27], few studies have focused on DBP gene polymorphisms in women with PCOS or androgen excess. The single work published to date showed similar genotype frequencies of SNP rs2282679 in PCOS and controls [25]. In other populations, polymorphisms of DBP gene were associated with several endocrine and metabolic parameters [28–30].
Therefore, the aim of the present study was to compare the frequency of SNPs rs2282679, rs4588, and rs7041 of the DBP gene and their haplotypes in women with PCOS and healthy controls with regular ovulatory cycles from Southern Brazil. We also aimed to investigate whether these genetic variants are related to MetS and 25(OH)D levels in PCOS women.
Materials and methods
Patients
Participants were enrolled by advertisement in the local media. The advertisement called for women of reproductive age with excess hair (hirsutism) and irregular menses and for volunteers without hirsutism and with regular menses. The study population comprised 291 women: 191 patients with PCOS and 100 non-hirsute women with regular ovulatory cycles (confirmed by progesterone levels higher than 3.8 ng/mL). Diagnostic investigation was performed for all enrolled participants at a university hospital (Hospital de Clínicas de Porto Alegre, state of Rio Grande do Sul).
Rotterdam criteria were used for the diagnosis of PCOS, which was defined in the presence of two out of three of the following traits: 1) oligo/amenorrhea and/or chronic anovulation (≤9 cycles/year and/or luteal phase progesterone ≤3.8 ng/mL), 2) clinical and/or biochemical hyperandrogenism, and 3) polycystic ovary appearance on ultrasound examination. Exclusion criteria were presence of hyperandrogenic disorders, having used drugs known to interfere with hormone levels (such as oral contraceptive pills, antiandrogens, progestins, metformin, fibrates, or statins) for 3 or more months before the study, pregnancy, liver disease, or kidney disease.
Approval for this study was obtained from the Institutional Review Board at Hospital de Clínicas de Porto Alegre. Written informed consent was obtained from all participants.
Study protocol
Anthropometric measurements included BMI and waist circumference (WC). Blood pressure (BP) was measured twice after a 10-minute rest [4, 5, 31–33].
Hirsutism was defined as a modified Ferriman-Gallwey score [34] ≥8. Homeostasis model assessment index to estimate insulin resistance (HOMA-IR) was calculated by multiplying insulin (mIU/mL) by glucose (mmol/L) and dividing this product by 22.5 [35]. Joint Scientific Statement criteria were used to define MetS [36].
Laboratory measurements
Blood samples for determination of hormone levels were drawn from an antecubital vein after a 12-h overnight fast, between 8:00 and 10:00 am. Samples were obtained between the 2nd and 10th days of the cycle, or on any day in amenorrheic women. Blood samples were also collected for genomic DNA extraction.
Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), triglycerides (TG), and fasting glucose levels were determined by colorimetric-enzymatic methods (Siemens Advia 1650, Deerfield, USA). Low-density lipoprotein cholesterol (LDL-c) was estimated indirectly with the Friedewald formula [37]. Total testosterone and insulin levels were measured by chemiluminescence (Siemens Advia Centaur XP, Deerfield, USA). Serum 25(OH)D levels were measured in a subset of 102 women by a chemiluminescence assay (Liaison, DiaSorin, Saluggia, Italy) with sensitivity of ≤4.0 ng/mL and intra- and inter-assay CV of 7.7% and 10.9%, respectively.
Genotype analysis
Genomic DNA was extracted from peripheral blood leukocytes [38]. DNA samples were diluted to 2 ng/mL. Duplicate measurements were performed in 10% of the samples to assess the internal quality of genotype data. Molecular genotyping for rs4588 (substitution of C for A), rs7041 (substitution of T for G), and rs2282679 (substitution of A for C) was performed through real-time polymerase chain reaction (PCR) (7500 Fast Real-Time Polymerase Chain Reaction System, Applied Biosystems, California, USA), using the allelic discrimination assay with TaqMan MGB primers and probes (Applied Biosystems, California, USA).
Statistical analysis
Data distribution was assessed by Kolmogorov-Smirnov test and descriptive statistics. Results are expressed as mean ± standard deviation (SD) for normally distributed variables, as median and interquartile range for variables with a non-Gaussian distribution, or as absolute numbers and percentages. Non-Gaussian variables were log-transformed for statistical analysis and back-transformed into their original units for reporting. Unpaired two-tailed Student’s t-test was used to compare group means. Pearson’s chi-square test (χ2) was applied to test categorical variables and the agreement of genotype frequencies with Hardy-Weinberg equilibrium.
Haplotypes were constructed from the combination of the rs4588 and rs7041 polymorphisms. Lewontin’s D’ statistic for linkage disequilibrium was calculated for each pair of polymorphisms. Linkage disequilibrium was inferred using the Phase 2.1.1 software [39], which employs Bayesian statistics. This software was also used to compare haplotype frequencies in PCOS and control women by permutation testing. Data were considered as statistically significant at p<0.05. The Statistical Package for the Social Sciences 18 (SPSS, Chicago, IL) was used for analyses.
Results
Participants were mostly white (93.9%), and some (6.1%) had mixed African and European ancestry. Clinical characteristics of the sample are shown in Table 1. The mean age of PCOS and control participants was 22.89±6.66 and 25.18±7.72 years respectively (p = 0.013, Student’s t test). As expected, women with PCOS had significantly higher BMI, WC, BP, HOMA-IR, triglycerides, Ferriman-Gallwey score, and total testosterone, as well as lower HDL-c than controls (p<0.05 for all variables, Student’s t test). MetS was more frequent in PCOS participants (p<0.001, Pearson’s χ2 test).
Table 1. Clinical and biochemical profile of PCOS and control participants.
| Variable | Controls (n = 100) | PCOS (n = 191) | p |
|---|---|---|---|
| BMI (kg/m2) | 27.04±6.09 | 29.70±6.40 | 0.001 |
| WC (cm) | 78.04±11.51 | 89.23±15.08 | <0.001 |
| Systolic BP (mmHg) | 109.52±12.90 | 121.10±15.50 | <0.001 |
| Diastolic BP (mmHg) | 70.83±9.39 | 78.06±11.53 | <0.001 |
| Fasting glucose (mg/dL) | 88.53±7.57 | 88.89±15.30 | 0.797 |
| HOMA-IR | 2.18 (1.42–3.14) | 3.52 (1.96–6.36) | <0.001 |
| TC (mg/dL) | 170.11±30.72 | 174.69±38.31 | 0.290 |
| HDL-c (mg/dL) | 52.84±12.28 | 48.85±10.87 | 0.007 |
| LDL-c (mg/dL) | 101.70±26.30 | 104.51±31.82 | 0.443 |
| Triglycerides (mg/dL) | 66.00 (50.00–99.00) | 89.00 (62.00–131.00) | <0.001 |
| Ferriman-Gallwey | 2.19±2.10 | 15.55±6.11 | <0.001 |
| TT (ng/mL) | 0.55 (0.42–0.64) | 0.82 (0.62–1.11) | <0.001 |
| Metabolic syndrome | 4.8% | 26.5% | <0.001 |
Data are expressed as mean ± SD, median (interquartile range) (Student’s t test), or percentage. p value by Pearson’s χ2 test. PCOS: polycystic ovary syndrome; BMI: body mass index; WC: waist circumference; BP: blood pressure; HOMA-IR: homeostasis model assessment index to estimate insulin resistance; TC: total cholesterol, HDL-c: high-density lipoprotein cholesterol, LDL-c: low-density lipoprotein cholesterol; TT: total testosterone.
Serum 25(OH)D levels were measured in a subset of participants (54 PCOS and 48 controls) who had an extra serum aliquot available for this measurement. Participants with and without measured 25(OH)D levels were similar regarding BMI (p = 0.545), HOMA-IR (p = 0.110), and presence of MetS (p = 0.540).
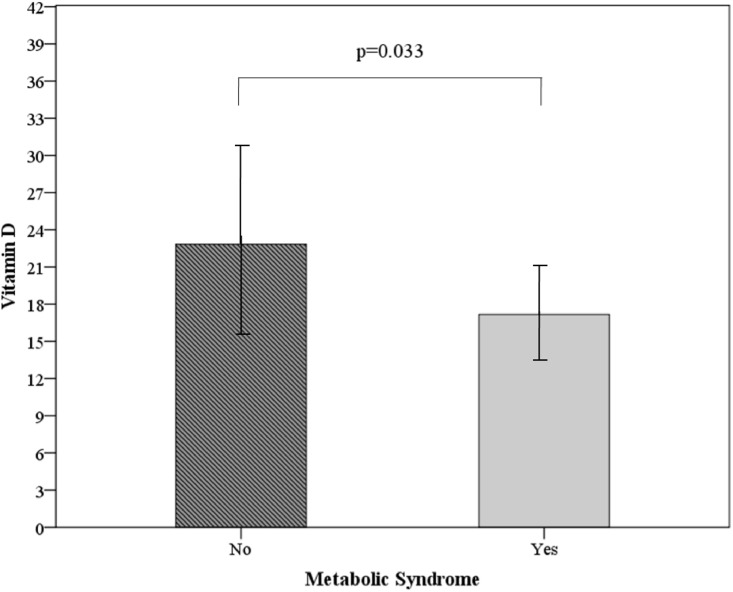
The mean 25(OH)D concentration in the subset was 21.48±7.25 ng/mL. Only 12.7% of this subset had adequate circulating levels of 25(OH)D (≥ 30 ng/mL). In 45.1%, 25(OH)D levels were insufficient (20–29 ng/mL), and 42.2% had vitamin D deficiency (<20 ng/mL). Sufficient vitamin D status was similar in the PCOS and control women included in the subset (14.8% vs. 10.4% respectively). Also, 25(OH)D values were similarly low in both subset groups (21.50±6.90, controls and 21.47±7.61, PCOS; p = 0.985). A separate analysis of the subset PCOS group revealed lower vitamin D levels in women with MetS (p = 0.018, Student’s t test), even after adjustment for age (p = 0.033) (Fig 1).
Fig 1. 25(OH)D levels according to presence of the metabolic syndrome in 54 PCOS participants.
Data are expressed as mean ± SD. p value by Student’s t test, adjusted for age. 25(OH)D: 25-hydroxyvitamin D.
Regarding DBP gene polymorphisms, all three SNPs were in Hardy-Weinberg equilibrium in the PCOS and control groups. Only three, two, and one participants were not genotyped for SNP rs2282679, rs7041, and rs4588 respectively. The genotype and allele frequencies of DBP gene variants are presented in Table 2. The genotype and allele distribution of all three polymorphisms was similar in PCOS and controls.
Table 2. Genotype, allele, and haplotype frequencies of DBP gene variants in PCOS and control women.
| SNP | Controls n (%) | PCOS n (%) | p |
|---|---|---|---|
| rs2282679 | |||
| AA | 61 (61%) | 103 (55%) | 0.360 |
| AC | 33 (33%) | 65 (35%) | |
| CC | 6 (6%) | 20 (10%) | |
| A | 155 (77%) | 271 (72%) | 0.159 |
| C | 45 (23%) | 105 (27%) | |
| rs4588 | |||
| CC | 61 (61%) | 104 (55%) | 0.479 |
| CA | 32 (32%) | 66 (35%) | |
| AA | 7 (7%) | 20 (10%) | |
| C | 154 (77%) | 274 (72%) | 0.203 |
| A | 46 (23%) | 106 (28%) | |
| rs7041 | |||
| TT | 24 (24%) | 47 (25%) | 0.911 |
| TG | 45 (45%) | 88 (47%) | |
| GG | 31 (31%) | 54 (28%) | |
| T | 93 (47%) | 182 (48%) | 0.706 |
| G | 107 (53%) | 196 (52%) | |
| Haplotypes | |||
| CG CG + CT CG | 54 (54%) | 94 (49.3%) | 0.740 |
| CG AT + CT CT | 29 (29%) | 61 (31.9%) | |
| CT AT + AT AT | 17 (17%) | 36 (18.8%) |
Data are expressed as percentage. p value by Pearson’s χ2 test. The rs4588-rs7041 haplotype is grouped by presence of the risk haplotype.
Polymorphism rs4588 (C→A) was in linkage disequilibrium with rs7041 (T→G) (|D’| = 1; r2 = 0.44). Three haplotypes were inferred in the sample: CT, CG, and AT, formally called GC-1f, GC-1s, and GC-2 respectively. The first letter of each haplotype refers to SNP rs4588 and the second to SNP rs7041. Haplotype frequencies were 0.21 for CT, 0.53 for CG, and 0.26 for AT. The three common haplotype variants (CT, CG, and AT) formed six diplotypes: CT-CT, CT-CG, CG-CG, CG-AT, CT-AT, and AT-AT, with frequencies of 0.06, 0.21, 0.30, 0.25, 0.09, and 0.09 respectively.
Taking into consideration the results of individual polymorphism analyses and previous data from the literature [19–24], AT was regarded as the risk haplotype and CG as the protective haplotype against lower 25(OH)D concentrations. Therefore, we grouped haplotype combinations accordingly: CG CG + CT CG, CG AT + CT CT, and CT AT + AT AT. Haplotype frequencies were similar in PCOS and control groups (Table 2).
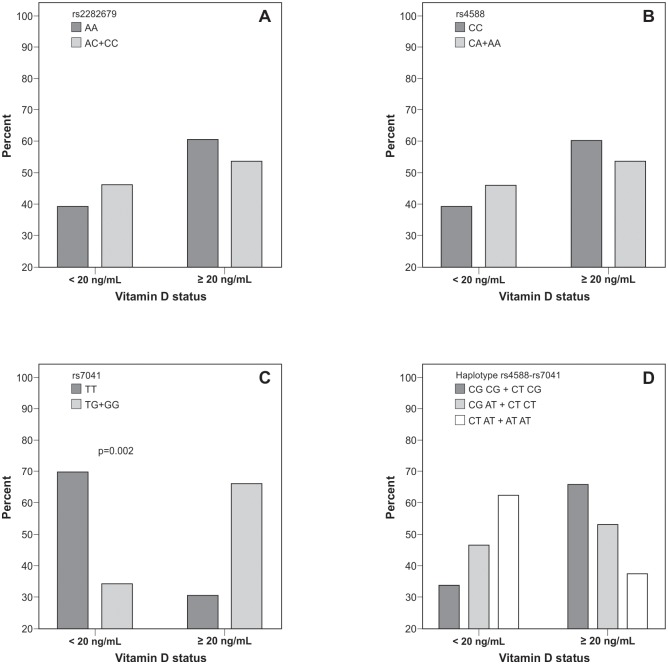
When all participants were analyzed (PCOS and controls), 69.6% of the women carrying the TT genotype of rs7041 were deficient in vitamin D (<20 ng/mL) and 30.4% presented vitamin D levels ≥20 ng/mL (OR: 4.402, 95% CI: 1.62–12.00; p = 0.002, Pearson’s χ2 test). Conversely, vitamin D status was similar for polymorphisms rs2282679 and rs4588 and haplotype variants (Fig 2).
Fig 2. Genotype and haplotype distribution of DBP gene according to vitamin D status in participants with and without PCOS.
Data are expressed as percentages. p value by Pearson’s χ2 test. DBP: vitamin D-binding protein. A: rs2282679 (p = 0.542). B: rs4588 (p = 0.542). C: rs7041 (p = 0.002). D: haplotype rs4588-rs7041, grouped according to the presence of the risk haplotype (p = 0.104).
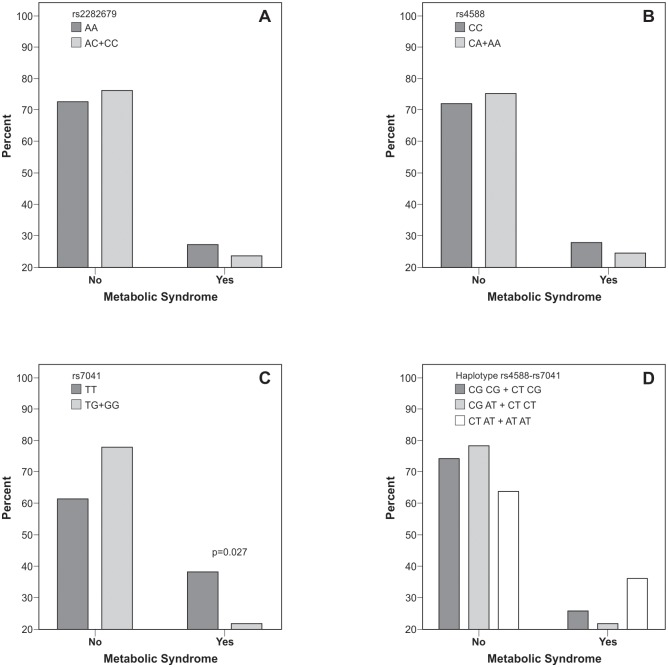
Within the PCOS group, a higher frequency of the TT genotype of rs7041 was observed in the presence of MetS (OR: 2.21, 95% CI: 1.08–4.52; p = 0.027, Pearson χ2 test). This was not observed for rs2282679, rs4588, and the rs4588-rs7041 haplotype, whose frequencies were similar in PCOS participants with or without MetS (Fig 3).
Fig 3. Genotype and haplotype distribution of DBP gene according to metabolic syndrome in PCOS participants.
Data are expressed as percentage. p value by Pearson’s χ2 test. DBP: vitamin D-binding protein; PCOS: polycystic ovary syndrome. A: rs2282679 (p = 0.593). B: rs4588 (p = 0.613). C: rs7041 (p = 0.027). D: haplotype rs4588-rs7041, grouped according to the presence of the risk haplotype (p = 0.294).
Discussion
In the present study, the frequency of SNPs rs2282679, rs4588, and rs7041 of the DBP gene and their haplotypes was similar in women with PCOS and healthy controls with regular ovulatory cycles from Southern Brazil. In turn, polymorphism rs7041 in the DBP gene was related to lower 25(OH)D levels in the overall group and to MetS in PCOS—women with PCOS carrying the TT genotype of rs7041 were twice as likely to present MetS. To the best of our knowledge, this is the first report to show an association between MetS and polymorphism rs7041 in a PCOS population.
Our results also show that 25(OH)D levels were lower in PCOS participants with MetS. A recent meta-analysis has reported that vitamin D levels are indeed related to metabolic and hormonal disturbances in PCOS women. In that study, women with PCOS and vitamin D deficiency were more likely to have dysglycemia compared to those without vitamin D deficiency [11]. In addition, a study comparing PCOS women and controls showed that vitamin D levels were lower in participants with both PCOS and MetS compared to those with PCOS but without MetS. Furthermore, vitamin D levels decreased as the number of risk factors for MetS increased [12]. Moreover, in PCOS women, lower vitamin D levels have been correlated with clinical traits, insulin resistance measures, and lipid profile [8, 12, 13].
Only a few studies have assessed SNPs rs4588 and rs7041 in relation to metabolic parameters in other populations. Some studies have shown an association of polymorphisms in exon 11 with circulating levels of insulin and HOMA-IR [28] and with glucose levels [29] in non-diabetic individuals. Nevertheless, these polymorphisms have not been associated with type 2 diabetes [29, 30].
Regarding polymorphisms rs4588 and rs7041 and vitamin D levels, one study reported no interaction between 25(OH)D and SNPs of DBP [40], while another showed a marginal interaction of 25(OH)D deficiency with rs7041 in white subjects [41]. In adult and elderly populations, two studies with Chinese participants have shown that both SNPs rs4588 and rs7041, as well as the AT-AT haplotype, were related to lower 25(OH)D levels [22, 23]. Similar results were reported in Canadian young adults [20] and elderly Caucasians [42]. Finally, lower 25(OH)D concentrations have been observed in premenopausal white women [19] and early postmenopausal women [43] carrying the AA genotype of rs4588 and the TT genotype of rs7041. Taken together, data from the literature and the present results showing an association between the TT genotype of rs7041 and vitamin D deficiency support the hypothesis that this DBP gene variant is related to 25(OH)D concentrations. In addition, the main finding of this study—that polymorphism rs7041 in the DBP gene is related to metabolic syndrome in PCOS and to lower 25(OH)D levels in the overall group—might signal a genetic link between metabolic disturbances in PCOS and low vitamin D levels.
In a previous study with 545 Austrian women with PCOS aged 16–45 years, no higher risk of PCOS was found in association with genotypes of rs2282679. However, anthropometric variables and lipid profile differed significantly among rs2282679 genotypes [25]. In contrast, we did not find associations between MetS and rs2282679 genotypes. Ethnic differences between the two populations, as well as the older age of participants in the study by Wehr et al. [25], may explain this disagreement.
The DBP gene encodes a multifunctional plasma transport protein, DBP, also known as a group-specific component, synthesized in the liver. DBP binds and transports vitamin D and its metabolites to target tissues. DBP exerts important biological functions, including the binding of mainly monounsaturated and saturated fatty acids [15]. A link between obesity and vitamin D has been described in PCOS [8] and in other populations [44–49]. The relationship with DBP is, however, less clear. In elderly men, a positive relationship has been described between DBP concentrations and BMI [50]. However, this association has not been confirmed in women aged 18–44 years [51]. Overall, the mechanisms underlying this association are still unknown, but deserve further investigation.
It should also be noted that polymorphisms rs4588 and rs7041, which are located in exon 11, were in linkage disequilibrium, making it difficult to discern the best single SNP surrogate to detect genetic variability for this region. Indeed, rs4588 and rs7041, described as having an association with 25(OH)D levels, were in linkage disequilibrium in a healthy population of girls from Southern Brazil [24].
One strength of our study is the focus on a less well represented ethnic group, PCOS women from Southern Brazil. Conversely, a limitation was the relatively small sample size of 291 participants, which does not allow supplemental analyses. However, the effect sizes observed in our sample are similar to those reported in other PCOS populations. Another limitation was the lack of data on DBP levels of participants. Nevertheless, DBP gene polymorphism seems to have no effect on the relationship between 25(OH)D and parathyroid hormone in infants and toddlers [21]. Other possible limitations are the lack of data on dietary vitamin D intake and daily sun exposure, even though it is well recognized that, below a latitude of approximately 35°, UVB radiation is sufficient for year-round vitamin D synthesis [52]; furthermore, the season of blood collection seems not to interfere with vitamin D levels, as we have previously shown in another group living in the same region as the present sample [24]. While DBP measurements were not available in the present study, previous studies have shown that DBP levels are associated with DBP gene polymorphisms [43, 53] and positively correlated with 25(OH)D levels [21].
Conclusions
The present study is the first to describe that genotype TT of SNP rs7041 is associated with MetS in PCOS and with lower 25(OH)D levels in both PCOS and healthy controls with regular ovulatory cycles. Our results indicate that polymorphisms rs2282679, rs4588, and rs7041 of the DBP gene, as well as their haplotypes, are not related to PCOS in southern Brazilian women. Further studies in PCOS populations of different ethnicities are needed to confirm these findings.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico/Brazilian National Institute of Hormones and Women's Health (CNPq/INCT 573747/2008-3) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Post-Doc grant to BRS). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2745–9. [DOI] [PubMed] [Google Scholar]
- 2.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar-Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. Journal of Clinical Endocrinology & Metabolism. 2000;85(7):2434–8. [DOI] [PubMed] [Google Scholar]
- 3.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human Reproduction. 2010;25(2):544–51. 10.1093/humrep/dep399 [DOI] [PubMed] [Google Scholar]
- 4.Graff SK, Mario FM, Alves BC, Spritzer PM. Dietary glycemic index is associated with less favorable anthropometric and metabolic profiles in polycystic ovary syndrome women with different phenotypes. Fertility and Sterility. 2013;100(4):1081–8. 10.1016/j.fertnstert.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 5.Di Domenico K, Wiltgen D, Nickel FJ, Magalhaes JA, Moraes RS, Spritzer PM. Cardiac autonomic modulation in polycystic ovary syndrome: does the phenotype matter? Fertility and Sterility. 2013;99(1):286–92. 10.1016/j.fertnstert.2012.08.049 [DOI] [PubMed] [Google Scholar]
- 6.Aydin Y, Hassa H, Burkankulu D, Arslantas D, Sayiner D, Ozerdogan N. What is the Risk of Metabolic Syndrome in Adolescents with Normal BMI who have Polycystic Ovary Syndrome? Journal of Pediatric and Adolescent Gynecology. 2015;28(4):271–4. 10.1016/j.jpag.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 7.Hahn S, Haselhorst U, Tan S, Quadbeck B, Schmidt M, Roesler S, et al. Low serum 25-hydroxyvitamin D concentrations are associated with insulin resistance and obesity in women with polycystic ovary syndrome. Experimental and Clinical Endocrinology & Diabetes. 2006;114(10):577–83. [DOI] [PubMed] [Google Scholar]
- 8.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. European Journal of Endocrinology. 2009;161(4):575–82. 10.1530/EJE-09-0432 [DOI] [PubMed] [Google Scholar]
- 9.Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Archives of Gynecology and Obstetrics. 2009;280(4):559–63. 10.1007/s00404-009-0958-7 [DOI] [PubMed] [Google Scholar]
- 10.Irani M, Merhi Z. Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertility and Sterility. 2014;102(2):460–8. 10.1016/j.fertnstert.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 11.He CL, Lin ZM, Robb SW, Ezeamama AE. Serum Vitamin D Levels and Polycystic Ovary syndrome: A Systematic Review and Meta-Analysis. Nutrients. 2015;7(6):4555–77. 10.3390/nu7064555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joham AE, Teede HJ, Cassar S, Stepto NK, Strauss BJ, Harrison CL, et al. Vitamin D in polycystic ovary syndrome: Relationship to obesity and insulin resistance. Molecular Nutrition & Food Research. 2016;60(1):110–8. [DOI] [PubMed] [Google Scholar]
- 13.Mishra S, Das AK, Das S. Hypovitaminosis D and Associated Cardiometabolic Risk in Women with PCOS. Journal of Clinical Diagnostic Ressearch. 2016;10(5):BC01–BC4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palomer X, Gonzalez-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obesity & Metabolism. 2008;10(3):185–97. [DOI] [PubMed] [Google Scholar]
- 15.Speeckaert M, Huang GM, Delanghe JR, Taes YEC. Biological and clinical aspects of the vitamin D binding protein (Gc-globulin) and its polymorphism. Clinica Chimica Acta. 2006;372(1–2):33–42. [DOI] [PubMed] [Google Scholar]
- 16.Chun RF. New perspectives on the vitamin D binding protein. Cell Biochemistry and Function. 2012;30(6):445–56. 10.1002/cbf.2835 [DOI] [PubMed] [Google Scholar]
- 17.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739–45. 10.1093/hmg/ddq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376(9736):180–8. 10.1016/S0140-6736(10)60588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinotte M, Diorio C, Berube S, Pollak M, Brisson J. Genetic polymorphisms of the vitamin D binding protein and plasma concentrations of 25-hydroxyvitamin D in premenopausal women. American Journal of Clinical Nutrition. 2009;89(2):634–40. 10.3945/ajcn.2008.26445 [DOI] [PubMed] [Google Scholar]
- 20.Gozdzik A, Zhu J, Wong BYL, Fu L, Cole DEC, Parra EJ. Association of vitamin D binding protein (VDBP) polymorphisms and serum 25(OH)D concentrations in a sample of young Canadian adults of different ancestry. J Steroid Biochem Mol Biol. 2011;127(3–5):405–12. 10.1016/j.jsbmb.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Carpenter TO, Zhang JH, Parra E, Ellis BK, Simpson C, Lee WL, et al. Vitamin D Binding Protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. Journal of Bone and Mineral Research. 2013;28(1):231–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu L, Sheng H, Li H, Gan W, Liu C, Zhu J, et al. Associations between common variants in GC and DHCR7/NADSYN1 and vitamin D concentration in Chinese Hans. Human Genetics. 2012;131(3):505–12. 10.1007/s00439-011-1099-1 [DOI] [PubMed] [Google Scholar]
- 23.Robien K, Butler LM, Wang R, Beckman KB, Walek D, Koh WP, et al. Genetic and environmental predictors of serum 25-hydroxyvitamin D concentrations among middle-aged and elderly Chinese in Singapore. British Journal of Nutrition. 2013;109(3):493–502. 10.1017/S0007114512001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos BR, Mascarenhas LPG, Boguszewski MCS, Spritzer PM. Variations in the Vitamin D-Binding Protein (DBP) Gene Are Related to Lower 25-Hydroxyvitamin D Levels in Healthy Girls: A Cross-Sectional Study. Hormone Research in Paediatrics. 2013;79(3):162–8. [DOI] [PubMed] [Google Scholar]
- 25.Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Obermayer-Pietsch B. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. European Journal of Endocrinology. 2011;164(5):741–9. 10.1530/EJE-11-0134 [DOI] [PubMed] [Google Scholar]
- 26.El-Shal AS, Shalaby SM, Aly NM, Rashad NM, Abdelaziz AM. Genetic variation in the vitamin D receptor gene and vitamin D serum levels in Egyptian women with polycystic ovary syndrome. Molecular Biology Reports. 2013;40(11):6063–73. 10.1007/s11033-013-2716-y [DOI] [PubMed] [Google Scholar]
- 27.Dasgupta S, Dutta J, Annamaneni S, Kudugunti N, Battini MR. Association of vitamin D receptor gene polymorphisms with polycystic ovary syndrome among Indian women. Indian Journal of Medical Research. 2015;142(3):276–85. 10.4103/0971-5916.166587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirai M, Suzuki S, Hinokio Y, Hirai A, Chiba M, Akai H, et al. Variations in vitamin D-binding protein (group-specific component protein) are associated with fasting plasma insulin levels in Japanese with normal glucose tolerance. Journal of Clinical Endocrinology & Metabolism. 2000;85(5):1951–3. [DOI] [PubMed] [Google Scholar]
- 29.Baier LJ, Dobberfuhl AM, Pratley RE, Hanson RL, Bogardus C. Variations in the vitamin D-binding protein (Gc locus) are associated with oral glucose tolerance in nondiabetic Pima Indians. Journal of Clinical Endocrinology & Metabolism. 1998;83(8):2993–6. [DOI] [PubMed] [Google Scholar]
- 30.Ye WZ, Dubois-Laforgue D, Bellanne-Chantelot C, Timsit J, Velho G. Variations in the vitamin D-Binding protein (Gc locus) and risk of type 2 diabetes mellitus in French Caucasians. Metabolism Clinical and Experimental. 2001;50(3):366–9. 10.1053/meta.2001.20172 [DOI] [PubMed] [Google Scholar]
- 31.Toscani M, Mighavacca R, Sisson de Castro JA, Spritzer PM. Estimation of truncal adiposity using waist circumference or the sum of trunk skinfolds: a pilot study for insulin resistance screening in hirsute patients with or without polycystic ovary syndrome. Metabolism Clinical and Experimental. 2007;56(7):992–7. 10.1016/j.metabol.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 32.Ramos RB, Spritzer PM. FTO gene variants are not associated with polycystic ovary syndrome in women from Southern Brazil. Gene. 2015;560(1):25–9. 10.1016/j.gene.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 33.Ramos RB, Wiltgen D, Spritzer PM. Polymorphisms of TCF7L2 gene in South Brazilian women with polycystic ovary syndrome: a cross-sectional study. European Journal of Endocrinology. 2013;169(5):569–76. 10.1530/EJE-13-0105 [DOI] [PubMed] [Google Scholar]
- 34.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. Journal of Clinical Endocrinology and Metabolism. 1961;21(11):1440–1447. [DOI] [PubMed] [Google Scholar]
- 35.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 36.Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 37.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical Chemistry. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 38.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):1215-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68(4):978–89. 10.1086/319501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michos ED, Misialek JR, Selvin E, Folsom AR, Pankow JS, Post WS, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241(1):12–7. 10.1016/j.atherosclerosis.2015.04.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takiar R, Lutsey PL, Zhao D, Guallar E, Schneider ALC, Grams ME, et al. The associations of 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms, and race with risk of incident fracture-related hospitalization: Twenty-year follow-up in a bi-ethnic cohort (the ARIC Study). Bone. 2015;78:94–101. 10.1016/j.bone.2015.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang Y, van Meurs JBJ, Arp P, van Leeuwen JPT, Hofman A, Pols HAP, et al. Vitamin D Binding Protein Genotype and Osteoporosis. Calcified Tissue International. 2009;85(2):85–93. 10.1007/s00223-009-9251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lauridsen AL, Vestergaard P, Hermann AP, Brot C, Heickendorff L, Mosekilde L, et al. Plasma concentrations of 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D are related to the phenotype of Gc (vitamin D-binding protein): A cross-sectional study on 595—Early postmenopausal women. Calcified Tissue International. 2005;77(1):15–22. 10.1007/s00223-004-0227-5 [DOI] [PubMed] [Google Scholar]
- 44.Reinchr T, de Sousa G, Alexy U, Kersting M, Andler W. Vitamin D status and parathyroid hormone in obese children before and after weight loss. European Journal of Endocrinology. 2007;157(2):225–32. 10.1530/EJE-07-0188 [DOI] [PubMed] [Google Scholar]
- 45.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism Clinical and Experimental. 2008;57(2):183–91. 10.1016/j.metabol.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 46.Ashraf A, Alvarez J, Saenz K, Gower B, McCormick K, Franklin F. Threshold for Effects of Vitamin D Deficiency on Glucose Metabolism in Obese Female African-American Adolescents. Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reis JP, von Muhlen D, Miller ER, Michos ED, Appel LJ. Vitamin D Status and Cardiometabolic Risk Factors in the United States Adolescent Population. Pediatrics. 2009;124(3):371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delvin EE, Lambert M, Levy E, O'Loughlin J, Mark S, Gray-Donald K, et al. Vitamin D Status Is Modestly Associated with Glycemia and Indicators of Lipid Metabolism in French-Canadian Children and Adolescents. Journal of Nutrition. 2010;140(5):987–91. 10.3945/jn.109.112250 [DOI] [PubMed] [Google Scholar]
- 49.Johnson MD, Nader NS, Weaver AL, Singh R, Kumar S. Relationships between 25-Hydroxyvitamin D Levels and Plasma Glucose and Lipid Levels in Pediatric Outpatients. Journal of Pediatrics. 2010;156(3):444–9. 10.1016/j.jpeds.2009.09.070 [DOI] [PubMed] [Google Scholar]
- 50.Taes YEC, Goemaere S, Huang GM, Van Pottelbergh I, De Bacquer D, Verhasselt B, et al. Vitamin D binding protein, bone status and body composition in community-dwelling elderly men. Bone. 2006;38(5):701–7. 10.1016/j.bone.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 51.Winters SJ, Chennubhatla R, Wang CX, Miller JJ. Influence of obesity on vitamin D-binding protein and 25-hydroxy vitamin D levels in African American and white women. Metabolism Clinical and Experimental. 2009;58(4):438–42. 10.1016/j.metabol.2008.10.017 [DOI] [PubMed] [Google Scholar]
- 52.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin-D3—exposure to winter sunlight in Boston and Edmonton will not promote vitamin-D3 synthesis in human-skin. Journal of Clinical Endocrinology & Metabolism. 1988;67(2):373–8. [DOI] [PubMed] [Google Scholar]
- 53.Lauridsen AL, Vestergaard P, Nexo E. Mean serum concentration of vitamin D-binding protein (Gc globulin) is related to the Gc phenotype in women. Clinical Chemistry. 2001;47(4):753–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.