Abstract
Emerging evidence suggests that regular physical activity can impact cortical function and facilitate plasticity. In the present study, we examined how physical activity levels influence corticospinal excitability and intracortical circuitry in motor cortex following a single session of moderate intensity aerobic exercise. We aimed to determine whether exercise-induced short-term plasticity differed between high versus low physically active individuals. Participants included twenty-eight young, healthy adults divided into two equal groups based on physical activity level determined by the International Physical Activity Questionnaire: low-to-moderate (LOW) and high (HIGH) physical activity. Transcranial magnetic stimulation was used to assess motor cortex excitability via motor evoked potential (MEP) recruitment curves for the first dorsal interosseous (FDI) muscle at rest (MEPREST) and during tonic contraction (MEPACTIVE), short-interval intracortical inhibition (SICI) and facilitation (SICF), and intracortical facilitation (ICF). All dependent measures were obtained in the resting FDI muscle, with the exception of AMT and MEPACTIVE recruitment curves that were obtained during tonic FDI contraction. Dependent measures were acquired before and following moderate intensity aerobic exercise (20 mins, ~60% of the age-predicted maximal heart rate) performed on a recumbent cycle ergometer. Results indicate that MEPREST recruitment curve amplitudes and area under the recruitment curve (AURC) were increased following exercise in the HIGH group only (p = 0.002 and p = 0.044, respectively). SICI and ICF were reduced following exercise irrespective of physical activity level (p = 0.007 and p = 0.04, respectively). MEPACTIVE recruitment curves and SICF were unaltered by exercise. These findings indicate that the propensity for exercise-induced plasticity is different in high versus low physically active individuals. Additionally, these data highlight that a single session of aerobic exercise can transiently reduce inhibition in the motor cortex regardless of physical activity level, potentially priming the system for plasticity induction.
Introduction
Regular aerobic exercise can influence cellular [1–6] and molecular [5, 7, 8] processes thereby altering hippocampal and sub-cortical loci [1–6] as well as increasing levels of key neurotrophic factors, such as brain derived neurotrophic factor (BDNF) [7], that mediate neuroplasticity [9]. Further, executive function, such as response inhibition and processing speed, is enhanced in physically active individuals [1, 2, 5, 10, 11]. Thus, long-term exercise appears to facilitate cognitive function and memory through neuroplasticity and neuroprotective mechanisms. Additionally, regular exercise leads to chronic physiological adaptions that impact the response to a single session of aerobic exercise (see review: [12]). These adaptions include increased metabolic efficiency at a given heart rate [12], increased stroke volume [13], increased brain perfusion [14], and alterations in individual responses to stress, anxiety, and perceived rate of exertion [12]. As such, individuals who regularly exercise may respond differently to a single session of exercise than those who do not.
A single session of aerobic exercise has the capacity to induce short-term neuroplasticity within the human motor cortex, as assessed via cortical circuits evoked by transcranial magnetic stimulation (TMS). For example, intracortical facilitation (ICF) increases [15] while short-interval intracortical inhibition (SICI) decreases [15, 16] or does not change [17] following a single session of exercise. ICF is thought to reflect N-methylD-aspartate (NMDA) receptors [18], while SICI is mediated via GABAA receptor activity [19, 20]. Both ICF and SICI have been implicated in cortical reorganization and plasticity within the primary motor cortex [15, 21]. Further, motor evoked potentials (MEPs), a measure of corticospinal excitability [22, 23], are unchanged following a single session of aerobic exercise in individuals who are relatively sedentary [15, 16]. Aerobic exercise, therefore, provides an opportunity to create short-term changes in specific TMS circuits that may serve as targets for promoting neuroplasticity. Finally, a single session of aerobic exercise can be used to supplement other plasticity inducing approaches to yield greater effects [24–26].
MEPs can be obtained in the resting or actively contracted muscle to assess distinct mechanisms of corticospinal excitability [22, 23]. To date, no studies have investigated whether exercise induces short-term changes in MEPs in actively contracted muscles. Compared to relaxed muscle, voluntarily activation reduces the threshold for TMS activation of motor neurons [23]. Therefore, quantifying MEPs in the active versus resting state assesses corticospinal output with and without the voluntary activation of motor neurons. For example, MEPs are reduced in individuals with spinal cord injury [27] and Parkinson’s disease (PD) [28] compared to controls when they are measured in the active but not resting muscle. Therefore, it is important to determine whether corticospinal excitability can be modulated with aerobic exercise via either of these mechanisms.
Short interval intracortical facilitation (SICF) is considered to reflect GABAA receptor activity [29] and is facilitated in PD [30] and Fabry’s disease [31]. SICF is unchanged following repetitive TMS [32, 33]. However, the natural stimulus of aerobic exercise may have the capacity to alter SICF and operate as a method to create neuroplasticity in these populations. To date, no studies have investigated the impact of a single session of aerobic exercise on the SICF circuit, yet this information may benefit clinical neuroscience research.
The type of exercise and the population tested may influence the opportunity for exercise-induced short-term plasticity. First, aerobic exercise is favored as it increases BDNF more frequently than resistance training (see review [34]). Second, physical activity levels influence the propensity for plasticity. For example, paired associative stimulation (PAS) induces short-term plasticity only in the corticospinal output of physically active individuals [35]. The effects of physical activity levels on exercise-induced plasticity have yet to be investigated.
In the present study, we aimed to identify the TMS circuits that are modulated following a single session of aerobic exercise and to determine if physical activity levels influence the magnitude of exercise-induced plasticity in these circuits. To assess plasticity, we measured TMS-evoked circuits including MEP recruitment curves in the resting and active states, SICI, ICF, and SICF in a resting hand muscle before and after aerobic exercise. To our knowledge, this is the first study to investigate the influence of physical activity levels on exercise-induced plasticity in these circuits. Our data indicate that a single session of aerobic exercise induces changes in resting MEP recruitment curves, SICI, and ICF, confirming the capability for exercise to induce short-term plasticity. These findings suggest that aerobic exercise, as a plasticity-inducing paradigm, has differential effects on corticospinal excitability depending on physical activity level.
Methods
Participants
Twenty-eight young adults who self-identified as physically active (HIGH: N = 14, 22.1 ± 2.8 years, 9 female) or sedentary (LOW: N = 14, 20.6 ± 0.84, 8 female) participated in this study. All participants were right-hand dominant as determined by the modified version of the Edinburgh Handedness Scale [36] and had no known history of neurological disease. Participants were screened for contraindications to TMS [37] and exercise using the Physical Activity Readiness Questionnaire (PAR-Q) [38]. Written informed consent was obtained prior to participation. This study was approved by the McMaster Research Ethics Board and conformed to the Declaration of Helsinki.
Physical activity levels were determined using the International Physical Activity Questionnaire (IPAQ) that quantifies the physical activity performed in the past week [39]. Participants who accumulated more than 3000 metabolic equivalents (METs) on IPAQ were considered highly physically active (HIGH), while those who accumulated less than 3000 METs were considered low-to-moderately active (LOW) [16, 40]. The IPAQ scores were significantly higher for the HIGH (METs 7631 ± 6120; 3231–21162) versus LOW (METs 1305 ± 773; 360–2892) group (p < 0.001). To verify the grouping of participants in the HIGH and LOW categories, the Minnesota Leisure Activity Questionnaire [41] was used to assess long term physical activity levels in each participant. These data confirm the division of HIGH (7441 ± 6157) and LOW (965 ± 1062) activity levels (p < 0.001) in the sample tested.
Electromyography (EMG) recording
Electromyography (EMG) was recorded using surface electrodes (9 mm diameter Ag-AgCl) over first dorsal interosseous (FDI) muscle of the right hand in a belly tendon montage. A wet ground electrode was placed around the forearm. EMG measurements were amplified (x1000), and filtered with a band pass (20 Hz—2.5 kHz) (Intronix Technologies Corporation Model 2024F with Signal Conditioning; Intronix Technologies Corporation, Bolton, Canada) and subsequently digitized at 5 kHz (Power1401, Cambridge Electronic Design, Cambridge, UK). EMG data was collected using Signal software version 6.02 (Cambridge Electronic Design, Cambridge, UK).
Maximum Voluntary Contraction (MVC)
Participants completed three maximal isometric contractions of the FDI against an immovable structure. Each contraction persisted for 5 s with a 30 s rest interval between trials. The largest maximum EMG activity obtained from the three trials was deemed the maximum voluntary contraction (MVC) of FDI for a given participant. The level of EMG corresponding to the MVC was displayed to the participant on an oscilloscope. The voltage that corresponded to 10% of MVC was calculated and displayed on the oscilloscope with a horizontal target line. The participant then performed a contraction of FDI to move a second horizontal line controlled by their EMG to match the position of the target line. Therefore, participants used their own visual feedback to maintain the 10% MVC during the acquisition of active motor threshold and active MEP recruitment curve (see below).
Transcranial Magnetic Stimulation (TMS)
Single and paired monophasic TMS pulses were delivered using a custom-built 50 mm diameter figure-of-eight branding coil connected to a Magstim Bistim stimulator (Magstim, Whitland, UK). The TMS coil was placed over the optimal location to elicit MEPs in the relaxed right FDI. Coil was positioned 45 degrees in relation to the parasagittal plane to induce posterior-to-anterior current in the cortex. The motor hotspot for FDI muscle of the right hand was determined within the left hemisphere motor cortex and defined as the location that consistently elicited MEPs in the relaxed FDI muscle. The motor hotspot was marked by digital registration using a standard MRI template via Brainsight Neuronavigation (Rogue Research, Canada). Resting motor threshold (RMT) and active motor threshold (AMT) were determined for the right FDI. RMT was defined as the lowest intensity required to evoke an MEP ≥ 50 μV in 5 out of 10 consecutive trials in relaxed FDI muscle [37]. AMT was defined as the lowest intensity required to evoke an MEP ≥ 200 μV in 5 out of 10 consecutive trials while participants maintained ~10% of their MVC in right FDI [37]. Visual feedback of the right FDI contraction was provided using an oscilloscope.
Motor Evoked Potential (MEP) recruitment curves
MEP recruitment curves were obtained in the resting and active (~10% MVC) right FDI muscle. For each curve, three stimuli were delivered at each of 90%, 100%, 110%, 120%, 130%, 140% and 150% RMT or AMT in a randomized order. The number of pulses delivered at each intensity reflects the results of recent studies examining the consistency of MEPs with few stimuli [42, 43].
Short-interval Intracortical Inhibition (SICI) and Intracortical Facilitation (ICF)
SICI and ICF were investigated using interstimulus intervals (ISI) of 2 ms and 10 ms between the conditioning stimulus (CS) and test stimulus (TS), respectively. Each circuit was tested with CS intensities of 80% and 90% AMT and with TS set to evoke MEPs with peak-to-peak amplitudes of ~1 mV in the right FDI at rest. For each circuit, 20 trials were acquired whereby equal numbers of unconditioned (i.e. MEPTS) and conditioned (MEPCS-TS) trials were randomly delivered.
Short-interval Intracortical Facilitation (SICF)
SICF was measured using a similar method to that described by Ziemann et al. [44]. SICF was investigated using ISIs of 1.2 ms and 2.5 ms and was recorded in two blocks of 20 trials for each ISI (20 at 1.2 ms, 20 at 2.5 ms; 10 TS and 10 CS/TS each block). CS intensity was set to 90% RMT for both ISIs. The TS intensity was set to evoke MEPs with peak-to-peak amplitudes of ~1 mV in the right FDI at rest.
Experimental design
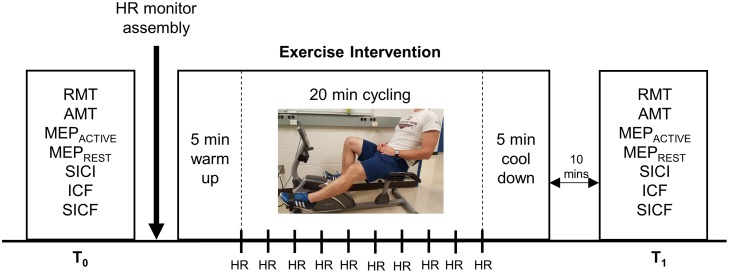
The experimental timeline is depicted in Fig 1. The session was divided into two time blocks: T0 (pre-exercise) and T1 (10 minutes post-exercise). All dependent measures were obtained in the resting FDI muscle, with the exception of AMT and MEPACTIVE recruitment curves that were obtained during tonic FDI contraction corresponding to ~10% MVC. The TMS intensity to elicit ~1 mV response in the resting FDI was re-assessed prior to the start of T1. The order of dependent measure acquisition was pseudorandomized (William Square Counterbalance).
Fig 1. Experimental timeline.
Measures of resting motor threshold (RMT), active motor threshold (AMT), motor evoked potential (MEP) recruitment curves obtained at rest (MEPREST) and during ~10% MVC (MEPACTIVE), short-interval intracortical inhibition (SICI), intracortical facilitation (ICF) and short-interval intracortical facilitation (SICF) were acquired prior to (T0) and ten minutes following the cessation of exercise (T1).The order of dependent measure acquisition was pseudo-randomized across participants using a Williams Square design. The exercise intervention began following the assembly of the heart rate (HR) monitor and involved 5 minutes of cycling warm-up, 20 minutes of moderate-intensity exercise (50–70% of age-predicted maximal heart rate (HR)) and 5 minutes of cycling cool-down. During the 20-minute exercise, resistance was adjusted online to maintain HR in the target range. HR was recorded every 2 minutes as shown.
Exercise procedures
Participants completed a lower limb exercise on a recumbent cycle ergometer (Exerpeutic Heavy Duty Magnetic Recumbent Bike with Pulse, PARADIGM Health & Wellness, USA). The exercise session included 5 minutes of warm up at a comfortable pace, 20 minutes of moderate intensity exercise at 50–70% age-predicted maximal heart rate (i.e. 220 –age) [45], followed by 5 minutes of cool down at a comfortable pace. All individuals were maintained at ~60% of their age-predicted maximal heart rate, as performed elsewhere [15, 40]. Heart rate was monitored using an FT1 Polar heart rate monitor (FT1/FT2 watch and chest strap—Polar, Australia) and recorded by the experimenter every two minutes during the 20-minute exercise period. This information was used to adjust the resistance on the ergometer to maintain the targeted age-predicted heart rate range. Participants were instructed to maintain a cycling speed of 8–12 miles per hour, as indicated on the ergometer display available to them. Throughout the exercise, participants kept their arms comfortably relaxed in their lap to ensure that the FDI muscle, the target muscle for TMS-evoked measures, was not active during the exercise intervention.
Data analysis and statistics
For MEP recruitment curves, the mean peak-to-peak amplitude was calculated for each RMT and AMT intensity (90–150%). All slopes were calculated in Microsoft Excel using linear regression of the entire recruitment curve and were subsequently tested for correlation with IPAQ scores. The area under the recruitment curve (AURC) was obtained by taking a trapezoidal integration of the recruitment curves. For paired-pulse TMS measures, the mean peak-to-peak MEP amplitude was calculated for the conditioned and unconditioned stimuli at each CS or ISI intensity separately. The percentage inhibition and facilitation was then calculated as a ratio of conditioned over unconditioned stimulus (CS-TS/TS). For each individual, the depth of the SICI and ICF was examined at T0 for each of the two CS intensities tested (80%, 90% AMT). The CS intensity at which the greatest depth was observed at T0 was chosen for further analyses. Since reductions in SICI were hypothesized from previous research [15, 16], participants were required to demonstrate a minimum of 5% SICI at T0 to be included in the SICI analysis. Additionally, since increases in facilitation were hypothesized [15] individuals were required to present with a minimum of 5% ICF or SICF at T0 to be included in the ICF or SICF analysis.
Group-level statistics included normality testing via the Shapiro-Wilk analysis. Outlier data were identified via SPSS, as 3 times above or below the interquartile range and were removed from further analyses. Specifically, one participant was removed from the HIGH group for resting and active MEP recruitment curves, and one participant was removed from the LOW group for SICI, ICF, and SICF1.2ms. Non-normally distributed data was ranked and a non-parametric Conover’s ANOVA was performed [46]. MEP recruitment curves were analyzed using three-way Conover’s ANOVA using within-subject factors TIME (2 levels: T0, T1) and factor INTENSITY (7 levels: 90, 100, 110, 120, 130, 140, 150% RMT/AMT) and between-subject factors GROUP (2 levels: LOW, HIGH). RMT, AMT, and SICI were analyzed using two-way ANOVA while AURC, ICF, and SICF were analyzed using a two-way Conover’s ANOVA with within-subject factor TIME (2 levels: T0, T1) and between-subject factor GROUP (2 levels: LOW, HIGH). For normally distributed dependent measures, post-hoc testing was performed using two-tailed t-tests. Post-hoc tests for non-parametric data included a Wilcoxon Signed-Rank for within group comparisons and Mann-Whitney U test for between group differences. All post-hoc testing was Bonferroni corrected. The significance level was set to p ≤ 0.05.
Results
All participants successfully completed the study and performed the aerobic exercise at ~60% of their age-predicted maximal heart rate (LOW: 60.5 ± 1.9%, HIGH: 58.9 ± 3.3%, p = 0.154). Table 1 displays the results of all statistical analyses with associated effect sizes and 95% confidence intervals.
Table 1. Statistical analyses.
| Dependent Measure | ANOVA |
|---|---|
| RMT | TIME (1,26) = 0.101, p = 0.753 |
| GROUP(1,26) = 0.042, p = 0.839 | |
| TIME x GROUP(1,26) = 9.449, p = 0.005 # | |
| L (N = 14): T0: 38.6 ± 1.62%MSO T1: 37.5 ± 1.79%MSO, d = 0.17 | |
| H (N = 14): T0: 37.1 ± 1.93%MSO T1: 38.0 ± 2.09%MSO, d = 0.12 | |
| MEPREST Amplitude* | TIME (1,25) = 0.577, p = 0.455 |
| GROUP(1,25) = 1.447, p = 0.240 | |
| INTENSITY(6,20) = 79.32, p = 0.000 | |
| TIME x GROUP(1,25) = 4.788, p = 0.038 | |
| H: T0<T1 p = 0.002, d = 0.17, 95% CI: -0.93 to 0.61 | |
| TIME x INTENSITY(6,20) = 0.761, p = 0.609 | |
| INTENSITY x GROUP(6,20) = 0.798, p = 0.583 | |
| TIME x GROUP x INTENSITY(6,20) = 1.594, p = 0.201 | |
| MEPREST AURC* | TIME (1,26) = 0.178, p = 0.676 |
| GROUP(1,26) = 3.914, p = 0.059 | |
| TIME x GROUP(1,26) = 6.10, p = 0.02 | |
| H: T0<T1 p = 0.044, d = 0.54, 95% CI: -1.27 to 0.23 (uncorrected) | |
| AMT | TIME (1,26) = 1.204, p = 0.283 |
| GROUP(1,26) = 0.278, p = 0.603 | |
| TIME x GROUP(1,26) = 1.873, p = 0.187 | |
| L (N = 14): T0: 26.1 ± 0.84%MSO T1: 24.7 ± 0.96%MSO, d = 0.42 | |
| H (N = 14): T0: 24.5 ± 1.31%MSO T1: 24.6 ± 1.45%MSO, d = 0.02 | |
| MEPACTIVE Amplitude* | TIME (1,25) = 3.554, p = 0.071 |
| GROUP(1,26) = 0.365 p = 0.551 | |
| INTENSITY(6, 20) = 55.17, p = 0.000 | |
| TIME x GROUP(1,25) = 0.001, p = 0.981 | |
| TIME x INTENSITY(6, 20) = 0.617, p = 0.714 | |
| INTENSITY x GROUP(6, 20) = 1.406, p = 0.261 | |
| TIME x GROUP x INTENSITY(6, 20) = 1.34, p = 0.286 | |
| MEPACTIVE AURC* | TIME (1,26) = 0.084, p = 0.775 |
| GROUP(1,26) = 0.453, p = 0.507 | |
| TIME x GROUP(1,26) = 0.44, p = 0.513 | |
| L (N = 14): T0: 6.08 ± 0.67 AURC T1: 4.73 ± 0.52 AURC, d = 0.60 | |
| H (N = 14): T0: 5.99 ± 1.01 AURC T1: 5.25 ± 0.76 AURC, d = 0.22 | |
| SICI | TIME(1,22) = 8.674, p = 0.007 |
| T0<T1, p = 0.007, d = 0.512, 95% CI: -1.08 to 0.07 | |
| GROUP(1,22) = 0.168, p = 0.686 | |
| TIME x GROUP(1,22) = 0.380, p = 0.544 | |
| SICI TS | TIME(1,23) = 0.036, p = 0.851 |
| L (N = 13): T0: 1.03 ± 0.04 mV T1: 1.13 ± 0.06 mV, d = 0.54 | |
| H (N = 11): T0: 1.16 ± 0.03 mV T1: 1.06 ± 0.06 mV, d = 0.57 | |
| ICF* | TIME(1,22) = 5.268, p = 0.032 |
| T0>T1, p = 0.04, d = 0.71, 95% CI: -0.14 to 1.51 | |
| GROUP(1,22) = 0.000, p = 0.983 | |
| TIME x GROUP(1,22) = 0.222, p = 0.642 | |
| ICF TS | TIME(1,23) = 0.060 p = 0.808 |
| L (N = 12): T0: 1.05 ± 0.08 mV T1: 1.12 ± 0.09 mV, d = 0.25 | |
| H (N = 12): T0: 1.20 ± 0.08 mV T1: 1.18 ± 0.06 mV, d = 0.07 | |
| SICF1.2ms * | TIME (1,24) = 3.681, p = 0.067 |
| GROUP(1,24) = 1.677, p = 0.208 | |
| TIME x GROUP(1,24) = 5.274, p = 0.031 # | |
| L (N = 13): T0: 1.61 ± 0.14 mV T1: 1.65 ± 0.13 mV, d = 0.08 | |
| H (N = 13): T0: 2.12 ± 0.22 mV T1: 1.76 ± 0.21 mV, d = 0.46 | |
| SICF1.2ms TS | TIME(1,25) = 1.579, p = 0.221 |
| L (N = 13): T0: 0.96 ± 0.08 mV T1: 0.96 ± 0.07 mV, d = 0.00 | |
| H (N = 13): T0: 0.99 ± 0.06 mV T1: 1.16 ± 0.07 mV, d = 0.74 | |
| SICF2.5ms* | TIME(1,22) = 1.247, p = 0.276 |
| GROUP(1,22) = 0.604, p = 0.445 | |
| TIME x GROUP(1,22) = 0.455, p = 0.507 | |
| L (N = 13): T0: 1.88 ± 0.21 mV T1: 1.91 ± 0.23 mV, d = 0.04 | |
| H (N = 11): T0: 1.68 ± 0.14 mV T1: 1.53 ± 0.17 mV, d = 0.40 | |
| SICF2.5ms TS | TIME(1,25) = 0.042, p = 0.840 |
| L (N = 13): T0: 0.98 ± 0.06 mV T1: 1.04 ± 0.06 mV, d = 0.28 | |
| H (N = 11): T0: 1.09 ± 0.09 mV T1: 1.05 ± 0.09 mV, d = 0.14 |
*Conover’s ANOVA (ranked data) and subsequent non-parametric post-hoc analyses.
#: post-hoc analyses did not pass Bonferroni corrections.
Bolded values indicate significance as shown. Means ± SE displayed. d: Cohen’s D, 95% CI: 95% confidence intervalof effect size, T0 (baseline), T1 (10 minutes post-exercise), L (LOW group), H (HIGH group).
Thresholds and MEP recruitment curves
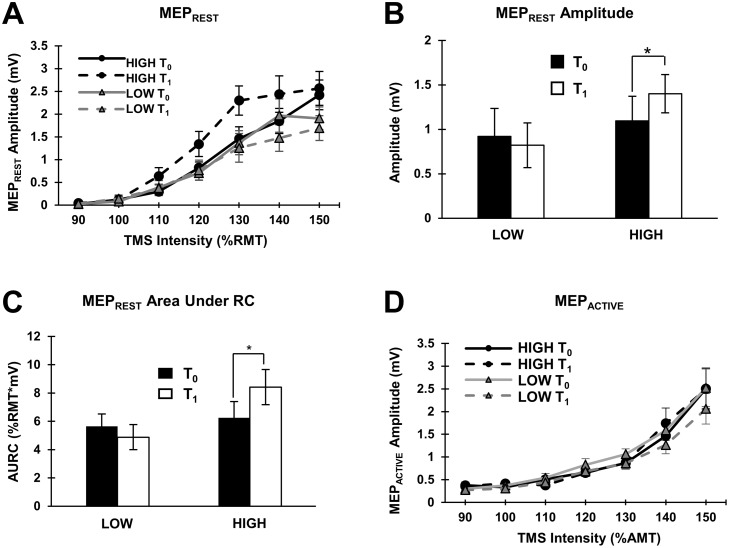
All participants were included in RMT analysis (N = 14 per group). The group-averaged RMT showed no significant differences among the means (Table 1). Thirteen and fourteen individuals were included in MEPREST analysis for the HIGH and LOW groups, respectively. Group-averaged MEPREST recruitment curves (with standard errors) are shown in Fig 2A and analyses revealed a significant TIME x GROUP interaction (p = 0.038; Table 1) such that amplitudes were significantly increased in the HIGH group following exercise (Wilcoxon: p = 0.002; Fig 2B). This effect was observed in nine individuals, with little to no change in three individuals and a decrease in MEPs following exercise in one individual. MEPREST AURC (N = 14 per group) also revealed a significant TIME x GROUP interaction (p = 0.02; Table 1) such that AURC increased following exercise in the HIGH group only (Wilcoxon: p = 0.04, Fig 2C). All participants were included in AMT analysis (N = 14 per group). AMT did not statistically differ between groups and was unchanged following exercise (Table 1). Thirteen and fourteen individuals were included in the MEPACTIVE analysis for the HIGH and LOW groups, respectively. Similarly, the MEPACTIVE recruitment curves (shown in Fig 2D) and MEPACTIVE AURC were unchanged following exercise (Table 1). IPAQ scores did not correlate with the percent change in slopes for MEPREST or MEPACTIVE recruitment curves (p > 0.05).
Fig 2. Thresholds and recruitment curves.
(A) Group-averaged MEPREST recruitment curves (with standard errors) at T0 and T1 for the LOW (N = 14) and HIGH (N = 13) groups. TMS intensity is defined as the percentage of RMT. Solid and dashed lines indicate pre (T0) and post (T1) values, respectively. (B) Histograms displaying TIME x GROUP interaction for group-averaged MEPREST amplitude (with standard error; LOW: N = 14, HIGH: N = 13). The asterisk indicates a significant increase in MEPREST amplitudes. (C) Histograms displaying TIME x GROUP interaction for group-averaged MEPREST AURC (with standard error; LOW: N = 14, HIGH: N = 14). The asterisk indicates a significant increase in MEPREST AURC. (D) Group-averaged MEPACTIVE recruitment curves (with standard errors) at T0 and T1 for the LOW (N = 14) and HIGH (N = 13) group. TMS intensity is defined as the percentage of the active motor threshold (AMT). Solid and dashed lines indicate pre (T0) and post (T1) values, respectively.
Intracortical circuits
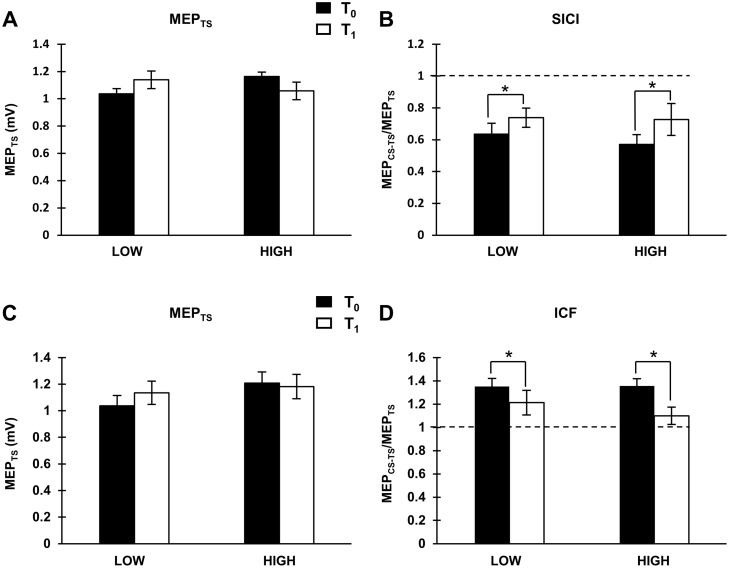
Eleven and thirteen individuals demonstrated SICI at T0 in the HIGH and LOW groups, respectively, and were included in subsequent analyses. Unconditioned MEPs were maintained at ~1 mV (Fig 3A). SICI was reduced following exercise regardless of physical activity level (Fig 3B, showing the main effect of TIME, Table 1, paired t-test: p = 0.007). Fifteen participants (7 HIGH, 8 LOW) demonstrated a reduction in SICI following exercise while three showed little to no change and six revealed increases in SICI. Twelve and twelve individuals demonstrated ICF at T0 in the HIGH and LOW groups, respectively, and were included in subsequent analyses. Unconditioned MEPs were maintained at ~1 mV (Table 1, Fig 3C). ICF was reduced following exercise regardless of physical activity level (Table 1, Wilcoxon: p = 0.04, Fig 3D). Fifteen participants (7 HIGH, 8 LOW) demonstrated a reduction in ICF following exercise while three showed little to no change and six revealed increases. SICF1.2ms and SICF2.5ms were not different between groups and were unchanged by exercise (Table 1).
Fig 3.
SICI: (A) Group-averaged unconditioned MEPs (i.e. MEPTS) (with standard errors) for both groups (LOW: N = 13; HIGH: N = 11) at both time points. (B) Group-averaged SICI (with standard errors) for each group (LOW: N = 13; HIGH: N = 11) displaying the main effect of TIME. The asterisk indicates a significant decrease in SICI. ICF: (C) Group-averaged unconditioned MEPs (i.e. MEPTS) (with standard errors) for both groups (LOW: N = 12; HIGH: N = 12) at both time points. (B) Group-averaged ICF (with standard errors) for each group (LOW: N = 12; HIGH: N = 12) displaying main effect of TIME. The asterisk indicates a significant decrease in ICF.
Discussion
This study revealed that exercise-induced short-term plasticity depends on the physical activity level of the individual. Exercise increased the amplitude of corticospinal output in the HIGH group, and in contrast, did not alter corticospinal output in the LOW group. This finding indicates that physical activity levels influence the propensity and direction of exercise-induced short-term plasticity. Our data also indicated that exercise reduces SICI, in support of previous literature [15, 16]. We extend this finding to indicate that SICI reduction occurs in both fit and relatively sedentary individuals. We discuss these findings and the implications for rehabilitation.
Exercise induces short-term plasticity in corticospinal output
A key finding in this study is that exercise alters corticospinal excitability depending on the level of physical activity. Corticospinal excitability in the HIGH group increased by ~28% following exercise while no changes were observed for the LOW group. Our findings are similar to the effects of PAS that increases MEPs in physically active but not sedentary groups [35]. While spike-timing dependent mechanisms mediate PAS effects [47], less is known about the neural mechanisms that mediate exercise-induced plasticity. Modulation of neurotransmitter concentration may participate in increasing corticospinal excitability. A single session of aerobic exercise upregulates the release of serotonin [48, 49], norepinephrine [50, 51], and upregulates [48, 50, 52–55] or does not change [56] dopamine. These neurotransmitters have been shown to modulate the excitability of motor neurons [57–60]. Exercise-induced increases in corticospinal output may also be due to an increase in glutamate. A single session of exercise increases glutamate [57, 58], as measured via 1H-MRS and a positive correlation exists such that greater cortical glutamate is associated with steeper MEP recruitment curves [59].
It has been shown that long-term physical activity is linked to improved cognitive function and memory [1, 2, 5, 60]. Although the mechanisms that underpin such improvements remain unclear, there is strong evidence to implicate BDNF as a mediator of neural plasticity. Higher fitness levels are associated with a lower concentration of peripheral BDNF [61, 62], suggesting that gains in fitness may yield more efficient uptake and utilization of BDNF in the central nervous system [63]. The exercise-induced facilitation of MEPs we observed in the HIGH group may relate to an increased uptake of BDNF within the central nervous system. Future studies may consider measuring BDNF and corticospinal excitability, simultaneously.
Increases in MEPs may also be associated with the physiological differences between the two groups. The HIGH group may have increased stroke volume [12], increased brain perfusion [14], and muscle adaptions that may reduce fatigue [12]. Comparatively, the LOW group lacks these chronic adaptions to exercise, which may reduce neuroplasticity induction. This is supported by the trend towards decreases in excitability seen in the LOW group. Since we opted to control heart rate only, differences in the resting MEPs between the groups may be contributed by variances in the workload performed, stress or anxiety associated with the exercise regime and/or the rate of perceived exertion associated with the exercise intervention. Future studies will need to address whether MEPs differ between HIGH and LOW groups when the exercise intervention controls for one or more of these variables. From the present results, we can conclude that exercise controlled by heart rate revealed differences in resting MEPs only and did not create changes in intracortical circuits. However, we note again, that we cannot exclude the contribution of workload, cortisol levels, anxiety and perceived exertion, which were not controlled and may also contribute to these differences.
Exercise induces short-term plasticity in intracortical inhibition and facilitation
We observed a reduction in SICI following exercise in support of previous literature [15, 16], but extend these findings to show that SICI is reduced irrespective of the physical activity level. Previous studies report reductions in SICI following moderate intensity exercise (~20% in [16] and ~35% in [15]). In our study, we observed an overall decrease of ~18%. The difference may reflect our less intense exercise (~60% age-predicted maximal heart rate vs. 65–70% in [15]) or the muscle studied (FDI vs. flexor carpi radialis). The mechanisms by which exercise reduces SICI may involve changes in GABAA receptor activity that are considered to mediate the circuit [19, 20]. Aerobic exercise increases serum BDNF [25, 61, 63–74] that in turn reduces GABAA receptor activity [75]. Additionally, in rat models, BDNF reduces GABAA receptor function [76]. Thus, as suggested elsewhere [16] and above, aerobic exercise may increase serum BDNF that acts to decrease SICI.
We also observed ~15% reduction in ICF following exercise irrespective of the physical activity level. Previous study reports increase in ICF following moderate intensity exercise [15]. The disparity between previous work and our study may be attributed to differences in exercise or TMS protocols (% AMT vs. % RMT). However, our findings are similar to the effects of continuous theta burst stimulation (cTBS) over M1 which reduced both ICF and SICI [77]. ICF is thought to be mediated via glutamatergic facilitation coupled with persisting GABAergic inhibition [29]. Previous research on drugs has demonstrated that administration of GABAA agonists and NMDA antagonists reduces ICF [78–80]. Further, some dopamine agonists, such as cabergoline, also reduce ICF [81] and hence, changes in ICF may be dependent on more than one neuronal circuit [82], as suggested previously [83].
Circuitry unaltered by exercise
Several measures of cortical activity were unchanged following exercise. RMT and AMT were unaltered by physical activity levels or exercise, in support of previous literature [16, 35, 84]. Therefore, a single session of aerobic exercise does not alter the membrane thresholds. Additionally, MEP recruitment curves obtained in the actively contracted FDI were unchanged by exercise, indicating that exercise only alters corticospinal output without the voluntary activation of motor neurons. Lastly, we did not observe significant changes in the SICF circuits following exercise, suggesting that moderate intensity aerobic exercise does not modulate early and late indirect waves (i.e. I-waves), similar to other plasticity inducing paradigms [32, 33].
Implications
One of the main implications of this work is that pre-existing physical activity levels determine the propensity for plasticity. In our sedentary group, exercise did not alter excitability, while in the active group the neuronal output to a hand muscle was enhanced, which is a major goal of rehabilitative approaches. Collectively our data suggest that physically active individuals demonstrate a propensity for increasing neuronal output to the hand muscle following a single session of exercise. We speculate that this may have ramifications for the success of rehabilitation protocols aiming to promote neural plasticity, such that individuals with greater physical activity levels may demonstrate increased propensity for plasticity. Indeed, evidence in the animal literature suggests that physically active animals show better recovery of behavioural performance than their sedentary counterparts [8]. It is important to note that exercise also protects from further neurodegeneration after injury and promotes better recovery. In humans, rehabilitation protocols involving exercise regimes have shown improvements in physical function [85], movement initiation [86], and activities of daily living [87]. Our results support the PAS results [35], indicating that short-term plasticity is observed only in the corticospinal output of physically active individuals. Therefore, regular physical activity may be a determinant for the success of rehabilitation approaches that aim to increase corticospinal output to impaired muscles. Further, our data suggests that exercise can be used to prime the motor cortex for plasticity via a reduction in inhibition, regardless of physical activity level. Recent studies have shown improvements in motor learning [88] and increased response to brain stimulation in healthy adults [24, 25, 40, 89] when exercise is used as a prime.
Limitations and future directions
Direct assessment of aerobic capacity may be achieved using maximal volume oxygen uptake (VO2 max). We use the IPAQ that provides a reliable self-report of physical activity in the past 7 days [39] as used elsewhere [16, 35, 40]. Future studies may confirm our findings using VO2 max. Additionally, the recumbent cycle ergometer used in our study did not provide wattage as a function of resistance level. Therefore, we cannot provide the workload achieved by the exercising lower limb muscles. However, we chose to control the aerobic intensity via heart rate as performed elsewhere [15, 40], as changes in heart rate yield modification in serum BDNF [90]. An alternative approach is to control for both workload and heart rate by altering the duration of the exercise. Future studies should examine how controlling for workload or altering exercise duration affects exercise-induced neuroplasticity. We collected MEP recruitment curves using 3 pulses per intensity to reduce the intrusion of TMS-induced plasticity. This is a small number of pulses to obtain an estimate of MEP amplitudes, that while used elsewhere [91] may benefit from a greater number of stimuli delivered [92–94]. Thus, future studies should consider using more pulses per intensity to reduce variability in this dependent measure. Further, we emphasize that these findings are achieved following a single session of exercise. Multiple sessions of exercise may increase the opportunity for plasticity to be observed in sedentary individuals [11]. Finally, we have tested young adults, and it remains unknown whether these findings will also be revealed across the lifespan.
Conclusions
The present study demonstrated that physical activity levels influence motor cortex excitability and the propensity for exercise-induced plasticity. First, corticospinal excitability is increased following exercise in highly active individuals only. Second, exercise reduces cortical inhibition regardless of physical activity level. A reduction of inhibitory input in the motor cortex creates a more favorable environment for plasticity induction. Therefore, we conclude that physical activity levels should be taken into consideration when investigating corticospinal excitability and plasticity induction within the motor cortex in healthy and clinical populations.
Acknowledgments
The authors thank the Natural Sciences and Engineering Research Council for funding to AJN.
Data Availability
All data files are available from the Figshare database at https://figshare.com (accession number 10.6084/m9.figshare.4270178).
Funding Statement
This study was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC RGPIN -2015-06309).
References
- 1.Chaddock L, Erickson KI, Prakash RS, Kim JS, Voss MW, Vanpatter M, et al. A neuroimaging investigation of the association between aerobic fitness, hippocampal volume, and memory performance in preadolescent children. Brain Res. 2010;1358:172–83. Epub 2010/08/26. 10.1016/j.brainres.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herting MM, Nagel BJ. Differences in brain activity during a verbal associative memory encoding task in high- and low-fit adolescents. J Cogn Neurosci. 2013;25(4):595–612. Epub 2012/12/20. 10.1162/jocn_a_00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killgore WDS, Olson EA, Weber M. Physical Exercise Habits Correlate with Gray Matter Volume of the Hippocampus in Healthy Adult Humans. Sci Rep. 2013. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon BA, Rykhlevskaia EI, Brumback CR, Lee Y, Elavsky S, Konopack JF, et al. Neuroanatomical correlates of aging, cardiopulmonary fitness level, and education. Psychophysiology. 2008;45(5):825–38. Epub 2008/07/17. 10.1111/j.1469-8986.2008.00676.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floel A, Ruscheweyh R, Kruger K, Willemer C, Winter B, Volker K, et al. Physical activity and memory functions: are neurotrophins and cerebral gray matter volume the missing link? Neuroimage. 2010;49(3):2756–63. Epub 2009/10/27. 10.1016/j.neuroimage.2009.10.043 [DOI] [PubMed] [Google Scholar]
- 6.Prakash RS, Snook EM, Motl RW, Kramer AF. Aerobic Fitness is Associated with Gray Matter Volume and White Matter Integrity in Multiple Sclerosis. Brain Res. 2010;1341C:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373(6510):109 Epub 1995/01/12. 10.1038/373109a0 [DOI] [PubMed] [Google Scholar]
- 8.Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21(15):5678–84. Epub 2001/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9(5):580–6. Epub 2006/09/28. [PubMed] [Google Scholar]
- 10.Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, et al. Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus. 2009;19(10):1030–9. Epub 2009/01/06. 10.1002/hipo.20547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–21. Epub 2004/02/24. 10.1073/pnas.0400266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petriz BA, Gomes CP, Almeida JA, de Oliveira GP Jr., Ribeiro FM, Pereira RW, et al. The Effects of Acute and Chronic Exercise on Skeletal Muscle Proteome. J Cell Physiol. 2017;232(2):257–69. Epub 2016/10/31. 10.1002/jcp.25477 [DOI] [PubMed] [Google Scholar]
- 13.Wilmore JH, Stanforth PR, Gagnon J, Rice T, Mandel S, Leon AS, et al. Cardiac output and stroke volume changes with endurance training: the HERITAGE Family Study. Med Sci Sports Exerc. 2001;33(1):99–106. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 14.Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3(1):15–23. Epub 2006/02/14. [DOI] [PubMed] [Google Scholar]
- 15.Singh AM, Duncan RE, Neva JL, Staines WR. Aerobic exercise modulates intracortical inhibition and facilitation in a nonexercised upper limb muscle. BMC Sports Sci Med Rehabil. 2014;6:23 Epub 2014/07/18. 10.1186/2052-1847-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AE, Goldsworthy MR, Garside T, Wood FM, Ridding MC. The influence of a single bout of aerobic exercise on short-interval intracortical excitability. Exp Brain Res. 2014;232(6):1875–82. Epub 2014/02/27. 10.1007/s00221-014-3879-z [DOI] [PubMed] [Google Scholar]
- 17.Mooney RA, Coxon JP, Cirillo J, Glenny H, Gant N, Byblow WD. Acute aerobic exercise modulates primary motor cortex inhibition. Exp Brain Res. 2016. Epub 2016/09/04. [DOI] [PubMed] [Google Scholar]
- 18.Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm (Vienna). 1997;104(11–12):1207–14. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 19.Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119(2):265–8. Epub 1998/04/16. [DOI] [PubMed] [Google Scholar]
- 20.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–19. Epub 1993/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80(6):2870–81. Epub 1998/12/24. [DOI] [PubMed] [Google Scholar]
- 22.Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015;233(3):679–89. Epub 2015/01/08. 10.1007/s00221-014-4183-7 [DOI] [PubMed] [Google Scholar]
- 23.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126(6):1071–107. Epub 2015/03/24. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh AM, Neva JL, Staines WR. Acute exercise enhances the response to paired associative stimulation-induced plasticity in the primary motor cortex. Exp Brain Res. 2014;232(11):3675–85. Epub 2014/08/07. 10.1007/s00221-014-4049-z [DOI] [PubMed] [Google Scholar]
- 25.Mang CS, Snow NJ, Campbell KL, Ross CJ, Boyd LA. A single bout of high-intensity aerobic exercise facilitates response to paired associative stimulation and promotes sequence-specific implicit motor learning. J Appl Physiol (1985). 2014;117(11):1325–36. Epub 2014/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ploughman M, Attwood Z, White N, Dore JJ, Corbett D. Endurance exercise facilitates relearning of forelimb motor skill after focal ischemia. Eur J Neurosci. 2007;25(11):3453–60. Epub 2007/06/08. 10.1111/j.1460-9568.2007.05591.x [DOI] [PubMed] [Google Scholar]
- 27.Bailey AZ, Mi YP, Nelson AJ. Short-latency afferentinhibition in chronic spinalcord injury. Translational Neuroscience. 2016;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leon-Sarmiento FE, Rizzo-Sierra CV, Bayona EA, Bayona-Prieto J, Doty RL, Bara-Jimenez W. Novel mechanisms underlying inhibitory and facilitatory transcranial magnetic stimulation abnormalities in Parkinson's disease. Arch Med Res. 2013;44(3):221–8. Epub 2013/03/26. 10.1016/j.arcmed.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 29.Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2014;126(10):1847–68. Epub 2014/12/24. 10.1016/j.clinph.2014.08.028 [DOI] [PubMed] [Google Scholar]
- 30.Ni Z, Bahl N, Gunraj CA, Mazzella F, Chen R. Increased motor cortical facilitation and decreased inhibition in Parkinson disease. Neurology. 2013;80(19):1746–53. Epub 2013/04/12. 10.1212/WNL.0b013e3182919029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortu E, Fancellu L, Sau G, Falchi P, Traccis S, Pes GM, et al. Primary motor cortex hyperexcitability in Fabry's disease. Clin Neurophysiol. 2013;124(7):1381–9. Epub 2013/03/12. 10.1016/j.clinph.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 32.McAllister SM, Rothwell JC, Ridding MC. Selective modulation of intracortical inhibition by low-intensity Theta Burst Stimulation. Clin Neurophysiol. 2009;120(4):820–6. 10.1016/j.clinph.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 33.Doeltgen SH, Ridding MC. Modulation of cortical motor networks following primed theta burst transcranial magnetic stimulation. Exp Brain Res. 2011;215(3–4):199–206. Epub 2011/10/04. 10.1007/s00221-011-2886-6 [DOI] [PubMed] [Google Scholar]
- 34.Huang T, Larsen KT, Ried-Larsen M, Moller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand J Med Sci Sports. 2014;24(1):1–10. Epub 2013/04/23. 10.1111/sms.12069 [DOI] [PubMed] [Google Scholar]
- 35.Cirillo J, Lavender AP, Ridding MC, Semmler JG. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol. 2009;587(Pt 24):5831–42. Epub 2009/10/28. 10.1113/jphysiol.2009.181834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. [DOI] [PubMed] [Google Scholar]
- 37.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research☆. Clin Neurophysiol. 2009;120(12):2008–39. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warburton DE, Gledhill N, Jamnik VK, Bredin SS, McKenzie DC, Stone J, et al. Evidence-based risk assessment and recommendations for physical activity clearance: Consensus Document 2011. Appl Physiol Nutr Metab. 2011;36 Suppl 1:S266–98. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 39.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. Epub 2003/08/06. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 40.McDonnell MN, Buckley JD, Opie GM, Ridding MC, Semmler JG. A single bout of aerobic exercise promotes motor cortical neuroplasticity. J Appl Physiol (1985). 2013;114(9):1174–82. Epub 2013/03/16. [DOI] [PubMed] [Google Scholar]
- 41.Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–55. Epub 1978/01/01. [DOI] [PubMed] [Google Scholar]
- 42.van de Ruit M, Perenboom MJL, Grey MJ. TMS Brain Mapping in Less Than Two Minutes. Brain Stimulation. 2015;8(2):231–9. 10.1016/j.brs.2014.10.020 [DOI] [PubMed] [Google Scholar]
- 43.Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim YH, et al. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol. 2016;127(8):2892–7. Epub 2016/05/10. 10.1016/j.clinph.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 1998;109(4):321–30. Epub 1998/09/29. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited Journal of the American College of Cardiology | American College of Cardiology Foundation. 2001. [DOI] [PubMed] [Google Scholar]
- 46.Conover WJ, Iman RL. Analysis of covariance using the rank transformation. Biometrics. 1982;38(3):715–24. Epub 1982/09/01. [PubMed] [Google Scholar]
- 47.Veniero D, Ponzo V, Koch G. Paired associative stimulation enforces the communication between interconnected areas. J Neurosci. 2013;33(34):13773–83. Epub 2013/08/24. 10.1523/JNEUROSCI.1777-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitaoka R, Fujikawa T, Miyaki T, Matsumura S, Fushiki T, Inoue K. Increased noradrenergic activity in the ventromedial hypothalamus during treadmill running in rats. J Nutr Sci Vitaminol (Tokyo). 2010;56(3):185–90. Epub 2010/07/24. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Merino D, Bequet F, Berthelot M, Chennaoui M, Guezennec CY. Site-dependent effects of an acute intensive exercise on extracellular 5-HT and 5-HIAA levels in rat brain. Neurosci Lett. 2001;301(2):143–6. Epub 2001/03/15. [DOI] [PubMed] [Google Scholar]
- 50.Meeusen R, Roeykens J, Magnus L, Keizer H, De Meirleir K. Endurance performance in humans: the effect of a dopamine precursor or a specific serotonin (5-HT2A/2C) antagonist. Int J Sports Med. 1997;18(8):571–7. Epub 1998/01/27. 10.1055/s-2007-972683 [DOI] [PubMed] [Google Scholar]
- 51.Zouhal H, Jacob C, Delamarche P, Gratas-Delamarche A. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–23. Epub 2008/04/18. [DOI] [PubMed] [Google Scholar]
- 52.Meeusen R, De Meirleir K. Exercise and brain neurotransmission. Sports Med. 1995;20(3):160–88. Epub 1995/09/01. [DOI] [PubMed] [Google Scholar]
- 53.Goekint M, Bos I, Heyman E, Meeusen R, Michotte Y, Sarre S. Acute running stimulates hippocampal dopaminergic neurotransmission in rats, but has no influence on brain-derived neurotrophic factor. J Appl Physiol (1985). 2012;112(4):535–41. Epub 2011/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hattori S, Naoi M, Nishino H. Striatal dopamine turnover during treadmill running in the rat: relation to the speed of running. Brain Res Bull. 1994;35(1):41–9. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 55.Sutoo D, Akiyama K. Regulation of brain function by exercise. Neurobiol Dis. 2003;13(1):1–14. Epub 2003/05/22. [DOI] [PubMed] [Google Scholar]
- 56.Wang GJ, Volkow ND, Fowler JS, Franceschi D, Logan J, Pappas NR, et al. PET studies of the effects of aerobic exercise on human striatal dopamine release. J Nucl Med. 2000;41(8):1352–6. Epub 2000/08/17. [PubMed] [Google Scholar]
- 57.Maddock RJ, Casazza GA, Buonocore MH, Tanase C. Vigorous exercise increases brain lactate and Glx (glutamate+glutamine): a dynamic 1H-MRS study. Neuroimage. 2011;57(4):1324–30. Epub 2011/06/07. 10.1016/j.neuroimage.2011.05.048 [DOI] [PubMed] [Google Scholar]
- 58.Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. Acute Modulation of Cortical Glutamate and GABA Content by Physical Activity. J Neurosci. 2016;36(8):2449–57. Epub 2016/02/26. 10.1523/JNEUROSCI.3455-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589(Pt 23):5845–55. Epub 2011/10/19. 10.1113/jphysiol.2011.216978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frederiksen KS, Verdelho A, Madureira S, Bazner H, O'Brien JT, Fazekas F, et al. Physical activity in the elderly is associated with improved executive function and processing speed: the LADIS Study. Int J Geriatr Psychiatry. 2015;30(7):744–50. Epub 2014/11/05. 10.1002/gps.4220 [DOI] [PubMed] [Google Scholar]
- 61.Cho H-c, Sungyeon JK, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neuroscience Letters. 2012;519(1):78–83. 10.1016/j.neulet.2012.05.025 [DOI] [PubMed] [Google Scholar]
- 62.Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M. Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett. 2009;451(2):152–5. Epub 2009/01/10. 10.1016/j.neulet.2008.12.043 [DOI] [PubMed] [Google Scholar]
- 63.Nofuji Y, Suwa M, Sasaki H, Ichimiya A, Nishichi R, Kumagai S. Different circulating brain-derived neurotrophic factor responses to acute exercise between physically active and sedentary subjects. J Sports Sci Med. 2012;11(1):83–8. Epub 2012/01/01. [PMC free article] [PubMed] [Google Scholar]
- 64.Tang SW, Chu E, Hui T, Helmeste D, Law C. Influence of exercise on serum brain-derived neurotrophic factor concentrations in healthy human subjects. Neurosci Lett. 2008;431(1):62–5. Epub 2007/12/11. 10.1016/j.neulet.2007.11.019 [DOI] [PubMed] [Google Scholar]
- 65.Winter B, Breitenstein C, Mooren FC, Voelker K, Fobker M, Lechtermann A, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87(4):597–609. Epub 2006/12/23. 10.1016/j.nlm.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 66.Bos I, Jacobs L, Nawrot TS, de Geus B, Torfs R, Int Panis L, et al. No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neurosci Lett. 2011;500(2):129–32. Epub 2011/06/29. 10.1016/j.neulet.2011.06.019 [DOI] [PubMed] [Google Scholar]
- 67.Goekint M, Heyman E, Roelands B, Njemini R, Bautmans I, Mets T, et al. No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Med Sci Sports Exerc. 2008;40(11):1990–6. Epub 2008/10/11. 10.1249/MSS.0b013e31817eee85 [DOI] [PubMed] [Google Scholar]
- 68.Goekint M, Roelands B, Heyman E, Njemini R, Meeusen R. Influence of citalopram and environmental temperature on exercise-induced changes in BDNF. Neuroscience Letters. 2011;494(2):150–4. 10.1016/j.neulet.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 69.Heyman E, Gamelin F-X, Goekint M, Piscitelli F, Roelands B, Leclair E, et al. Intense exercise increases circulating endocannabinoid and BDNF levels in humansâ” Possible implications for reward and depression. Psychoneuroendocrinology. 2012;37(6):844–51. 10.1016/j.psyneuen.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 70.Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40(9):765–801. Epub 2010/08/24. 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 71.Rojas Vega S, Struder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Res. 2006;1121(1):59–65. Epub 2006/10/03. 10.1016/j.brainres.2006.08.105 [DOI] [PubMed] [Google Scholar]
- 72.Rojas Vega S, Hollmann W, Vera Wahrmann B, Strüder HK. pH Buffering Does not Influence BDNF Responses to Exercise. International Journal of Sports Medicine. 2012;33:8–12. 10.1055/s-0031-1285929 [DOI] [PubMed] [Google Scholar]
- 73.Vega SR, Kleinert J, Sulprizio M, Hollmann W, Bloch W, Struder HK. Responses of serum neurotrophic factors to exercise in pregnant and postpartum women. Psychoneuroendocrinology. 2011;36(2):220–7. Epub 2010/08/10. 10.1016/j.psyneuen.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 74.Griffin ÃaW, Mullally S, Foley C, Warmington SA, O'Mara SM, Kelly ÁM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiology & Behavior. 2011;104(5):934–41. [DOI] [PubMed] [Google Scholar]
- 75.Jovanovic JN, Thomas P, Kittler JT, Smart TG, Moss SJ. Brain-Derived Neurotrophic Factor Modulates Fast Synaptic Inhibition by Regulating GABAA Receptor Phosphorylation, Activity, and Cell-Surface Stability. 2004. [DOI] [PMC free article] [PubMed]
- 76.Brunig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13(7):1320–8. Epub 2001/04/12. [DOI] [PubMed] [Google Scholar]
- 77.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45(2):201–6. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 78.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40(3):367–78. 10.1002/ana.410400306 [DOI] [PubMed] [Google Scholar]
- 79.Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51(5):1320–4. Epub 1998/11/18. [DOI] [PubMed] [Google Scholar]
- 80.Schwenkreis P, Witscher K, Janssen F, Addo A, Dertwinkel R, Zenz M, et al. Influence of the N-methyl-D-aspartate antagonist memantine on human motor cortex excitability. Neurosci Lett. 1999;270(3):137–40. Epub 1999/08/26. [DOI] [PubMed] [Google Scholar]
- 81.Korchounov A, Ilic TV, Ziemann U. TMS-assisted neurophysiological profiling of the dopamine receptor agonist cabergoline in human motor cortex. J Neural Transm (Vienna). 2007;114(2):223–9. Epub 2006/07/27. [DOI] [PubMed] [Google Scholar]
- 82.Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, et al. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998;509 (Pt 2):607–18. Epub 1998/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tergau F, Geese R, Bauer A, Baur S, Paulus W, Reimers CD. Motor cortex fatigue in sports measured by transcranial magnetic double stimulation. Med Sci Sports Exerc. 2000;32(11):1942–8. Epub 2000/11/18. [DOI] [PubMed] [Google Scholar]
- 84.Dai W, Pi YL, Ni Z, Tan XY, Zhang J, Wu Y. Maintenance of balance between motor cortical excitation and inhibition after long-term training. Neuroscience. 2016;336:114–22. Epub 2016/09/08. 10.1016/j.neuroscience.2016.08.053 [DOI] [PubMed] [Google Scholar]
- 85.Teri L, Gibbons LE, McCurry SM, Logsdon RG, Buchner DM, Barlow WE, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. Jama. 2003;290(15):2015–22. Epub 2003/10/16. 10.1001/jama.290.15.2015 [DOI] [PubMed] [Google Scholar]
- 86.Bergen JL, Toole T, Elliott RG 3rd, Wallace B, Robinson K, Maitland CG. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson's disease patients. NeuroRehabilitation. 2002;17(2):161–8. Epub 2002/06/26. [PubMed] [Google Scholar]
- 87.Crizzle AM, Newhouse IJ. Is physical exercise beneficial for persons with Parkinson's disease? Clin J Sport Med. 2006;16(5):422–5. Epub 2006/10/04. 10.1097/01.jsm.0000244612.55550.7d [DOI] [PubMed] [Google Scholar]
- 88.Snow NJ, Mang CS, Roig M, McDonnell MN, Campbell KL, Boyd LA. The Effect of an Acute Bout of Moderate-Intensity Aerobic Exercise on Motor Learning of a Continuous Tracking Task. PLoS One. 2016;11(2):e0150039 Epub 2016/02/24. 10.1371/journal.pone.0150039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mang CS, Brown KE, Neva JL, Snow NJ, Campbell KL, Boyd LA. Promoting Motor Cortical Plasticity with Acute Aerobic Exercise: A Role for Cerebellar Circuits. Neural Plast. 2016;2016:6797928 Epub 2016/04/30. 10.1155/2016/6797928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schmolesky MT, Webb DL, Hansen RA. The Effects of Aerobic Exercise Intensity and Duration on Levels of Brain-Derived Neurotrophic Factor in Healthy Men. J Sports Sci Med. 2013. 12 p. 502–11. [PMC free article] [PubMed] [Google Scholar]
- 91.Thirugnanasambandam N, Khera R, Wang H, Kukke SN, Hallett M. Distinct interneuronal networks influence excitability of the surround during movement initiation. J Neurophysiol. 2015. 114 p. 1102–8. 10.1152/jn.00791.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cuypers K, Thijs H, Meesen RLJ. Optimization of the Transcranial Magnetic Stimulation Protocol by Defining a Reliable Estimate for Corticospinal Excitability. PLoS One. 2014. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schmidt S, Cichy RM, Kraft A, Brocke J, Irlbacher K, Brandt SA. An initial transient-state and reliable measures of corticospinal excitability in TMS studies. Clin Neurophysiol. 2009;120(5):987–93. Epub 2009/04/11. 10.1016/j.clinph.2009.02.164 [DOI] [PubMed] [Google Scholar]
- 94.Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–9. Epub 2016/02/14. 10.1016/j.neuroscience.2016.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data files are available from the Figshare database at https://figshare.com (accession number 10.6084/m9.figshare.4270178).