Treatment for advanced metastatic colorectal cancer has improved over the past decade with the addition of biologic agents to standard chemotherapy regimens, leading to increases in median overall survival. FOLFOX plus bevacizumab is now widely accepted as the standard first‐line treatment. To determine whether onartuzumab has a beneficial role in either unselected or MET‐selected populations with metastatic colorectal cancer, this phase II study was initiated to evaluate the combination of bevacizumab and mFOLOFOX6 with or without onartuzumab
Keywords: Bevacizumb, FOLFOX, Metastatic colorectal cancer, Onartuzumab, Phase II, Randomized
Abstract
Background.
Dysregulated hepatocyte growth factor/mesenchymal‐epithelial transition (MET) signaling is associated with poor prognosis and resistance to vascular endothelial growth factor inhibition in metastatic colorectal cancer (mCRC). We report outcomes from a double‐blind, multicenter phase II trial of the MET inhibitor onartuzumab in combination with mFOLFOX‐6 and bevacizumab for mCRC (GO27827; NCT01418222).
Materials and Methods.
Patients were randomized 1:1 to receive onartuzumab (10 mg/kg intravenously [IV]) or placebo plus mFOLFOX‐6 and bevacizumab (5 mg/kg IV). Oxaliplatin was given for 8–12 cycles; other agents were continued until disease progression, unacceptable toxicity, or death. The primary endpoint was progression‐free survival (PFS) in the intent‐to‐treat (ITT) and MET immunohistochemistry (IHC) expression‐positive populations.
Results.
Between September 2011 and November 2012, 194 patients were enrolled. In September 2013, an interim analysis recommended stopping onartuzumab treatment due to lack of efficacy. At the time of the final analysis in February 2014, no significant improvement in PFS was seen with onartuzumab versus placebo in either the ITT or MET IHC‐positive populations. An improvement in PFS was noted in the MET IHC‐negative population. Neither overall survival nor response rate was improved with onartuzumab. The incidence of fatigue, peripheral edema, and deep vein thrombosis was increased with onartuzumab relative to placebo.
Conclusion.
Onartuzumab combined with mFOLFOX‐6 and bevacizumab did not significantly improve efficacy outcomes in either the ITT or MET IHC‐positive populations. MET expression by IHC was not a predictive biomarker in this setting.
Implications for Practice.
The addition of onartuzumab to mFOLFOX‐6 plus bevacizumab did not improve outcomes in patients with previously untreated metastatic colorectal cancer in this randomized, phase II study. Although initial results with onartuzumab were promising, a number of phase II/III clinical trials have reported a lack of improvement in efficacy with onartuzumab combined with standard‐of‐care therapies in several tumor types. Furthermore, negative study data have been published for rilotumumab and ficlatuzumab, both of which block hepatocyte growth factor binding to the mesenchymal‐epithelial transition (MET) receptor. MET immunohistochemistry was not a predictive biomarker. It remains to be seen if other biomarkers or small molecule inhibitors may be more appropriate for inhibiting this oncogenic pathway.
摘要
背景. 肝细胞生长因子调节异常/间质‐上皮转化(MET)信号传导与转移性结直肠癌(mCRC)预后不良和对血管内皮细胞生长因子抑制剂耐药有关。我们报告了一项MET抑制剂Onartuzumab联合mFOLFOX‐6和贝伐珠单抗治疗mCRC的双盲、多中心、II期试验的结果(GO27827; NCT01418222)。
材料和方法. 患者按1:1的比例随机接受Onartuzumab[10mg/kg静脉注射(IV)]或安慰剂与mFOLFOX‐6和贝伐珠单抗(5mg/kg IV)联合治疗。奥沙利铂给药8‐12个周期, 其它药物持续给药至出现疾病进展、不可接受的毒性或死亡。主要终点为意向性治疗(ITT)人群和MET免疫组化(IHC)表达阳性人群的无进展生存期(PFS)。
结果. 2011年9月至2012年11月期间入组了194例患者。2013年9月进行了一项中期分析, 结果显示缺乏疗效, 故建议停止Onartuzumab治疗。2014年2月最终分析时, 在ITT或MET IHC阳性人群中均未观察到Onartuzumab与安慰剂相比显著改善PFS。在MET IHC阴性人群中观察到PFS改善。Onartuzumab治疗时总生存期和缓解率均无改善。Onartuzumab治疗时疲乏、外周水肿和深静脉血栓的发生率与安慰剂相比有所增加。
结论. 在ITT或MET IHC阳性人群中, Onartuzumab与mFOLFOX‐6和贝伐珠单抗联用未显著改善疗效结果。在这种情况下, IHC测得的MET表达并非预测性生物标志物。
对临床实践的提示:在本项随机、II期研究中, 既往未经治疗的转移性结直肠癌患者接受Onartuzumab与mFOLFOX‐6和贝伐珠单抗联合治疗未能改善预后。虽然Onartuzumab的初步结果前景良好, 但随后的多项II/III期临床试验报告称Onartuzumab与标准疗法联合治疗多种肿瘤时疗效未见改善。此外, 已发表了对Rilotumumab和Ficlatuzumab不利的研究数据, 二者均是阻断肝细胞生长因子与MET受体结合的药物。MET免疫组化并非预测性生物标志物。其它生物标志物或小分子抑制剂是否更适合抑制该致癌通路仍需拭目以待。
Introduction
Metastatic colorectal cancer (mCRC) is a common and highly morbid malignancy, representing the second leading cause of cancer‐related deaths worldwide [1]. Treatment for advanced mCRC has improved considerably over the past decade with the addition of biologic agents to standard chemotherapy regimens, leading to increases in median overall survival (OS), which has increased from about 12 months in the mid‐1990s to more than 30 months in recent studies [2], [3]. Reflecting this, 5‐fluorouracil (5‐FU), leucovorin (LV), and oxaliplatin (FOLFOX), plus the vascular endothelial growth factor (VEGF) inhibitor bevacizumab, is a widely accepted standard first‐line treatment [4]. Despite improved clinical outcomes, the five‐year survival rate of patients with mCRC remains less than 10% [5], necessitating new treatment options.
Mesenchymal‐epithelial transition (MET) is a cell membrane receptor that binds hepatocyte growth factor (HGF) [6]. Signaling through HGF/MET stimulates tissue repair and regeneration in normal tissue but can be co‐opted by tumors to promote proliferation, survival, metastasis, and resistance to VEGF inhibition in tumor cells [7], [8]. In addition, oncogenic crosstalk between the HGF/MET pathway and the VEGF angiogenic pathway has been reported, suggesting a synergistic role between the two [9]. In mCRC, MET overexpression has a proposed role in both tumorigenesis and metastasis, with dysregulation of the HGF/MET pathway being associated with poor prognosis and aggressive biologic tumor characteristics [10], [11]. Furthermore, elevated levels of HGF have been observed in the plasma of patients with mCRC undergoing treatment with bevacizumab prior to disease progression (PD) [12].
Onartuzumab is a recombinant, fully humanized, monovalent monoclonal antibody that binds to the extracellular domain of MET, thereby preventing it from binding with HGF and restricting cellular signaling via the MET pathway [13]. Results of a phase II study demonstrated that second‐/third‐line treatment with onartuzumab in combination with erlotinib improved progression‐free survival (PFS) and OS versus placebo plus erlotinib in patients with MET‐positive non‐small cell lung cancer (NSCLC) [14]. To determine whether onartuzumab has a beneficial role in either unselected or MET‐selected populations with mCRC, the GO27827 randomized, phase II study was initiated to evaluate the combination of bevacizumab and mFOLFOX‐6 with or without onartuzumab.
Materials and Methods
Study Design
GO27827 (ClinicalTrials.gov identifier: NCT01418222) was a randomized, double‐blind, placebo‐controlled, phase II trial conducted at 22 sites in the U.S. and designed to compare mFOLFOX‐6/bevacizumab plus onartuzumab versus mFOLFOX‐6/bevacizumab plus placebo in stage IV mCRC.
Patients, investigators, study team members (except for the unblinded mixing pharmacist/nurse), and any other persons involved with the conduct of the study from the time of randomization until the interim analysis were blinded to the identity of the treatment assignment. The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All patients provided written informed consent as approved by local institutional review boards.
Patients
Eligible patients were aged ≥18 years with histologically or cytologically confirmed stage IV adenocarcinoma of the colon or rectum in the first‐line setting for metastatic disease. Patients had an Eastern Cooperative Oncology Group performance status of 0 or 1, life expectancy of ≥3 months, adequate organ system function, and confirmed availability of archival tissue (either a paraffin‐embedded tissue block or 20 unstained slides) for evaluation of MET expression and pathway‐related biomarkers.
Patients were excluded if they had received prior systemic or radiation therapy for mCRC (including chemotherapy and bevacizumab), had received chemotherapy for colorectal carcinoma within 12 months prior to the date of diagnosis of metastatic disease, and had previously untreated brain metastases and grade ≥1 peripheral neuropathy.
Treatment
Patients were randomized in a 1:1 ratio according to an interactive voice/web response system to receive onartuzumab (10 mg/kg intravenously [IV]) or placebo, plus mFOLFOX‐6 (oxaliplatin [85 mg/m2 IV], 5‐FU [400 mg/m2 IV bolus], and LV [400 mg/m2 IV]) and bevacizumab (5 mg/kg IV) every 2 weeks. Patients were stratified by prior administration of adjuvant chemotherapy (yes versus no).
All treatments were given on days 1–3 of a 2‐week cycle. Oxaliplatin was given for 8–12 cycles (at the discretion of the treating physician), following which patients received maintenance 5‐FU 400 mg/m2 IV bolus (after the administration of LV) and then 2,400 mg/m2 5‐FU in a continuous IV infusion over 46 hours, LV 400 mg/m2 IV, bevacizumab 5 mg/kg IV, and study agent (onartuzumab or placebo) 10 mg/kg IV on day 1 of each 14‐day cycle. Both treatment regimens were repeated at 14‐day intervals (defined as one treatment cycle). Treatment was continued until PD, unacceptable toxicity, or death.
Biomarker Methods
MET status was determined centrally by immunohistochemistry (IHC) using the CONFIRM SP44 anti‐MET monoclonal antibody (Ventana Medical Systems, Inc., Tucson, AZ, USA; http://www.ventana.com), with scores of 2+/3+ (≥50% moderate or strong intensity staining in tumor cells) considered MET IHC‐positive and scores of 1+/0 considered MET IHC‐negative. Provision of fresh or archival tissue to determine MET expression and evaluation of other biomarkers was mandatory. HGF expression in tissue samples was assessed using IHC. An enzyme‐linked immunosorbent assay was utilized for HGF expression in plasma samples.
Study Endpoints
The primary endpoint was to compare PFS between the two treatment arms in both the intent‐to‐treat (ITT) and the MET IHC‐positive populations. Secondary endpoints included OS and overall response rate (ORR) in the ITT and MET IHC‐positive populations as well as safety.
Assessments
Tumor response and progression were assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, with scans repeated every 8 weeks. PFS was calculated from the date of randomization until the date of first PD or death, whichever occurred first. OS was calculated from the date of randomization until death from any cause. ORR was defined as the percentage of patients with a complete or partial response, according to RECIST.
The ITT population comprised all randomized patients, and the safety population comprised all patients who received at least one dose of any study drug. The MET IHC‐positive population comprised all patients who had tissue with ≥50% of tumor cells with strong staining (3+ score) or ≥50% of tumor cells with either moderate or strong staining, but <50% of cells with strong staining (2+ score) for MET expression.
Adverse events (AEs) were recorded and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and classified according to the Medical Dictionary for Regulatory Activities version 16.1.
Statistical Analysis
A total of 178 evaluable patients were planned (89 in each arm) to achieve a target of 90% power at a significance level of 10% in order to detect a hazard ratio (HR) of 0.625 given a median PFS of 10.0 months in the placebo arm. Kaplan–Meier methodology was used to estimate median PFS and median OS for each treatment arm. A stratified Cox regression model was used to estimate HR and 95% confidence intervals (CIs) of PFS and OS; a log‐rank test was used to calculate the p‐value. The nonparametric subpopulation treatment effect pattern plot (STEPP) methodology was used to assess treatment effect differences according to the IHC assay.
An interim analysis of PFS and safety was planned to be performed when 50% of all patients in the ITT population experienced a PFS event or 6 months after the final patient was enrolled in the study, whichever occurred later. The final analysis was conducted on February 6, 2014.
Results
Patients
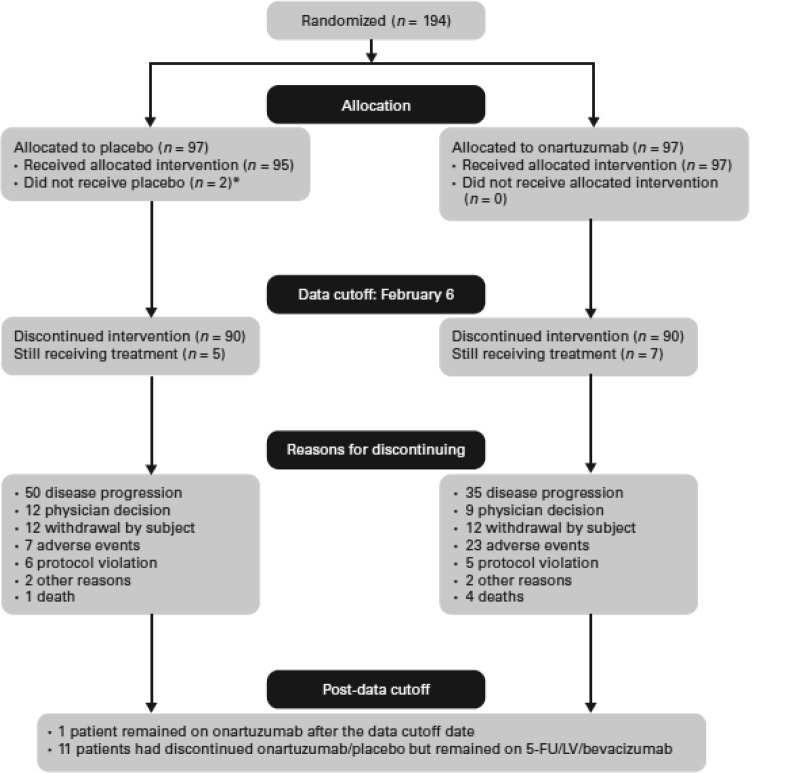
Between September 2011 and November 2012, 194 patients were randomized to treatment, with 97 patients allocated to each of the onartuzumab and placebo arms (ITT population). A number of patients were in screening at the time that the target of 178 evaluable patients was met, thus all 194 patients were enrolled onto the study. Two patients in the placebo arm did not receive treatment, and another two patients inadvertently received onartuzumab, leaving 192 patients in the safety population (Fig. 1). Overall, 79 patients (41%) were included in the MET IHC‐positive population (42 received onartuzumab and 37 received placebo), and 108 patients (56%) were included in the MET‐negative population (51 received onartuzumab and 57 received placebo). Five patients had inadequate tissue available for IHC assessment and were therefore not evaluable for MET determination.
Figure 1.
CONSORT diagram. *, One patient due to serious adverse event; one patient due to medical costs. Abbreviations: 5‐FU, 5‐fluorouracil; LV, leucovorin.
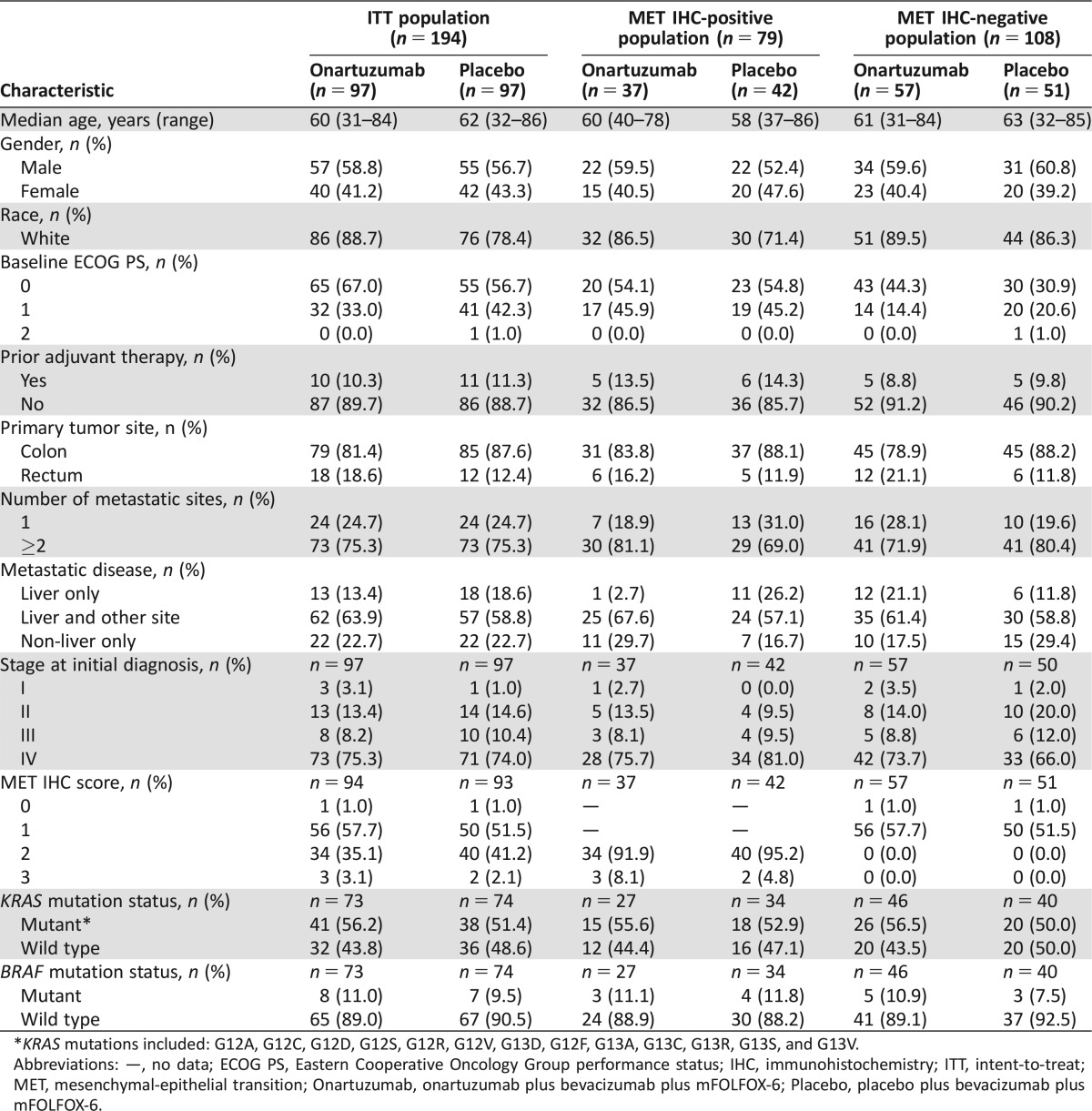
Patient characteristics were generally balanced between treatment arms for the ITT population (Table 1). For the MET IHC‐positive population, the onartuzumab arm had a higher proportion of males compared with the placebo arm (59.5% versus 52.4%).
Table 1. Baseline characteristics of the ITT, MET IHC‐positive, and MET IHC‐negative populations.
*KRAS mutations included: G12A, G12C, G12D, G12S, G12R, G12V, G13D, G12F, G13A, G13C, G13R, G13S, and G13V.
Abbreviations: —, no data; ECOG PS, Eastern Cooperative Oncology Group performance status; IHC, immunohistochemistry; ITT, intent‐to‐treat; MET, mesenchymal‐epithelial transition; Onartuzumab, onartuzumab plus bevacizumab plus mFOLFOX‐6; Placebo, placebo plus bevacizumab plus mFOLFOX‐6.
Dose intensity of onartuzumab/placebo was 98.3% (50–116) and 99.6% (66–112) for onartuzumab versus placebo, respectively; median treatment duration was 6.4 and 7.1 months for onartuzumab (n = 99) and placebo (n = 93), respectively. Reasons for patient withdrawal are listed in Figure 1.
Efficacy
On September 19, 2013, after the planned interim analysis, investigators were recommended to discontinue onartuzumab due to lack of efficacy and the observed increased incidence of venous thromboembolism.
ITT Population.
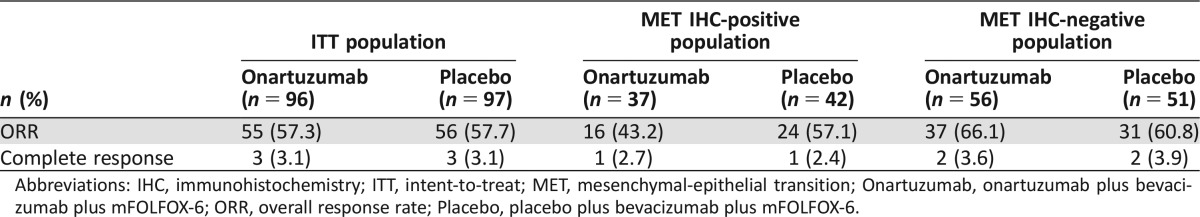
At the final data analysis of February 6, 2014, with a median follow‐up of 19.2 months, there was no significant improvement in PFS with onartuzumab versus placebo in the ITT population (HR, 0.75; 95% CI, 0.52–1.08; p = .12; median PFS, 11.0 versus 10.3 months, respectively, Fig. 2A). Onartuzumab also did not demonstrate an improvement in OS compared with placebo (HR, 0.96; 95% CI, 0.61–1.50; p = .85; median OS, 22.2 months versus not reached, respectively, Fig. 3A). There was no significant difference in ORR between the treatment arms (p = 1.00, Table 2).
Figure 2.
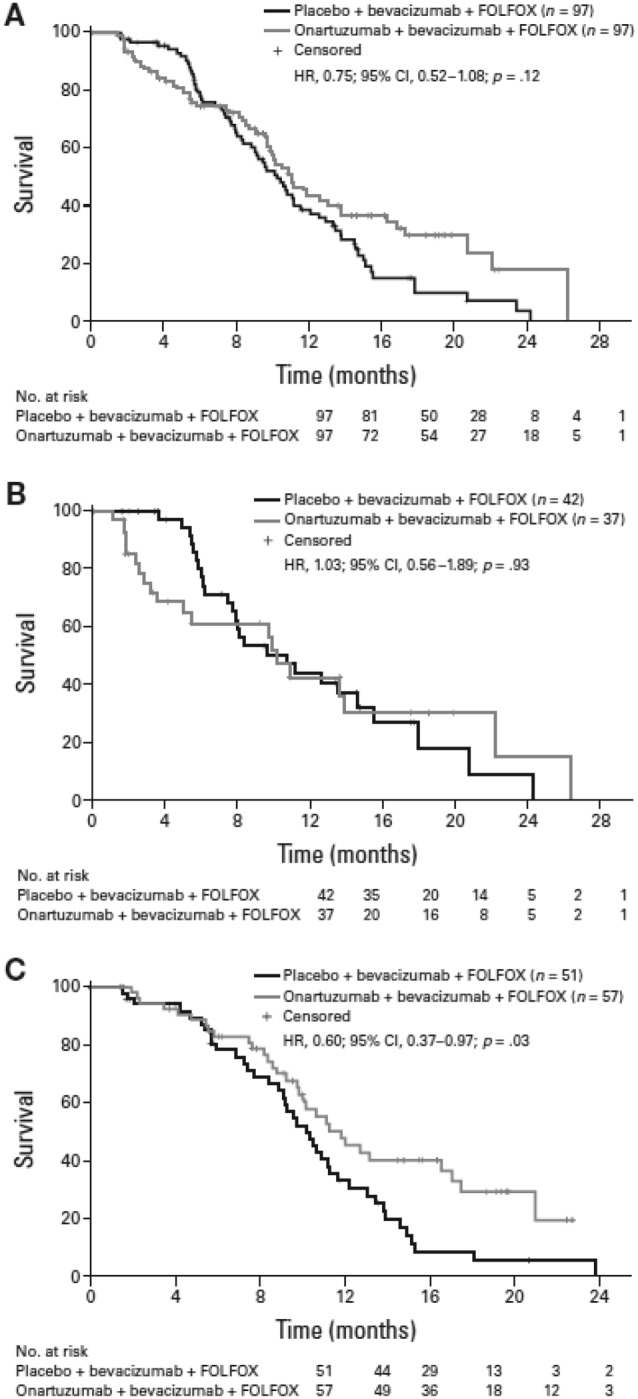
Progression‐free survival. (A): Intent‐to‐treat population. (B): mesenchymal‐epithelial transition (MET) immunohistochemistry (IHC)‐positive population. (C): MET IHC‐negative population. Abbreviations: CI, confidence interval; HR, hazard ratio.
Figure 3.

Overall survival. (A): Intent‐to‐treat population. (B): mesenchymal‐epithelial transition (MET) immunohistochemistry (IHC)‐positive population. (C): MET IHC‐negative population. Abbreviations: CI, confidence interval; HR, hazard ratio.
Table 2. Overall response rates in patients with tumor assessment at baseline.
Abbreviations: IHC, immunohistochemistry; ITT, intent‐to‐treat; MET, mesenchymal‐epithelial transition; Onartuzumab, onartuzumab plus bevacizumab plus mFOLFOX‐6; ORR, overall response rate; Placebo, placebo plus bevacizumab plus mFOLFOX‐6.
MET IHC‐Positive Population.
At the final data analysis, there was no significant difference in PFS between the onartuzumab and placebo arms in the MET IHC‐positive population (HR, 1.03; 95% CI, 0.56–1.89; p = .93; median PFS, 10.2 versus 10.7 months, respectively, Fig. 2B). Median OS was also not improved with onartuzumab versus placebo (HR, 1.24; 95% CI, 0.63–2.43; p = .54; median OS, 19.2 versus 19.7 months, respectively, Fig. 3B). Furthermore, there was no significant difference in ORR between the treatment arms (p = .26, Table 2).
MET IHC‐Negative Population.
Onartuzumab prolonged PFS compared with placebo in the MET‐negative population (HR, 0.60; 95% CI, 0.37–0.97; p = .03; median PFS, 11.7 versus 10.2 months, respectively, Fig. 2C). However, there was no significant difference in OS between the treatment arms (HR, 0.83; 95% CI, 0.44–1.56; p = .56; median OS not reached in either arm, Fig. 3C) and no statistical difference in ORR (p = .69, Table 2).
Exploratory Biomarker Data.
STEPPs for HGF and MET showed no association between MET IHC or HGF expression at any level (supplemental online Figs. 1 and 2). Exploratory PFS and OS analyses revealed no significant differences between the treatment arms in patient subgroups defined by KRAS or BRAF mutation status (supplemental online Fig. 3).
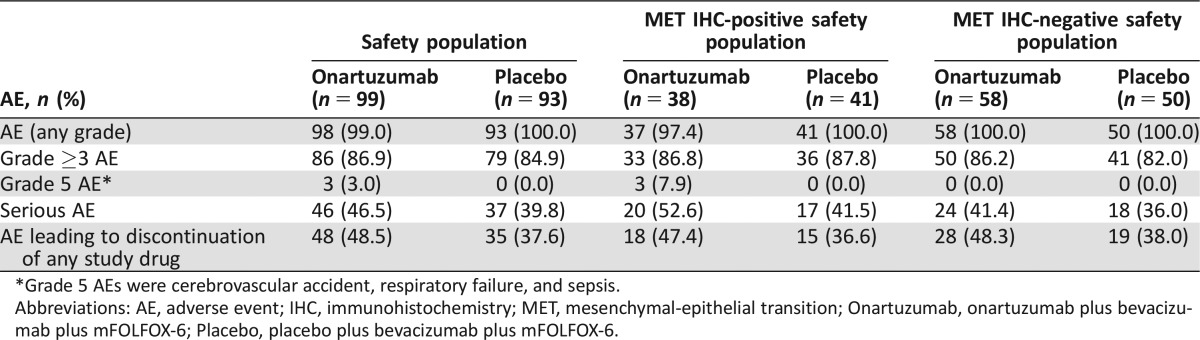
Safety
Overall, the frequency of AEs was similar between the two treatment arms in the ITT and MET populations (Table 3). Serious AEs (SAEs; safety population: 46.5% versus 39.8%; MET IHC‐positive: 52.6% versus 41.5%; MET IHC‐negative: 41.4% versus 36.0%) and AEs leading to discontinuation of any study drug (ITT: 48.5% versus 37.6%; MET IHC‐positive: 47.4% versus 36.6%; MET IHC‐negative: 48.3% versus 38.0%) were numerically higher with onartuzumab than with placebo. Median duration of treatment was comparable between the onartuzumab and placebo arms, respectively, for bolus 5‐FU (5.5 versus 6.7 months), bevacizumab (5.1 versus 6.9 months), LV (6.0 versus 7.1 months), onartuzumab/placebo (6.4 versus 7.1 months), and oxaliplatin (3.2 versus 3.3 months).
Table 3. Summary of AEs in the safety and MET subgroup populations.
*Grade 5 AEs were cerebrovascular accident, respiratory failure, and sepsis.
Abbreviations: AE, adverse event; IHC, immunohistochemistry; MET, mesenchymal‐epithelial transition; Onartuzumab, onartuzumab plus bevacizumab plus mFOLFOX‐6; Placebo, placebo plus bevacizumab plus mFOLFOX‐6.
Grade ≥3 AEs with an incidence of >5% in either arm in the safety population are shown in Table 4. In general, fatigue (23.2% versus 8.6%), peripheral edema (11.1% versus 0%), and deep vein thrombosis (5.1% versus 0%) occurred at a higher frequency in the onartuzumab arm compared with the placebo arm.
Table 4. Grade ≥3 AEs with an incidence of >5% in either arm (safety population).
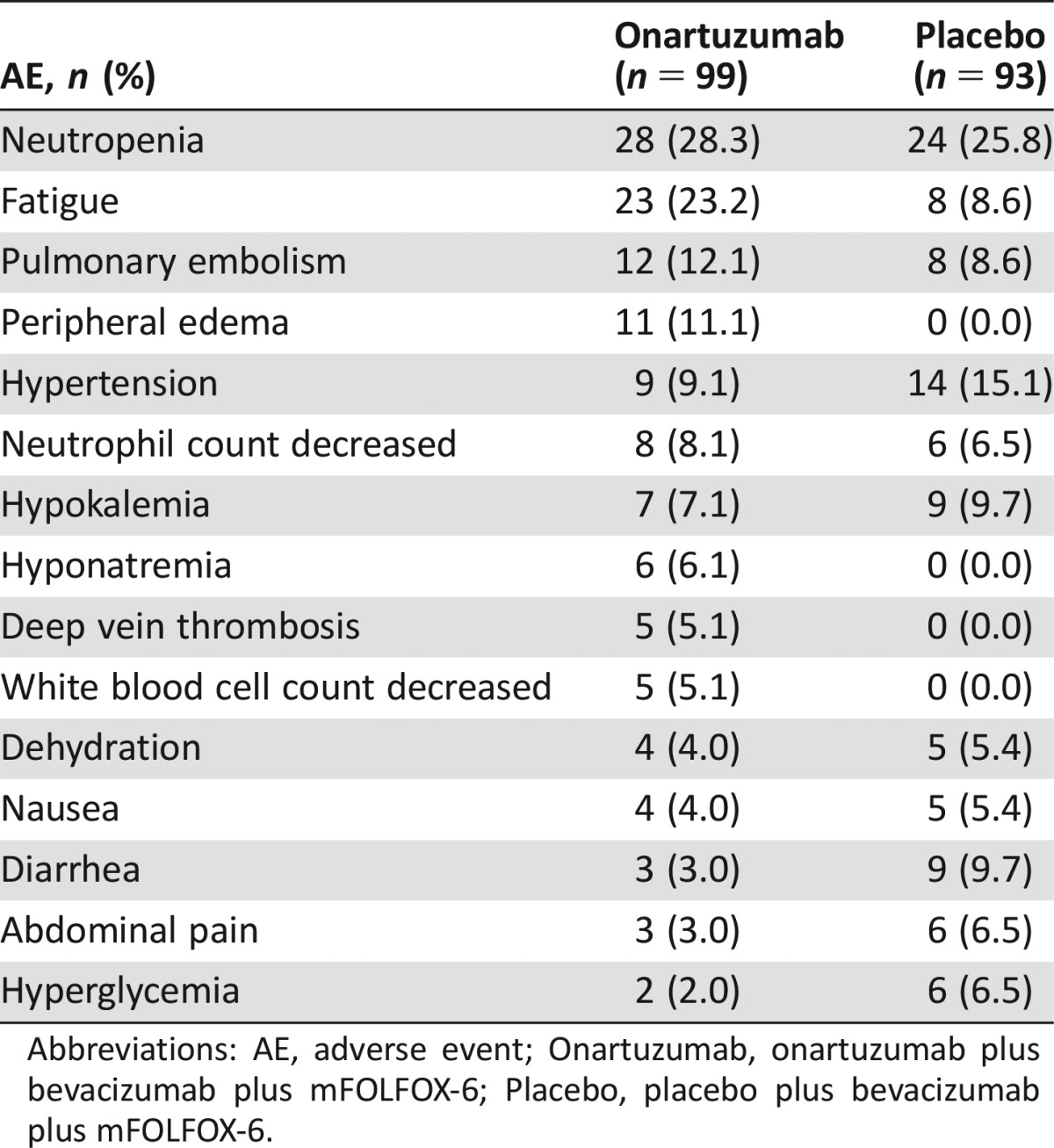
Abbreviations: AE, adverse event; Onartuzumab, onartuzumab plus bevacizumab plus mFOLFOX‐6; Placebo, placebo plus bevacizumab plus mFOLFOX‐6.
Discussion
In the present study, the addition of onartuzumab to mFOLFOX‐6 plus bevacizumab in patients with previously untreated mCRC did not improve PFS, OS, or ORR in either the ITT population or the MET IHC‐positive population. Somewhat surprisingly, a significant prolongation of PFS with onartuzumab was noted in the MET IHC‐negative population, although this is of uncertain significance. Exploratory biomarker analyses revealed no association between MET IHC or HGF expression at any level and no significant survival differences between the treatment arms in patient subgroups defined by KRAS or BRAF mutation status.
Although initial preclinical and early clinical results with onartuzumab showed promising efficacy data [15], a number of phase II/III clinical trials have since reported a lack of improvement in efficacy with onartuzumab combined with standard‐of‐care therapies in several tumor types. For example, METLung, a randomized phase III study of erlotinib with or without onartuzumab in patients with NSCLC and MET IHC‐positive tumors, was halted for futility following a planned interim analysis because the combination did not confirm the efficacy results observed in the phase II NSCLC trial [16]. In other tumors, including gastroesophageal adenocarcinoma [17], triple‐negative breast cancer [18], and recurrent glioblastoma [19], the addition of onartuzumab to standard treatment regimens reported similarly disappointing results.
These results, coupled with the lack of efficacy with onartuzumab reported in the present study and other negative studies of the MET antibodies rilotumumab [20] and ficlatuzumab [21] (both of which are fully humanized monoclonal antibodies that block HGF binding to the MET receptor [15]), suggest that monoclonal antibodies that compete with or interfere with HGF binding to the MET receptor may not be suitable for targeting HGF/MET dysregulation. Alternative means of inhibiting the MET pathway may be more successful in controlling oncogenic MET/HGF signaling, which can occur through several mechanisms, including gene amplification or mutation, protein overexpression, or abnormal gene splicing [22]. The anaplastic lymphoma kinase (ALK) inhibitor crizotinib, which also targets MET tyrosine kinase (TK), has shown promising efficacy in patients with lung cancer and de novo genomic MET amplification but who have no ALK gene rearrangements [23]. Alternatively, targeting the MET pathway for control of signal transduction in cancer may require multiple points of blockade.
An unexpected finding of this study was a trend toward improved PFS benefit in patients with MET IHC‐negative mCRC, which is contrary to the detrimental outcomes seen with MET inhibitors in previous phase II studies of patients with MET IHC‐negative tumors [14], [24], [25]. Although this finding could be due to chance, it is in line with an exploratory analysis of the monoclonal antibody ficlatuzumab in NSCLC, which demonstrated that the addition of ficlatuzumab to gefitinib appeared to benefit patients with low tumoral MET expression [15]. In addition, a subgroup analysis of a phase Ib study in colorectal cancer showed that patients with low MET expression tumors derived a statistically significant improvement in PFS with the possible anti‐MET TK inhibitor tivantinib [26], [27]. These results indicate a complex and as yet not understood relationship between MET expression and tumor response to anti‐MET agents. However, it should be noted that because secondary endpoints were similar between arms in the MET IHC‐negative subgroup, the PFS finding could represent type 1 error.
Onartuzumab combined with mFOLFOX‐6 plus bevacizumab was generally well tolerated in patients with mCRC. The incidence of grade ≥3 deep vein thrombosis, fatigue, and peripheral edema was higher with onartuzumab than with placebo, which was expected [14]. Peripheral edema has also been frequently reported as a common toxicity associated with anti‐HGF/MET antibodies across multiple tumor types and combination regimens [28], [29]. In addition, higher rates of SAEs and AEs leading to study withdrawal were recorded in the onartuzumab arm compared with placebo, although these are in line with previous studies of onartuzumab [14], [18]. Overall, the safety profile was as expected, with no new safety signals for onartuzumab.
Conclusion
In this randomized phase II study, onartuzumab combined with mFOLFOX‐6/bevacizumab failed to improve PFS, OS, or ORR in patients with mCRC. Collective experience across multiple trials with onartuzumab suggests that MET IHC was not an appropriate biomarker for this agent. It remains to be seen if other biomarkers might have worked better, or, alternatively, small molecule inhibitors might be a more appropriate approach to inhibiting this important oncogenic pathway.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
Third‐party medical writing assistance, under the direction of the authors, was provided by Fiona Fernando, Ph.D., of Gardiner‐Caldwell Communications, and was funded by F. Hoffmann‐La Roche Ltd. This work was sponsored by F. Hoffmann‐La Roche Ltd.
Author Contributions
Conception/Design: Johanna C. Bendell, Lowell L. Hart, Irfan Firdaus
Provision of study material or patients: Johanna C. Bendell, Joseph R. Mace, Joshua J. McFarlane, Mark Kozloff, Daniel Catenacci
Collection and/or assembly of data: Johanna C. Bendell, Howard Hochster, Lowell L. Hart, Irfan Firdaus, Joseph R. Mace, Joshua J. McFarlane, Daniel Catenacci, Jessie J. Hsu, David S. Shames, See‐Chun Phan, Hartmut Koeppen, Allen L. Cohn
Data analysis and interpretation: Johanna C. Bendell, Lowell L. Hart, Irfan Firdaus, Mark Kozloff, Daniel Catenacci, Jessie J. Hsu, Stephen P. Hack, David S. Shames, See‐Chun Phan, Hartmut Koeppen, Allen L. Cohn
Manuscript writing: Johanna C. Bendell, Howard Hochster, Lowell L. Hart, Joshua J. McFarlane, Mark Kozloff, Daniel Catenacci, Jessie J. Hsu, Stephen P. Hack, David S. Shames, See‐Chun Phan, Hartmut Koeppen, Allen L. Cohn
Final approval of manuscript: Johanna C. Bendell, Howard Hochster, Lowell L. Hart, Irfan Firdaus, Joseph R. Mace, Joshua J. McFarlane, Mark Kozloff, Daniel Catenacci, Jessie J. Hsu, Stephen P. Hack, David S. Shames, See‐Chun Phan, Hartmut Koeppen, Allen L. Cohn
Disclosures
Howard Hochster: Genentech (C/A); Mark Kozloff: Genentech/Roche (C/A, H); Daniel V. Catenacci: Genentech/Roche (C/A, RF, H); Jessie J. Hsu: Genentech (E, OI); Stephen P. Hack: Genentech/Roche (E, OI); David S. Shames: Genentech/Roche (E, OI); See-Chun Phan: Genentech (E, OI); Hartmut Koeppen: Genentech/Roche (E); Allen L. Cohn: Ipsen, Taiho, Merrimack (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supplementary Information
References
- 1. Luo HY, Xu RH. Predictive and prognostic biomarkers with therapeutic targets in advanced colorectal cancer. World J Gastroenterol 2014;20:3858–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Divitiis C, Nasti G, Montano M et al. Prognostic and predictive response factors in colorectal cancer patients: Between hope and reality. World J Gastroenterol 2014;20:15049–15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Zouhairi M, Charabaty A, Pishvaian MJ. Molecularly targeted therapy for metastatic colon cancer: Proven treatments and promising new agents. Gastrointest Cancer Res 2011;4:15–21. [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN clinical practice guidelines in oncology: Colon cancer. V5.2015 . Available at http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed May 11, 2016.
- 5. Kopetz S, Chang GJ, Overman MJ et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu X, Yao W, Newton RC et al. Targeting the c‐MET signaling pathway for cancer therapy. Expert Opin Investig Drugs 2008;17:997–1011. [DOI] [PubMed] [Google Scholar]
- 7. Ma PC, Maulik G, Christensen J et al. c‐Met: Structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev 2003;22:309–325. [DOI] [PubMed] [Google Scholar]
- 8. Blumenschein GR Jr, Mills GB, Gonzalez‐Angulo AM. Targeting the hepatocyte growth factor‐cMET axis in cancer therapy. J Clin Oncol 2012;30:3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. You WK, McDonald DM. The hepatocyte growth factor/c‐Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep 2008;41:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng ZS, Weiser MR, Kuntz E et al. c‐Met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett 2008;265:258–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samamé Pérez‐Vargas JC, Biondani P, Maggi C et al. Role of cMET in the development and progression of colorectal cancer. Int J Mol Sci 2013;14:18056–18077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kopetz S, Hoff PM, Morris JS et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010;28:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merchant M, Ma X, Maun HR et al. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti‐tumor activity as a therapeutic agent. Proc Natl Acad Sci USA 2013;110:E2987–E2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spigel DR, Ervin TJ, Ramlau RA et al. Randomized phase II trial of onartuzumab in combination with erlotinib in patients with advanced non‐small‐cell lung cancer. J Clin Oncol 2013;31:4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smyth EC, Sclafani F, Cunningham D. Emerging molecular targets in oncology: Clinical potential of MET/hepatocyte growth‐factor inhibitors. Onco Targets Ther 2014;7:1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spigel DR, Edelman MJ, O'Byrne K et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo‐controlled METLung (OAM4971g) global trial. J Clin Oncol 2014;32(suppl):Abstract 8000. [DOI] [PubMed] [Google Scholar]
- 17. Shah MA, Cho JY, Huat ITB et al. Randomized phase II study of FOLFOX ± MET inhibitor, onartuzumab (O), in advanced gastroesophageal adenocarcinoma (GEC). J Clin Oncol 2015;33(suppl):Abstract 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diéras V, Campone M, Yardley DA et al. Randomized, phase II, placebo‐controlled trial of onartuzumab and/or bevacizumab in combination with weekly paclitaxel in patients with metastatic triple‐negative breast cancer. Ann Oncol 2015;26:1904–1910. [DOI] [PubMed] [Google Scholar]
- 19. Cloughesy T, Finocchiaro G, Belda‐Iniesta C et al. Phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma. Neuro Oncol 2014;81(suppl 5):Abstract ET‐12. [Google Scholar]
- 20. Cunningham D, Tebbutt NC, Davidenko I et al. Phase III, randomized, double‐blind, multicenter, placebo (P)‐controlled trial of rilotumumab (R) plus epirubicin, cisplatin and capecitabine (ECX) as first‐line therapy in patients (pts) with advanced MET‐positive (pos) gastric or gastroesophageal junction (G/GEJ) cancer: RILOMET‐1 study. J Clin Oncol 2015;33(suppl):Abstract 4000. [Google Scholar]
- 21. Mok TSK, Park K, Geater SL et al. A randomized phase (Ph) 2 study with exploratory biomarker analysis of ficlatuzumab (F) a humanized hepatocyte growth factor (HGF) inhibitory MAB in combination with gefitinib (G) versus G in Asian patients (pts) with lung adenocarcinoma (LA). Ann Oncol 2012;23(suppl 9):Abstract 1198P. [Google Scholar]
- 22. Gherardi E, Birchmeier W, Birchmeier C et al. Targeting MET in cancer: Rationale and progress. Nat Rev Cancer 2012;12:89–103. [DOI] [PubMed] [Google Scholar]
- 23. Awad MM, Shaw AT. ALK inhibitors in non‐small cell lung cancer: Crizotinib and beyond. Clin Adv Hematol Oncol 2014;12:429–439. [PMC free article] [PubMed] [Google Scholar]
- 24. Oliner KS, Tang R, Anderson A et al. Evaluation of MET pathway biomarkers in a phase II study of rilotumumab (R, AMG 102) or placebo (P) in combination with epirubicin, cisplatin, and capecitabine (ECX) in patients (pts) with locally advanced or metastatic gastric (G) or esophagogastric junction (EGJ) cancer. J Clin Oncol 2012;30(suppl):Abstract 4005. [Google Scholar]
- 25. Ryan CJ, Rosenthal M, Ng S et al. Targeted MET inhibition in castration‐resistant prostate cancer: A randomized phase II study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin Cancer Res 2013;19:215–224. [DOI] [PubMed] [Google Scholar]
- 26. Eng C, Bessudo A, Gabrail N. Phase I/II study of tivantinib (ARQ197), irinotecan, and cetuximab in patients with KRAS wild type, previously treated, metastatic colorectal cancer patients. Ann Oncol 2012;23(suppl):Abstract PD‐0018. [Google Scholar]
- 27. Eng C, Hart LL, Severtsev A et al. A randomized, placebo‐controlled, phase I/II study of tivantinib (ARQ 197) in combination with cetuximab and irinotecan in patients (pts) with KRAS wild‐type (WT) metastatic colorectal cancer (CRC) who had received previous front‐line systemic therapy. J Clin Oncol 2013;31(suppl):Abstract 3508. [Google Scholar]
- 28. Maroun CR, Rowlands T. The Met receptor tyrosine kinase: A key player in oncogenesis and drug resistance. Pharmacol Ther 2014;142:316–338. [DOI] [PubMed] [Google Scholar]
- 29. Morley R, Cardenas A, Hawkins P et al. Safety of onartuzumab in patients with solid tumors: Experience to date from the onartuzumab clinical trial program. PLoS One 2015;10:e0139679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.