Abstract
Lessons Learned.
The safety profile in the gemcitabine/simtuzumab group was similar to that in the gemcitabine/placebo group.
The addition of simtuzumab to gemcitabine does not improve clinical outcomes in patients with metastatic pancreatic adenocarcinoma
Background.
The humanized IgG4 monoclonal antibody simtuzumab inhibits the extracellular matrix‐remodeling enzyme lysyl oxidase‐like 2 maintaining pathological stroma in tumors.
Methods.
Adult patients with metastatic pancreatic adenocarcinoma (mPaCa) were randomly assigned to receive intravenous gemcitabine, 1,000 mg/m2, in combination with 200 or 700 mg simtuzumab or placebo. Primary endpoint was progression‐free survival (PFS), secondary endpoints included overall survival (OS), objective response rate (ORR), and safety.
Results.
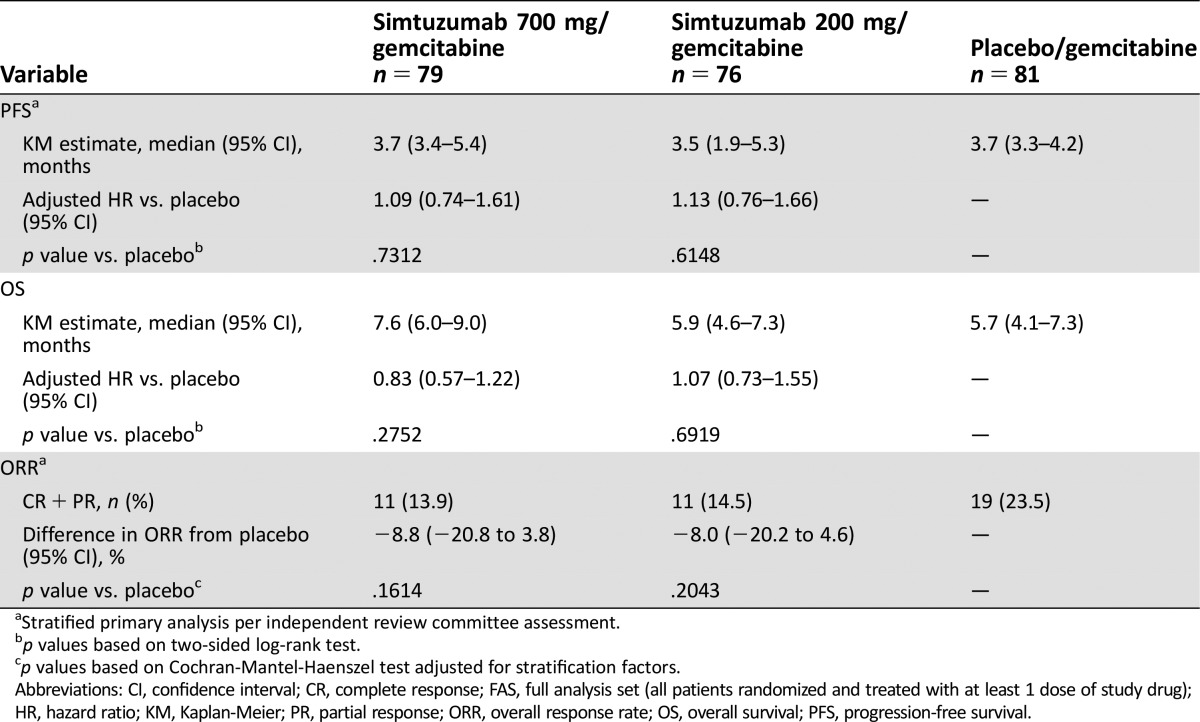
Of 240 patients, 80 were randomly assigned to gemcitabine/simtuzumab 700 mg, 79 to gemcitabine/simtuzumab 200 mg, and 81 to gemcitabine/placebo. After a median follow‐up of 3.0, 1.9, and 3.4 months for gemcitabine/simtuzumab 700 mg, gemcitabine/simtuzumab 200 mg, and gemcitabine/placebo, respectively, the median PFS was 3.7 months (adjusted hazard ratio [HR], 95% confidence interval [CI], p value vs placebo: 1.09 [0.74–1.61]; p = .73), 3.5 months (1.13 [0.76–1.66], p = .61]), and 3.7 months, respectively. Median OS was 7.6 months (0.83 [0.57–1.22]; p = .28), 5.9 months (1.07 [0.73–1.55]; p = .69), and 5.7 months, respectively. ORRs were 13.9%, 14.5%, and 23.5%, respectively. Simtuzumab was well tolerated.
Conclusion.
The addition of simtuzumab to gemcitabine did not improve clinical outcomes in patients with mPaCa.
Abstract
经验总结
• 吉西他滨/Simtuzumab组的安全性特征与吉西他滨/安慰剂组相似。
• Simtuzumab与吉西他滨联用未改善转移性胰腺腺癌患者的临床预后。
摘要
背景. 人源化IgG4单克隆抗体Simtuzumab可抑制赖氨酰氧化酶样蛋白‐2这一细胞外基质重塑酶, 该酶负责维系肿瘤的病理性基质。
方法. 转移性胰腺腺癌 (mPaCa) 成年患者随机接受静脉注射用吉西他滨 1,000mg/m2 联合 Simtuzumab 200 或 700mg 或者联合安慰剂治疗。主要终点为无进展生存期 (PFS), 次要终点包括总生存期 (OS)、客观缓解率 (ORR) 和安全性。
结果. 240例患者中有80例随机分配至吉西他滨/Simtuzumab 700 mg组, 79例分配至吉西他滨/Simtuzumab 200 mg组, 81例分配至吉西他滨/安慰剂组。分别对吉西他滨/Simtuzumab 700 mg组、吉西他滨/Simtuzumab 200 mg组和吉西他滨/安慰剂组进行为期3.0个月、1.9个月和3.4个月的中位随访后, 三组的中位PFS依次为3.7个月[校正风险比 (HR), 95%置信区间 (CI), 与安慰剂比较的p值:1.09 (0.74‐1.61); p = 0.73]、3.5个月 [1.13 (0.76‐1.66); p = 0.61] 和 3.7个月。中位OS分别为7.6个月[0.83 (0.57‐1.22); p = 0.28]、5.9 个月 [1.07 (0.73‐1.55); p = 0.69] 和5.7个月。ORR分别为13.9%、14.5%和23.5%。Simtuzumab耐受性良好。
结论. Simtuzumab与吉西他滨联用未改善mPaCa患者的临床预后。
Discussion
Tumor‐associated activated fibroblasts secrete oncogenic growth factors, produce extracellular matrix (ECM), and contribute to the desmoplastic reaction and epithelial cell transformation. Lysyl oxidase‐like 2 (LOXL2), an enzyme that remodels ECM, is expressed in desmoplastic tumors and supports maintenance of the pathologic stromal microenvironment in cancer and fibrotic diseases.
Simtuzumab is a humanized IgG4 monoclonal antibody that inhibits enzymatic activity of LOXL2. In preclinical studies, inhibition of LOXL2 expression reduced numbers of activated fibroblasts, decreased ECM deposition, inhibited angiogenesis, and prevented tumor cell invasion and metastases. In a phase I study in patients with advanced solid tumors (NCT01323933), simtuzumab monotherapy led to reduction in size of several solid tumors.
This multicenter, randomized, double‐blind, placebo‐controlled phase II study assessed the additive efficacy of simtuzumab versus placebo in combination with gemcitabine as a first‐line therapy in patients with metastatic pancreatic adenocarcinoma (mPaCa) (NCT01472198). Adult patients with measurable mPaCa diagnosed within 6 weeks of screening, Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1, and no prior systemic therapy for mPaCa were eligible. Patients were randomly assigned 1:1:1 to receive intravenous gemcitabine, 1,000 mg/m2, in combination with 200 or 700 mg simtuzumab or placebo in cycles of 28 days, until disease progression/death or unacceptable toxicity; randomization was stratified by ECOG PS 0 or 1 and prior therapy for primary pancreatic tumor (yes, yes including surgical resection, or no). Tumor progression was assessed by computed tomography or magnetic resonance imaging every 8 weeks. Primary analyses were performed on the basis of independent review committee (IRC) assessments, and sensitivity analyses were performed on the basis of investigator's assessments.
From December 2011 to May 2013, 240 patients (mean age, 63 years) were randomly assigned and 236 received at least one cycle of study drug, with a median number of three cycles. Median duration of treatment exposure was 2 months. Overall, 221 (92%) patients had discontinued study drug, mainly because of disease progression (162 [68%]) and adverse events (26 [11%]).
A sequential stepwise hypothesis and a Hochberg testing procedure were used to establish differences between simtuzumab dosing groups versus placebo for progression‐free survival (PFS), overall survival (OS), and overall response rate (ORR). Despite results from preclinical studies and the phase I study results indicating a positive response in select patients with solid tumors, this study did not meet the prespecified primary and secondary endpoints of improvement in PFS, OS, and ORR in patients treated with simtuzumab compared with placebo, as evidenced by the stratified primary analyses (Table 1). Sensitivity analyses based on investigator's assessments were consistent with the results obtained from the primary IRC assessments. The safety profile in the patient groups that received gemcitabine/simtuzumab did not differ from the profile in the gemcitabine/placebo group.
Table 1. The efficacy endpoints (FAS analysis set).
Stratified primary analysis per independent review committee assessment.
p values based on two‐sided log‐rank test.
p values based on Cochran‐Mantel‐Haenszel test adjusted for stratification factors.
Abbreviations: CI, confidence interval; CR, complete response; FAS, full analysis set (all patients randomized and treated with at least 1 dose of study drug); HR, hazard ratio; KM, Kaplan‐Meier; PR, partial response; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival.
Trial Information
- Disease
Pancreatic cancer
- Stage of disease/treatment
Metastatic/Advanced
- Prior Therapy
None
- Type of study – 1
Phase II
- Type of study – 2
Randomized
- ORR
Difference in ORR from placebo, stratified (primary) analysis was −8.8% for the simtuzumab 700 mg arm (p = .16) and −8% for the simtuzumab 200 mg arm (p = .20)
- PFS
The median PFS was 3.7 months, (HR 1.09, 0.74–1.61, p = .73) in the 700 mg simtuzumab arm, 3.5 months (HR 1.13, 0.76–1.66, p = .61) in the 200 mg simtuzumab arm, and 3.7 months in the control arm.
- Primary Endpoint
Progression-Free Survival
- Secondary Endpoint
Overall Response Rate
- Secondary Endpoint
Overall Survival
- Secondary Endpoint
Safety
- Additional Details of Endpoints or Study Design
-
Patients who received prior radiotherapy or chemoimmunotherapy given as preoperative neoadjuvant or radio sensitizer therapies were eligible for enrollment. During each 28‐day cycle, patients received intravenous simtuzumab infused on days 1 and 15 and gemcitabine on days 1, 8, and 15. Efficacy was analyzed in all randomly assigned patients who received at least one dose of study drug, with treatment assignments designated according to the study drug initially randomized (full analysis set [FAS]). Tumor assessments were analyzed primarily on the basis of IRC assessment; sensitivity analyses were performed according to investigator's tumor assessments. Safety analysis set included patients who received at least one dose of study drug grouped for analyses, with treatment assignments designated according to the actual study drug received. Safety assessments included the incidence of adverse events (AEs), injection site reactions, clinically relevant changes in laboratory values, and vital signs. Clinical and laboratory AEs were coded by using the Medical Dictionary for Regulatory Activities (MedDRA), version 16.1, and graded by using the National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03). The pharmacokinetic analysis set included patients who had the necessary baseline and on‐study measurements to provide interpretable results for simtuzumab plasma concentrations. Samples to measure simtuzumab serum concentrations were collected before infusion on day 1 and day 15 of each cycle.
ORR was assessed by IRC per Response Evaluation Criteria In Solid Tumors (RECIST version 1.1) as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) for patients with measurable target lesions at baseline; as CR, non‐CR/non‐PD, or PD for patients without measurable target lesion identified at baseline; or as no disease or PD for patients with no disease identified at baseline. The response of not evaluable was recorded for patients who dropped out early because of toxicity, death, or other reasons before the scheduled imaging was performed or for patients with poor‐quality images. The difference in PFS and OS among the treatment groups was assessed by using Kaplan‐Meier methods and the stratified log‐rank test, adjusted for the stratification factors ECOG PS 0 or 1 and prior therapy for primary tumor. The Cochran‐Mantel‐Haenszel test was used for assessing the difference in ORR from placebo. A sequential stepwise hypothesis and a Hochberg testing procedure were used for multiple hypothesis testing to compare between two simtuzumab dosing groups versus placebo on multiple endpoints: PFS, OS, and ORR. A number of sensitivity analyses for PFS, OS, and ORR were also performed to confirm the results of primary analyses. A total of 150 PFS events had to be observed in this study to detect a hazard ratio of 0.57 with approximately 90% power at a two‐sided .05 significance level based on log‐rank test and Hochberg procedure to claim that at least one of the simtuzumab treatment groups improved PFS significantly compared with placebo. The overall sample size needed was estimated to be 234 patients.
- Investigator's Analysis
Level of activity did not meet planned endpoint.
Drug Information Control Arm
- Drug 1
- Generic/Working name
Gemcitabine
- Trade name
Gemzar
- Company name
Lilly USA, LLC
- Drug type
Cytotoxic
- Drug class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
Drug Information Experimental Arm A: Simtuzumab 200 mg
- Drug 1
- Generic/Working name
Simtuzumab (GS‐6624)
- Trade name
- Company name
Gilead Sciences, Inc.
- Drug type
Antibody
- Drug class
Inhibitor of lysyl oxydase-like 2 enzyme
- Dose
200 milligrams (mg) per flat dose
- Route
IV
- Schedule of Administration
Day 1 and 15 of each 28‐day cycle
- Drug 2
- Generic/Working name
Gemcitabine
- Trade name
Gemzar
- Company name
Lilly USA, LLC
- Drug type
Cytotoxic
- Drug class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
Drug Information Experimental Arm B: Simtuzumab 700 mg
- Drug 1
- Generic/Working Name
Simtuzumab (GS‐6624)
- Trade name
- Company name
Gilead Sciences, Inc.
- Drug type
Antibody
- Drug class
Inhibitor of lysyl oxydase-like 2 enzyme
- Dose
700 milligrams (mg) per flat dose
- Route
IV
- Schedule of Administration
Day 1 and 15 of each 28‐day cycle
- Drug 2
- Generic/Working name
Gemcitabine
- Trade name
Gemzar
- Company name
Lilly USA, LLC
- Drug type
Cytotoxic
- Drug class
Antimetabolite
- Dose
1,000 milligrams (mg) per squared meter (m2)
- Route
IV
- Schedule of Administration
Days 1, 8, and 15 of each 28‐day cycle
Patient Characteristics
- Number of patients, male
138
- Number of patients, female
98
- Stage
Advanced, metastatic
- Age
Median (range): Simtuzumab 700 mg: 61 (37–86) years; simtuzumab 200 mg: 64 (34–88) years; placebo: 63 (40–87) years
- Number of prior systemic therapies
Median (range): None
- Performance Status: ECOG
-
0 — 65
1 — 167
2 — 4
Primary Assessment Method
- Number of patients enrolled
81
- Number of patients evaluable for toxicity
81
- Number of patients evaluated for efficacy
81
- Response assessment CR
n =1 (1%)
- Response assessment PR
n = 18 (22%)
- Response assessment SD
n = 24 (30%)
- Response assessment PD
n = 13 (16%)
- Response assessment OTHER
n = 25 (31%)
- (Median) duration assessments PFS
3.7 months, CI: 3.3–4.2
- (Median) duration assessments OS
5.7 months, CI: 4.1–7.3
- Experimental Arm A: Simtuzumab 200 mg
- Number of patients enrolled
79
- Number of patients evaluable for toxicity
76
- Number of patients evaluated for efficacy
6
- Response assessment CR
n = 1 (1%)
- Response assessment PR
n = 10 (13%)
- Response assessment SD
n = 27 (36%)
- Response assessment PD
n = 21 (28%)
- Response assessment OTHER
n = 17 (22%)
- (Median) duration assessments PFS
3.5 months, CI: 1.9–5.3
- (Median) duration assessments OS
5.9 months, CI: 4.6–7.3
- Experimental Arm B: Simtuzumab 700 mg
- Number of patients enrolled
80
- Number of patients evaluable for toxicity
79
- Number of patients evaluated for efficacy
79
- Response assessment CR
n = 2 (3%)
- Response assessment PR
n = 9 (11%)
- Response assessment SD
n = 35 (44%)
- Response assessment PD
n = 14 (18%)
- Response assessment OTHER
n = 19 (24%)
- (Median) duration assessments PFS
3.7 months, CI: 3.4–5.4
- (Median) duration assessments OS
7.6 months, CI: 6.0–9.0
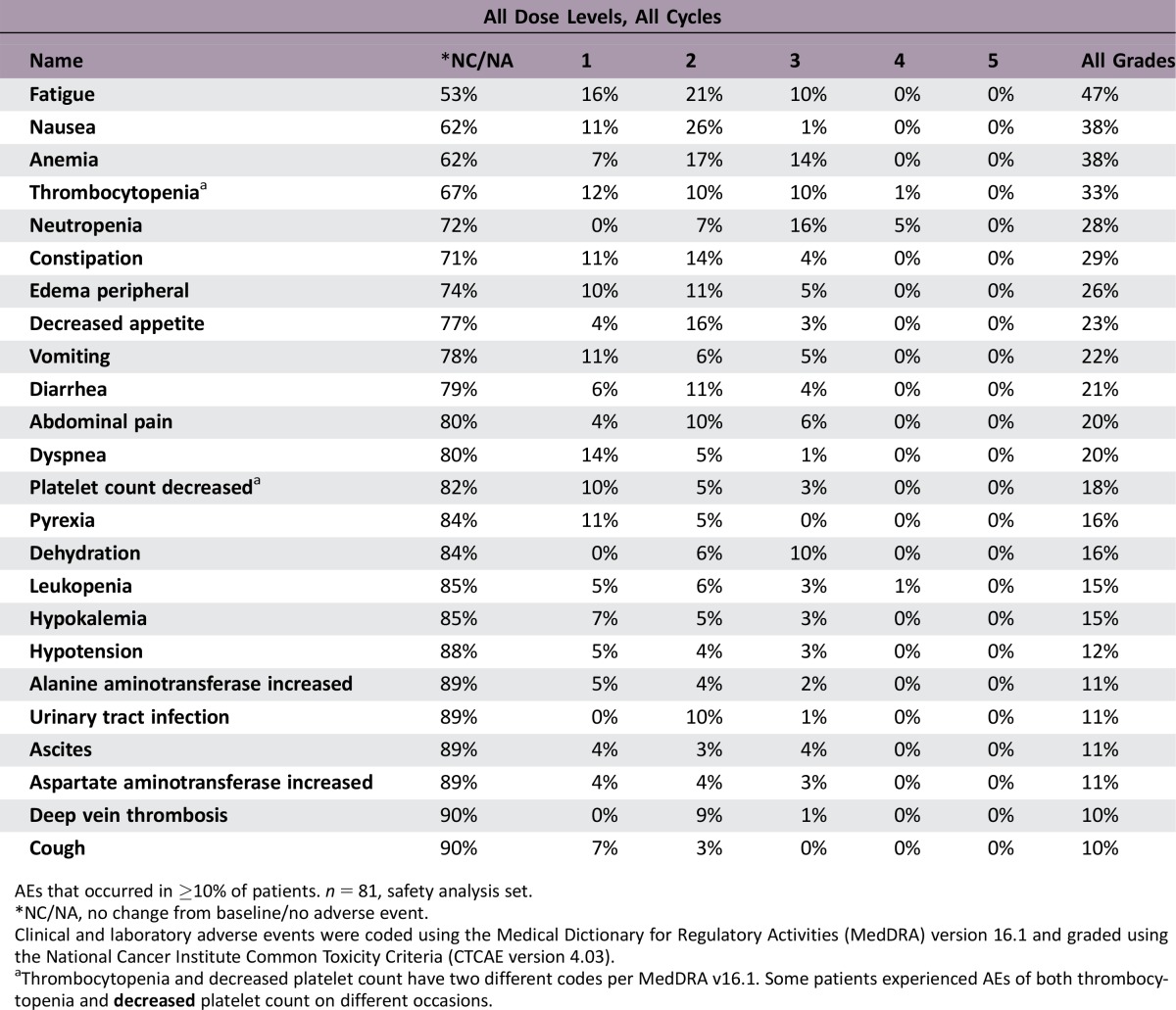
Adverse Events Control Arm
AEs that occurred in ≥10% of patients. n = 81, safety analysis set.
*NC/NA, no change from baseline/no adverse event.
Clinical and laboratory adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1 and graded using the National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03).
aThrombocytopenia and decreased platelet count have two different codes per MedDRA v16.1. Some patients experienced AEs of both thrombocytopenia and decreased platelet count on different occasions.
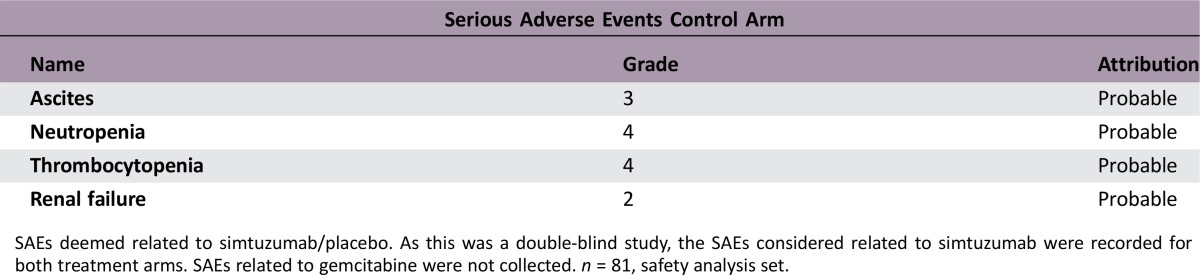
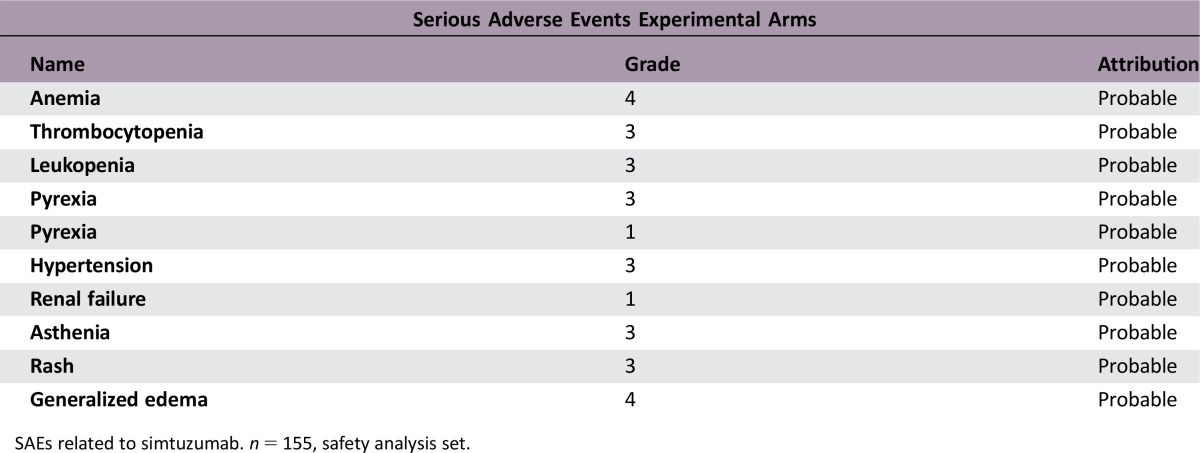
SAEs deemed related to simtuzumab/placebo. As this was a double‐blind study, the SAEs considered related to simtuzumab were recorded for both treatment arms. SAEs related to gemcitabine were not collected. n = 81, safety analysis set.
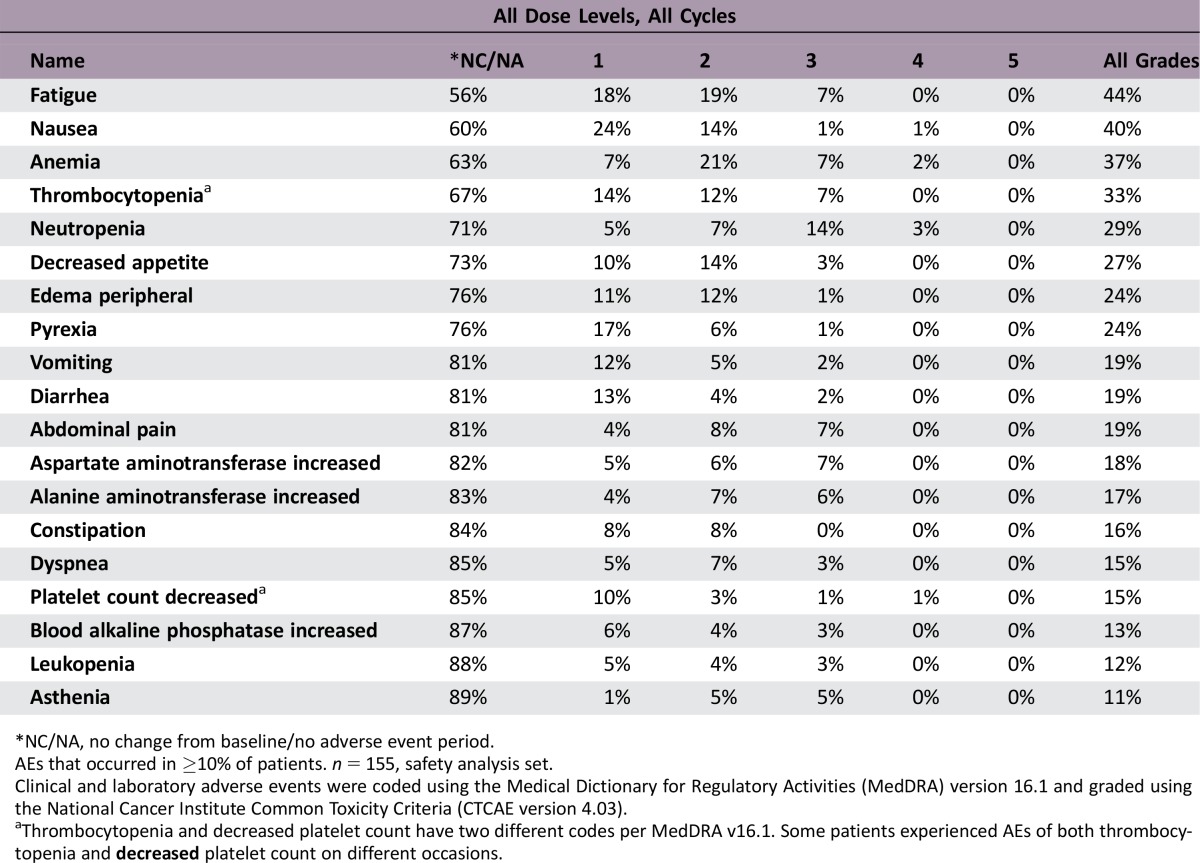
Adverse Events Experimental Arms
*NC/NA, no change from baseline/no adverse event period.
AEs that occurred in ≥10% of patients. n = 155, safety analysis set.
Clinical and laboratory adverse events were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 16.1 and graded using the National Cancer Institute Common Toxicity Criteria (CTCAE version 4.03).
aThrombocytopenia and decreased platelet count have two different codes per MedDRA v16.1. Some patients experienced AEs of both thrombocytopenia and decreased platelet count on different occasions.
SAEs related to simtuzumab. n = 155, safety analysis set.
Assessment, Analysis, and Discussion
- Study completed
- Pharmacokinetics/Pharmacodynamics
Pharmacokinetics were assessed.
- Investigator's Assessment
Level of activity did not meet planned endpoint
Pancreatic adenocarcinoma (PaCa) is the seventh most common cause of death from cancer, with 330,400 deaths reported worldwide annually [1]. Owing to the lack of early detection tests and aggressive tumor growth, PaCa has a very poor prognosis, with a 5‐year survival rate of 7% or less [2], [3].
In early stages of PaCa development, activated fibroblasts often proliferate and produce an excess collagen‐rich extracellular matrix (ECM) [3]. As a result, the basement membrane surrounding pancreatic epithelial ducts may be disrupted, promoting invasion of tumor cells and subsequent propagation of desmoplastic reaction comprising vascular proliferation, infiltration of inflammatory cells, and further ECM synthesis [3], [4]. Desmoplastic reaction may also limit the chance of successful surgical removal of the primary tumor, with only 15% of diagnosed patients considered suitable for the procedure [4]. The most effective nonsurgical first‐line treatment for advanced or metastatic PaCa (mPaCa) includes gemcitabine alone or in combination with chemotherapy [5]. This therapy is not curative [2], thus emphasizing the need for more effective treatment options.
Lysyl oxidase‐like 2 (LOXL2), an extracellular matrix enzyme that catalyzes the cross‐linking of collagen and elastin [6], is expressed in desmoplastic tumors [7], and inhibition of LOXL2 was shown to reduce the desmoplastic reaction [6], [8]. LOXL2 is thought to contribute to the pathologic stromal microenvironment in cancer and fibrotic diseases [6] and to promote tumor angiogenesis [9] and metastasis [10].
Simtuzumab, a humanized IgG4 monoclonal antibody, binds to LOXL2 and inhibits its enzymatic activity [11]. In preclinical studies with antibody precursors to simtuzumab, inhibition of LOXL2 reduced numbers of activated fibroblasts, decreased ECM deposition [6], reduced angiogenesis [12], and inhibited tumor growth and metastases [13]. In a phase I study, treatment with simtuzumab led to a decrease in size of several solid tumors in select patients [14].
This multicenter, randomized, double‐blind, placebo‐controlled phase II study evaluated the additive efficacy of simtuzumab or placebo in combination with gemcitabine as a first‐line therapy in patients with mPaCa (NCT01472198). The key endpoints were progression‐free survival (PFS), overall survival (OS), and objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, along with safety. Eligible for enrollment were adult patients with a histologically confirmed recent diagnosis (≤6 weeks before enrollment) of mPaCa; measurable disease per RECIST version 1.1, an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and adequate hematopoietic, hepatic, and renal organ function.
Patients were randomly assigned 1:1:1 to receive i.v. infusions of simtuzumab 700 mg or 200 mg, or placebo, and gemcitabine, 1,000 mg/m2, in 28‐day cycles until disease progression/death or unacceptable toxicity. Randomization was stratified by ECOG status 0 or 1 and prior primary tumor therapy. Tumor assessments were performed by computed tomography or magnetic resonance imaging every 8 weeks by independent review committee (IRC). Primary statistical analyses were performed on the basis of IRC assessments, and sensitivity analyses were performed according to investigator's assessments.
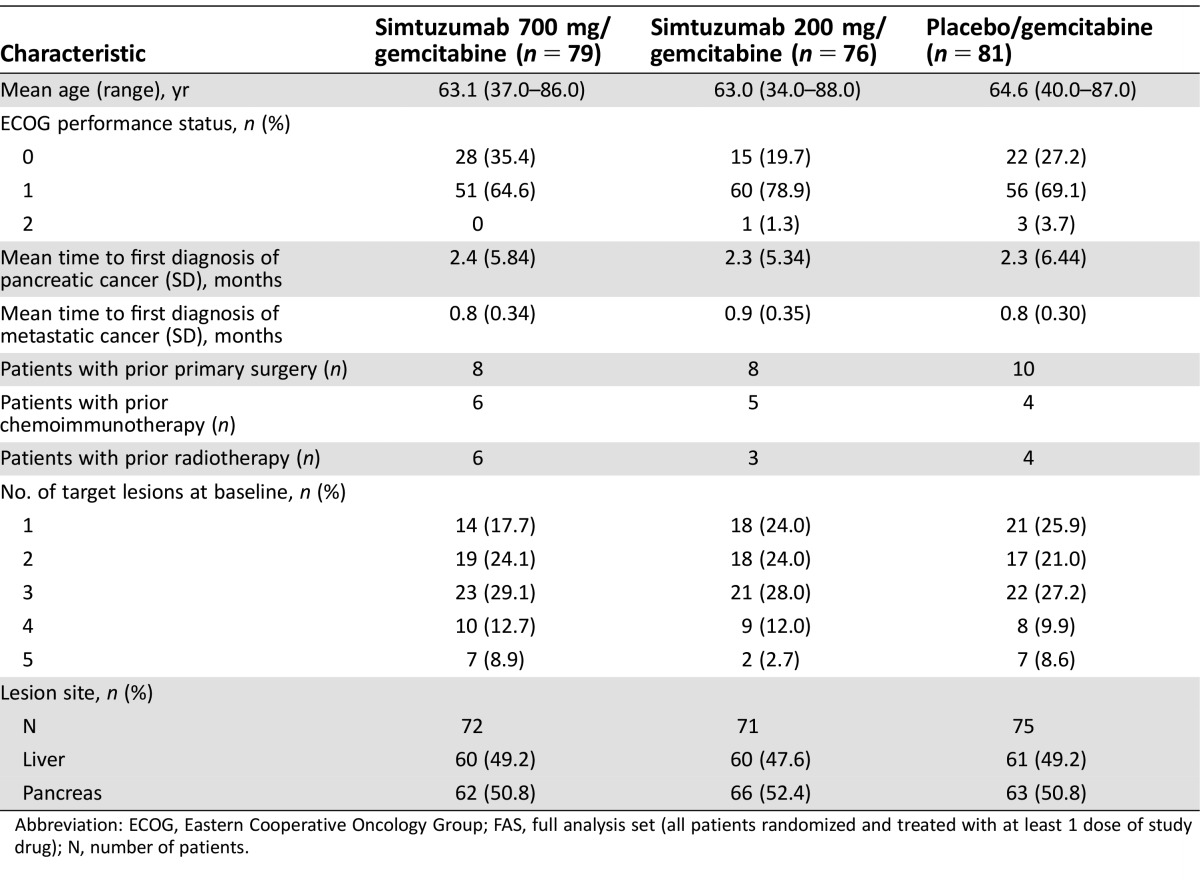
From December 2011 to May 2013, 240 patients were randomly assigned. Overall, 221 (92%) patients had discontinued study and 19 patients continued to receive treatment. The reasons for discontinuation included disease progression (162 [68%]), adverse events (26 [11%]), withdrawal of consent (20 [8.3%]), investigators decision (7 [2.9%]), or death (1 [0.4%]). The majority of enrolled patients were male (138/236, [59%]) and white (212/236 [90%]), with a mean age (range) of 63 (34.0–88.0) years. Baseline tumor burden was similar across treatment groups (Table 2).
Table 2. Demographic and baseline characteristics (FAS analysis set).
Abbreviation: ECOG, Eastern Cooperative Oncology Group; FAS, full analysis set (all patients randomized and treated with at least 1 dose of study drug); N, number of patients.
The pharmacokinetic analysis set included 134 patients. The mean serum simtuzumab trough levels increased dose‐proportionally with increased numbers of cycles, from 13,282 ng/mL after cycle 1 to 47,251 ng/mL after cycle 8 in patients on simtuzumab 200 mg, and from 49,483 ng/mL after cycle 1 to 109,281 after cycle 4 in patients on simtuzumab 700 mg. Thereafter, simtuzumab serum levels remained stable for both doses.
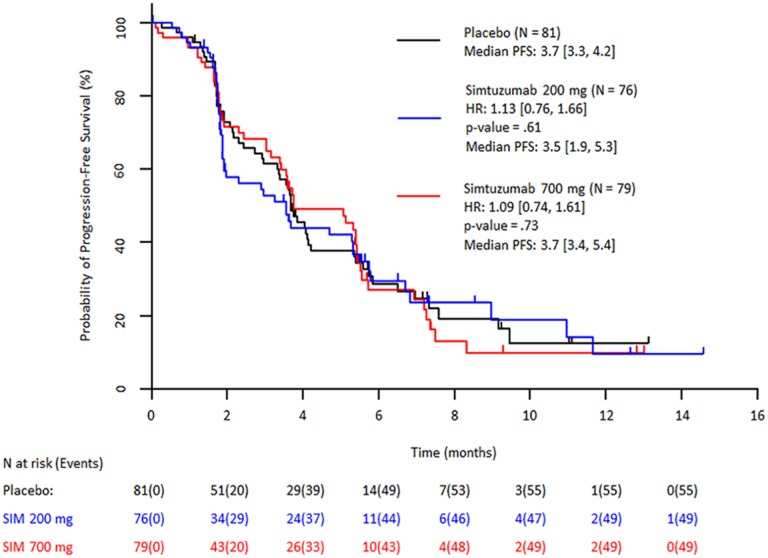
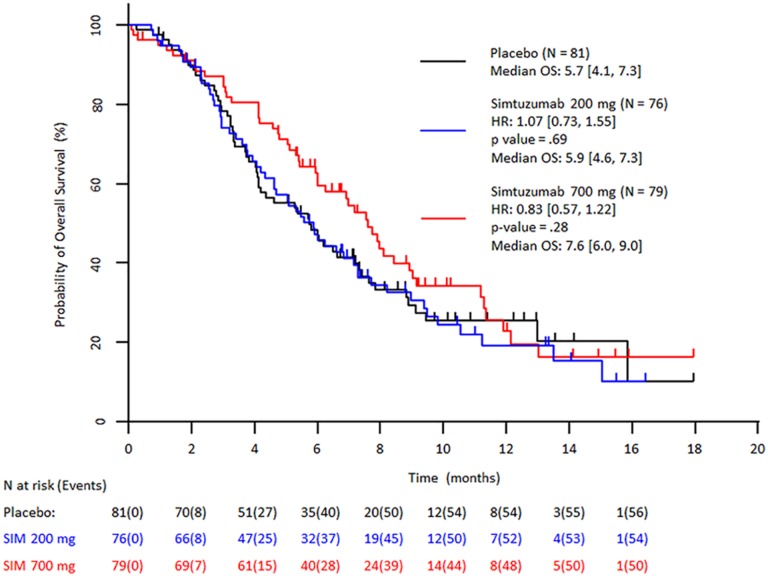
Despite preclinical and preliminary clinical results indicating positive response to simtuzumab, the prespecified primary and secondary efficacy endpoints of improvement in PFS, OS, and ORR in patients treated with simtuzumab/gemcitabine compared with placebo/gemcitabine were not reached in this study. After a median follow‐up of 3.0, 1.9, and 3.4 months for gemcitabine/simtuzumab 700 mg, gemcitabine/simtuzumab 200 mg, and gemcitabine/placebo, respectively, median PFS was 3.7 months (adjusted hazard ratio [HR] with 95% confidence interval [CI] for the stratified primary analysis, p value vs. placebo: 1.09 [0.74–1.61]; p = .73]), 3.5 months (1.13 [0.76–1.66]; p = .61), and 3.7 months, respectively (Figure 1). Median OS for the gemcitabine/simtuzumab 700 mg, gemcitabine/simtuzumab 200 mg, and gemcitabine/placebo was 7.6 months (adjusted HR, 95% CIs for the stratified primary analysis, p value vs. placebo, 0.83 [0.57–1.22]; p = .28), 5.9 months (1.07 [0.73–1.55]; p = .69), and 5.7 months, respectively (Figure 2). The ORRs for gemcitabine/simtuzumab 700 mg, gemcitabine/simtuzumab 200 mg, and gemcitabine/placebo were 13.9%, 14.5%, and 23.5%, respectively. The difference (95% CI) in ORR for patients treated with gemcitabine/simtuzumab 700 mg from patients treated with gemcitabine/placebo was −8.8% (−20.8% to 3.8%; p = .16) and for patients treated with gemcitabine/simtuzumab 200 mg from patients treated with gemcitabine/placebo was −8.0% (−0.2% to 4.6%; p = .20). Results from sensitivity analyses were consistent with the stratified primary analyses.
Figure 1.
Stratified Kaplan–Meier plot of progression‐free survival by independent review committee assessment (FAS population). Abbreviations: CI, confidence interval; FAS, full analysis set (all patients randomized and treated with 1 dose of study drug); HR, hazard ratio (numbers in brackets are 95% CIs); PFS, progression‐free survival; SIM, simtuzumab.
Figure 2.
Stratified Kaplan–Meier plot of overall survival (FAS population). Abbreviations: CI, confidence interval; FAS, full analysis set (all patients randomized and treated with 1 dose of study drug); HR, hazard ratio (numbers in brackets are 95% CIs); OS, overall survival; SIM, simtuzumab.
The safety analysis data set included 236 patients, 197 (84%) of whom received 2 or more cycles, with a median number of 3 cycles. Median duration of exposure to study treatment was 3.25 months in the gemcitabine/simtuzumab 700 mg arm and 1.75 months in the gemcitabine/simtuzumab 200 mg and gemcitabine/placebo arms.
The safety profile in the gemcitabine/simtuzumab group was similar to that in the gemcitabine/placebo group. The most common adverse events (AEs) included fatigue, nausea, anemia, thrombocytopenia, and neutropenia and occurred with similar frequency in all treatment groups. Similarly, the frequencies of AEs grade 3 or higher were 67%, 63%, and 70% in the gemcitabine/simtuzumab 700 mg, gemcitabine/simtuzumab 200 mg, and gemcitabine/placebo groups, respectively. None of the AEs resulting in deaths that occurred during the study were deemed related to study treatment.
In conclusion, the addition of simtuzumab to gemcitabine was tolerable but did not improve PFS, OS, or ORR in patients with mPaCa.
Figures and Tables
Acknowledgments
We acknowledge medical writing assistance funded by Gilead Sciences, Inc., and provided by Ewa Wandzioch, Ph.D., from AlphaBioCom, LLC.
Footnotes
ClinicalTrials.gov Identifier: NCT01472198
Sponsor(s): Gilead Sciences, Inc., Foster City, California, USA
Principal Investigator: Al B. Benson III
IRB Approved: Yes
Disclosures
Al B. Benson III: Genentech/Roche, Sanofi, Bristol‐Myers Squibb, Merck Serono, Merck/Schering Plough, Spectrum Pharmaceuticals, Lilly/ImClone, Celgene, Genomic Health, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono (C/A), Genentech/Roche, Sanofi, Bristol‐Myers Squibb, Merck Serono, Merck/Schering Plough, Spectrum Pharmaceuticals, Lilly/ImClone, Celgene, Genomic Health, Vicus Therapeutics, Pharmacyclics, Precision Therapeutics, Taiho Pharmaceutical, Bayer, Alchemia, Infinity Pharmaceuticals, Boehringer Ingelheim, Astellas Pharma, EMD Serono, AVEO, Gilead Sciences (H), Genentech, Gilead Sciences, Amgen, Astellas Pharma, Bayer/Onyx, Novartis, Alchemia, AVEO, Infinity Pharmaceuticals, Merck Serono, EMD Serono (RF); Zev A. Wainberg: Genentech, Sirtex, Taiho (C/A), Novartis, Gilead (RF); J. Randolph Hecht: Amgen, Armo, Boston Biomedical, Cornerstone, Genentech, Halozyme, Lexicon, Novocure, Symphogen (C/A), Amgen, Celgene, Gilead, GlaxoSmithKline, Merrimack, OncoMed, Symphogen (RF), Amgen, Genentech, Cornerstone, Armo, Novocure, Halozyme, Lexicon, Symphogen (H); Hua Dong: Gilead (E, OI); Johanna Bendell: Gilead, Genentech, Bristol‐Myers Squibb, Five Prime, Lilly, Merck, MedImmune, Celgene, EMD Serono, Taiho, Macrogenics, GlaxoSmithKline, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Bayer, Incyte, Apexigen, Roche, Koltan, SynDevRx, Forty Seven (RF); Fred Kudrik: Flatiron Health (CA). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Pancreatic Cancer Facts . 2015. Pancreatic Cancer Action Network. Available at https://www.pancan.org/wp-content/uploads/2015/06/2015-GAA-PC-Facts.pdf. Accessed May 12, 2016.
- 3. Chu GC, Kimmelman AC, Hezel AF et al. Stromal biology of pancreatic cancer. J Cell Biochem 2007;101:887–907. [DOI] [PubMed] [Google Scholar]
- 4. Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res 2008;149:319–328. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Pancreatic adenocarcinoma. 2016. Available at https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed May 12, 2016.
- 6. Barry‐Hamilton V, Spangler R, Marshall D et al. Allosteric inhibition of lysyl oxidase‐like‐2 impedes the development of a pathologic microenvironment. Nat Med 2010;16:1009–1017. [DOI] [PubMed] [Google Scholar]
- 7. Fong SF, Dietzsch E, Fong KS et al. Lysyl oxidase‐like 2 expression is increased in colon and esophageal tumors and associated with less differentiated colon tumors. Genes Chromosomes Cancer 2007;46:644–‐655. [DOI] [PubMed] [Google Scholar]
- 8. Benson A, Bendell J, Wainberg Z et al. A phase 2 randomized, double‐blind, placebo controlled study of simtuzumab or placebo in combination with gemcitabine for the first line treatment of pancreatic adenocarcinoma. Ann Oncol 2014;25;iv210–iv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zaffryar‐Eilot S, Marshall D, Voloshin T et al. Lysyl oxidase‐like‐2 promotes tumour angiogenesis and is a potential therapeutic target in angiogenic tumours. Carcinogenesis 2013;34:2370–2379. [DOI] [PubMed] [Google Scholar]
- 10. Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer—a prospect. J Cell Biochem 2007;101:1338–1354. [DOI] [PubMed] [Google Scholar]
- 11. Rodriguez HM, Vaysberg M, Mikels A et al. Modulation of lysyl oxidase‐like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem 2010;285:20964–20974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Bergen T, Marshall D, Van de Veire S et al. The role of LOX and LOXL2 in scar formation after glaucoma surgery. Invest Ophthalmol Vis Sci 2013;54:5788–5796. [DOI] [PubMed] [Google Scholar]
- 13. Peng L, Ran YL, Hu H et al. Secreted LOXL2 is a novel therapeutic target that promotes gastric cancer metastasis via the Src/FAK pathway. Carcinogenesis 2009;30:1660–1669. [DOI] [PubMed] [Google Scholar]
- 14. LoRusso P, Hecht J, Thai D et al. Phase I and IIa studies of simtuzumab alone and in combination with FOLFIRI in patients with advanced solid tumors. J Clin Oncol 2014;32:abstract 554. [Google Scholar]