With the aging of the population, the management of complexity of cancer in the elderly is an increasingly common challenge. A personalized approach to treatment of older cancer patients is proposed in this article.
Keywords: Aging and Cancer, Geriatric Oncology, Personalized Treatment, Complexity of Cancer in the Elderly
Abstract
The management of cancer in older aged people is becoming a common problem due to the aging of the population. There are many variables determining the complex situation that are interconnected. Some of them can be assessed, such as risk of mortality and risk of treatment complications, but many others are still unknown, such as the course of disease, the host‐related factors that influence cancer aggressiveness, and the phenotype heralding risk of permanent treatment‐related damage.
This article presents a dynamic and personalized approach to older people with cancer based on our experience on aging, cancer, and their biological interactions. Also, novel treatments and management approaches to older individuals, based on their functional age and their social and emotional needs, are thoughtfully explored here.
Implications for Practice.
The goal of this article is to suggest a practical approach to complexity, a clinical situation becoming increasingly common with the aging of the population. Beginning with the analysis of two clinical cases, the authors offer an algorithm for approaching cancer in the older person that involves the assessment of life expectancy without cancer, the risk that cancer might compromise a patient's survival, function, or quality of life, and the potential benefits and risks of the treatments based on a clinical evaluation. The authors then review possible laboratory assessment of functional age and the importance of rapid‐learning databases in the study of cancer and age.
Introduction
The Ramon Areces Foundation organized an International Workshop on cancer and aging entitled “Cancer as a result of aging: possible solutions,” which took place in Madrid, Spain, on November 3, 2015. This opinion paper represents the consensus reached after one day of discussion. The full list of the participants is provided in the acknowledgments.
With the aging of the population, the management of complexity represents an increasingly common problem [1], [2], [3], [4]. Complexity derives from the Latin “cum plexere,” which means “to weave together” [5]. In a complex situation, the variables are interwoven like the threads of a carpet. The practitioner is given the task to correct a flawed carpet design without unraveling the whole fabric.
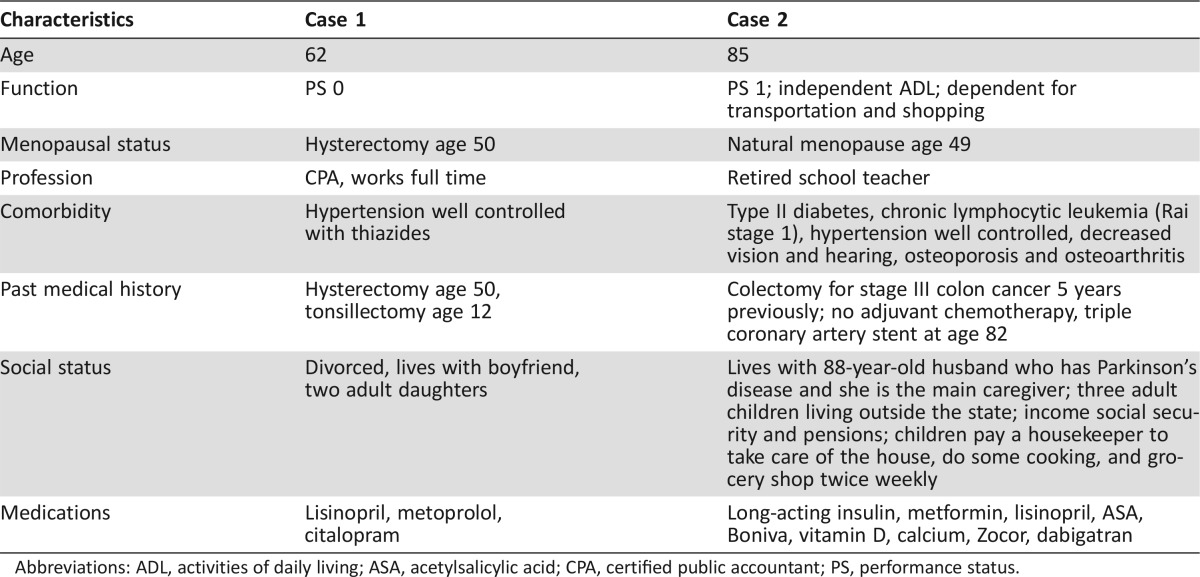
This article explores approaches to complexity in managing older persons with cancer, because cancer disproportionately affects older individuals [6], [7], [8], [9]. We propose a personalized approach to older cancer patients, using as a springboard two clinical cases (Table 1). Appropriately for an era of rapid scientific progresses and globalization our model is dynamic, to accommodate medical advances, flexible, to encompass the values of individual patients, and transferable to different cultures and countries.
Table 1. Comparison of two clinical cases.
Abbreviations: ADL, activities of daily living; ASA, acetylsalicylic acid; CPA, certified public accountant; PS, performance status.
Analysis of the Clinical Cases
Both patients were diagnosed with cancer of the left breast 2.1 cm in diameter and involving 2/17 axillary lymph nodes. In the first case, the balance of treatment benefits and risks has been established in clinical trials. Modern combination chemotherapy reduces the risk of breast cancer death from 50% to 35% with a likelihood of acute complications lower than 2% [10]. Despite some risk of late complications [11], [12], [13], there is consensus that the benefits of adjuvant chemotherapy trump the risks.
The management of the second case is based on inference and assumptions, as there are not and seemingly there will not be clinical trials proving the benefits of adjuvant chemotherapy in an older woman with comorbidities and functional dependence.
Most medical oncologists would handle the case in two different ways: the expectant and the split‐risk approaches. The expectant approach, inspired by the “first do no harm” principle, avoids chemotherapy for the following reasons: (a) there is at least a 50% chance that the cancer will not recur; (b) the benefit of aggressive treatment is marginal at most, given the limited life expectancy due to age, comorbidity, and function [14], [15]; and (c) cancer recurrence can be managed with single‐agent chemotherapy that may prolong life and palliate symptoms with minimal risk of complications; the risk of chemotherapy‐related toxicity increases with age [16]. Additional risk factors include diabetes, coronary artery disease, and polypharmacy [17].
The split‐risk approach assumes some adjuvant chemotherapy may reduce the risk of recurrence without compromising health and function and may decrease the risk of breast cancer‐related death and disability. This approach may include weekly treatments, such as a combination of carboplatin and paclitaxel, easily titrated to the emergence of complications.
Few practitioners would administer full treatment. Assuming cancer is the most likely cause of death in this woman [18], the risks of chemotherapy may be mitigated by antidotes and one course of chemotherapy may assess her tolerance of stress.
In the absence of better evidence, all three approaches appear legitimate. They may be better individualized thanks to genomic analysis of the cancer [19]. Yet it is legitimate to ask whether a more evidence‐based approach may be devised for a situation likely to become more and more common.
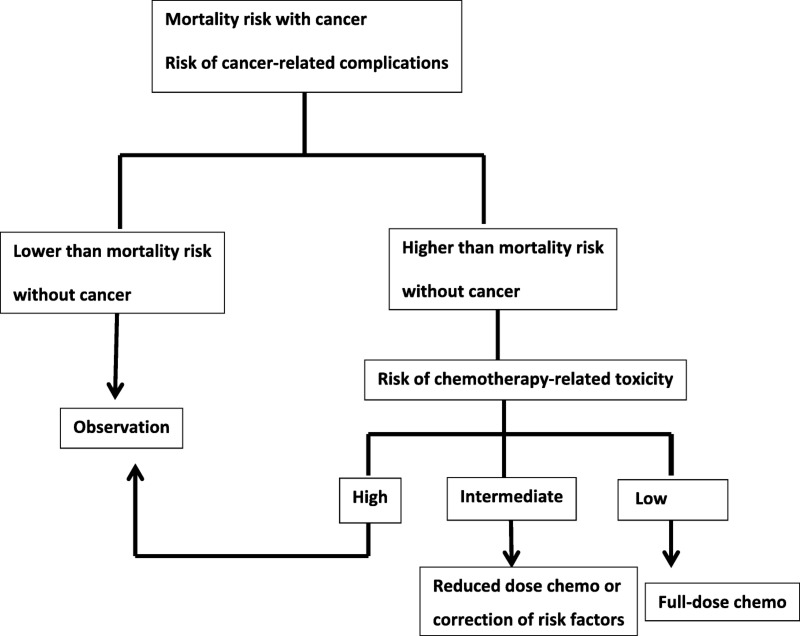
The assessment of benefits and risks in this situation is a complex endeavor. The decisional process is summarized in Figure 1. Risk‐adverse patients are more likely than risk‐takers to accept substantial chemotherapy‐related toxicity for a small incremental benefit [20].
Figure 1.
A simple model to decide which treatment to offer to older cancer patients. Risk or mortality without cancer is established calculated with the e‐prognosis instrument [14]; risk of chemotherapy‐related toxicity is calculated with the Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) [21] or the Classification and Regression Tree (CART) [22] instruments; and risk of surgical complication is calculated with the Preoperative Assessment in elderly Cancer Patients (PACE) [29] instrument. No instruments are available yet to estimate the risk of radiation therapy, combined chemoradiation, or targeted and biological therapy. All instruments may be fine‐tuned when integrated with biological markers of aging and with the new definition of frailty.
Following the carpet metaphor, the assessment of risks and benefits in the second patient is the carpet design. Figure 1 reveals the threads contributing to the design: risk of recurrence, risk of death and of disabling complications from metastatic cancer, patient's life expectancy and treatment tolerance, risk of treatment‐related long‐term disability, availability of an effective home caregiver to compensate for the couple's dependence in instrumental activities of daily living (IADL), timely response to emergencies, and risk of drug–drug and drug–diseases interaction. In the following discussion, we will review the information we already have and weave together these different threads.
The Information We Have
Functional Age Aging is universal, but individualized. The assessment of functional age, that is, of individual life expectancy and functional reserve, is central to medical decisions.
We have instruments able to predict a person's life expectancy [14] with and without cancer and the risk of chemotherapy‐related toxicity in older individuals [21], [22]. We also have information on how polypharmacy may affect the course of the disease and treatment‐related toxicity [23].
Life Expectancy and Treatment‐Related Toxicity.
Aging involves a progressive decline in functional reserve of multiple organ systems and an increased prevalence of chronic diseases [21], [22] that lead to reduced life expectancy and stress tolerance. When older individuals are dependent in some IADL and basic activities of daily living, health maintenance, quality of life, and even their very survival may be contingent on a caregiver.
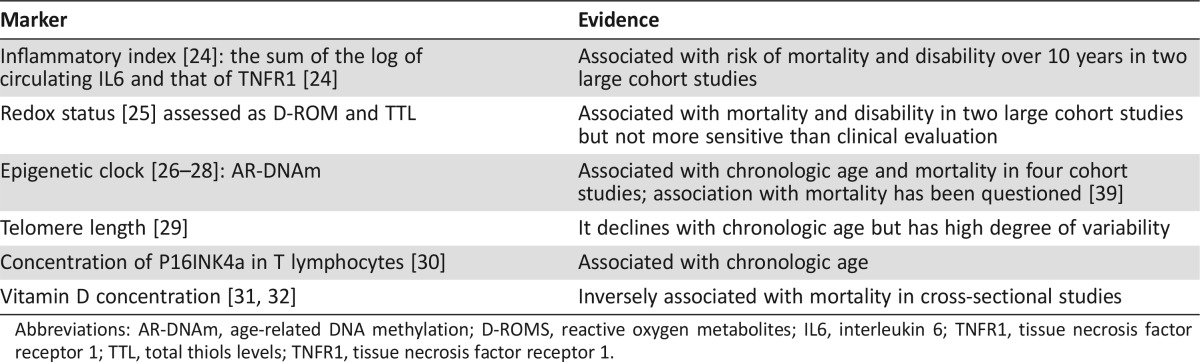
A number of biological changes underlie these clinical events: generalized chronic and progressive inflammation [24], cellular damage by reactive oxygen species and alterations in redox status [25], epigenetic changes, including DNA hyper‐ and hypomethylation [26], [27], [28], [30], and a progressive shortening of telomeres [31]. Proliferative senescence, the irreversible arrest of cellular proliferation, is mediated by the P16INK4a/Rb system [32], [33]. A decline of vitamin D serum levels was also observed with age [34]. A recent meta‐analysis found three gene clusters negatively and four clusters positively correlated with age [35].
The instruments to assess the risk of mortality and cancer treatment complications in older individuals are based on a comprehensive geriatric assessment (CGA) [36], [37], [38], [39]. One of the most important domains of the CGA is functional status and physical performance, which are powerful “biomarkers” of the risk of death, disability, and hospitalization [37], [38], [39], [40]. The site e‐prognosis.com summarizes all CGA‐based clinical models assessing the risk of mortality from 1 to 9 years for older individuals [14]. The preoperative assessment of elderly cancer patients estimates the risk of surgical [29], whereas other two instruments estimate that of chemotherapy complications [21], [22].
These instruments may be fine‐tuned with the inclusion of biological markers of aging (Table 2) [23], [24], [25], [26], [27], [28], [30], [31], [32], [33], although the value of these markers has not been tested yet in patients with cancer. The use of these biological indices currently should be limited to a research context.
Table 2. Potential biological markers of aging.
Abbreviations: AR‐DNAm, age‐related DNA methylation; D‐ROMS, reactive oxygen metabolites; IL6, interleukin 6; TNFR1, tissue necrosis factor receptor 1; TTL, total thiols levels; TNFR1, tissue necrosis factor receptor 1.
A novel promising approach to unravel the complexity of age is the “principal component analysis” (PCA). PCA may simplify the determination of functional age by identifying a few variables that best reflect the risk of mortality and functional dependence [69].
An effective caregiver improves treatment adherence and reduces the risk of serious complications [42], [43]. In the case we examine, the patient was herself the caregiver of an older disabled spouse. This common situation represents an example of “social” fragility [43]: a minor disease making the older caregiver temporarily incapacitated may result in loss of couple's independence.
The characteristics of an effective home caregiver include availability for regular care and for emergencies, access to transportation, and ability to provide emotional support. The ideal caregiver may have an important role as spokesperson for the patient and the family in mediating familial conflicts. The pool of home caregivers is becoming increasingly more limited due to the disappearance of the extended family and the entrance of women into the workforce. Currently, most of the home caregivers of older individuals are either older spouses with health problems of their own or grown‐up children [44] who need to accommodate the demands of their job, their own families, and their elderly parents (Aeneas syndrome) [45]. The home caregiver saves a substantial amount of money to the health care system [46], [47] and is susceptible to conditions, such as depression, that may lead to early mortality [43].
Polypharmacy may be defined as the intake of five or more daily drugs or drugs that are contraindicated, as the presence of drug interactions, or as redundancy [23]. Polypharmacy is of concern for two reasons: risk of complications from individual medications and potential drug–drug and drug–disease interactions [17], [22], [48], [49].
What We Don't Know Yet
Critical unknown information includes the course of cancer in the older aged person, the management of common cancers with unusual presentation, and the identification of individuals with critically reduced functional reserve for whom cancer treatment may cause death or permanent disability. The mortality of most neoplasms increases with aging, including the mortality from breast [50] and prostate [51] cancer and from chronic lymphocytic leukemia [52].
The cancer growth is determined by three elements: the “seed,” or the neoplastic cell, the “ground,” or the tumor host, and the “gardener,” or the provider. With aging, the prevalence of unfavorable cytogenetics, genomic abnormalities, and multidrug resistance increases in acute myelogenous leukemia [53]. Likewise, the prevalence of Epstein‐Barr virus‐related, activated B‐cell, and “double hit” large cell lymphomas increases with age [54]. In the majority of cases, it is not evident that the seed is different in older and younger patients. In the case of breast cancer, age is associated with increased prevalence of low‐grade, hormone receptor‐rich, human epidermal growth receptor 2‐negative disease, which is with a seed harbinger of a more indolent neoplasm [55]. Thus, the difference of “seed” alone cannot explain the poorer prognosis of cancer with age.
Aging is associated with endocrine, immunologic, and proliferative senescence, as well as chronic inflammation. Endocrine senescence includes insulin resistance and increased levels of circulating insulin [56], a powerful tumor growth factor. The use of metformin, which reduces the levels of circulating insulin, is associated with a more prolonged survival in women with metastatic breast cancer [57], while insulin resistance increases the risk of pancreatic cancer [58]. Proliferative senescence of fibroblasts is associated with the so‐called senescence‐associated secretory phenotype [59], [60]. The senescent cells acquire proinflammatory capacity and secrete a number of products, including heregulin, that stimulate tumor growth and facilitate metastatic spreading [61]. Chronic inflammation through mechanisms that are only partly known favors neoplastic proliferation and invasion [62], [63] and may reduce the efficacy and enhance the toxicity of antineoplastic agents [64]. Immune senescence [65] includes a decline in cell‐mediated immunity that may favor cancer growth.
To some extent, providers may be responsible for the poorer prognosis of older cancer patients. In a meta‐analysis, Lee et al. [66] demonstrated that patients aged 60 years and older with large cell lymphoma receiving CHOP in full doses had the same outcome as younger patients. The outcome was much worse when the dose of treatment was reduced. Similar results were reported from a single institution experience [67].
Also wanted is information related to the management of unusual presentations of common cases, such as a newly diagnosed breast cancer in an 86‐year‐old woman with left hemiplegia from a cerebrovascular accident (CVA) of the right middle cerebral artery. The CVA occurred in the presence of thrombocytosis ascribed to a myeloproliferative disorder for which the patient received hydroxyurea for 10 years. Eventually, diagnosis of BCR/ABL chronic myelogenous leukemia was established and treatment with imatinib instituted. Three years before the diagnosis of breast cancer, the patient had a resection of a stage III colonic cancer for which she received no adjuvant chemotherapy. Additional problems included type II diabetes and recurring kidney stones.
An instrument is wanted to recognize patients whose functional reserve is so exhausted that even a minimal stress can produce permanent disability. These patients are sometimes referred to as frail, but the term is confusing [68]. The accepted definition of frailty encompasses individuals at increased risk of mortality, disability, and hospitalization over a 10‐year period. In the cardiovascular health study that included 8,500 persons aged 65 years and older, a frailty phenotype (Panel 1) has been defined [72]. The role of this phenotype in the management of cancer has not been fully established. Recently, Cohen et al. [71] proposed a frailty definition of older cancer patients based on a CGA and the deficit‐accumulation conceptual framework that correlates to the risk of cytotoxic chemotherapy complications. This promising instrument needs to be prospectively evaluated.
Controversy lingers as to the chronologic landmark of when physiologic age and frailty should be assessed. Although the risk of mortality and the depletion of functional reserve with age may start early in life, these processes appear to accelerate by the age 70 years [73], and the National Cancer Center Network guidelines recommend that the assessment of physiologic age in older patients with cancer begins at age 70 years [6].
A Blueprint to the Management of Complexity
In this section, we will examine three issues: (a) how to improve the instruments already in place for assessing physiologic age, (b) how to obtain missing information, and (c) how to structure medical decisions in face of uncertainty.
At this point, it is useful to review different types of medical evidence that may be utilized in the study of a highly diverse population, such as the elderly. Varadhan et al. [74] described four different types of heterogeneity of treatment–effect analysis. First, confirmatory heterogeneity analysis is used to confirm the efficacy of an intervention in a few well‐defined subgroups of patients and is the cornerstone of the analysis of randomized controlled trials (RCTs). Second, descriptive heterogeneity analysis is used to establish the outcome of an intervention in a highly heterogeneous population, such as registry‐based population studies [75]. Third, exploratory heterogeneity analysis is hypothesis generating as utilized in phase II studies. Fourth, predictive heterogeneity analysis establishes the balance of benefits and risks of an intervention in individual patients based on prospectively collected rapid learning databases that include patient characteristics prior to the intervention as well as the outcome. This type of analysis was used to generate the model predicting the risks of surgery [29] and of cytotoxic chemotherapy [21], [22] in older cancer patients.
In combination with the results of RCTs, predictive heterogeneity analysis is the most promising form of evidence to support medical decisions in the elderly. RCTs [76], [77] are essential to prove a principle, such as the value of adjuvant chemotherapy or the equivalence of total and partial mastectomy. Conducted in a very select population, RCTs provide limited information on the benefit of an intervention in the general population [78]. Three types of RCT designs have already proven very helpful in addressing age‐related questions: (a) trials aimed to demonstrate that chronologic age is not a contraindication to a specific medical intervention—the seminal trial of Muss et al. [79], demonstrating that adjuvant chemotherapy of breast cancer is effective irrespective of age, is a paradigm; (b) trials in which older individuals of the same physiologic ages are randomly assigned to different forms of treatment (with this design, Goede et al. [80] demonstrated that treatment with chlorambucil and obinutuzumab was superior to chlorambucil and rituximab in prolonging the survival of chronic lymphocytic leukemia patients aged 70 years and older); and (c) trials that try to accommodate the diversity of the older population by tailoring the treatment to individual patients’ characteristics. By comparing a fixed treatment approach with an individually tailored approach, the FOCUS trial showed that intermittent chemotherapy is preferable to the continuous one for some older patients with metastatic colorectal cancer [81], and Tucci et al. [82] demonstrated that a reduced dose of chemotherapy is preferable to full doses of CHOP in lymphoma patients with severe comorbidity and advanced disability.
The recruitment of older patients to RCTs may be facilitated by removing some restrictions in eligibility criteria. For example, Lichtman et al. [83] demonstrated that moderate renal dysfunction should not exclude older patients from some clinical trials, as it does not seem to affect the outcome nor the treatment toxicity. Yet, a global scope of older cancer patients may be best obtained by complementing the results of RCT with prospectively collected rapid learning databases
How to Improve the Instruments Already in Place.
The instruments currently used for assessing physiologic age may be calibrated with the inclusion of more information, such as biological markers of aging, polypharmacy, and the risk of drug interactions. Of special interest is to establish whether the frail phenotype purports increased risk of acute and late treatment complications and whether the frail phenotype itself represents a complication of cancer treatment. Equally important is to test current instruments in combined modality treatments, such as chemoradiation, and in newly emerging forms of cancer treatment.
In the previous discussion, we acknowledged the role of the home caregiver. To assure consistent home support to older cancer patients, it is necessary to define the ideal caregiver profile and the interventions that may prevent the caregiver's stress and burnout. It is also important to define the societal obligations to the caregiver for the resources he/she saves to the health care system.
How to Collect the Information We Don't Have.
The rapid learning database‐based approach to individual medical decisions in older patients has been advocated by epidemiologists and gerontologists [74]. These databases are essential to establish the course of the disease in older cancer patients. Ideally the outcome should be related to the following factors: molecular characteristics of each tumor, biological markers of aging, physiologic age determined by clinical assessment, comorbidity, frailty, and treatment.
Databases are also essential to establish the outcome of unusual situations, such as the case of an older woman with breast cancer, hemiparesis, and a myeloproliferative disorder. The American Society of Clinical Oncology has instituted the CancerLinQ program [84] to study unusual neoplasms that are not amenable to randomized clinical trials due to their rarity. Through CancerLinQ, these cases are registered and closely followed, and the outcome of different treatments may be compared. Finally, databases may help identify the phenotype of patients with almost exhausted functional reserve for whom a minimal stress may precipitate death or lasting disability.
How to Structure Medical Decisions in the Presence of Uncertainty.
Undoubtedly, more information will lead to more evidence‐based decisions in the management of cancer in the older aged person. A wide range of uncertainty is likely to persist, however, particularly in the short term.
Decision analysis models have been used to structure decisions in the presence of uncertainty. Of these, the most commonly used include a Markov simulation system calculating the probability that every individual patient may transition from a state of disease to a state of health or to death over a specific time interval [70]. The results are then verified in a Monte Carlo sensitivity analysis [70] that evaluates the probability of different outcomes based on the simultaneous change of multiple variables. Decision analysis is helpful in establishing the value of an intervention, such as screening for cancer in a large population. It may help the variables affecting a complex situation. After examining an adequate sample of cases of women aged 80 years or older with triple negative breast cancer, such as the one described in case 2, decision analysis may estimate the range of neoplastic genomic changes, concentration of aging markers, and dependence in IADL over which adjuvant chemotherapy may be beneficial and safe, independent of other variables. In other words, it may lead to PCA.
Figure 1 offers the scaffold to store and the model to apply emerging information to case 2. Any threshold of the risk of mortality and serious complications needs to be negotiated with the patients, except in extreme cases. For illustrating the interpretation of the model, we will consider a 50% threshold. If the risk of mortality is higher than 50% over the subsequent 2–3 years, adjuvant chemotherapy should be discouraged. Chances are that the patient would die of other causes even if the cancer recurs. If the patient has a risk of cancer‐independent death of 50% or less in 5 years, but the risk of grade 4 complications is higher than 50%, the risk of chemotherapy probably overwhelms the marginal benefits. Chemotherapy could be beneficial if the risk of mortality at 5 years and the risk of grade 4 complications are both lower than 50%, as long as the patient is willing to accept the complications (that, in her case, may be social as well as medical) for a small but definite benefit. In this scenario, the availability of a caregiver may be determinant for the decision to treat. This model can be modified according to different clinical situations and, as such, is flexible and reproducible.
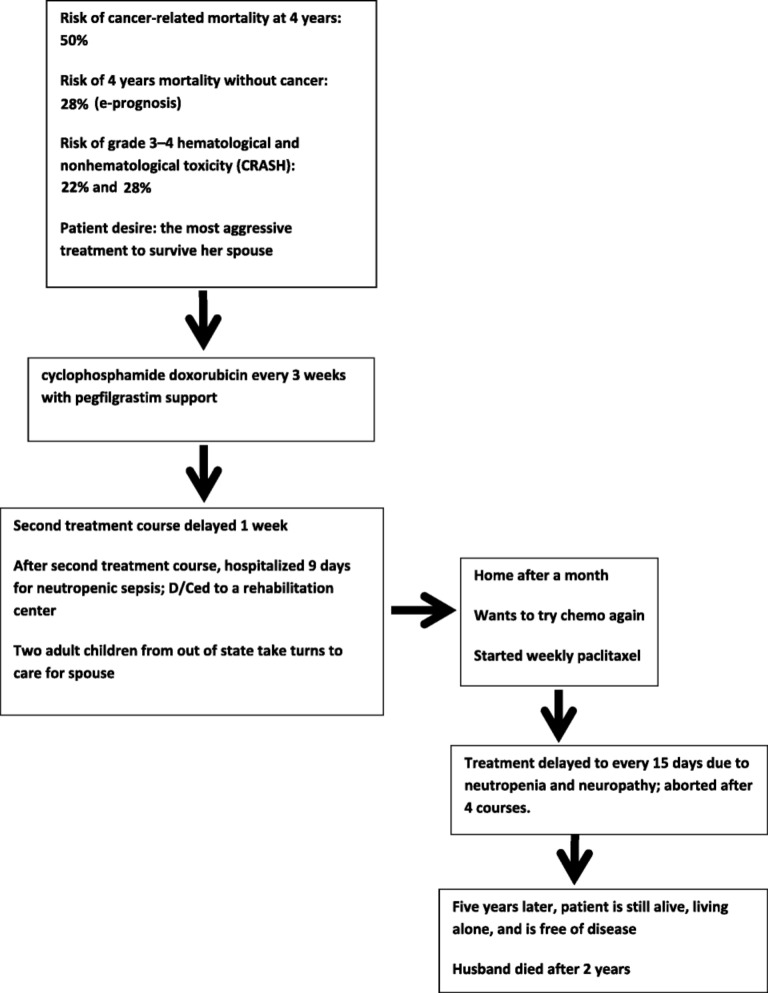
In Figure 2, we describe the decisional process utilized in the case of the 85‐year‐old woman with stage IIb breast cancer reported at the beginning of the article. A determinant factor in choosing the most aggressive chemotherapy was the patient's expressed desire to survive her demented spouse and to care for him up to the end. The treatment had to be aborted due to myelotoxic and non‐myelotoxic complications. The collection of an adequate number of cases like this in a rapid learning database may allow the investigators to establish the potential benefits and risk of chemotherapy in this population and whether a reduced course of treatment may be as beneficial as a full‐dose treatment.
Figure 2.
Treatment of case 2, based on the Figure 1 model.
Even if some estimates are not precise, we believe our model represents a useful frame of reference capable to accommodate new information related both to cancer treatment and to older patients. If this approach becomes standard in the management of uncertainties and the information related to risk of mortality and complications are recorded in the database, the model may eventually be validated by outcome data.
Conclusion
The management of cancer in the older aged person is a complex issue that is becoming more and more common with the aging of the population. Some interwoven variables such as risk of mortality and of treatment complications may be assessed with existing instruments. Other information, such as the course of the disease, the host‐related factors that influence cancer aggressiveness, and the phenotype heralding risk of permanent treatment related damage, is wanted. Also wanted is the pertinence of frailty on cancer treatment. Existing instruments are based on clinical evaluation of aging and may be fine‐tuned with biological markers of aging. For collecting the missing information, several different types of medical evidence are required, including (a) RCTs exploring specific problems, such as the influence of chronologic and physiologic age on cancer treatment outcome or the value of a personalized approach to treatment; (b) phase II studies exploring the pharmacology of new drugs in older patients; and (c) rapid learning database studies in which the information is prospectively collected to explore the influence of individual patient profile on treatment outcome (predictive heterogeneity analysis). In Figure 1, we present a simple, inclusive, reproducible, and flexible model to approach the management‐related decisions in older patients with cancer.
Acknowledgments
This study was supported by grant no. CSO2010‐11384‐E from the Ministerio de Economía y Competitividad (Spain) and the Ramón Areces Foundation (Spain). Participants in the International Workshop “Cancer as a result of ageing: possible solutions,” which took place in Madrid, Spain, on November 3, 2015, include the following: María Vallet Regí, Universidad Complutense, CIBER‐BBN, Red Agening, Madrid, Spain; Mariano Barbacid, Centro Nacional de Investigaciones Oncológicas, Madrid, Spain; Theresa A. Guise, Department of Medicine, Division of Endocrinology at Indiana University, Bloomington, Indiana, USA; Lodovico Balducci, H. Lee Moffitt Cancer Center & Research Institute, Tampa General Hospital, Tampa, Florida, USA; Alfredo Carrato Mena, Head of Oncology, Hospital Universitario Ramón y Cajal, Madrid, Spain, and Profesor at Universidad de Alcalá de Henares, Madrid, Spain; Pablo L. Ortiz Romero, Dermatology Department, Hospital Universitario 12 de Octubre, Madrid, Spain; Manuel Ramírez Orellana, Department of Hematology and Pedriatic Oncology, Hospital Infantil Universitario Niño Jesús, Madrid, Spain; Leocadio Rodríguez Mañas, Head of the Service of Geriatrics, Hospital Universitario de Getafe, Madrid, Spain, Networks Spanish Collaborative Research Network on Aging and Frailty (RETICEF) and Agening (Spanish Network of Excellence for prevention and local treatment of osteoporotic fractures). The authors thank funding from the European Research Council (Advanced Grant VERDI; ERC‐2015‐AdG Proposal no. 694160).
Contributor Information
María Vallet‐Regí, Email: vallet@ucm.es.
Collaborators: Spanish Collaborative Research Network on Aging and Frailty (RETICEF), Mariano Barbacid, Theresa A. Guise, Lodovico Balducci, Alfredo Carrato Mena, Pablo L. Ortiz Romero, Manuel Ramírez Orellana, and Leocadio Rodríguez Mañas
Author Contributions
Conception/Design: María Vallet‐Regí, Miguel Manzano, Leocadio Rodriguez‐Mañas, Marta Checa López, Matti Aapro, Lodovico Balducci
Manuscript writing: María Vallet‐Regí, Miguel Manzano, Leocadio Rodriguez‐Mañas, Marta Checa López, Matti Aapro, Lodovico Balducci
Final approval of manuscript: María Vallet‐Regí, Miguel Manzano, Leocadio Rodriguez‐Mañas, Marta Checa López, Matti Aapro, Lodovico Balducci
Disclosures
Matti Aapro: Amgen, Roche, Sandoz, Pfizer (C/A, H), Helsinn, Pierre Fabre, Hospira (RF), Amgen (ET); Lodovico Balducci: TEVA (C/A), TEVA, AMGEN, Johnson & Johnson, Celgene (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Balducci L, Aapro M. Complicated and complex: Helping the older patient with cancer to exit the labyrinth. J Geriatr Oncol 2014;5:116–118. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AA. Complex systems dynamics in aging: New evidence, continuing questions. Biogerontology 2016;17:205–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zullig LL, Whitson HE, Hastings SN et al. A systematic review of conceptual frameworks of medical complexity and new model development. J Gen InternMed 2016;31:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wyatt KD, Stuart LM, Brito JP et al. Out of context: Clinical practice guidelines and patients with multiple chronic conditions: A systematic review. Med Care 2014;52:S92–S100. [DOI] [PubMed] [Google Scholar]
- 5. Santoro A, Balducci L. The complexity of cancer survivorship: A case for personalized medicine. Report of the 2014 Grandangolo conference. J Med Person 2014;12:37–43. [Google Scholar]
- 6. Shih YC, Hurria A. Preparing for an epidemic: Cancer care in an aging population. Am Soc Clin Oncol Educ Book 2014:133–137. [DOI] [PubMed] [Google Scholar]
- 7. Chouliara Z, Kearney N, Stott D et al. Perceptions of older people with cancer of information, decision making and treatment: A systematic review of selected literature. Ann Oncol 2004;15:1596–1602. [DOI] [PubMed] [Google Scholar]
- 8. Molina‐Garrido MJ, Guillén‐Ponce C. Development of a cancer‐specific comprehensive geriatric assessment in a university hospital in Spain. Crit Rev Oncol Hematol 2011;77:148–161. [DOI] [PubMed] [Google Scholar]
- 9. Swaminathan D, Swaminathan V. Geriatric oncology: Problems with under‐treatment within this population. Cancer Biol Med 2015;12:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists' Collaborative Group , R Peto, C Davies et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta‐analyses of long‐term outcome among 100,000 women in 123 randomised trials. Lancet 2012;379:432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patt DA, Duan Z, Fang S et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: Understanding risk. J Clin Oncol 2007;25:3871–3876. [DOI] [PubMed] [Google Scholar]
- 12. Caram ME, Guo C, Leja M et al. Doxorubicin‐induced cardiac dysfunction in unselected patients with a history of early‐stage breast cancer. Breast Cancer Res Treat 2015;152:163–172. [DOI] [PubMed] [Google Scholar]
- 13. Balducci L, Fossa SD. Rehabilitation of older cancer patients. Acta Oncol 2013;52:233–238. [DOI] [PubMed] [Google Scholar]
- 14. Yourman LC, Lee SJ, Schonberg MA et al. Prognostic indices for older adults: A systematic review. JAMA 2012;307:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McClymont KM, Lee SJ, Schonberg MA et al. Usefulness and effect of online prognostic calculators. J Am Geriatr Soc 2014;62:2444–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gillison TL, Chatta GS. Cancer chemotherapy in the elderly patient. Oncology (Williston Park) 2010;24:76–85. [PubMed] [Google Scholar]
- 17. Popa MA, Wallace KJ, Brunello A et al. Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 2014;5:307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wallwiener CW, Hartkopf AD, Grabe E et al. Adjuvant chemotherapy in elderly patients with primary breast cancer: Are women ≥65 undertreated? J Cancer Res Clin Oncol 2016;142:1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Myers MB. Targeted therapies with companion diagnostics in the management of breast cancer: Current perspectives. Pharmacogenomics Pers Med 2016;9:7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H, Haley WE, Robinson BE et al. Decisions for hospice care in patients with advanced cancer. J Am Geriatr Soc 2003;51:789–797. [DOI] [PubMed] [Google Scholar]
- 21. Extermann M, Boler I, Reich RR et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High‐Age Patients (CRASH) score. Cancer 2012;118:3377–3386. [DOI] [PubMed] [Google Scholar]
- 22. Hurria A, Togawa K, Mohile SG et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 2011;29:3457–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Balducci L, Goetz‐Parten D, Steinman MA, Polypharmacy and the management of the older cancer patient. Ann Oncol 2013;24(suppl 7):36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Varadhan R, Yao W, Matteini A et al. Simple biologically informed inflammatory index of two serum cytokines predicts 10 year all‐cause mortality in older adults. J Gerontol A Biol Sci Med Sci 2014;69:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schöttker B, Brenner H, Jansen EH et al. Evidence for the free radical/oxidative stress theory of ageing from the CHANCES consortium: A meta‐analysis of individual participant data. BMC Med 2015;13:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell JT, Tsai PC, Yang TP et al. Epigenome‐wide scans identify differentially methylated regions for age and age‐related phenotypes in a healthy ageing population. PLoS Genet 2012;8:e1002629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weidner CI, Lin Q, Koch CM et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol 2014;15:R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Marioni RE, Shah S, McRae AF et al. DNA methylation age of blood predicts all‐cause mortality in later life. Genome Biol 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moore AZ, Hernandez DG, Tanaka T et al. Change in epigenome‐wide DNA methylation over 9 years and Subsequent Mortality: Results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2016; 71:1029–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aubert G. Telomere dynamics and aging. Prog Mol Biol Transl Sci 2014;125:89–111. [DOI] [PubMed] [Google Scholar]
- 31. LaPak KM, Burd CE. The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res 2014;12:167–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sanoff HK, Deal AM, Krishnamurthy J et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst 2014;106:dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schöttker B, Jorde R, Peasey A et al. Vitamin D and mortality: Meta‐analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ 2014;348:g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peters MJ, Joehanes R, Pilling LC et al. The transcriptional landscape of age in human peripheral blood. Nat Commun 2015;6:8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wildiers H, Heeren P, Puts M et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hamaker ME, Jonker JM, de Rooij SE et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol 2012;13:e437–e444. [DOI] [PubMed] [Google Scholar]
- 37. Boult C, Boult LB, Morishita L et al. A randomized clinical trial of outpatient geriatric evaluation and management. J Am Geriatr Soc 2001;49:351–359. [DOI] [PubMed] [Google Scholar]
- 38. Extermann M. Integrating a geriatric evaluation in the clinical setting. Semin Radiat Oncol 2012;22:272–276. [DOI] [PubMed] [Google Scholar]
- 39. Pallis AG, Hatse S, Brouwers B et al. Evaluating the physiological reserves of older patients with cancer: The value of potential biomarkers of aging? J Geriatr Oncol 2014;5:204–218. [DOI] [PubMed] [Google Scholar]
- 40.PACE participants , Audisio RA, Pope D et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–163. [DOI] [PubMed] [Google Scholar]
- 41. Cohen AA, Milot E, Li Q et al. Detection of a novel, integrative aging process suggests complex physiological integration. PLoS One 2015;10:e0116489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fletcher BS, Miaskowski C, Given B et al. The cancer family caregiving experience: An updated and expanded conceptual model. Eur J Oncol Nurs 2012;16:387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Svendsboe E, Terum T, Testad I et al. Caregiver burden in family carers of people with dementia with Lewy bodies and Alzheimer's disease. Int J Geriatr Psychiatry 2016;31:1075–1083. [DOI] [PubMed] [Google Scholar]
- 44. Makizako H, Shimada H, Tsutsumimoto K et al. Social frailty in community‐dwelling older adults as a risk factor for disability. J Am Med Dir Assoc 2015;16:1003.e7–1003.e11. [DOI] [PubMed] [Google Scholar]
- 45. Wiet SG. Future of caring for an aging population: Trends, technology, and caregiving. Stud Health Technol Inform 2005;118:220–230. [PubMed] [Google Scholar]
- 46. Dominguez L.J. Medicine and the arts. L'incendio di Borgo. Commentary. Acad Med 2009;84:1260–1261. [DOI] [PubMed] [Google Scholar]
- 47. Arno PS, Levine C, Memmott MM. The economic value of informal caregiving. Health Aff (Millwood) 1999;18(2): 182–188. [DOI] [PubMed] [Google Scholar]
- 48. Rhee Y, Degenholtz HB, Lo Sasso AT et al. Estimating the quantity and economic value of family caregiving for community‐dwelling older persons in the last year of life. J Am Geriatr Soc 2009;57:1654–1659. [DOI] [PubMed] [Google Scholar]
- 49. Morabito F, Gentile M, Seymour JF et al. Ibrutinib, idelalisib and obinutuzumab for the treatment of patients with chronic lymphocytic leukemia: Three new arrows aiming at the target. Leuk Lymphoma 2015;56:3250–3256. [DOI] [PubMed] [Google Scholar]
- 50. Nightingale G, Hajjar E, Schwartz K et al. Evaluation for a pharmacist‐led medication assessment used to identify prevalence of and associations with polypharmacy and potentially inappropriate medication use among ambulatory senior adults with cancer. J Clin Oncol 2015;33:1453–1459 [DOI] [PubMed] [Google Scholar]
- 51. van de Water W, Markopoulos C, van de Velde CJ et al. Association between age at diagnosis and disease‐specific mortality among postmenopausal women with hormone receptor‐positive breast cancer. JAMA 2012;307:590–597. [DOI] [PubMed] [Google Scholar]
- 52. McDavid K, Lee J, Fulton JP et al. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep 2004;119:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balducci L, Dolan D. Chronic lymphocytic leukemia in the elderly: Epidemiology and proposed patient‐related approach. Cancer Control 2015;22:3–6. [DOI] [PubMed] [Google Scholar]
- 54. Dickson GJ, Bustraan S, Hills RK et al. The value of molecular stratification for CEBPA(DM) and NPM1(MUT) FLT3(WT) genotypes in older patients with acute myeloid leukaemia. Br J Haematol 2016;172:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morrison VA, Hamlin P, Soubeyran P et al. Approach to therapy of diffuse large B‐cell lymphoma in the elderly: The International Society of Geriatric Oncology (SIOG) expert position commentary. Ann Oncol 2015;26:1058–1068. [DOI] [PubMed] [Google Scholar]
- 56. VanderWalde A, Hurria A. Early breast cancer in the older woman. Clin Geriatr Med 2012;28:73–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hirose H, Takayama M, Iwao Y et al. Effects of aging on visceral and subcutaneous fat areas and on homeostasis model assessment of insulin resistance and insulin secretion capacity in a comprehensive health checkup. J Atheroscler Thromb 2016;23:207–215. [DOI] [PubMed] [Google Scholar]
- 58. Xu H, Chen K, Jia X et al. Metformin use Is associated with better survival of breast cancer patients with diabetes: A meta‐analysis. The Oncologist 2015;20:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wolpin BM, Bao Y, Qian ZR et al. Hyperglycemia, insulin resistance, impaired pancreatic β‐cell function, and risk of pancreatic cancer. J Natl Cancer Inst 2013;105:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Loaiza N, Demaria M. Cellular senescence and tumor promotion: Is aging the key? Biochim Biophys Acta 2016;1865:155–167. [DOI] [PubMed] [Google Scholar]
- 61. Bhatia‐Dey N, Kanherkar RR, Stair SE et al. Cellular Senescence as the Causal Nexus of Aging. Front Genet 2016;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chang J, Wang Y, Shao L et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 2016;22:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henry CJ, Casás‐Selves M, Kim J et al. Aging‐associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest 2015;125:4666–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Serrano M. The InflammTORy powers of senescence. Trends Cell Biol 2015;25:634–636. [DOI] [PubMed] [Google Scholar]
- 65. Slaviero KA, Clarke SJ, Rivory LP. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol 2003;4:224–232. [DOI] [PubMed] [Google Scholar]
- 66. Hazeldine J, Lord JM. Innate immunesenescence: underlying mechanisms and clinical relevance. Biogerontology 2015;16:187–201. [DOI] [PubMed] [Google Scholar]
- 67. Lee KW, Kim DY, Yun T et al. Doxorubicin‐based chemotherapy for diffuse large B‐cell lymphoma in elderly patients: Comparison of treatment outcomes between young and elderly patients and the significance of doxorubicin dosage. Cancer 2003;98:2651–2656. [DOI] [PubMed] [Google Scholar]
- 68. Ha H, Keam B, Kim TM et al. Reduced Dose Intensities of Doxorubicin in Elderly Patients with DLBCL in Rituximab Era. Cancer Res Treat 2016;48:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Whitson HE, Duan‐Porter W, Schmader KE et al. Physical resilience in older adults: Systematic review and development of an emerging construct. J Gerontol A Bio Sci Med Sci 2016;71:489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 71. Cohen HJ, Smith D, Sun CL et al. Frailty as determined by a comprehensive geriatric assessment‐derived deficit‐accumulation index in older patients with cancer who receive chemotherapy. Cancer 2016; 122:3865–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Balducci L. Frailty: A common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72. [DOI] [PubMed] [Google Scholar]
- 73. Varadhan R, Segal JB, Boyd CM et al. A framework for the analysis of heterogeneity of treatment effect in patient‐centered outcomes research. J Clin Epidemiol 2013;66:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shahinian VB, Kuo YF, Freeman JL et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154–164. [DOI] [PubMed] [Google Scholar]
- 75. Hurria A, Dale W, Mooney M et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32:2587–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hurria A, Levit LA, Dale W et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology statement. J Clin Oncol 2015;33:3826–3833 [DOI] [PubMed] [Google Scholar]
- 77. Evans JG. Evidence‐based and evidence‐biased medicine. Age Ageing 1995;24:461–463. [DOI] [PubMed] [Google Scholar]
- 78. Muss HB, Berry DA, Cirrincione CT et al. Adjuvant chemotherapy in older women with early‐stage breast cancer. N Engl J Med 2009;360:2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Goede V, Fischer K, Busch R et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014;370:1101–1110. [DOI] [PubMed] [Google Scholar]
- 80. Seymour MT, Thompson LC, Wasan HS et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open‐label, randomised factorial trial. Lancet 2011;377:1749–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tucci A, Ferrari S, Bottelli C et al. A comprehensive geriatric assessment is more effective than clinical judgment to identify elderly diffuse large cell lymphoma patients who benefit from aggressive therapy. Cancer 2009;115:4547–4553. [DOI] [PubMed] [Google Scholar]
- 82. Lichtman SM, Cirrincione CT, Hurria A et al. Effect of pretreatment renal function on treatment and clinical outcomes in the adjuvant treatment of older women with breast cancer: Alliance A171201, an ancillary study to CALGB/CTSU 49907. J Clin Oncol 2016;34:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Shah A, Stewart AK, Kolacevski A et al. Building a rapid learning health care system for oncology: Why CancerLinQ collects identifiable health information to achieve its vision. J Clin Oncol 2016;34:756–763. [DOI] [PubMed] [Google Scholar]
- 84. Green PL, Worden K. Bayesian and Markov chain Monte Carlo methods for identifying nonlinear systems in the presence of uncertainty. Philos Trans A Math Phys Eng Sci 2015;373:20140405. [DOI] [PMC free article] [PubMed] [Google Scholar]