Abstract
It has been long recognized that the mammalian heart loses its proliferative capacity soon after birth, yet, the molecular basis of this loss of cardiac proliferation postnatally is largely unknown. In this study, we found that cardiac ErbB2, a member of the epidermal growth factor receptor family, exhibits a rapid and dramatic decline in expression at the neonatal stage. We further demonstrate that conditional ablation of ErbB2 in the ventricular myocardium results in upregulation of negative cell cycle regulators and a significant reduction in cardiomyocyte proliferation during the narrow neonatal proliferative time window. Together, our data reveal a positive correlation between the expression levels of ErbB2 with neonatal cardiomyocyte proliferation and suggest that reduction in cardiac ErbB2 expression may contribute to the loss of postnatal cardiomyocyte proliferative capacity.
Keywords: ErbB2, Neonatal, Cardiomyocyte, Proliferation
1. Introduction
Cardiovascular diseases are the leading causes of morbidity and mortality worldwide. In contrast to lower vertebrates and neonatal mammals (Poss et al., 2002; Porrello et al., 2011b), the adult mammals exhibit limited cardiac regenerative capacity (Laflamme and Murry, 2011), and are therefore susceptible to massive cardiomyocyte (CM) loss due to myocardial infarction and subsequent adverse remodeling. To address this issue, a number of experimental strategies have been developed to restore functional myocardium; these include cell therapy approach that utilizes diverse cell types such as embryonic stem cells, induced pluripotent stem cells, cardiac progenitor cells to regenerate functional CMs in injured hearts. An alternative approach involves directly converting resident cardiac fibroblasts into functional CMs in vivo by forced expression of the cardiac reprogramming factors (Qian et al., 2012; Song et al., 2012). In addition, recent mouse studies have suggested that stimulating existing CMs to re-enter cell cycle and proliferate could be a promising strategy to replenish lost CMs for regenerative purposes (Reiss et al., 1996; Zhao et al., 1998; Liao et al., 2001; Bersell et al., 2009; Porrello et al., 2011a; Sdek et al., 2011; Mahmoud et al., 2013; Xin et al., 2013; Puente et al., 2014; D’Uva et al., 2015). For instance, the epidermal growth factor–like ligand Neuregulin1 (Nrg1) and its ErbB4/2 tyrosine kinase receptors have been manipulated to promote CM proliferation and cardiac regeneration (Bersell et al., 2009; D’Uva et al., 2015; Gemberling et al., 2015).
ERBB2 is found to be amplified or over-expressed in many cancer types, suggesting that its level of expression that could profoundly influence cellular behaviors should be tightly regulated. Nrg1/ErbB signaling also plays an important role in organ development by regulating multiple cellular processes, including cell migration, proliferation and differentiation. However, an early lethality phenotype of Nrg1, ErbB2 or ErbB4 mutant mice (Gassmann et al., 1995; Lee et al., 1995; Meyer and Birchmeier, 1995) has hindered a detailed analysis of the function for this signaling pathway in later cardiac development and/or homeostasis.
In this study, we show that cardiac ErbB2 exhibits a decrease in expression from E13.5 to adulthood with a steep decline at the neonatal stage. We further demonstrate that, in the absence of ErbB2, neonatal CM proliferation is significantly reduced, which is accompanied by an upregulation of negative cell cycle regulators. Together, our data reveals a dynamic expression of ErbB2 in the heart and provide evidence that ErbB2 expression level positively correlates with CM proliferative capacity.
2. Materials and methods
2.1. Animals
ErbB2 cardiac-specific conditional knockout mice ErbB2Cko were obtained by crossing ErbB2fl/fl mice and Mlc2v-Cre mice. Animal care was performed in accordance with the guidelines established by NIH and UNC-Chapel Hill. All mouse protocols were approved by UNC-Chapel Hill DLAM.
2.2. Immunohistochemistry
Murine hearts were excised and rinsed with PBS after euthanasia, fixed in 0.5% paraformaldehyde overnight and embedded in OCT compound. Histological section was performed according to the standard procedures (Ma et al., 2015). All sections were blocked in 10% serum for 30 min at room temperature, and then incubated with primary antibodies against α-Actinin (1:400, Sigma Aldrich) and Ki-67 (1:400, Abcam) or pH 3 (1:200, Millipore) for 1 h at room temperature. After wash with PBS plus 0.1% TritonX-100, sections were stained with fluorescent secondary antibodies (ThermoFisher Scientific) for another 1 h at room temperature followed by mounted in Vectashield with DAPI (Vector Laboratories). Slides were analyzed with EVOS FL cell imaging system.
2.3. Isolation of neonatal mouse CM
Primary CMs were isolated from P0 ErbB2Cko mice or their control littermates ErbB2fl/fl as previously described (Song et al., 2000). Ventricles were rinsed with ice-cold HBSS and then minced into small pieces. All tissue pieces were transferred into glass beaker and subjected to 0.5 mg/ml collagenase II digestion in HBSS under constant agitation. Cell suspension was harvested every 10 min and replaced with fresh digestion solution. CMs were enriched after 2-hour cell attachment.
2.4. Western blot
Murine hearts or CMs were harvested and homogenized in RIPA buffer (89901, ThermoFisher Scientific) using bullet blender (Next Advance) according to the manufacturer’s instructions. 40 μg protein of each sample was separated by 4–12% NuPAGE gels (ThermoFisher Scientific) and transferred to nitrocellulose membranes. After blocked with 5% BSA, the membranes were incubated with primary antibodies against ErbB2 (1:200, Santa Cruz) and Gapdh (1:1000, Cell Signaling Technology). The films were developed by chemiluminescent system (RPN2106, GE) and band intensities were quantified using ImageJ software.
2.5. qPCR
RNA was extracted from murine hearts or CMs using Trizol reagent (ThermoFisher Scientific) according to the manufacturer’s instructions. RT-PCR was performed with SuperScript III kit (ThermoFisher Scientific). qPCR was performed with SYBR Green Real-Time PCR mater mix (ThermoFisher Scientific) following the manufacturer’s protocols.
2.6. Quantification and statistics
All data were presented as mean ± SEM. The significance of differences between groups was analyzed using student’s t-test or ANOVA. P < 0.05 was considered as statistically significant.
3. Results
3.1. ErbB2 is downregulated during heart development and postnatal stages
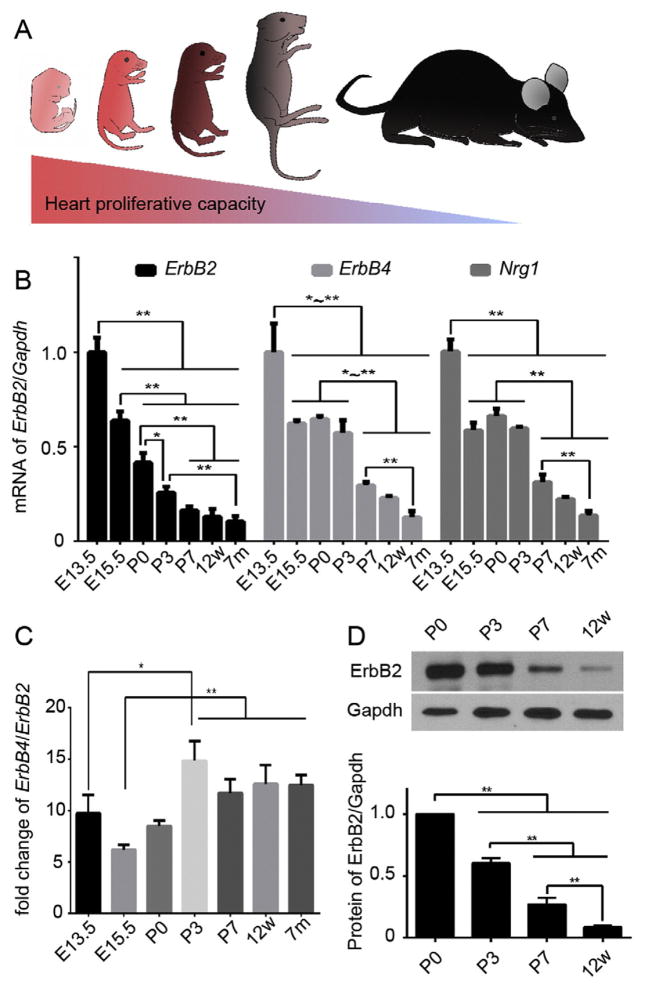
Accumulating evidence suggests that murine CMs are actively proliferating during embryonic stage but exit from cell cycle soon after birth (Walsh et al., 2010) (Fig. 1A). Since the level of ERBB2 expression appears to influence cell growth and proliferation, we first determined ErbB2 transcript levels in both fetal and postnatal hearts. To this end, we harvested the ventricles from mice at different developmental stages. Interestingly, ErbB2 exhibited a progressive reduction in expression with age (Fig. 1B). ErbB2 was highly expressed in the ventricles at embryonic day 13.5 (E13.5). This cardiac expression decreased significantly by about 58% at postnatal day 0 (P0). By P7, it was further reduced to about 15% of that at E13.5, and this low level of ErbB2 expression in the ventricles remained relatively unaltered from P7 to 7-month-old mice.
Fig. 1.
The expression profile of ErbB2 in the heart correlates with cardiac proliferative capacity. (A) A schematic illustration showing the difference of cardiac proliferative capacity over time. (B) qRT-PCR analysis of ErbB2, ErbB4, Nrg1 mRNA expression in murine hearts of different ages. (C) The relative expression of ErbB4 to ErbB2 from late embryonic to adult stages. (D) Western blots and quantification of ErbB2 protein expression in postnatal murine hearts. GADPH is used as a loading control. Data are presented as mean ± SEM. Statistical significance was determined by ANOVA test. *p < 0.05, **p < 0.01.
In CMs, ErbB4 is the receptor for endothelium derived Nrg1 and heterodimers with ErbB2 upon activation (Zhao et al., 1998). We next determined the transcript levels of ErbB4 and Nrg1 in the heart. We found that although it showed a trend of decreasing expression overtime, ErbB4, unlike ErbB2, maintained a relatively stable expression from late embryonic stage through early postnatal stage (Fig. 1B). Furthermore, ErbB4 in general exhibits much higher expression level of about 6–15 folds than ErbB2 depending on the stage (Fig. 1C). Likewise, the expression of Nrg1 decreased as the heart underwent a transition from hyperplastic to hypertrophic growth (Fig. 1B). Thus, the downregulation of Nrg1/Erbb expression in the heart correlates temporally with CM exiting from active cell cycle.
Previous studies suggest that murine CMs exit from active cell cycle around P3 (Walsh et al., 2010). Concurrently, active cell cycle markers show a dramatic decrease and are barely detectable after P21. This cell-cycle exit is associated with an upregulation of cell cycle inhibitors including p21, p27 (Walsh et al., 2010). Consequently, the normal growth of the postnatal heart become primarily driven by CM hypertrophy rather than hyperplasia starting from the first week of postnatal life (Li et al., 1996). Since ErbB2 exhibits a rapid and dramatic decrease in expression from late embryonic stage through early postnatal stage, we next focused on analyzing ErbB2 protein expression at the narrow neonatal time window. As shown in Fig. 1D, western blot analysis revealed that ErbB2 protein expression level was significantly downregulated right after what was previously shown to be the transient neonatal regenerative period. ErbB2 was about 4-fold more abundant at P0 than at P7 and became barely detectable in adult ventricles (postnatal 12 weeks). These results indicate that ErbB2 expression at both transcript and protein levels correlates with the proliferative capacity of the murine hearts.
3.2. ErbB2 is required for CM proliferation in neonatal hearts
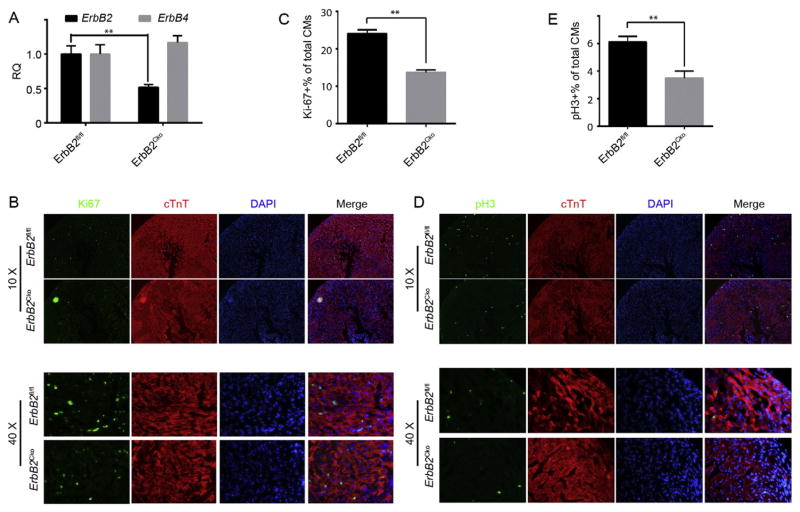
The loss of regenerative capacity of the heart corresponds with a significant decline in the ability of CM to re-enter cell cycle and proliferate upon injury. Thus, we wanted to determine if ErbB2 is required for neonatal CM proliferation. We crossed Mlc2v-Cre with ErbB2fl/fl mice to generate ErbB2 cardiac conditional knockout mice (ErbB2Cko). qPCR analysis indicated that ErbB2 expression in the ErbB2Cko CMs was significantly diminished compared to that in the ErbB2fl/fl control CMs (Fig. 2A), whereas ErbB4 expression remained unaltered in the ErbB2Cko CMs. ErbB2Cko hearts were morphologically indistinguishable from the littermate control hearts (data not shown), we thus wanted to determine if the mutant hearts could display any defects at the cellular level. To this end, we sectioned through ErbB2Cko and ErbB2fl/fl ventricles at P0 and counterstained the sections with antibodies against Ki67, a marker for all active phases of cell cycle, and phosphorylated histone H3 (pH 3), a maker for the onset of mitosis. Quantification of Ki67+ CMs from sections covering multiple layers of left ventricles revealed a significant decrease in the percentage of Ki67+ CMs in ErbB2Cko ventricles compared to that of ErbB2fl/fl ventricles (from 24.1% to 13.7%, Fig. 2B,C). Likewise, the percentage of pH 3+ CMs was also dramatically reduced in the ErbB2Cko hearts compared to that in the ErbB2fl/fl hearts (6.1% to 3.5%, Fig. 2D,E). These data suggest that ErbB2 is required for neonatal CM proliferation.
Fig. 2.
ErbB2 is required to neonatal CM proliferation. (A) qRT-PCR to determine the expression level of ErbB2 in CMs from ErbB2Cko. (B) Immunofluorescence analysis of CM proliferation of the ErbB2fl/fl and ErbB2Cko neonatal hearts by double staining of Ki67 (green) and cTnT (Red) on P0. Representative images obtained under 10× and 40× magnification respectively. (C) Quantification of immunofluorescence analysis in (B). (D) Immunofluorescence analysis of CM karyokinesis of the ErbB2fl/fl and ErbB2Cko hearts by double staining of pH 3 (green) and cTnT (Red) at P0. Representative images obtained under 10× and 40× magnification respectively. (E) Quantification of immunofluorescence analysis in (D). Data are presented as mean ± SEM. Statistical significance was determined by student’s t-test (between two groups). *p < 0.05, **p < 0.01.
3.3. Altered expression of cell cycle regulators in ErbB2Cko hearts
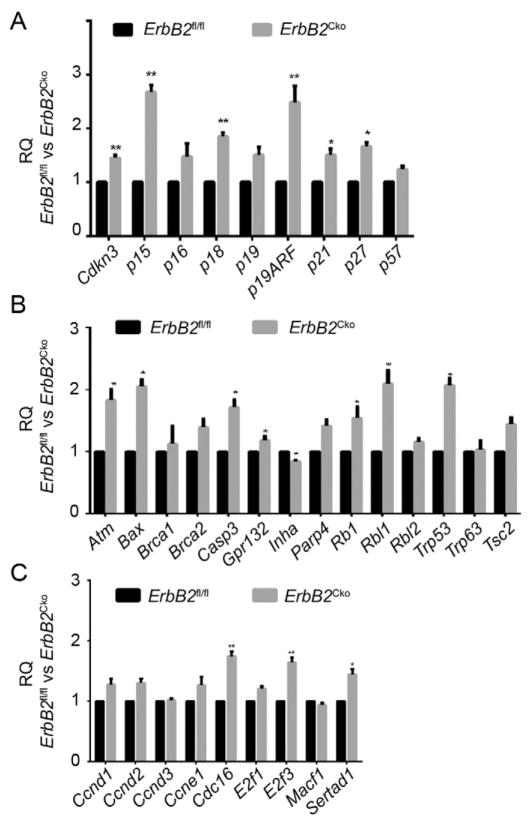
Next, we sought to explore the molecular mechanism underlying the essential role of ErbB2 in CM proliferation. We postulated that the expression of cell cycle regulators might be altered in the ErbB2-depleted CMs. To test this possibility, we selected a wide range of cell cycle regulator genes including cyclins, cyclin-dependent kinases (CDKs), CDK inhibitors (CDKIs), and checkpoint regulators, and examined their mRNA expression in ErbB2Cko and control ErbB2fl/fl CMs. We freshly isolated primary CMs from both control and conditional mutant hearts and performed qRT-PCR to assess the expression of these cell cycle genes. Six out of 9 selected CDKIs, including Cdkn3, p15, p18, p19ARF, p21, and p27, exhibited significantly 1.4 folds to 2.7 folds upregulation in their expression in ErbB2Cko CMs (Fig. 3A). The expression of other negative regulators of cell cycle, such as Atm, Bax, Casp3, Rbl1 and Trp53, were also significantly increased by >2.5 folds (Fig. 3B). In contrast, conditional ablation of ErbB2 in CMs resulted in changes in the expression levels of only a few positive cell cycle regulators, including Ccnd1, Ccnd2, Ccne1, Cdc16, E2f3 and Sertad1 (Fig. 3C, and data not shown). These results suggest that impaired proliferation of ErbB2Cko CMs may result from derepression of genes that negatively regulate cell cycle progression.
Fig. 3.
ErbB2 ablation results in an upregulation in the expression of the negative cell cycle regulators. (A) qRT-PCR analysis of the expression of selected CDKIs in ErbB2fl/fl and ErbB2Cko CMs at P0. (B) qRT-PCR analysis of the expression of negative regulators of cell cycle in ErbB2fl/fl and ErbB2Cko CMs at P0. (C) qRT-PCR analysis of the expression of positive regulators of cell cycle in ErbB2fl/fl and ErbB2Cko CMs at P0. Data are presented as mean ± SEM. Statistical significance was determined by student’s t-test (between two groups). *p < 0.05, **p < 0.01.
Together, our data suggest that the expression level of ErbB2, which rapidly declines soon after birth, is positively associated with neonatal CM proliferation possibly through its role in repressing a set of negative cell cycle regulators.
4. Discussion
The mammalian CMs exit cell cycle shortly after birth and retain limited proliferative potential, thereby rendering the adult hearts incapable to regenerate after myocardial insults, and consequently develop heart failure (Fig. 1A). In contrast, in response to cardiac injury CMs of the lower vertebrates and neonatal mammalian hearts re-enter cell cycle and actively proliferate to restore lost myocardium and myocardial function (Poss, 2010). Though efforts have been made to stimulate existing adult CMs to re-enter cell cycle and proliferate for regenerative purposes (Reiss et al., 1996; Liao et al., 2001; Bersell et al., 2009; Sdek et al., 2011; Mahmoud et al., 2013; Xin et al., 2013; Puente et al., 2014; D’Uva et al., 2015; Gemberling et al., 2015), mechanistic insight into the loss of proliferative potential of the adult mammalian CMs is still lacking. Herein, we provided evidence that ErbB2 expression is dramatically downregulated in the murine heart soon after birth (Fig. 1B, D). We further demonstrate that conditional cardiac ablation of ErbB2 resulted in a significant reduction in neonatal CM proliferation (Fig. 2B–E), which is accompanied with an overall significant upregulation of negative cell cycle regulators (Fig. 3A–B). Thus, our study suggests that the reduction in cardiac ErbB2 expression may be one of the contributing factors to the loss of postnatal CM proliferative capacity. It has been well documented that ErbB2 is an orphan receptor that heterodimerizes with other ErbB receptors, such as ErbB3 and ErbB4 to exert its biological functions (Brennan et al., 2000). During cardiac development, ErbB3 is mainly required for valvulogenesis but not any other aspects of cardiac development, including CM proliferation (Camenisch et al., 2002). In addition, the expression of ErbB3 is not detectable in neonatal hearts (Zhao et al., 1998), which is in sharp contrast to that of ErbB2 and ErbB4. Therefore, ErbB2/4 likely form heterodimer to regulate proliferation in neonatal CMs.
Previous studies have suggested that administration of recombinant Nrg1 improves cardiac function of injured hearts. This beneficial effect of Nrg1 can be attributed at least in part to its role in promoting CM proliferation after cardiac injury. Since ablating ErbB4 significantly diminished the proliferative effects of Nrg1 (Bersell et al., 2009; D’Uva et al., 2015; Gemberling et al., 2015), reduced activities or expression levels of ErbB4/2 in the adult heart could limit a potentially greater beneficial effect of Nrg1. Indeed, we and others found that the expression of ErbB2 in the heart decreases over time until it reaches a relatively low level in the adult heart (Fig. 1B, D) (Lee et al., 1995; Zhao et al., 1998). More interestingly, the expression profile of ErbB2 in the neonatal heart correlates nicely with the heart transitioning from being regenerative to non-regenerative. Functionally, ablating ErbB2 resulted in a significant reduction in neonatal CM proliferation (Fig. 2B–E). In contrast, overexpressing a constitutively active form of ErbB2 led to extensive proliferation of both neonatal and adult CMs, and appear to have a great effect on promoting CM proliferation than administration of recombinant NRG1 (D’Uva et al., 2015) (Bersell et al., 2009). Overall, our study provided clear evidence that ErbB2 is one of the important regulators of CM proliferation, and the downregulation of its postnatal expression correlates with the decline of CM proliferation. Thus, identifying the factors or pathways that downregulate ErbB2 expression in the adult heart may provide novel avenues to improve the therapeutic potential of recombinant NRG1.
5. Conclusion
The data provided here suggest that the steep decline in ErbB2 expression postnatally may account for the decreased cardiac proliferative capacity soon after birth. Yet, it still remains to be determined the molecular mechanism that downregulate cardiac ErbB2 expression after birth.
Acknowledgments
We are grateful for the expert technical assistance from the UNC Histology Core and UNC Microscopy Core. We thank members of the Liu lab and the Qian lab for helpful discussions and critical reviews of the manuscript. This study was supported by National Natural Science Foundation of China 81200192 to Dr. Ma, AHA Scientist Development Grant 13SDG17060010 and the Ellison Medical Foundation (EMF) New Scholar Grant AG-NS-1064-13 to Dr. Qian, and NIH/NHLBI R00 HL109079 grant to Dr. Liu.
Abbreviations
- Atm
ATM serine/threonine kinase
- Bax
BCL2-associated x protein
- Casp3
caspase 3
- CDK
cyclin-dependent kinases
- CDKIs
CDK inhibitors
- Cdkn3
cyclin-dependent kinase inhibitor 3
- Cko
cardiac conditional knockout
- CM
cardiomyocyte
- ErbB2
erb-b2 receptor tyrosine kinase 2
- ErbB4
erb-b2 receptor tyrosine kinase 4
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Nrg1
neuregulin1
- Rbl1
retinoblastoma-like 1
- Trp53
tumor protein p53
Footnotes
Conflict of interest
None.
References
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Brennan PJ, Kumagai T, Berezov A, Murali R, Greene MI. HER2/neu: mechanisms of dimerization/oligomerization. Oncogene. 2000;19:6093–101. doi: 10.1038/sj.onc.1203967. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, Weisinger K, Bassat E, Rajchman D, Yifa O, Lysenko M, Konfino T, Hegesh J, Brenner O, Neeman M, Yarden Y, Leor J, Sarig R, Harvey RP, Tzahor E. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gemberling M, Karra R, Dickson AL, Poss KD. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife. 2015:4. doi: 10.7554/eLife.05871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473:326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28:1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- Liao HS, Kang PM, Nagashima H, Yamasaki N, Usheva A, Ding B, Lorell BH, Izumo S. Cardiac-specific overexpression of cyclin-dependent kinase 2 increases smaller mononuclear cardiomyocytes. Circ Res. 2001;88:443–450. doi: 10.1161/01.res.88.4.443. [DOI] [PubMed] [Google Scholar]
- Ma H, Wang L, Yin C, Liu J, Qian L. In vivo cardiac reprogramming using an optimal single polycistronic construct. Cardiovasc Res. 2015;108:217–219. doi: 10.1093/cvr/cvv223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud AI, Kocabas F, Muralidhar SA, Kimura W, Koura AS, Thet S, Porrello ER, Sadek HA. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497:249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, 2nd, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011a;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011b;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD. Advances in understanding tissue regenerative capacity and mechanisms in animals. Nat Rev Genet. 2010;11:710–722. doi: 10.1038/nrg2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, Santos CX, Thet S, Mori E, Kinter MT, Rindler PM, Zacchigna S, Mukherjee S, Chen DJ, Mahmoud AI, Giacca M, Rabinovitch PS, Aroumougame A, Shah AM, Szweda LI, Sadek HA. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell. 2014;157:565–579. doi: 10.1016/j.cell.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Cheng W, Ferber A, Kajstura J, Li P, Li B, Olivetti G, Homcy CJ, Baserga R, Anversa P. Overexpression of insulin-like growth factor-1 in the heart is coupled with myocyte proliferation in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:8630–8635. doi: 10.1073/pnas.93.16.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdek P, Zhao P, Wang Y, Huang CJ, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. J Cell Biol. 2011;194:407–423. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Lu X, Feng Q. Tumor necrosis factor-alpha induces apoptosis via inducible nitric oxide synthase in neonatal mouse cardiomyocytes. Cardiovasc Res. 2000;45:595–602. doi: 10.1016/s0008-6363(99)00395-8. [DOI] [PubMed] [Google Scholar]
- Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Ponten A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo–an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010;86:365–373. doi: 10.1093/cvr/cvq005. [DOI] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]