Natural and accelerated vascular aging. Involved mechanisms and factors
The vascular aging process
In 2006, Dzau et al. presented the cardiovascular disease (CVD) continuum, represented by successive events/stages of disease progression from the incidence of known risk factors until death.1 This whole concept had the genesis and progression of atherosclerosis as its nuclear mechanism of progression to underlying CVD. In 2010, Dzau et al. gave new emphasis to the importance of age-related structural changes in the middle layer of the arterial wall (arteriosclerosis) as a contributing mechanism for the risk of development of CVD.2
There is a natural process of wear and progressive modification of the arterial wall structure that arises from the mechanical stress of distension induced at each cardiac cycle in connection with the pulse wave amplitude and incident and reflex pressure.3 In the absence of any other factor, this mechanism alone will produce wear on the arterial wall, promoting thickness reduction, fragmentation, and disorganization of the elastin layers. In parallel, this damaged elastic component is replaced by collagen and protein matrix, which is less capable of accommodating the incident pulse wave pressure. In addition, there is loss of integrative and functional connection between elastin layers and smooth muscle vascular cells,4 resulting in reduced distensibility and increased stiffness of the large artery wall, which can be measured by an increase in the transmission of the pulse wave velocity (PWV) and the return of the reflex wave. Thus, there is an influence on the central systolic blood pressure (cSBP), central pulse pressure, "augmentation index", and other ventricular-vascular integration indices.5
The factors accelerating arterial aging are multiple: fetal programming, genetic factors, hypertension, dyslipidemias, diabetes mellitus, chronic renal disease, chronic diseases with an inflammatory component, and smoking, among others.
Accelerated vascular aging
The identification of individuals with accelerated vascular aging may allow an earlier specific intervention, with control of the various risk factors. For each carotid-femoral PWV (cfPWV) increase of 1 m/s, the risk of cardiovascular death, cardiovascular event, or mortality from other causes increases between 14 and 15%.6 The publication of cfPWV7 reference values for different age groups has allowed an easier identification of individuals with early signs of arterial stiffness. However, ethnic and/or environmental exposure aspects that may also contribute to the arterial aging process should be taken into account in the definition of "normal".8
Arterial aging: relationship between microcirculation and macrocirculation, and between arteriosclerosis and atherosclerosis
We can identify four key milestones in the vascular aging process: 1) a progressive reduction in the distensibility of large muscular arteries; 2) a progressive increase in the reflected pressure wave, with a consequent increase in the various components of central arterial pressure; 3) a loss of the arterial stiffness gradient between the central and peripheral arteries; and 4) a progressive elimination of the impedance differential between the arterial macrocirculation and microcirculation.9-11 This set of structural and functional changes in the arterial tree following the deterioration of the structure and function of the middle layer of the arterial wall (arteriosclerosis) is associated with the appearance and concomitant development of atherosclerosis lesions in the vessel wall, having endothelial dysfunction as a unifying mechanism.12
Measures of Central and Peripheral Pressures: Differences and Advantages
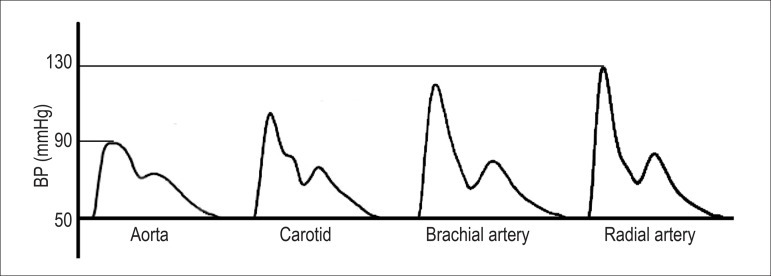
Brachial blood pressure (BP) measured with a sphygmomanometer cannot be considered equivalent to aortic pressure since the latter has invariably lower values. The BP varies continuously during the cardiac cycle, although in practice only the maximum value during systole and the minimum value during diastole are measured. Furthermore, the shape of the pulse wave varies along the arterial tree. With the advancement of the pulse wave from the more elastic central arteries to the more rigid peripheral arteries, the systolic peak becomes narrower and more elevated (Figure 1). Considering that the diastolic BP (DBP) and the mean BP are relatively constant, the brachial systolic BP (SBP) can be 30 mmHg higher than the central systolic aortic pressure in young individuals. This phenomenon, known as amplification of systolic pressure (or pulse pressure), occurs due to several reasons, among them the smaller caliber and greater stiffness of the peripheral arteries. In addition, pulse wave reflections occur at several sites in the arterial network, such as areas with greater collagen/elastin gradient, with greater vasomotor tone and, especially, at bifurcation points. Multiple reflected pulse waves integrate into a single reflected wave that is added to the incident pulse wave, caused by the ventricular ejection. When the reflected wave reaches the incident wave earlier, there is an increase in the central systolic pressure and, consequently, a reduction in the amplification of the pulse pressure. In fact, this increase in pressure depends on several variables, especially age, gender, height, and heart rate.13-15 Female gender, advanced age, short stature, and bradycardia are associated with a lower pulse pressure amplification. Even with the control of these variables, only about 70% of the variability in the pulse pressure amplification can be explained in multiple regression models.13,16 This indicates that central pressure cannot be accurately estimated from the brachial pressure using statistical models, but it actually needs to be determined directly through appropriate methods.
Figure 1.
Amplification of systolic pressure from central to peripheral arteries.
Advantages
Measurement of central pressure could result in greater accuracy in the diagnosis of hypertension, greater safety in the therapeutic decision, and better definition of the prognosis.17,18 Some authors have identified that central pressure, compared with brachial pressure, correlates better with intermediate cardiovascular risk markers such as carotid intima-media thickness and left ventricular hypertrophy.19,20 Several studies have reported an independent relationship between central pressure and future cardiovascular events, including in elderly patients with coronary disease and chronic kidney disease.14,21-23 However, other studies have not found a superior predictive value for central pressure over brachial pressure.24 This controversy exists because the methodology is still heterogeneous and the peripheral pressure, necessary for the final result, explains more than 90% of the variation in central pressure. Furthermore, derivation of central pressure requires an additional measurement, usually radial tonometry, which is also subject to errors that may contribute to the remaining 10% of the variation.18 Therefore, before recommending central pressure measurement for wide clinical use, standardization of the method and the calibration system, and technical limitations of the various devices available must be resolved.
Definition, evaluation and normal values of the main central parameters (central aortic pressure and carotid-femoral pulse wave velocity)
The SBP values vary considerably according to the place where they are obtained. The SBP is greater in the brachial artery when compared with the aorta. This difference in pressure values between the aorta and the brachial artery is a consequence of the phenomenon of peripheral amplification, which results from the difference in impedance between the large-, medium- and small-caliber arteries, especially in the bifurcations, and also the presence of several factors of interference, such as age, comorbidities (dyslipidemia, smoking, diabetes mellitus, etc.) and environmental factors (sodium).25 Recent evidence indicates that central aortic pressure, the augmentation index, and cfPWV are robust markers for future cardiovascular events.21,26
An important aspect in relation to central systolic pressure concerns pressure values obtained with commercial equipment by noninvasive methods. Although these values correlate well with invasive studies, they do not fully represent the central systolic pressure values, but they correctly reflect the amplification phenomenon. This static measurement is considered insufficient for a definitive validation of these methods in the stratification of cardiovascular risk.27
Measurement of cfPWV is an appropriate method of assessing arterial aging with an excellent correlation with the risk of cardiovascular death, cardiovascular events, and mortality from other causes.6 The stiffening of the distal aorta and large arteries, such as the carotid and iliac arteries, occurs due to the early return of the reflection wave, secondary to structural and functional alterations of the distal vascular wall.
Therefore, the great arteries differ from medium and small arteries in relation to histology, physiology, and elastic properties, which is why it is extremely important to define the anatomical target for the action of a drug and the therapeutic target to be achieved. Evidence regarding drug treatment points to a greater ease of reversal of alterations in small-caliber arteries (muscle) than in large arteries (elastic). Thus, results obtained in one arterial segment cannot be extrapolated to other segments in the same arterial tree. Tables 1 and 2 show the central aortic systolic pressure, augmentation index, and cfPWV values in the normal population.28,29
Table 1.
Central systolic aortic pressure values and the augmentation index in normal individuals28
| Central aortic pressure (mmHg) | Augmentation index (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | |||||
| Age (years) | Mean | Percentile (10-90) |
Mean | Percentile (10-90) |
Mean | Percentile (10-90) |
Mean | Percentile (10-90) |
| <20 | 97 | 86 -109 | 105 | 96 - 113 | 14 | 9 - 20 | 19 | 11 - 24 |
| 20 - 29 | 95 | 80 - 110 | 103 | 92 - 115 | 12 | 5 - 19 | 15 | 6 - 24 |
| 30 - 39 | 98 | 84 - 119 | 103 | 88 - 120 | 8 | 0 - 17 | 13 | 4 - 23 |
| 40 - 49 | 102 | 87 - 123 | 106 | 90 - 123 | 6 | 0 - 15 | 11 | 2 - 21 |
| 50 - 59 | 110 | 93 - 127 | 110 | 96 - 126 | 5 | 0 - 13 | 9 | 2 - 18 |
| 60 - 69 | 114 | 97 - 129 | 114 | 97 - 128 | 6 | 1 - 12 | 8 | 2 - 17 |
| > 70 | 118 | 100 - 131 | 116 | 99 - 130 | 6 | 1 - 13 | 8 | 1 - 17 |
% = percentage increase.
Table 2.
Carotid-femoral pulse wave velocity values (m/s) in normal individuals29
| Age | Mean ± 2SD | Median (percentile 10 - 90) |
|---|---|---|
| <30 a | 6.6 (4.9 - 8.2) | 6.4 (5.7 - 7.5) |
| 30 - 39 a | 6.8 (4.2 - 9.4) | 6.7 (5.3 - 8.2) |
| 40 - 49 a | 7.5 (5.1 - 10.0) | 7.4 (6.2 - 9.0) |
| 50 - 59 a | 8.4 (5.1 - 11.7) | 8.1 (6.7 - 10.4) |
| 60 - 69 a | 9.7 (5.7 -13.6) | 9.3 (7.6 - 12.1) |
| > 70 a | 11.7 (6.0 - 17.5) | 11.1 (8.6 - 15.5) |
SD: standard deviation.
Evaluation methodology - available devices and their validations
The cfPWV, which directly reflects arterial stiffness, has a predictive value in cardiovascular morbidity and mortality and is currently considered the gold standard method to assess arterial stiffness.5
The devices used to measure cfPWV have evolved over the last two decades, and their new versions have received systematic validation. Numerous studies have been published comparing invasive and noninvasive methods in different populations and among several existing noninvasive cfPWV measurement devices such as oscillometric, piezoelectric, and tonometric. Most of them have a good correlation with the methods most used in epidemiological studies, such as Complior® or SphymoCor®, among others. Currently, these methods involve little operator training and the ease of use and time consumed in the exam have been optimized so that they are becoming more available for use in clinical practice with good intraobserver and interobserver correlations.30
Some differences have been found in studies comparing devices, with higher values of systematically hemodynamic parameters obtained with one device in particular. The mathematical models used in different devices can lead to different results. However, in most cases, this has no translation in clinical practice, since it does not imply a change in the risk class of the individual. Nevertheless, it is prudent that the same type of equipment is used in multicenter research studies.31
In addition to the validation of different equipment, different procedures for measuring cfPWV have also been proposed. These different procedures, such as measuring the carotid-femoral distance, can influence the results obtained if they are not also standardized. In this case, there are arguments that 80% of the direct carotid-femoral distance is the most accurate estimate for this same distance.5
Central parameters: differences according to age, sex, and ethnicity
The best way to define normal values for central aortic pressure would be a correlation between the central aortic pressure levels obtained and the cardiovascular risk, as known for the BP obtained by the conventional or brachial method. However, these data are not yet available as results of prospective studies designed for this specific purpose, although some publications have sought to obtain these correlations between cardiovascular outcomes and central aortic pressure.21
One strategy would be to obtain correlations between central aortic pressure values that correspond to conventional pressure values obtained in the casual brachial artery or in the clinic. Following this strategy, population studies suggest that an optimal systolic central aortic pressure would be represented by values < 110 mmHg, which would be equivalent to 120 mmHg when obtained by the conventional BP measurement. Likewise, a central aortic pressure < 120 mmHg would correspond to a brachial SBP of 140 mmHg, defining as stage 1 systemic arterial hypertension a systolic central aortic pressure ≤ 120 mmHg.32
Applicability and cost-benefit relationship of the measurement of central parameters
Although it is not part of the stratification routine in hypertensive patients, the central aortic pressure has attracted increasing interest due to its predictive value for the occurrence of cardiovascular events, as well as for the differential evaluation of the different anti-hypertensive drugs, when compared with the traditional determination of the brachial pressure.33 The augmentation index and the pulse pressure measured by carotid tonometry have been considered independent predictors of cardiovascular mortality in end-stage renal disease. However, the predictive value of the central aortic pressure, when compared with that of the brachial BP showed no significant differences.21 Nevertheless, the recommendation for its routine use requires further studies. As an exception and as an added value, isolated systolic hypertension is observed in youths, since the brachial artery SBP in these individuals may be increased due to an exaggerated amplification of the central pressure wave, which would be normal.34
There are no data verifying the cost-benefit relationship of central aortic pressure determination, extrapolating it from small studies with the use of angiotensin II receptor blocker (e.g., losartan), which reduces central aortic pressure and may bring some additional benefit when using it in addition to the reduction of brachial BP.35
Isolated systolic hypertension in young adults: true hypertension and spurious hypertension
The pathophysiological mechanism of isolated systolic hypertension in elderly and young individuals is not the same. In addition, information on the prognosis of both is scarce and the guidelines currently available offer different recommendations on how to address these situations depending on the age group.34
Isolated systolic hypertension in young adults (ISHY) was described in 1999 as a "spurious" elevation of the SBP or pseudo-elevation of the SBP (> 140 mmHg) with normal values of diastolic pressure (< 90 mmHg) resulting from a phenomenon of amplification of the peripheral arterial pulse waveform.36 ISHY is more common in male athletes, in individuals who are taller, and in those with higher body mass index.37 The prevalence of ISHY shows a significant variation (between 2% and 16%) in exclusively male cohorts and has obesity and tobacco as two of the main determinants.38 The noninvasive evaluation of the central pressure and pulse wave amplification in the upper limbs has a precise indication in these cases, since it allows the identification of young adults with "spurious" isolated systolic hypertension, sparing them from being labeled as "hypertensive patients".39 The identification of patients with ISHY should be complemented by outpatient monitoring to exclude white coat hypertension.40
ISHY has increased in prevalence and, given the lack of information about it, there are controversies about how to intervene in this situation. If on the one hand the values of central aortic pressure in individuals with ISHY are lower than those found in true hypertensive patients, they are higher than those obtained in normotensive patients.39 The study by Yano et al.,41 of 2015, showed a higher cardiovascular risk in this group when compared with individuals with optimal BP, but the study did not include an assessment of the central pressure for a possible differentiation between the groups.41 With the information available, the management is to carefully monitor with nonpharmacological measures, with a more aggressive management reserved for situations of greater associated cardiovascular risk, at least until new data are available.42
Prognostic value of the ambulatory arterial stiffness index
The ambulatory arterial stiffness index (AASI) is used for the evaluation of arterial stiffness and is calculated based on the slope of the diastolic pressure versus the values from the systolic pressure in outpatient monitoring, evaluating the dynamic relationship between the DBP and the SBP in 24 hours.43
Thus, for any increase in the distension of the artery wall, the SBP and DBP values tend to increase in parallel, whereas in a rigid artery, there is an increase in the value of SBP accompanied by a lower elevation or even a decrease in DBP. Li et al.44 confirmed in a healthy Chinese population that there was a significant correlation coefficient between AASI and cfPWV, which is the gold standard method.44
The AASI depends on the degree of functional and structural integrity of the arteries, and may also depend on the ejected systolic volume and the reflection waves.45
Because the AASI is dependent on the mechanical properties of small arteries and reflection waves, this index correlates well with pulse pressure and augmentation index, and has a good correlation with some markers of lesion in target organs (ventricular hypertrophy, carotid lesion, and microalbuminuria).46
Some studies have shown a relationship between AASI and global and cardiovascular mortality, as well as a relationship with stroke in normotensive individuals.47 Nevertheless, this prognostic value is still debatable and is related to the degree of decrease during sleep and other factors, such as heart rate and peripheral vascular resistance.48 Moreover, its reproducibility is poor (around 50-68%).49
Central parameters as predictors of arterial hypertension
There is evidence that increased arterial stiffness is a precursor to the occurrence of hypertension and not a consequence of increased BP. The increase in cfPWV preceded the appearance of hypertension over 7 years in an analysis of the Framingham Heart Study.50 The Baltimore Longitudinal Study of Aging also demonstrated an association between increased cfPWV and a higher incidence of hypertension.51 Other central parameters emerged as predictors of hypertension, such as increased brachial-ankle pulse wave velocity, increased proximal aortic stiffness assessed by echocardiography, and increased carotid artery stiffness, as demonstrated in the Atherosclerosis Risk in Communities (ARIC) study.52-54
The increase in aortic stiffness correlated with a lower sensitivity of the baroreflex, a precursor mechanism for the development of hypertension, as well as an increase in the BP variability.55,56
Central parameters and cardiovascular risk
Role of the carotid-femoral pulse wave velocity as a predictor of cardiovascular outcomes
The cfPWV is the most studied central parameter; consequently, there is a greater amount of evidence related to this parameter. Thus, it has been demonstrated that the cfPWV has an independent predictive value for different cardiovascular outcomes in different subgroups, as in patients with hypertension, type 2 diabetes, elderly and in those with end-stage renal disease.57 Even in apparently healthy individuals, cfPWV is an independent predictor of coronary disease and stroke.58,59 When the predictive values for cfPWV and peripheral pressure have been compared, the cfPWV showed an infallible superiority.60 A systematic review including 16 studies with 17,635 participants revealed that for each increase of one standard deviation in cfPWV, the risk ratio was 1.35 (95% confidence interval [95%CI] 1.22 - 1.50, p < 0.001) for coronary disease, 1.54 (95%CI, 1.34 - 1.78, p < 0.001) for stroke, and 1.45 (95%CI, 1.30 - 1.61, p < 0.001) for cardiovascular disease. These risk ratios were even higher in younger participants and remained significant even after adjustment for the presence of conventional cardiovascular risk factors.59
Small studies have shown that the persistent elevation of pulse wave velocity during the treatment of hypertension or cardiovascular disease is associated with a high risk for a cardiovascular event.60
Role of the carotid-femoral pulse wave velocity in the stratification of cardiovascular risk
Studies have shown that the addition of cfPWV to traditional risk factors involved in scores such as Framingham and SCORE, and even atherosclerosis measures, significantly increases the predictive value for cardiovascular outcomes.61-64 They also indicated that cfPWV aggregates information for the stratification of cardiovascular risk, with the potential for clinical applicability. The use of cfPWV allowed to reclassify the cardiovascular risk range of the individuals and was able to improve the evaluation of the prognosis of cardiovascular risk in 10 years in individuals with intermediate risk by 13%, according to a recent systematic review.59,65 Thus, the presence of an elevated cfPWV measurement added to classic risk factors indicates an excess of cardiovascular risk and suggests the need for a more rigorous multifactorial approach.
Role of central aortic pressure as a predictor of cardiovascular outcomes
One of the first publications to draw the attention of the scientific community to the role of central aortic pressure in cardiovascular outcomes, regardless of the peripheral BP values, was the Conduit Artery Function Evaluation (CAFE) study in 2006. In this analysis, the hypertensive patients who presented a greater reduction of the systolic component of the central aortic pressure to the same level of reduction of the BP values obtained by the conventional evaluation had a lower incidence of cardiovascular outcomes.66 In that same year, the European Society of Cardiology published a position drawing attention to the fact that brachial measurements overestimate the central BP values and that the systolic component of central aortic pressure, as well as central pulse pressure, are better predictors of cardiovascular outcomes, especially in patients with hypertension and chronic kidney disease.37 Other publications have also drawn attention to this superiority when comparing central measurements with brachial ones obtained from ambulatory BP monitoring (ABPM).67 On the other hand, a meta-analysis of 11 longitudinal studies showed that both central aortic systolic pressure and central pulse pressure were independent markers of outcome and cardiovascular mortality, but were not superior to the values obtained by conventional measurement (peripheral pressure assessment, p = 0.057).21
Relationship of central parameters with target-organ lesions and associated clinical conditions
Numerous studies have demonstrated that central BP measurement is promising in terms of better correlation with cardiovascular events.68 Differences in central and peripheral arterial pulsatility are difficult to be attributed to cardiovascular events.69 No studies have so far demonstrated robust evidence that central BP adds a new model of cardiovascular risk stratification in relation to the conventional SBP and DBP measurement. A recent analysis of data from the Framingham Offspring Cohort70 demonstrated a strong correlation between central aortic pressure and the incidence of cardiovascular events. A follow-up of up to 6.8 years in a population of 2,492 individuals (mean age 66 ± 9 years) has shown that 6% had a cardiovascular event. In a multivariate analysis, the measurement of central aortic pressure in this population correlated significantly with cardiovascular events. The CAFE study66 recruited 2,199 patients from the five centers of the ASCOT study and performed tonometry by radial artery applanation for analysis of central BP and pulse wave. Although the two arms of the study presented similar brachial pressure reduction (difference of 0.7 mmHg, 95%CI 0.4 - 1.7, p = 0.2), there was a reduction in central aortic pressure with statistical significance in the group that used amlodipine (central aortic systolic pressure 4.3 mmHg, 95%CI 3.3 - 5.4, p < 0.0001; and central aortic pulse pressure 3.0 mmHg, 95%CI 2.1 - 3.9, p < 0.0001). A post hoc analysis of this study demonstrated that central BP was significantly associated with combined cardiovascular outcomes and the development of renal failure (p < 0.05).
Implication of the central parameters in the strategy for the treatment of hypertension
Despite the adequate reduction of (peripheral) BP with anti-hypertensive treatment, the results on clinical outcomes have shown a significant difference attributed to the pleiotropic effects of anti-hypertensive drugs on the elastic properties of large arteries (aorta), on the central aortic pressure, and on the cfPWV.71 Table 3 shows the effects of different classes of anti-hypertensive drugs on central hemodynamics.
Table 3.
Comparative effect of different classes of anti-hypertensive drugs on central hemodynamics
| Classes of anti-hypertensive drugs | CSaP | CDaP | Amplification | Reflection | cfPWV | PAP |
|---|---|---|---|---|---|---|
| Beta-blockers | ↑↑ | ←→ | ↓ | ↑ | ←→ | ↓ |
| Calcium channel blockers | ↓ | ↓/←→ | ↑ | ↓ | ↓ | ↓ |
| Angiotensin-converting enzyme inhibitors | ↓↓ | ↓ | ↑ | ↓ | ↓ | ↓ |
| Angiotensin II AT1 receptor blockers | ↓ | ↓/←→ | ↑/←→ | ↓ | ↓ | ↓ |
| Diuretics | ←→ | ←→ | ←→/↓ | ←→ | ←→ | ↓ |
| Nitrates | ↓ | ↓ | ↓ | ↓ | ←→ | ←→/↓ |
CSaP: central systolic aortic pressure; CDaP: central diastolic aortic pressure; cfPWV: carotid femoral pulse wave velocity; PAP: peripheral arterial pressure.
Beta-blockers
The CAFE study compared the effect of beta-blockers on the central pressure for a similar peripheral BP, and the atenolol/thiazide group showed higher aortic central pressure values when compared with the amlodipine/perindopril group.66
Nebivolol (a beta-blocker with a vasodilatory effect) and carvedilol (an anti-hypertensive with alpha- and beta-blocking effects) compared with atenolol promoted a greater reduction in central aortic pressure and pulse amplification.72,73 Nebivolol reduces central aortic pressure and the augmentation index in mildly hypertensive patients after 3 months of treatment.74
Calcium channel blockers
Calcium channel blockers reduce oxidative stress in experimental models and decrease central aortic pressure.66 The AORTA study compared the addition of azelnidipine or amlodipine to hypertensive patients using olmesartan and demonstrated that the azelnidipine group achieved a greater reduction in central aortic pressure and in the augmentation index, and a greater regression in left ventricular hypertrophy and left ventricular diastolic dysfunction.75,76
Angiotensin converting enzyme inhibitors
The reduction in central aortic pressure demonstrated in comparative studies with angiotensin converting enzyme inhibitors (ACEi) can be attributed to possible mechanisms involving reduction in compliance and oxidative stress, structural remodeling of the vascular wall, collagen/elastin relationship, anti-inflammatory effect and consequent relaxation of the vascular smooth muscle.77,78
Angiotensin II AT1 receptor blockers
Valsartan and captopril reduce to a similar extent the central aortic pressure and the cfPWV.79 The EXPLOR study compared valsartan/amlodipine versus amlodipine/atenolol for a similar BP reduction in the peripheral artery. Central aortic pressure and cfPWV showed a greater reduction in the valsartan/amlodipine group.80 Studies with other AT1 receptor blockers have shown similar results.81,82
Diuretics
Diuretics appear to have no beneficial effect on central hemodynamics.83,84
Nitrates
The effects of nitrates on central aortic pressure are attributed to the relaxation of the vascular smooth muscle of medium-caliber arteries that result in a reduction in the reflection wave amplitude, a reduction in the pulse wave velocity, and an increase in the effective reflection distance. Isosorbide mononitrate has also been evaluated in hypertensive patients and demonstrated a greater reduction in central aortic pressure than in peripheral BP and a greater reduction in the augmentation index without a significant change in the heart rate. On the other hand, nitrates do not influence cfPWV.
Footnotes
Sources of Funding
There were no external funding sources for this study.
Study Association
This study is not associated with any thesis or dissertation work.
References
- 1.Dzau VJ, Antman EM, Black HR, Hayes DL, Manson JE, Plutzky J, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part II: Clinical trial evidence (acute coronary syndromes through renal disease) and future directions. Circulation. 2006;114(25):2871–2891. doi: 10.1161/CIRCULATIONAHA.106.655761. 19. [DOI] [PubMed] [Google Scholar]
- 2.O'Rourke MF, Safar ME, Dzau V. The cardiovascular continuum extended: aging effects on the aorta and microvasculature. Vasc Med. 2010;15(6):461–468. doi: 10.1177/1358863X10382946. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. 2Am J Hypertens. 2005;18(1):3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Laurent S. Defining vascular aging and cardiovascular risk. J Hypertens. 2012;30(Suppl):S3–S8. doi: 10.1097/HJH.0b013e328353e501. [DOI] [PubMed] [Google Scholar]
- 5.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 6.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 7.Miyashita H, Aizawa A, Hashimoto J, Hirooka Y, Imai Y, Kawano Y, et al. Cross-sectional characterization of all classes of antihypertensives in terms of central blood pressure in Japanese hypertensive patients. Am J Hypertens. 2010;23(3):260–268. doi: 10.1038/ajh.2009.255. [DOI] [PubMed] [Google Scholar]
- 8.Cunha PG, Cotter J, Oliveira P, Vila I, Boutouyrie P, Laurent S, et al. Pulse wave velocity distribution in a cohort study: from arterial stiffness to early vascular aging. J Hypertens. 2015;33(7):1438–1445. doi: 10.1097/HJH.0000000000000565. [DOI] [PubMed] [Google Scholar]
- 9.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35(44):3122–3133. doi: 10.1093/eurheartj/ehu293. [DOI] [PubMed] [Google Scholar]
- 10.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46(1):200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S, Briet M, Boutouyrie P. Large and small artery cross-talk and recent morbidity-mortality trials in hypertension. Hypertension. 2009;54(2):388–392. doi: 10.1161/HYPERTENSIONAHA.109.133116. [DOI] [PubMed] [Google Scholar]
- 12.Humphrey JD. Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension. 2008;52(2):195–200. doi: 10.1161/HYPERTENSIONAHA.107.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camacho F, Avolio A, Lovell NH. Estimation of pressure pulse amplification between aorta and brachial artery using stepwise multiple regression models. Physiol Measurement. 2004;25(4):879–889. doi: 10.1088/0967-3334/25/4/008. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson IB, Mohammad NH, Tyrrell S, Hall IR, Webb DJ, Paul VE, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Pt1Am J Hypertens. 2002;15(1):24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 15.McEniery CM Yasmin, Hall IR Qasem A, Wilkinson IB Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46(9):1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 16.McEniery CM Yasmin, McDonnell B Munnery M, Wallace SM Rowe CV, et al. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008 Jun; doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 17.Sharman JE, Laurent S. Central blood pressure in the management of hypertension: soon reaching the goal? J Hum Hypertens. 2013;27(7):405–411. doi: 10.1038/jhh.2013.23. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF. Central pressure should not be used in clinical practice. Artery Res. 2015;9:8–13. doi: 10.1016/j.artres.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28(2):384–388. doi: 10.1097/HJH.0b013e328333d228. [DOI] [PubMed] [Google Scholar]
- 20.Wohlfahrt P, Wichterle D, Seidlerova J, Filipovsky J, Bruthans J, Adamkova V, et al. Relation of central and brachial blood pressure to left ventricular hypertrophy. The Czech Post-MONICA Study. J Hum Hypertens. 2012;26(1):14–19. doi: 10.1038/jhh.2011.78. [DOI] [PubMed] [Google Scholar]
- 21.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 22.Pini R, Cavallini MC, Palmieri V, Marchionni N, Di Bari M, Devereux RB, et al. Central but not brachial blood pressure predicts cardiovascular events in an unselected geriatric population: the ICARe Dicomano Study. J Am Coll Cardiol. 2008;51(25):2432–2439. doi: 10.1016/j.jacc.2008.03.031. 24. [DOI] [PubMed] [Google Scholar]
- 23.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, et al. Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51(4):848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benetos A, Thomas F, Joly L, Blacher J, Pannier B, Labat C, et al. Pulse pressure amplification a mechanical biomarker of cardiovascular risk. J Am Coll Cardiol. 2010;55(10):1032–1037. doi: 10.1016/j.jacc.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 26.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, et al. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27(3):461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayan O, Casan J, Szarski M, Dart AM, Meredith IT, Cameron JD. Estimation of central aortic blood pressure: a systematic meta-analysis of available techniques. J Hypertens. 2014;32(9):1727–1740. doi: 10.1097/HJH.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 28.Herbert A, Cruickshank JK, Laurent S, Boutouyrie P. Establishing reference values for central blood pressure and its amplification in a general healthy population and according to cardiovascular risk factors. Eur Heart J. 2014;35(44):3122–3133. doi: 10.1093/eurheartj/ehu293. [DOI] [PubMed] [Google Scholar]
- 29.Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: 'establishing normal and reference values'. Eur Heart J. 2010;31(19):2338–2350. doi: 10.1093/eurheartj/ehq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ring M, Eriksson MJ, Zierath JR, Caidahl K. Arterial stiffness estimation in healthy subjects: a validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques. Hypertens Res. 2014;37(11):999–1007. doi: 10.1038/hr.2014.115. [DOI] [PubMed] [Google Scholar]
- 31.Hickson SS, Butlin M, Broad J, Avolio AP, Wilkinson IB, McEniery CM. Validity and repeatability of the Vicorder apparatus: a comparison with the SphygmoCor device. Hypertens Res. 2009;32(12):1079–1085. doi: 10.1038/hr.2009.154. [DOI] [PubMed] [Google Scholar]
- 32.Berbari E. Special issues in hypertension. In: Mancia A, editor. Special issues in hypertension. London: Springer; 2012. [Google Scholar]
- 33.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 34.O'Rourke MF, Adji A. Guidelines on guidelines: focus on isolated systolic hypertension in youth. J Hypertens. 2013;31(4):649–654. doi: 10.1097/HJH.0b013e32835d8230. [DOI] [PubMed] [Google Scholar]
- 35.Mancia G. Prevention and treatment of stroke in patients with hypertension. Clin Ther. 2004;26(5):631–648. doi: 10.1016/s0149-2918(04)90065-3. [DOI] [PubMed] [Google Scholar]
- 36.O'Rourke MF, Vlachopoulos C, Graham RM. Spurious systolic hypertension in youth. Vasc Med. 2000;5(3):141–145. doi: 10.1177/1358836X0000500303. [DOI] [PubMed] [Google Scholar]
- 37.Shim CY, Park S, Choi D, Yang WI, Cho IJ, Choi EY, et al. Sex differences in central hemodynamics and their relationship to left ventricular diastolic function. J Am Coll Cardiol. 2011;57(10):1226–1233. doi: 10.1016/j.jacc.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 38.Grebla RC, Rodriguez CJ, Borrell LN, Pickering TG. Prevalence and determinants of isolated systolic hypertension among young adults: the 1999-2004 US National Health And Nutrition Examination Survey. J Hypertens. 2010;28(1):15–23. doi: 10.1097/HJH.0b013e328331b7ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saladini F, Santonastaso M, Mos L, Benetti E, Zanatta N, Maraglino G, et al. Isolated systolic hypertension of young-to-middle-age individuals implies a relatively low risk of developing hypertension needing treatment when central blood pressure is low. J Hypertens. 2011;29(7):1311–1319. doi: 10.1097/HJH.0b013e3283481a32. [DOI] [PubMed] [Google Scholar]
- 40.Krzesinski JM, Saint-Remy A. Spurious systolic hypertension in youth: what does it really mean in clinical practice? J Hypertens. 2006;24(6):999–1001. doi: 10.1097/01.hjh.0000226184.98439.7d. [DOI] [PubMed] [Google Scholar]
- 41.Yano Y, Stamler J, Garside DB, Daviglus ML, Franklin SS, Carnethon MR, et al. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol. 2015;65(4):327–335. doi: 10.1016/j.jacc.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Protogerou AD, Blacher J, Safar ME. Isolated systolic hypertension: 'to treat or not to treat' and the role of central haemodynamics. J Hypertens. 2013;31(4):655–658. doi: 10.1097/HJH.0b013e32835f7e2b. [DOI] [PubMed] [Google Scholar]
- 43.Dolan E, Thijs L, Li Y, Atkins N, McCormack P, McClory S, et al. Ambulatory arterial stiffness index as a predictor of cardiovascular mortality in the Dublin Outcome Study. Hypertension. 2006;47(3):365–370. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Wang JG, Dolan E, Gao PJ, Guo HF, Nawrot T, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension. 2006;47(3):359–364. doi: 10.1161/01.HYP.0000200695.34024.4c. [DOI] [PubMed] [Google Scholar]
- 45.Adiyaman A, Dechering DG, Boggia J, Li Y, Hansen TW, Kikuya M, et al. Determinants of the ambulatory arterial stiffness index in 7604 subjects from 6 populations. Hypertension. 2008;52(6):1038–1044. doi: 10.1161/HYPERTENSIONAHA.108.119511. [DOI] [PubMed] [Google Scholar]
- 46.Leoncini G, Ratto E, Viazzi F, Vaccaro V, Parodi A, Falqui V, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension. 2006;48(3):397–403. doi: 10.1161/01.HYP.0000236599.91051.1e. [DOI] [PubMed] [Google Scholar]
- 47.Kikuya M, Staessen JA, Ohkubo T, Thijs L, Metoki H, Asayama K, et al. Ambulatory arterial stiffness index and 24-hour ambulatory pulse pressure as predictors of mortality in Ohasama, Japan. Stroke. 2007;38(4):1161–1166. doi: 10.1161/01.STR.0000259604.67283.69. [DOI] [PubMed] [Google Scholar]
- 48.Laurent S. Surrogate measures of arterial stiffness: do they have additive predictive value or are they only surrogates of a surrogate? Hypertension. 2006;47(3):325–326. doi: 10.1161/01.HYP.0000200701.43172.9a. [DOI] [PubMed] [Google Scholar]
- 49.Li Y, Hansen TW, Staessen JA. More information on the reproducibility of the ambulatory arterial stiffness index. Am J Hypertens. 2010;23(2):113–114. doi: 10.1038/ajh.2009.239. [DOI] [PubMed] [Google Scholar]
- 50.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Najjar SS, Scuteri A, Shetty V, Wright JG, Muller DC, Fleg JL, et al. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J Am Coll Cardiol. 2008;51(14):1377–1383. doi: 10.1016/j.jacc.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takase H, Dohi Y, Toriyama T, Okado T, Tanaka S, Sonoda H, et al. Brachial-ankle pulse wave velocity predicts increase in blood pressure and onset of hypertension. Am J Hypertens. 2011;24(6):667–673. doi: 10.1038/ajh.2011.19. [DOI] [PubMed] [Google Scholar]
- 53.Dernellis J, Panaretou M. Aortic stiffness is an independent predictor of progression to hypertension in nonhypertensive subjects. Hypertension. 2005;45(3):426–431. doi: 10.1161/01.HYP.0000157818.58878.93. [DOI] [PubMed] [Google Scholar]
- 54.Liao D, Arnett DK, Tyroler HA, Riley WA, Chambless LE, Szklo M, et al. Arterial stiffness and the development of hypertension. The ARIC Study. Hypertension. 1999;34(2):201–206. doi: 10.1161/01.hyp.34.2.201. [DOI] [PubMed] [Google Scholar]
- 55.Mattace-Raso FU, van den Meiracker AH, Bos WJ, van der Cammen TJ, Westerhof BE, Elias-Smale S, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25(7):1421–1426. doi: 10.1097/HJH.0b013e32811d6a07. [DOI] [PubMed] [Google Scholar]
- 56.Okada Y, Galbreath MM, Shibata S, Jarvis SS, VanGundy TB, Meier RL, et al. Relationship between sympathetic baroreflex sensitivity and arterial stiffness in elderly men and women. Hypertension. 2012;59(1):98–104. doi: 10.1161/HYPERTENSIONAHA.111.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 58.Sabovic M, Safar ME, Blacher J. Is there any additional prognostic value of central blood pressure wave forms beyond peripheral blood pressure? Curr Pharm Des. 2009;15(3):254–266. doi: 10.2174/138161209787354249. [DOI] [PubMed] [Google Scholar]
- 59.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orlova IA, Nuraliev EY, Yarovaya EB, Ageev FT. Prognostic value of changes in arterial stiffness in men with coronary artery disease. Vasc Health Risk Manag. 2010;6:1015–1021. doi: 10.2147/VHRM.S13591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- 62.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002 Jan;39(1):10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- 63.Sehestedt T, Jeppesen J, Hansen TW, Wachtell K, Ibsen H, Torp-Pedersen C, et al. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31(7):883–891. doi: 10.1093/eurheartj/ehp546. [DOI] [PubMed] [Google Scholar]
- 64.Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 65.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241(2):507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Williams B Lacy PS, Thom SM Cruickshank K, Stanton A Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- 67.Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, et al. Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens. 2011;29(3):454–459. doi: 10.1097/HJH.0b013e3283424b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55(3):799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mitchell GF. Does measurement of central blood pressure have treatment consequences in the clinical praxis? Curr Hypertens Rep. 2015;17(8):66–66. doi: 10.1007/s11906-015-0573-x. [DOI] [PubMed] [Google Scholar]
- 70.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, et al. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131(4):354–361. doi: 10.1161/CIRCULATIONAHA.114.011357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 72.Dhakam Z Yasmin, McEniery CM Burton T, Brown MJ Wilkinson IB. A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens. 2008;26(2):351–356. doi: 10.1097/HJH.0b013e3282f283c9. [DOI] [PubMed] [Google Scholar]
- 73.Shah NK, Smith SM, Nichols WW, Lo MC, Ashfaq U, Satish P, et al. J Clin Hypertens. 12. Vol. 13. Greenwich: 2011. Carvedilol reduces aortic wave reflection and improves left ventricular/vascular coupling: a comparison with atenolol (CENTRAL Study) pp. 917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaz-de-Melo RO, Giollo-Junior LT, Martinelli DD, Moreno-Junior H, Mota-Gomes MA, Cipullo JP, et al. Nebivolol reduces central blood pressure in stage I hypertensive patients: experimental single cohort study. Sao Paulo Med J. 2014;132(5):290–296. doi: 10.1590/1516-3180.2014.1325704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takami T, Saito Y. Effects of Azelnidipine plus OlmesaRTAn versus amlodipine plus olmesartan on central blood pressure and left ventricular mass index: the AORTA study. Vasc Health Risk Manag. 2011;7:383–390. doi: 10.2147/VHRM.S21991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takami T, Saito Y. Azelnidipine plus olmesartan versus amlodipine plus olmesartan on arterial stiffness and cardiac function in hypertensive patients: a randomized trial. Drug Design, Development and Therapy. 2013;7:175–183. doi: 10.2147/DDDT.S42338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Protogerou AD, Stergiou GS, Vlachopoulos C, Blacher J, Achimastos A. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part II: Evidence for specific class-effects of antihypertensive drugs on pressure amplification. Curr Pharm Des. 2009;15(3):272–289. doi: 10.2174/138161209787354186. [DOI] [PubMed] [Google Scholar]
- 78.Manisty CH, Hughes AD. Meta-analysis of the comparative effects of different classes of antihypertensive agents on brachial and central systolic blood pressure, and augmentation index. Br J Clin Pharmacol. 2013;75(1):79–92. doi: 10.1111/j.1365-2125.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.London GM, Pannier B, Vicaut E, Guerin AP, Marchais SJ, Safar ME, et al. Antihypertensive effects and arterial haemodynamic alterations during angiotensin converting enzyme inhibition. J Hypertens. 1996;14(9):1139–1146. doi: 10.1097/00004872-199609000-00015. [DOI] [PubMed] [Google Scholar]
- 80.Boutouyrie P, Achouba A, Trunet P, Laurent S. Amlodipine-valsartan combination decreases central systolic blood pressure more effectively than the amlodipine-atenolol combination: the EXPLOR Study. Hypertension. 2010;55(6):1314–1322. doi: 10.1161/HYPERTENSIONAHA.109.148999. [DOI] [PubMed] [Google Scholar]
- 81.Agnoletti D, Zhang Y, Borghi C, Blacher J, Safar ME. Effects of antihypertensive drugs on central blood pressure in humans: a preliminary observation. Am J Hypertens. 2013;26(8):1045–1052. doi: 10.1093/ajh/hpt081. [DOI] [PubMed] [Google Scholar]
- 82.Asmar R. Effect of telmisartan on arterial distensibility and central blood pressure in patients with mild to moderate hypertension and Type 2 diabetes mellitus. J Renin Angiotensin Aldosterone Syst. 2001;2(Suppl 2):S8–11. doi: 10.3317/jraas.2001.031. [DOI] [PubMed] [Google Scholar]
- 83.Morgan T, Lauri J, Bertram D, Anderson A. Effect of different antihypertensive drug classes on central aortic pressure. Am J Hypertens. 2004;17(2):118–123. doi: 10.1016/j.amjhyper.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Mackenzie IS, McEniery CM, Dhakam Z, Brown MJ, Cockcroft JR, Wilkinson IB. Comparison of the effects of antihypertensive agents on central blood pressure and arterial stiffness in isolated systolic hypertension. Hypertension. 2009;54(2):409–413. doi: 10.1161/HYPERTENSIONAHA.109.133801. [DOI] [PubMed] [Google Scholar]
- 85.Stokes GS, Barin ES, Gilfillan KL. Effects of isosorbide mononitrate and AII inhibition on pulse wave reflection in hypertension. Hypertension. 2003;41(2):297–301. doi: 10.1161/01.hyp.0000049622.07021.4f. [DOI] [PubMed] [Google Scholar]