Abstract
Case evidence is presented that highlights the clinical relevance and significance of a novel sound therapy-based treatment. This intervention has been shown to be efficacious in a randomized controlled trial for promoting expansion of the dynamic range for loudness and increased sound tolerance among persons with sensorineural hearing losses. Prior to treatment, these individuals were unable to use aided sound effectively because of their limited dynamic ranges. These promising treatment effects are shown in this article to be functionally significant, giving rise to improved speech understanding and enhanced hearing aid benefit and satisfaction, and, in turn, to enhanced quality of life posttreatment. These posttreatment sound therapy effects also are shown to be sustained, in whole or part, with aided environmental sound and to be dependent on specialized counseling to maximize treatment benefit. Importantly, the treatment appears to be efficacious for hearing-impaired persons with primary hyperacusis (i.e., abnormally reduced loudness discomfort levels [LDLs]) and for persons with loudness recruitment (i.e., LDLs within the typical range), which suggests the intervention should generalize across most individuals with reduced dynamic ranges owing to sensorineural hearing loss. An exception presented in this article is for a person describing the perceptual experience of pronounced loudness adaptation, which apparently rendered the sound therapy inaudible and ineffectual for this individual. Ultimately, these case examples showcase the enormous potential of a surprisingly simple sound therapy intervention, which has utility for virtually all audiologists to master and empower the adaptive plasticity of the auditory system to achieve remarkable treatment benefits for large numbers of individuals with sensorineural hearing losses.
Keywords: Adaptive plasticity, sound therapy, dynamic range expansion, hearing aid benefit, hyperacusis, loudness recruitment, sensorineural hearing loss, sound tolerance.
Learning Outcomes: As a result of this activity, the participant will be able to: (1) describe the treatment efficacy of a low-level, broadband, sound therapy-based protocol for expanding the auditory dynamic ranges of persons with sensorineural hearing losses; and (2) describe the application of the protocol for hearing-impaired persons with narrow dynamic ranges, including individuals with abnormally reduced loudness discomfort levels (i.e., hyperacusis) and with normal discomfort levels (i.e., loudness recruitment).
The purpose of this article is to present case examples and evidence to showcase the clinical relevance and significance of an efficacious sound therapy-based intervention that promotes expansion of the dynamic range (DR) for loudness and increased sound tolerance. The intervention protocol uses low-level broadband sound therapy, delivered via bilateral sound generators (SGs), in combination with specialized counseling described later, to achieve remarkable treatment effects highlighted in this article. We previously described the efficacy of this intervention in a randomized placebo-controlled trial that targeted prospective hearing aid candidates who, prior to treatment, were limited in the use of and benefit from amplification because of their reduced DRs.1 These individuals, with mostly mild to moderately severe sensorineural hearing losses, were similar to many sound-sensitive difficult-to-fit patients whom audiologists routinely encounter in a typical hearing aid practice. On average, they had lower, borderline-normal loudness discomfort levels (LDLs) of ∼85 to 90 dB hearing level (HL) across frequency, bilaterally, and reduced DRs at higher audiometric frequencies, typically ≤30 to 40 dB.
Formby et al considered only group findings in their original description of the efficacious treatment effects.1 We now focus in this article on selected case evidence. These individual results are perhaps more compelling and revealing of the clinical utility of the treatment than were the group findings. We begin our article by highlighting a pair of cases that represent two ends of a continuum of hearing aid candidates, both highlighting the significance of the intervention for clinical practice in individuals with reduced DRs. The first case, a patient with high-frequency hearing loss and mild to moderate (loudness-based) hyperacusis, provides a dramatic replication of the sound therapy results described originally by Hazell and Sheldrake for tinnitus patients with primary hyperacusis.2 Suffice to say, there is no consensus definition for hyperacusis,3 4 but the problem may be described as an intolerance to the loudness of sounds that most individuals deem to be tolerable. Hyperacusis can occur with or without hearing loss and is often associated with tinnitus (i.e., ∼40 to 50% of patients who report tinnitus suffer hyperacusis,5 6 whereas almost 90% of patients with hyperacusis report tinnitus7). Audiologically, the diagnosis of loudness-based hyperacusis may be considered when LDLs are <90 dB HL; usually, all or most audiometric frequencies are affected relatively uniformly and bilaterally.8 9 Associated pain, discomfort, and/or related distress to the offending sound, which would not be bothersome for most listeners, also are requisite symptoms of primary hyperacusis.10
The second and perhaps more interesting case we consider is that of a successfully treated individual, also with bilateral high-frequency sensorineural hearing loss and typical LDLs at most audiometric frequencies. The reader will recognize this case as the classical patient with loudness recruitment.11 12 13 His associated reduced DR at higher frequencies is primarily due to elevated audiometric thresholds, typical of those universally managed in hearing aid practices.
For those readers who may be unfamiliar with the phenomenological distinction between hyperacusis and loudness recruitment, consider the corresponding idealized examples of loudness growth shown in Fig. 1. Classical loudness-based primary hyperacusis,4 in association with sensorineural hearing loss, is characterized by loudness growth over a narrow DR between the elevated hearing threshold of ∼65 dB HL and an abnormally reduced uncomfortable loudness judgment at 80 dB HL. The corresponding loudness-growth function in primary hyperacusis therefore is much steeper than normal and the corresponding DR much narrower than normal. In contrast, loudness growth for a hearing-impaired listener experiencing loudness recruitment is accelerated for sounds just above threshold, which at higher levels is similar to that reported by typical listeners without hearing loss. The DR for the sensorineural listener with recruitment therefore is reduced because of the elevated hearing threshold, but not because of the reduced uncomfortable loudness (UCL) judgment, which is synonymous with and corresponds closely to the LDL.14 Thus, the judgments of loudness by a hearing-impaired listener who experiences primary hyperacusis reflect a steeper growth in loudness than that for either a normal or recruiting listener, and there is no overlapping of the hyperacusis growth function with the loudness-growth functions for either the normal or recruiting listener at high sound levels. Consequently, the DR for a person with primary hyperacusis is much smaller than that of a recruiting listener with similarly elevated hearing thresholds because the UCL judgment for the former is lower than that of the latter.
Figure 1.
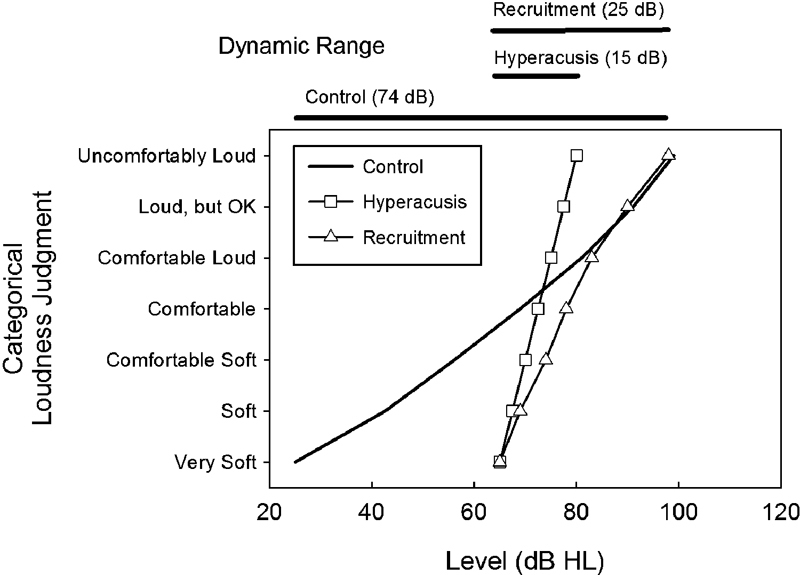
Idealized loudness-growth functions (categorical loudness judgment versus presentation level) for a typical (control) listener with normal-hearing sensitivity (solid line) and for a pair of hearing-impaired listeners (with similar hearing thresholds) representative of primary hyperacusis (square symbols) and classical loudness recruitment (triangle symbols). The reduced dynamic ranges (i.e., the difference between the level judged as “uncomfortably loud” and the hearing threshold, assumed here to correspond closely to the level judged as “very soft”) are indicated for each hearing-impaired listener by the associated bar shown at the top of the panel. The corresponding control dynamic range also is depicted. Abbreviation: HL, decibels in hearing level.
The examples of primary hyperacusis and loudness recruitment depicted in Fig. 1 fit within a pair of classification schemes proposed by Goldstein and Shulman for grading the severity of a sound sensitivity/hyperacusis condition.3 These two classification schemes are shown in Table 1. The first scheme, shown on the left side of the table, uses the listener's LDL values. It is directly relevant for classifying the severity of classical loudness-based (primary) hyperacusis.4 Goldstein and Shulman proposed that two or more audiometric frequencies must be affected in this scheme for there to be a problem, which they categorized as mild, moderate, and severe for the corresponding ranges of LDL values, 80 to 90, 65 to 75, ≤60-dB HL, respectively. Their second scheme (right side of Table 1) graded the severity of the problem in terms of the usable DR between the audiometric threshold and the LDL for a given frequency. Specifically, Goldstein and Shulman proposed that a DR at any frequency of 50 to 55, 40 to 45, and ≤35 dB denoted mild, moderate, and severe hyperacusis, respectively. Accordingly, using their LDL-based criteria, our example of primary hyperacusis in Fig. 1, with a UCL judgment of 80 dB HL, falls at the low end of their mild range of 80 to 90 dB, whereas the example of loudness recruitment in Fig. 1, with a UCL judgment of 110 dB HL, would be graded as normal. By contrast, the example of primary hyperacusis in Fig. 1, with a DR of only 15 dB, would be graded as a severe (if not a profound) problem in Goldstein and Shulman's DR-based classification scheme, whereas the example of loudness recruitment in Fig. 1, with a DR of 45 dB, would be categorized as a moderate hyperacusis problem. Thus, both examples of hearing impairment in Fig. 1 would be considered problematic for sound sensitivity/hyperacusis in their DR classification scheme.
Table 1. Goldstein and Shulman's3 Classification of the Degree of Hyperacusis (Sound Sensitivity) Based on Associated Ranges of LDL and DR.
| LDL-Based (dB HL) | DR-Based (dB) | |
|---|---|---|
| Mild hyperacusis | 80–90 | 50–55 |
| Moderate hyperacusis | 65–75 | 40–45 |
| Severe hyperacusis | ≤60 | ≤35 |
Abbreviations: DR, dynamic range; HL, hearing level; LDL, loudness discomfort level.
Goldstein and Shulman's latter classification scheme for hyperacusis, based on DR categories <60 dB, is not widely recognized nor used clinically today. Instead classical loudness-based hyperacusis is diagnosed by associated sound tolerance complaints and/or related distress in combination with atypically reduced LDL measures.4 10 In principle, however, Goldstein and Shulman's DR-based classification scheme may be functionally meaningful and of substantive utility for considering sound tolerance and loudness-based problems of listeners with sensorineural hearing losses, most notably those with elevated audiometric thresholds that account primarily for their abnormally reduced DRs.
To wit, Goldstein and Shulman's basic principles and classification scheme are generally consistent with the early thinking of Davis et al in the Harvard Hearing Aid Report.15 Davis and colleagues proposed that if a hearing aid candidate had a usable DR ≥ 60 dB, then the likelihood of successfully fitting the patient with linear amplification was high for aided benefit, and he or she would not typically require customized fitting.15 Accordingly, in the absence of modern signal-processing strategies to compress the amplified signal into the reduced DR of the hearing aid user, Davis et al championed the idea of sound tolerance training to expand the listener's DR. The training consisted of brief weekly exposures to high-level pure tones and speech stimuli presented just below an individual's LDLs. Their notion was that these toughening training exposures would promote increased tolerance, leading to DR expansion. This increased tolerance for amplified sound and the corresponding expanded DR would, in turn, enhance aided benefit, especially for prospective hearing aid candidates with DRs < 40 dB. Davis commissioned Silverman to implement and assess a sound tolerance training protocol of the kind described previously to determine the utility of this strategy.16 Silverman reported that tolerance training led to average increases in the DR of ∼10 dB for both normal and hearing-impaired listeners, usually after two or three weekly exposure sessions. Subsequent efforts to replicate Silverman's tolerance-training effects or to demonstrate their functional significance for improved speech understanding have not been successful.17 Accordingly, sound tolerance training with high-level sound exposures is not used in clinical practice today. Suffice to say, we will demonstrate in our case examples that the idealized tolerance-training effects sought by Davis et al can be achieved with clinically practical low-level sound therapy principles described originally by Hazell and Sheldrake and extended by Formby et al.1 2 This evidence indicates that the high-level toughening sound exposures that Davis et al advocated 70 years ago are not needed to expand the DR. Moreover, we will show that these treatment effects are functionally significant and meaningful for improved communication and enhanced hearing aid benefit.
In the other case examples that follow, we present convincing evidence that: (1) both counseling and sound therapy from SGs are needed in a combined protocol for maximizing treatment benefit to expand the DR and (2) positive posttreatment effects (i.e., an expanded DR) appear to be sustained, at least in part, when hearing aids are subsequently used and the SGs are discontinued. We conclude this series of case presentations with an example of a participant who did not benefit from his treatment. Based on his subjective self-reports of the perceptual experience to the sound therapy, his phenomenological account provides a plausible and sensible explanation for his negative treatment outcome. This explanation, which may be relevant for understanding the negative treatment results of others who did not benefit from the full-treatment protocol implemented by Formby et al,1 suggests the need to consider additional inclusion/exclusion criteria for participants in related future research. These criteria also may be relevant for devising screening strategies to predict patient success for this and other sound therapy protocols in clinical practice.
OVERVIEW OF THE TREATMENT AND OUTCOME MEASUREMENT PROTOCOL
Preliminary to presenting the selected case examples, we review briefly the full-treatment protocol used to achieve the results. The full protocol, described originally by Formby et al,1 consisted of two primary components:
A structured counseling protocol, which adhered to a checklist format that encompassed the following components: (a) an initial introduction to the counseling protocol, including the content, objectives, and the end goal of the treatment within the context of each participant's audiometric results, subjective complaints, and desire to use amplified sound from hearing aids successfully; (b) a brief overview of the auditory anatomy and physiology, as relevant to each participant's sound tolerance condition; (c) an introduction to auditory gain control processes, focusing on the adaptive plasticity of these processes and their presumed contribution to the participant's sound tolerance issues and reduced DR; and (d) the application of sound therapy as a clinical tool to recalibrate auditory pathway gain, thereby expanding the participant's DR, enhancing her or his sound tolerance, and, ultimately, improving the use of amplified sound and hearing aid benefit. (See Gold and Formby in this issue for a detailed description of the counseling protocol.18)
Continuous, low-level broadband sound therapy, delivered bilaterally by in-the-ear open-canal SGs, which emitted soft but comfortable broadband sound reminiscent of a gentle “sea shell-like” noise. The SGs were recommended for use at least 8 hours each day, with supplementation from enriched environmental sound, especially when devices were not in use. The interested reader can find a full description of the SG devices, sound therapy rationale, and fitting strategy in Formby et al.1
Other treatments evaluated by Formby et al included counseling combined with a short-acting placebo SG, SG alone, and the placebo SG alone.1 None of these treatment options was found to be as efficacious as the full-treatment protocol, which is the primary focus of this article in most of the case examples.
Participants assigned to the full, partial, and control treatments were evaluated at baseline, just prior to treatment onset, and then repeatedly over intervention periods of 4 to 12 months in a battery of primary and secondary outcome measures, including: repeated measures of pure tone thresholds; LDLs for pure tones (1,000 and 8,000 Hz) and white noise; categorical loudness judgments for FM pulsed (warble) tones (500, 2,000, and 4,000 Hz) and for recorded spondaic words as measured per the Contour test protocol19; and NU-6 word recognition scores (WRS) measured for speech presentation levels corresponding to categorical loudness judgments for comfortable and loud, but OK and associated Articulation Index (AI) predictions shown as performance intensity (PI) functions.20 These predictions of WRS as a function of presentation level enable a comparison of a given listener's performance with that expected for an average person with the same hearing loss (software and system to perform this is known as Speech Test Evaluation and Presentation System (STEPS)).21 These AI predictions of WRS effectively reveal the amount of audible information in the speech signal available to and usable by the listener. Additionally, auditory brainstem and middle latency responses were measured simultaneously for 500- and 2,000-Hz tone bursts across the repeated measurement sessions. The latter measures will be considered in a separate report highlighting selected case examples for participants who exhibited large positive treatment effects in terms of their DR expansion. (See Formby et al in this issue.22)
INDIVIDUAL SUBJECT DATA
The group loudness judgments that were presented in summary format in Formby et al1 are broken down and shown in Fig. 2 for individual participants by treatment group. Shown are their 1,000-Hz LDL and Contour test categorical UCL judgments; the latter judgments represent the average levels for 500, 2,000, and 4,000 Hz. Their pretreatment baseline and change in judgments after treatment are denoted in Fig. 2. The dashed line for each participant is the prediction of the level for that individual at which he or she would no longer report a sound tolerance problem (if he or she had hyperacusis) based on his or her audiometric characteristics (audiometric threshold at 2,000, 4,000 and 8,000 Hz)23:
Figure 2.
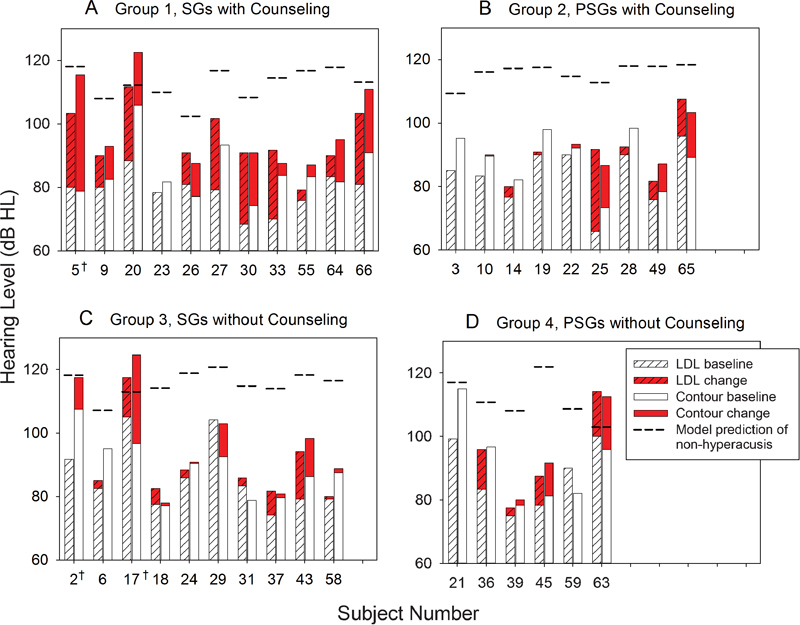
Individual subject data for LDL judgments at 1,000 Hz and for Contour test uncomfortable loudness judgments (averaged for 500, 2,000, and 4,000 Hz) for the as treated assignment groups from Formby et al1: (A) counseling and SGs; (B) counseling and PSGs; (C) SGs; and (D) PSGs. The shaded red bar depicts the respective change in performance with treatment (if any) relative to baseline performance, and the dashed lines denote the prediction of the level at which that subject, if hyperacusic, would no longer report hyperacusis based on the model by Hawley et al.23 See text for details. Abbreviations: HL, hearing level; LDL, loudness discomfort level; PSG, placebo sound generator; SG, sound generator.
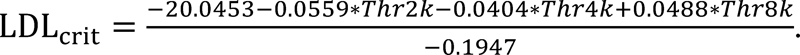 |
Notice that the predicted level for each participant varies; target values LDL criterion (LDLcrit) ranged from 102 to 121 dB HL. During their treatment, some participants exceeded their target, but most did not. As part of the protocol reported by Formby et al,1 those participants with ≥10 dB incremental shifts in their UCL judgments were offered hearing aids after 4 to 6 months in their assigned treatment group. They may not have had the opportunity to achieve their full potential change in response to the sound therapy, which we know may be progressive over a year or more for some individuals.24
CASE EXAMPLES
Primary Hyperacusis
One of the most dramatic case examples of positive sound therapy treatment outcomes from Formby et al is that shown in the outcomes profile for participant 5T in Fig. 3.1 This participant, with mild to moderate primary hyperacusis based on her pretreatment loudness judgments (i.e., LDL and equivalent categorical UCL judgments) and a severe sound sensitivity problem based on her pretreatment DR, achieved large positive treatment-related effects and substantial expansion of her DR. Her sizeable intervention effects were larger than those of most participants in the full-treatment group of Formby et al (see Fig. 2A).1
Figure 3.
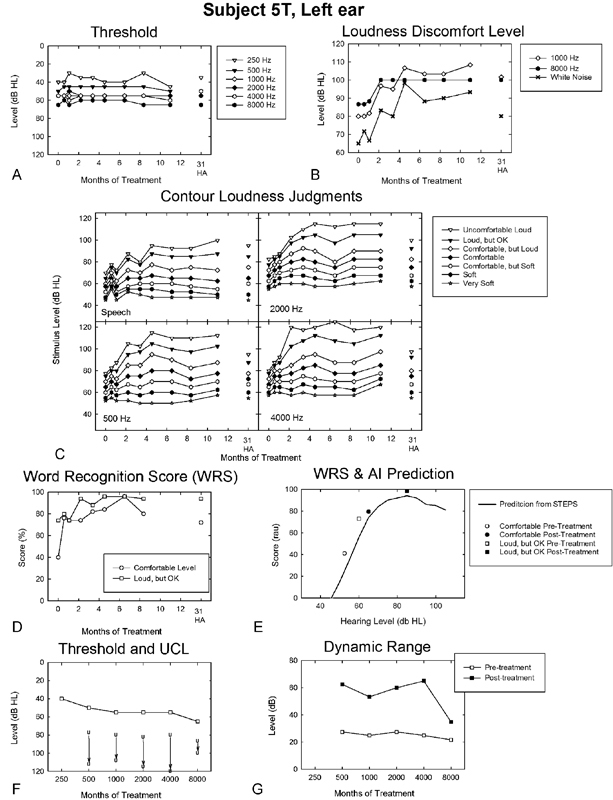
Outcomes profile for the left ear of a hearing-impaired participant (5T) with primary hyperacusis, followed across the period of her full-treatment protocol (counseling in combination with sound generators) and posttreatment for unaided listening after transition to HAs. Repeated-measures audiometric pure tone thresholds (A) and loudness discomfort levels measured at 1,000 and 8,000 Hz and for white noise stimulation (B); repeated-measures categorical loudness judgments for spondee speech stimuli and for warble tones presented at 500, 2,000, and 4,000 Hz reveal the dynamics of treatment change (presentation level versus months of treatment) for each response category designated in the inset legend (C); repeated-measures NU-6 WRS measured at presentation levels judged to be comfortable and loud, but OK, respectively, for spondee speech stimuli (D); WRS (in rationalized arcsine units) measured pre- and posttreatment for comfortable and loud, but OK presentation levels, respectively, superimposed on the corresponding AI prediction (shown as a performance intensity function) from the STEPS algorithm (see text for details) (E); pretreatment audiometric pure tone thresholds shown together with corresponding UCL judgments measured pre- and posttreatment (F); and corresponding pre- and posttreatment dynamic ranges (G). The number (31) in A to D above the HA label represents months post–onset treatment for 5T at the time of the measurement. Abbreviations: AI, Articulation Index; HA, hearing aid; dB HL, decibels hearing level; RAU, rationalized arcsine units; STEPS, Speech Test Evaluation and Presentation System; UCL, uncomfortable loudness; WRS, word recognition score.
5T was 69 years of age at the onset of treatment. She reported owning and occasionally using one hearing aid over the past 24 years, but related limited benefit because both aided and unaided listening were uncomfortable for moderate and louder sounds. 5T noted a gradual onset of her tolerance problem, which she reported was greater in her left ear and was associated with stress-related conditions (i.e., a mood-related symptom requisite for diagnosing primary hyperacusis per Baguley13). 5T denied using hearing protection or needing it for sound-induced pain, which she also denied. She related a history of dizziness and balance problems, difficulty understanding speech, and sensitivity to light. She also reported intermittent bilateral tinnitus, worse in the right ear, with sudden onset 2 years prior. The tinnitus apparently was a secondary issue for 5T, which she did not rank with respect to her hearing loss or tolerance problems.
The outcomes profile for 5T's left ear (the corresponding results for her right ear were similar and therefore are not shown in Fig. 3) includes repeated-measures pure tone thresholds (Fig. 3A), LDL (Fig. 3B), categorical loudness judgments (Fig. 3C), and WRS scores (Fig. 3D). Also shown are her obtained and predicted AI results; the latter is depicted as a PI function. The obtained results are presented in rationalized arcsine units (RAU)25 pre- and posttreatment for presentation levels corresponding to her comfortable and loud, but OK judgments (Fig. 3E). Her pretreatment audiometric thresholds and corresponding LDL and categorical judgments of UCL levels measured pre- and posttreatment (Fig. 3F), along with her corresponding DR values (Fig. 3G), are presented in the bottom profile panels of Fig. 3.
First, note that 5T's audiometric thresholds (see Fig. 3A, F) reveal her high-frequency loss. These values, shown in Fig. 3A, reflect little or no change over time of treatment, whereas her LDLs and the UCL levels, corresponding to her categorical judgments of uncomfortable loudness for warble tones, white noise, and spondee words (shown in Fig. 3B, C), changed by as much as 40 dB, usually becoming asymptotic after 4 months into treatment. These changes in loudness judgments over the course of treatment were similar in pattern across frequency from 500 to 8,000 Hz. Her loudness judgments for spondee words and white noise stimuli revealed similar trends to those measured for tonal stimuli.
A second observation of note is that the categorical loudness judgments for the warble tone and spondee speech stimuli (in Fig. 3C) increased systematically in level over time of treatment (until asymptotic) as the magnitude of the loudness response category was increased. Similarly, her repeated-measures WRS also reflect improved performance over ∼8 months of sound therapy, increasing from 40 to 72% for presentation levels judged as comfortable and from 74 to 94% for presentation levels corresponding to loud, but OK judgments. These substantial improvements in word understanding reflect the ability of 5T at treatment end to listen at comfortable and loud, but OK presentation levels 12.5 and 25 dB higher, respectively, than at pretreatment. These compelling results are shown in terms of the corresponding AI predictions via STEPS procedure21( in Fig. 3E), which reveal 5T's improved WRS can be explained by increased audibility of the NU-6 words for the comfortable and loud, but OK presentation levels. Thus, 5T was able to listen at higher and more favorable sensation levels as a consequence of her expanded DR and improved sound tolerance at the end of treatment. Accordingly, her measured WRS agree closely with her predicted AI performance (shown in Fig. 3E by the PI function). These are remarkable results. They reveal the treatment effects from the sound therapy protocol are functionally significant for 5T, as her posttreatment WRS for comfortable presentation levels now exceed her pretreatment WRS for loud, but OK presentation levels.
Thus, 5T's increased UCL judgments (Fig. 3F) and expanded DR (Fig. 3G), shown by incremental changes of 30 to 40 dB between pre- and posttreatment judgments across most of the audiometric frequency range (excluding 8,000 Hz for which the audiometer output limits constrained her judgments), are both substantive and functionally meaningful for her enhanced communication performance posttreatment. 5T's expanded DR posttreatment more than doubles her pretreatment DR, which at higher frequencies was only ∼20 to 25 dB at treatment onset. Consequently, over the frequency range from 500 to 4,000 Hz, 5T completed treatment in the normal range for both of Goldstein and Shulman's hyperacusis/sound sensitivity classification schemes.3
Subsequent to completing treatment, 5T was fitted successfully with hearing aids, bilaterally (see below). Her unaided audiometric thresholds (Fig. 3A), LDLs (Fig. 3B), categorical loudness judgments (Fig. 3C), and WRS (Fig. 3D) after 20 months of using amplification (31 months after onset of the sound therapy and counseling treatment) are shown in Fig. 3. As expected, her unaided audiometric thresholds and WRS were little changed from the end of sound therapy (11 months after treatment onset), whereas many of 5T's loudness judgments, while still revealing evidence of DR expansion measured at the end of treatment, partially declined and were measured at somewhat lower levels than had been measured for 5T at the end of the sound therapy treatment protocol. This regression of her loudness judgments toward pretreatment values suggests that amplified sound from the hearing aids alone may be insufficient for 5T for sustaining fully the broad DR achieved at the end of the sound therapy treatment with low-level broadband sound from bilateral SGs and counseling.
Participant 5T reported occasional, but unsuccessful use of analog amplification prior to entering the sound therapy protocol. She was underamplified based on her prescriptive National Acoustics Laboratory-Revised (NAL-R) gain targets,26 but she still perceived amplified sound to be too loud. Posttreatment, 5T achieved a 100% aided Hearing In Noise Test (HINT) sentence score in quiet (presentation at 50 dB HL) relative to a 0% score unaided,27 and a substantially improved aided signal-to-noise (SNR) ratio for the HINT in noise versus her unaided performance (+6 versus +14 dB, respectively). That 5T was pleased with her aided outcome posttreatment may be best documented anecdotally by the fact that she has sent recurrent Christmas cards to the study audiologist. In her cards, 5T has expressed continued thanks for her enhanced hearing aid benefit as a consequence of the sound therapy treatment.
Loudness Recruitment
Participant 20T represents a typical hearing-impaired listener with bilaterally symmetric high-frequency sensorineural hearing loss and loudness recruitment. He was 71 years old at the onset of treatment. 20T denied previous use of hearing aids, which he stated would be of limited benefit because of his tolerance problems. He noted using hearing protection rarely, only in noisy conditions. 20T reported no history of significant noise exposure. He described buzzing tinnitus in the right ear, intermittent and of uncertain origin, beginning ∼15 years prior. Tinnitus was not reported as a primary issue by 20T. At treatment onset, 20T was normal in Goldstein and Shulman's LDL-based hyperacusis scheme and severely sound sensitive based on his 25-dB DR at 8,000 Hz.3
The outcomes profile for 20T is shown in Fig. 4. His audiometric thresholds for the left ear (right ear was similar) reveal a slight to moderate sloping hearing loss (Fig. 4A), which is little changed over a treatment period of ∼4 months. His LDLs (Fig. 4B) reflect large and dramatic treatment-related incremental shifts for pure tones presented at 1,000 and 8,000 Hz and for white noise stimulation. These positive treatment effects, which were on the order of 25 to 30 dB, were rapid and mostly asymptotic for 20T after only 2 months of treatment. 20T's categorical loudness judgments (Fig. 4C), primarily for comfortable and the louder response categories, also reflect treatment-related shifts to higher levels, often occurring after the first month of treatment. These effects were evident for both the speech and warble tone stimulation and were similar for all warble tone frequencies tested. In some cases, particularly for the higher-level response categories, loudness judgments for the warble tones were continuing to increase in level even at the time 20T met the criterion for treatment termination (i.e., ≥10 dB increases in LDL or UCL categorical judgments at two consecutive follow-up measurement sessions); his incremental shifts for the higher (i.e., louder) response categories often reached or exceeded 20 dB. 20T's WRS (Fig. 4D) and AI predicted performance (Fig. 4E) were at or near the measurement ceiling level of 100% at treatment onset for both the comfortable and loud, but OK presentation levels, and these remained mostly unchanged over the full-treatment period.
Figure 4.
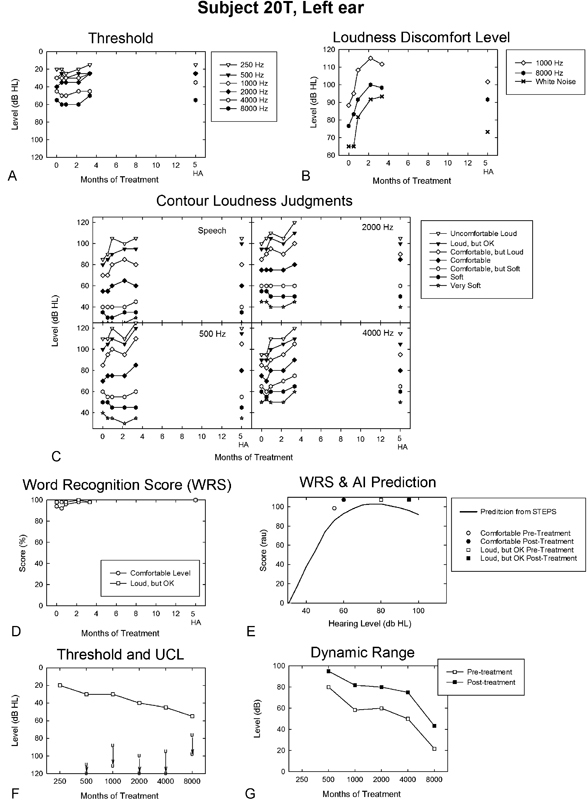
Outcomes profile for the left ear of a participant (20T) with classical loudness recruitment across the period of his full-treatment protocol and unaided subsequent to completing treatment and transitioning to HAs. The legend is otherwise the same as that described in Fig. 3. Abbreviations: AI, Articulation Index; HA, hearing aid; dB HL, decibels hearing level; RAU, rationalized arcsine units; STEPS, Speech Test Evaluation and Presentation System; UCL, uncomfortable loudness; WRS, word recognition score.
His pretreatment audiometric thresholds, along with the corresponding pre- and posttreatment LDL judgments (Fig. 4F), reveal substantive increases in sound tolerance as a result of treatment. The corresponding expansion of his DR, as a consequence of full treatment with the sound therapy protocol, was consistently on the order of 20 dB or more. His minimum DR exceeded 40 dB posttreatment at 8,000 Hz. Otherwise, most of 20T's DR values approached or exceeded 60 dB posttreatment at and below 4,000 Hz, reflecting normal sound tolerance for both of Goldstein and Shulman's classification schemes.3
20T's unaided audiometric thresholds (Fig. 4A) were unchanged over a period of 2 months subsequent to discontinuing the sound therapy protocol and moving to hearing aids, but his LDLs (Fig. 4B) revealed partial decline, appreciably more pronounced for the white noise stimulation than for either the 1,000- or 8,000-Hz tones. 20T's categorical loudness judgments (Fig. 4C), with the exception of 2,000 Hz, sustained most of the sound therapy treatment effects for the louder, higher-level response categories after transition to hearing aids. His WRS also continued to be at ceiling values after the transition to hearing aids. 20T's unaided versus aided benefit was not fully documented posttreatment because he acquired hearing aids elsewhere.
Treatment Crossover
Participant 25T offers a compelling case example of the importance of combining counseling together with low-level broadband sound therapy from SGs to optimize DR expansion. 25T was 58 years of age at treatment onset. She denied prior use or trial of hearing aids, in part, because of a bilateral tolerance problem for moderate and loud sounds beginning around 1992. 25T had not previously sought help for her condition. She described the use of earplugs rarely, only in unhealthy noise conditions, which she noted caused her problems for as long as 3 hours postexposure if she did not use sound-attenuating plugs. However, she denied any history of significant noise exposure. She rated her tolerance problem in terms of severity, distress, and effect on life as 3, 4, and 2 (on a scale of 0 to 10), respectively. 25T indicated associated problems with balance, speech understanding, and sensitivity to light and pain. She reported ringing tinnitus, bilaterally, constant in the left ear and intermittent in the right, with gradual onset also beginning in 1992. She rated her tolerance, hearing, and tinnitus problems as 2, 5, and 7, respectively, on a 0 to 10 scale prior to treatment.
25T, who was originally randomized to the combined counseling and placebo SG treatment, had a relatively uniform symmetric mild to moderate sensorineural hearing loss, which is shown for the left ear in Fig. 5A and F. In the Goldstein and Shulman3 categorization schemes she was classified as having a moderate hyperacusis problem based on her reduced pretreatment LDLs (declining from 75 to 60 dB HL with increasing frequency) and a moderate to severe hyperacusis condition based on her reduced DR (≤40 dB) pretreatment (see Fig. 5G).
Figure 5.
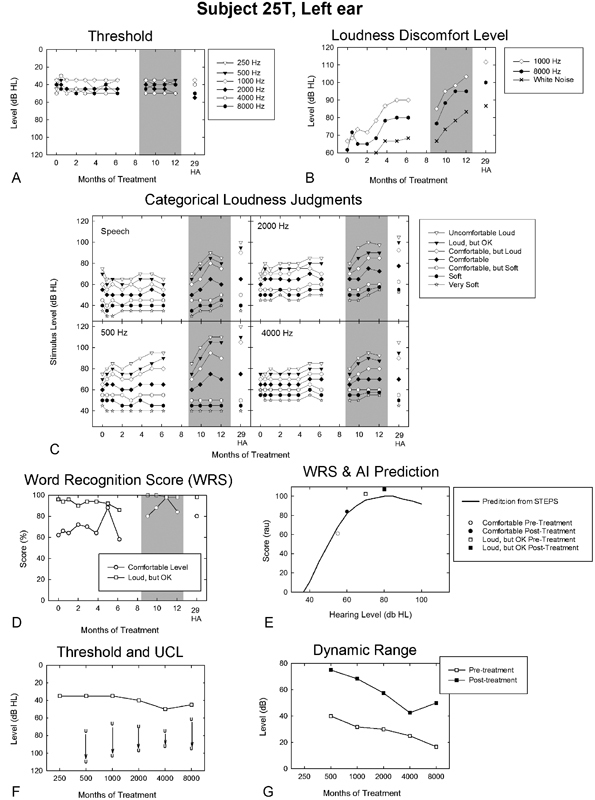
Outcomes profile for the left ear of a participant (25T) who crossed over from her initial treatment assignment of counseling combined with placebo sound generators to the full-treatment protocol (full-treatment period shaded in A to D). Posttreatment results (E to F) are those measured at completion of the full-treatment protocol. The legend is otherwise the same as that described in Fig. 3. Abbreviations: AI, Articulation Index; HA, hearing aid; dB HL, decibels hearing level; RAU, rationalized arcsine units; STEPS, Speech Test Evaluation and Presentation System; UCL, uncomfortable loudness; WRS, word recognition score.
During the intervention period of 25T's original assignment, her audiometric thresholds were unchanged; however, she achieved some positive treatment effect as evidenced by her increased LDLs (Fig. 5B). These effects were most notable at 1,000 Hz for which her LDLs increased by 25 dB over the 6-month treatment period. Similar positive effects of the intervention were apparent in her categorical loudness judgments for warble tones (Fig. 5C). These treatment effects were most prominent for her categorical UCL judgments at 500 Hz, which increased in level by 20 dB over the treatment period. Less change was generally observed in her judgments for the other loudness response categories at 500 Hz or for the other warble tone test frequencies. 25T was one of only two participants assigned to counseling and placebo SGs to meet the “graduation” criterion for treatment efficacy (see Formby et al1). It is noteworthy, however, that little or no change (or even a subtle negative change) was evident in her categorical loudness judgments for the spondee speech stimuli (Fig. 5C). 25T's WRS (Fig. 5D) were consistent with the absence of a treatment effect in her categorical loudness judgments for speech stimuli; her WRS either were unchanged or declined slightly (excluding one measurement session for the comfortable presentation level at 5 months into treatment) at both the comfortable and loud, but OK speech presentation levels over the initial 6-month treatment period.
25T was given an opportunity to receive the full treatment after completing her initial treatment assignment. She agreed to crossover to full treatment and was recounseled and fitted with conventional SGs for sound therapy. Her full-treatment effects after crossover are denoted by the shaded portions in Fig. 5A to D. Her audiometric thresholds (Fig. 5A), beginning with their measurement ∼8 months after the onset of her original treatment assignment, remained constant during the 4-month full-treatment period. These values were similar to her audiometric thresholds measured over the course of her original treatment assignment. However, her LDL (Fig. 5B) and categorical loudness judgments (Fig. 5C) systematically and rapidly increased for all forms of stimulation, including the spondee speech stimuli, over the initial 2-month period of the full-treatment protocol. Afterwards, her loudness judgments were mostly asymptotic over the final 2 months of the full-treatment protocol. Twenty decibel increases in the LDL and categorical loudness judgments for the louder, higher-level response categories for both the warble tone and speech stimuli were routinely measured for 25T during full treatment; incremental shifts in her categorical loudness judgments for the 500-Hz warble tones reached as much as 35 dB. These highly positive full-treatment effects appear to be largely additive to those (if any) achieved during the initial treatment assignment. This evidence of additivity is best seen in Fig. 5B when comparing 25T's final LDL judgments in her initial treatment assignment and her corresponding LDL judgments at the onset of the full-treatment protocol.
The full-treatment increases in the levels of the categorical loudness judgments for the spondee speech stimuli appear to be represented in corresponding improvements in 25T's WRS for the comfortable and the loud, but OK speech presentation levels. Her WRS for the comfortable presentation level exceeded 80%, and the corresponding measures for the loud, but OK presentation level were at measurement ceiling, near 100%, before and after full treatment. By comparison, her initial-treatment WRS, measured pretreatment and at treatment end, were ∼20% lower for the comfortable presentation level. This significant improvement in 25T's comfortable WRS after full treatment is consistent with her enhanced ability to listen more comfortably at higher speech presentation levels. Her measured WRS and AI predicted performance, shown by the PI function in Fig. 5E, reveal that 25T was able to listen at speech presentation levels that were ∼10 dB higher at the end of the full-treatment protocol than those levels that were judged as comfortable prior to onset of her initial treatment assignment. Note also that her DR, shown in Fig. 5G, was substantially expanded across frequency after full treatment relative to her original baseline (pre–initial treatment) DR values. That is, her DR after full treatment was expanded by 20 dB at 4,000 Hz and by 35 to 40 dB at 500 and 1,000 Hz (Fig. 5G). This substantial expansion of her DR across frequency, giving rise to a DR typically greater than 50 dB following full treatment, appears to have contributed directly to her improved speech understanding. This substantially larger DR after completing the full-treatment protocol enabled 25T to benefit from and listen more comfortably, with greater ease, across a much wider range of dynamically changing speech levels (often exceeding 50 dB in normal conversational speech27). By comparison, her baseline DR values were mostly on the order of 20 to 30 dB prior to the start of her original treatment assignment. Thus, her full-treatment effects were not only substantial, but also functionally significant for improved communication. Moreover, her full-treatment effects were either sustained or even enhanced after successful transition to hearing aids (measured 29 months after the original treatment assignment and 20 months after onset of the full treatment).
Based on the positive treatment effects described previously, 25T attained clinically significant aided posttreatment benefit, which was not possible to achieve pretreatment. Her HINT SNR improved from 0 (unaided) to −4 dB (aided),28 and her satisfaction with amplification in daily life (SADL) results reflected excellent aided satisfaction (global score 5.6).29 25T indicated in her Glasgow hearing aid benefit profile (GHABP) responses that the “hearing aid is a great help” in all basic listening conditions (i.e., television, quiet, background noise, and group conversations),30 whereas unaided, she had “great difficulty” in these basic conditions. 25T also reported regular daily use of her new amplification posttreatment.
Loudness Adaptation
At trial onset, 10T was among our most motivated participants to use hearing aids. He was a 73-year-old salesman who reported no prior use of hearing aids, despite a symmetric sloping slight to profound sensorineural hearing loss. He described a gradually and progressively worse sound tolerance problem, bilaterally. He denied pain or untoward discomfort associated with moderate and loud sounds, and reported no primary history of using hearing protection. He rated his tolerance problem in terms of severity, distress, and effect on life as 7, 9, and 9, respectively (on a scale of 0 to 10). He noted primary problems in social settings for most sounds, including the understanding of speech. He also noted increased sensitivity to light. He denied significant history of either noise exposure or tinnitus, but related surgical procedures for back problems. He ranked his issues with tinnitus, sound tolerance, and hearing loss as 0, 8, and 10, respectively (on a scale of 0 to 10). In Goldstein and Shulman's schemes,3 10T had a mild low-frequency (≤1,000 Hz) loudness-based hyperacusis problem in terms of his LDL/UCL judgments, but a severe (if not profound) sound sensitivity problem across frequency (excluding 500 Hz) based on his DR values.
10T was initially assigned to counseling and placebo SGs for 6 months, but received no benefit from the treatment. Upon completion of his initial assignment, 10T agreed to crossover to full treatment. He was fitted with conventional SGs, but was unable to hear the “seashell-like” sound therapy and was refitted with high-powered SGs. 10T was recounseled and followed for the full-treatment period across 6 months while using the power SGs.
10T's outcomes profile for the left ear is shown in Fig. 6 across the full-treatment period. Suffice to say, what is most compelling about his profile is the lack of change in virtually any measure shown in Fig. 6, even after transitioning to hearing aids, which he tried satisfactorily (notwithstanding the absence of an apparent treatment benefit). His surprising failure to benefit from full treatment with power SGs led us to question him about his treatment experience. Participant 10T reported he was unable to hear the sound therapy noise from his SGs except when the devices were initially activated. That is, he described rapid loudness adaptation to the output of the SGs, which operationally was like the decay operation of the ineffectual placebo SGs in Formby et al.1 Loudness adaptation to proprietary sound therapy has been reported elsewhere by Chang and Zeng in the treatment of tinnitus patients,31 but we are not aware that loudness adaptation has been described previously for applications of low-level broadband sound therapy of the kind used here or that implemented for treatment of tinnitus and hyperacusis patients with TRT.5 Perhaps 10T's subjective observation of apparent loudness adaptation to the SG outputs explains one or both of the full-treatment participant failures reported in Formby et al.1 These latter participants may have suffered a similar adaptation problem that went unrecognized and unreported.
Figure 6.
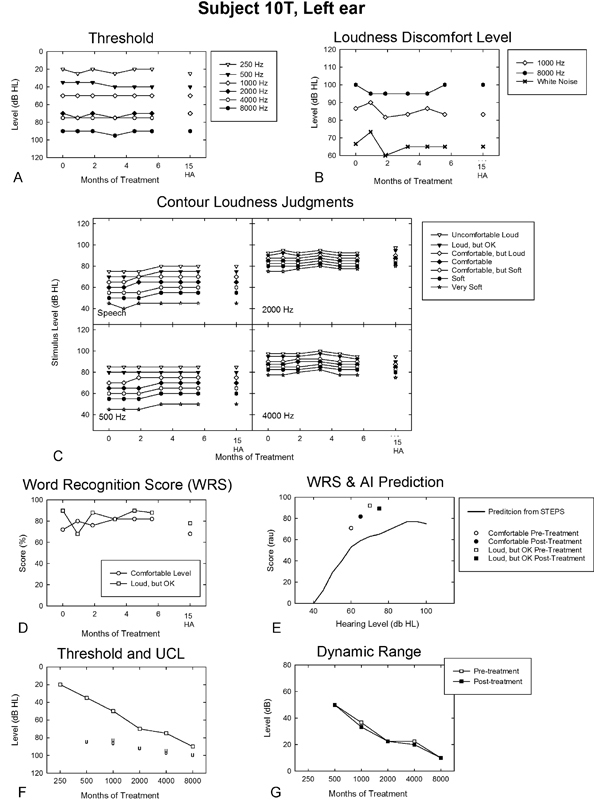
Outcomes profile for the left ear of a participant (10T) in the full-treatment protocol who described pronounced loudness adaptation to sound therapy after initial activation of his power sound generators. The legend is otherwise the same as that described in Fig. 3. Abbreviations: AI, Articulation Index; HA, hearing aid; dB HL, decibels hearing level; RAU, rationalized arcsine units; STEPS, Speech Test Evaluation and Presentation System; UCL, uncomfortable loudness; WRS, word recognition score.
DISCUSSION
The selected case examples presented in this article highlight clinically significant virtues and limitations of a promising interventional tool for facilitating auditory DR expansion among hearing-impaired individuals with sensorineural hearing losses typical of those seen in traditional audiological and hearing aid practices. Our first two case examples, those of 5T and 20T, showcased the fact that the full-treatment approach, implemented with structured counseling (targeted for hearing-impaired persons with reduced DRs) and low-level sound therapy, was efficacious for expanding the DRs of individuals with primary hyperacusis and classical loudness recruitment, respectively. The resulting expanded DRs for both cases enabled them to transition to hearing aids. The latter example is perhaps more interesting than the former inasmuch as we have previously documented applications of sound therapy in TRT leading to resolution of hyperacusis and the successful transition to hearing aids by individuals with hearing impairments.24 The latter recruitment case is compelling because it reveals for the first time that this promising sound therapy approach may be broadly applicable for treating sensorineural hearing loss. That is, our approach is not limited solely to individuals with dramatically reduced LDLs, associated distress, and chronic sound tolerance complaints, which characterize primary hyperacusis; rather, this promising tool also can be used efficaciously to extend the DRs of sensorineurally impaired individuals with LDLs in the normal listening range typical of classical loudness recruitment.
Compare and contrast the loudness-growth functions for 5T (top panel) and 20T (bottom panel) shown in Fig. 7 pre- and posttreatment. The pretreatment loudness-growth function measured for 5T at 4,000 Hz clearly depicts her hyperacusis problem, which is characterized by very steep loudness growth over a limited DR of 22.5 dB and by loudness judgments well below those normally judged to be comfortable (shown for comparison by the solid-growth function) at higher sound levels. Posttreatment her loudness-growth function is shifted to higher levels for all loudness categories, even for very soft sounds. This shift is most prominent at higher presentation levels for the louder response categories for which the expansion of her DR is as much as 52.5 dB for her UCL judgments. Her posttreatment loudness-growth function therefore is much shallower and extends to appreciably higher levels, virtually in parallel, above the normal loudness-growth function. This dramatic increase in her DR and change in loudness growth, in parallel with the normal growth function, reflects an almost linear (upwardly shifted) “normalized” loudness-growth response for 5T posttreatment. This large increase in her DR accounts for 5T's subsequent success in transitioning comfortably to hearing aids posttreatment.
Figure 7.
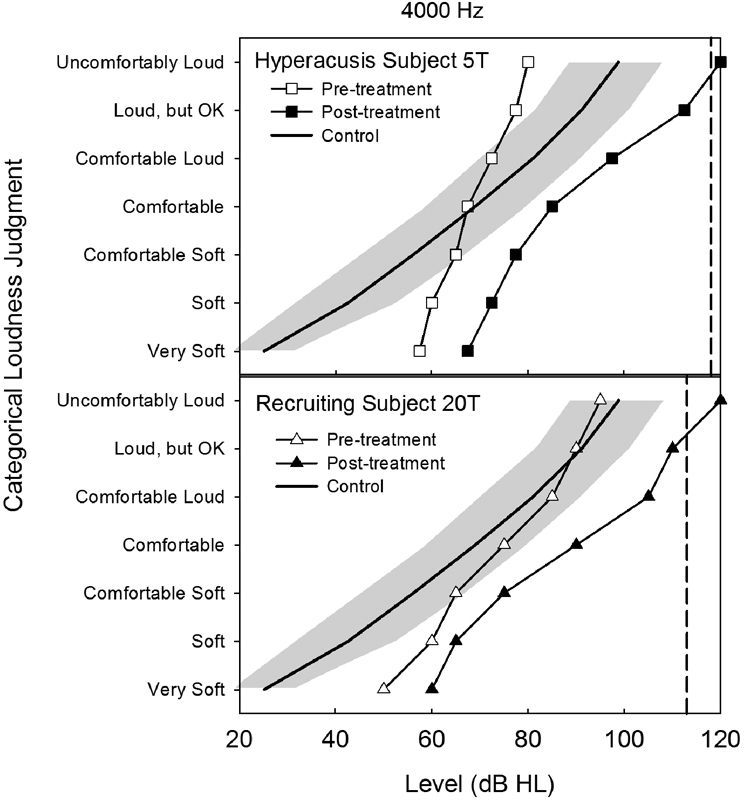
Pre- and posttreatment loudness-growth functions are shown in the top and bottom panels, respectively, for the 4,000-Hz warble tone condition for hyperacusis participant 5T (outcomes profile shown in Fig. 3) and for loudness recruitment participant 20T (outcomes profile shown in Fig. 4). The dashed vertical line in each panel denotes the predicted LDLcrit for normal sound tolerance for that participant based on a mathematical model (see Eq. 1) described by Hawley et al.23 Posttreatment LDLs for both of these participants exceeded their normal sound tolerance predictions at 4,000 Hz. The average control function (±1 standard deviation denoted by the shaded region) represents the mean loudness-growth response measured at 4,000 Hz from 10 typical normal-hearing listeners described in this issue by Hawley et al.56 Abbreviation: HL, hearing level.
The pretherapy loudness-growth function measured for 20T typifies classical loudness recruitment.11 His categorical loudness judgments, shown in the bottom panel of Fig. 7, reveal rapid loudness growth for softer sounds near threshold and normal loudness growth for higher level sounds that he judged to be uncomfortably loud. Thus, because his judgments of uncomfortably loud sounds are similar to those of typical listeners, 20T's reduced DR is primarily, if not solely, the result of his elevated hearing thresholds as a consequence of the sensorineural impairment. His posttreatment loudness-growth function reveals a much shallower growth pattern and clear separation from his pretreatment growth function. This separation between his pre- and posttreatment functions increases systematically at and above the comfortable loudness response category such that the difference in loudness growth is greatest between the two functions at the highest presentation levels. Accordingly, his DR is expanded most for the UCL judgments, as measured by an increase of 25 dB between his pre- and posttreatment loudness judgments. Subsequently, 20T also transitioned into hearing aids upon completing his treatment.
With the exception of 10T, all of the case examples highlighted in this article revealed the same positive treatment effects after completing the full sound therapy protocol. That is, in each case their DRs were expanded as a consequence of an enhanced ability to listen more comfortably and/or to tolerate, with greater ease, sounds of higher level. These clinically significant treatment effects typically increased systematically as a function of increasing loudness across the corresponding response categories and were most prominent at higher sound levels for which the expanded DR was largest posttreatment. As a consequence of DR expansion and improved sound tolerance, each case demonstrated functional benefits in speech understanding as measured by improved spondee reception and/or WRS posttreatment. In turn, each case was then able to transition comfortably to hearing aids. (Even 10T made a satisfactory transition posttreatment despite little or no evidence of DR expansion.)
Participant 10T's treatment failure provides insight for selecting candidates for treatment success. His observation that he was unable to hear the low-level broadband sound therapy, beyond the brief period initially following activation of his power SGs, suggests that we should be evaluating loudness adaptation as an inclusion/exclusion criterion for prospective candidates prior to study entry in future sound therapy research. Likewise, inclusion of some measure of tone decay or loudness adaption should be part of the evaluation protocol for clinical applications of sound therapy to ensure the low-level stimulation remains audible and, therefore, offers the possibility for an effective therapy.
Small masked-audibility effects from the broadband sound therapy may partially offset treatment-related DR expansion (see Formby et al1). However, the treatment-related incremental DR expansion reported here for 5T, 20T, and 25T, measured uniformly across most frequencies, dwarfed the small masked-threshold shifts they experienced from the sound therapy (typically ≤5 dB except for the ear-canal resonance frequencies for which masked-threshold shift was on the order of ∼10 dB). However, even small amounts of masked-threshold shift represent an added handicap for a hearing-impaired listener struggling to hear in the face of elevated hearing thresholds, especially prior to beginning hearing aid usage. Accordingly, it will be important in future research to determine whether ongoing or intermittent dosing of the sound therapy in some form is necessary to sustain treatment-induced DR expansion posttreatment. Ideally, ongoing sound therapy would not be needed after the start of aided listening. However, these case examples, excluding that of 25T, indicate otherwise as the treatment effects appear to be only partially sustained after sound therapy is ended and replaced with aided environmental sound alone. Thus, a combination of hearing aid and SG devices, enabling the application of an appropriate dosing protocol to sustain the efficacious sound therapy, may ultimately be needed to maintain a broad DR and optimum hearing aid benefit for the aided listener with a limited DR. If so, then the fitting audiologist will play a new and significant role in the future in treating and prescribing a monitored and controlled sound therapy treatment for aided hearing loss.
Our sound therapy protocol appears to be effective for expanding the DRs for hearing-impaired persons with hyperacusis and for those with loudness recruitment, notwithstanding the fact that the two phenomena are clearly different problems. In recent years the relation between loudness recruitment and primary hyperacusis, and the origins of these perceptual phenomena, have become increasingly muddled.32 33 There is growing evidence that loudness recruitment originates, or at least manifests, at anatomical sites central to the auditory nerve,34 35 36 37 and most current models of hyperacusis assume central origins,33 38 39 but both may have peripheral trigger sources as noted later. Diminished cochlear compression processes, which are commonly associated with the phenomenon of loudness recruitment, are not represented in auditory nerve responses of cochlear-damaged animal models.32 This latter finding suggests a problem of central origins. However, persons with hyperacusis and normal audiometric thresholds also exhibit diminished cochlear compression processes,40 notwithstanding that the condition tends to be bilateral and to affect loudness perception relatively uniformly across frequency, indicative of a centrally mediated auditory problem.8 9 Furthermore, primary hyperacusis may occur either with or without measurable hearing loss,4 9 and may be triggered by peripheral auditory insults of the kind that often give rise to loudness recruitment (e.g., exposures to ototoxic medications and episodes of noise exposure or acoustic shock).41 42 43 44 Loudness recruitment is characteristically associated with hearing loss of cochlear origin, reflecting frequency-specific localized cochlear damage.11 32 Moreover, loudness recruitment is independent of mood,13 which is regarded by some as a hallmark of hyperacusis.10 Hazell and Sheldrake suggested hyperacusis may be a consequence of untoward efferent effects upon the cochlear processes, also perhaps triggered by peripheral insults.2 Additionally, there is evidence to suggest that peripheral and central auditory processes may, together, give rise to a mixed deficit for some sound intolerant conditions.10 45 46 Thus, although primary hyperacusis appears to be predominately a central auditory problem,24 its triggers may often be traced to influences on peripheral auditory processes that give rise to cochlear damage and loudness recruitment.
Regardless of the nature and origins of these underlying problems, a growing body of evidence from multiple sources suggests that adaptive and plastic processes, involving auditory pathway gain, play a key role in the mechanism and success of the sound therapy treatment. Low-level sound therapy protocols for treatment of primary hyperacusis and related sound tolerance problems have as their motivation the goal of resetting abnormally elevated auditory gain within the (central) auditory pathways.2 10 24 45 47 48 49 50 51 Specifically, the goal of sound therapy is to downregulate the suprathreshold sensitivity of the auditory pathways to restore a “normal” set point for the listener. This treatment strategy is consistent with the theoretical notion of homeostatic plasticity.38 52 Homeostatic plasticity assumes that targeted neuronal processes have the capacity to up- or downregulate their activity with respect to the larger neuronal network activity within the central nervous system. This plastic neuronal activity achieves equilibrium between opposing excitatory and inhibitory mechanisms through an imposed change in the targeted neuronal activity.53 Thus, in theory, a viable model of the treatment mechanism underlying the empirically measured incremental shifts in the loudness judgments is one operating through equilibrating compensatory changes in neuronal excitation induced over days to weeks by chronic exposure to the low-level broadband noise stimulation from the sound therapy.
The success of sound therapy in driving down auditory pathway gain in Formby et al1 and in the case examples in this article is quantifiable in the large, relatively uniform, bilateral, treatment-induced incremental shifts in the LDLs and loudness-growth functions. The resulting values approached and, in some cases, appreciably exceeded typical LDLs/UCLs of ∼100-dB HL.5 8 14 This result suggests the gain setting may be driven downward past the normal set point for most persons. Thus, in practice, this set point and the dynamics of recalibrating the adaptive plastic gain processes, ostensibly of central auditory origins,22 can be monitored, controlled, and manipulated for clinical purposes with judicious use of sound therapy. Fortunately, the current intensive basic science interest in plastic auditory gain control processes (see reviews by Robinson and McAlpine,54 Auerbach et al,33 Knipper et al,55 Brotherton et al50) and the growing recognition of their clinical relevance, as described previously, portends well for significant breakthroughs in the scientific understanding of the underlying mechanisms and related interventions in future research.
CONCLUSIONS
The selected case examples in this article highlight the clinical viability of a low-level broadband sound therapy approach, requiring specialized counseling, for treating individuals with reduced DRs (owing to their elevated hearing thresholds as a consequence of sensorineural hearing loss and/or reduced sound tolerance as measured by abnormally low LDLs or diminished judgments of uncomfortable loudness). These individuals, after successful treatment, can achieve large and functionally meaningful increases (>30 dB) in their DRs, enabling them anew to make a comfortable transition to amplification and/or achieve improved hearing aid benefit. This promising treatment approach appears to be effective for most hearing-impaired persons with sensorineural hearing losses, including persons with primary hyperacusis and those with classical loudness recruitment. The enormous potential for the mastery and control of plastic and adaptive processes, which appear to underlie treatment success, offers audiologists a unique opportunity for the future—in ways not previously imagined with strategies never before envisioned—to devise new and improved clinical protocols and interventions to enhance clinical outcomes and treatment benefit for large numbers of persons with sensorineural hearing losses.
ACKNOWLEDGMENTS
This research was supported by the Public Health Service under a parent grant award (R01DC04678) from NIDCD. We thank Dr. David Wark of the University of Memphis, Memphis, TN for analyzing our data using the STEPS program. We thank Karen Tucker for editorial assistance and General Hearing Instruments, Inc. for providing the sound therapy treatments.
References
- 1.Formby C, Hawley M L, Sherlock L P. et al. A sound therapy-based intervention to expand the auditory dynamic range for loudness among persons with sensorineural hearing losses: a randomized placebo-controlled clinical trial. Semin Hear. 2015;36(2):77–110. doi: 10.1055/s-0035-1546958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hazell J WP Sheldrake J B Hyperacusis and tinnitus Amsterdam, Netherlands/New York, NY: Kugler Publications; 1992: 245–248. [Google Scholar]
- 3.Goldstein B, Shulman A. Tinnitus—hyperacusis and the loudness discomfort level test—a preliminary report. Int Tinnitus J. 1996;2:83–89. [PubMed] [Google Scholar]
- 4.Tyler R S, Pienkowski M, Roncancio E R. et al. A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol. 2014;23(4):402–419. doi: 10.1044/2014_AJA-14-0010. [DOI] [PubMed] [Google Scholar]
- 5.Jastreboff P J, Jastreboff M M. Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol. 2000;11(3):162–177. [PubMed] [Google Scholar]
- 6.Schecklmann M Landgrebe M Langguth B; TRI Database Study Group Phenotypic characteristics of hyperacusis in tinnitus PLoS One 201491e86944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound—questionnaire data, audiometry and classification. Scand Audiol. 1999;28(4):219–230. doi: 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- 8.Formby C, Gold S L, Keaser M L. et al. Secondary benefits from Tinnitus Retraining Therapy: clinically significant increases in loudness discomfort level and expansion of the auditory dynamic range. Semin Hear. 2007;28:227–260. [Google Scholar]
- 9.Sheldrake J, Diehl P U, Schaette R. Audiometric characteristics of hyperacusis patients. Front Neurol. 2015;6:105. doi: 10.3389/fneur.2015.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baguley D M, Andersson G. San Diego, CA: Plural Publishing Inc.; 2007. Hyperacusis: Mechanisms, Diagnosis, and Therapies. [Google Scholar]
- 11.Fowler E P. The recruitment of loudness phenomenon. Laryngoscope. 1950;60(7):680–695. doi: 10.1288/00005537-195007000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Buus S, Florentine M. Growth of loudness in listeners with cochlear hearing losses: recruitment reconsidered. J Assoc Res Otolaryngol. 2002;3(2):120–139. doi: 10.1007/s101620010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baguley D M. Hyperacusis. J R Soc Med. 2003;96(12):582–585. doi: 10.1258/jrsm.96.12.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherlock L P, Formby C. Estimates of loudness, loudness discomfort, and the auditory dynamic range: normative estimates, comparison of procedures, and test-retest reliability. J Am Acad Audiol. 2005;16(2):85–100. doi: 10.3766/jaaa.16.2.4. [DOI] [PubMed] [Google Scholar]
- 15.Davis H, Hudgins C V, Marquis R J. et al. The selection of hearing aids. Laryngoscope. 1946;56:135–163. [PubMed] [Google Scholar]
- 16.Silverman S R. Tolerance for pure tones and speech in normal and defective hearing. Ann Otol Rhinol Laryngol. 1947;56(3):658–677. doi: 10.1177/000348944705600310. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz H D. Loudness discomfort level modification. J Speech Hear Res. 1969;12(4):807–817. doi: 10.1044/jshr.1204.807. [DOI] [PubMed] [Google Scholar]
- 18.Gold S L, Formby C. Structured counseling for dynamic range expansion. Semin Hear. 2017;38(1):113–127. doi: 10.1055/s-0037-1598068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox R M, Alexander G C, Taylor I M, Gray G A. The contour test of loudness perception. Ear Hear. 1997;18(5):388–400. doi: 10.1097/00003446-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Tillman T W Carhart R An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66–55 [technical report]. SAM-TR USAF School of Aerospace Medicine; 19661–12. [DOI] [PubMed] [Google Scholar]
- 21.Studebaker G A, Gray G A, Branch W E. Prediction and statistical evaluation of speech recognition test scores. J Am Acad Audiol. 1999;10(7):355–370. [PubMed] [Google Scholar]
- 22.Formby C, Korczak P A, Sherlock L P, Gold S L, Hawley M. Auditory brainstem and middle latency responses measured pre- and posttreatment for hyperacusic hearing-impaired persons successfully treated to improve sound tolerance and expand the dynamic range for loudness: case evidence. Semin Hear. 2017;38(1):69–91. doi: 10.1055/s-0037-1598066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley M L, Keaser M L, Formby C. Predicting hyperacusis in tinnitus patients. Semin Hear. 2007;28:261–275. [Google Scholar]
- 24.Formby C, Gold S L. Modification of loudness discomfort levels: evidence for adaptive chronic gain and its clinical relevance. Semin Hear. 2002;23:21–34. [Google Scholar]
- 25.Studebaker G AA. A “rationalized” arcsine transform. J Speech Hear Res. 1985;28(3):455–462. doi: 10.1044/jshr.2803.455. [DOI] [PubMed] [Google Scholar]
- 26.Dillon H. New York, NY: Thieme; 2012. Hearing Aids. 2nd ed. [Google Scholar]
- 27.Zeng F G Grant G Niparko J et al. Speech dynamic range and its effect on cochlear implant performance J Acoust Soc Am 2002111(1 Pt 1):377–386. [DOI] [PubMed] [Google Scholar]
- 28.Nilsson M, Soli S D, Sullivan J A. Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise. J Acoust Soc Am. 1994;95(2):1085–1099. doi: 10.1121/1.408469. [DOI] [PubMed] [Google Scholar]
- 29.Cox R M, Alexander G C. Measuring satisfaction with amplification in daily life: the SADL scale. Ear Hear. 1999;20(4):306–320. doi: 10.1097/00003446-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Gatehouse S. Glasgow hearing aid benefit profile: derivation and validation of a client-centered outcome measure for hearing aid services. J Am Acad Audiol. 1999;10:80–103. [Google Scholar]
- 31.Chang J E, Zeng F G. Tinnitus suppression by electric stimulation of the auditory nerve. Front Syst Neurosci. 2012;6:19. doi: 10.3389/fnsys.2012.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joris P X. Recruitment of neurons and loudness. Commentary on “Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment” by Cai et al. J. Assoc. Res. Otolaryngol. DOI: 10.1007/s10162-008-0142-y. J Assoc Res Otolaryngol. 2009;10(1):1–4. doi: 10.1007/s10162-009-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auerbach B D, Rodrigues P V, Salvi R J. Central gain control in tinnitus and hyperacusis. Front Neurol. 2014;5:206. doi: 10.3389/fneur.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips D P. Stimulus intensity and loudness recruitment: neural correlates. J Acoust Soc Am. 1987;82(1):1–12. doi: 10.1121/1.395547. [DOI] [PubMed] [Google Scholar]
- 35.Heinz M G, Issa J B, Young E D. Auditory-nerve rate responses are inconsistent with common hypotheses for the neural correlates of loudness recruitment. J Assoc Res Otolaryngol. 2005;6(2):91–105. doi: 10.1007/s10162-004-5043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinz M G, Young E D. Response growth with sound level in auditory-nerve fibers after noise-induced hearing loss. J Neurophysiol. 2004;91(2):784–795. doi: 10.1152/jn.00776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai S, Ma W L, Young E D. Encoding intensity in ventral cochlear nucleus following acoustic trauma: implications for loudness recruitment. J Assoc Res Otolaryngol. 2009;10(1):5–22. doi: 10.1007/s10162-008-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diehl P U, Schaette R. Abnormal auditory gain in hyperacusis: investigation with a computational model. Front Neurol. 2015;6:157. doi: 10.3389/fneur.2015.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng F G. An active loudness model suggesting tinnitus as increased central noise and hyperacusis as increased nonlinear gain. Hear Res. 2013;295:172–179. doi: 10.1016/j.heares.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartnik G, Hawley M L, Rogowski M. et al. Distortion product otoacoustic emission levels and input/output growth functions in normal-hearing individuals with tinnitus and/or hyperacusis. Semin Hear. 2007;28:303–318. [Google Scholar]
- 41.Qiu C, Salvi R, Ding D, Burkard R. Inner hair cell loss leads to enhanced response amplitudes in auditory cortex of unanesthetized chinchillas: evidence for increased system gain. Hear Res. 2000;139(1–2):153–171. doi: 10.1016/s0378-5955(99)00171-9. [DOI] [PubMed] [Google Scholar]
- 42.Salvi R J, Henderson D, Fiorino F, New York, NY: Thieme; 1996. Auditory System Plasticity and Regeneration. [Google Scholar]
- 43.Westcott M. Acoustic shock injury (ASI) Acta Otolaryngol Suppl. 2006;(556):54–58. doi: 10.1080/03655230600895531. [DOI] [PubMed] [Google Scholar]
- 44.McFerran D J, Baguley D M. Acoustic shock. J Laryngol Otol. 2007;121(4):301–305. doi: 10.1017/S0022215107006111. [DOI] [PubMed] [Google Scholar]
- 45.Jastreboff P J, Hazell J WP. Cambridge, UK: Cambridge University Press; 2004. Tinnitus Retraining Therapy: Implementing the Neurophysiological Model. [Google Scholar]
- 46.Jastreboff P J, Jastreboff M M. Ontario, Canada: Hamilton Decker; 2004. Decreased sound tolerance; pp. 8–15. [Google Scholar]
- 47.Hazell J WP. London, UK: Churchill Livingston; 1987. Tinnitus masking therapy; pp. 96–117. [Google Scholar]
- 48.Jastreboff P J, Hazell J W. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 1993;27(1):7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 49.Noreña A J, Chery-Croze S. Enriched acoustic environment rescales auditory sensitivity. Neuroreport. 2007;18(12):1251–1255. doi: 10.1097/WNR.0b013e3282202c35. [DOI] [PubMed] [Google Scholar]
- 50.Brotherton H, Plack C J, Maslin M, Schaette R, Munro K J. Pump up the volume: could excessive neural gain explain tinnitus and hyperacusis? Audiol Neurootol. 2015;20(4):273–282. doi: 10.1159/000430459. [DOI] [PubMed] [Google Scholar]
- 51.Pienkowski M, Tyler R S, Roncancio E R. et al. A review of hyperacusis and future directions: part II. Measurement, mechanisms, and treatment. Am J Audiol. 2014;23(4):420–436. doi: 10.1044/2014_AJA-13-0037. [DOI] [PubMed] [Google Scholar]
- 52.Schaette R Tinnitus Related Hyperactivity through Homeostatic Plasticity in the Auditory Pathway Humboldt-Universität Berlin; 2007 [Google Scholar]
- 53.Turrigiano G G, Leslie K R, Desai N S, Rutherford L C, Nelson S B. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391(6670):892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- 54.Robinson B L, McAlpine D. Gain control mechanisms in the auditory pathway. Curr Opin Neurobiol. 2009;19(4):402–407. doi: 10.1016/j.conb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Knipper M, Van Dijk P, Nunes I, Rüttiger L, Zimmermann U. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. 2013;111:17–33. doi: 10.1016/j.pneurobio.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 56.Hawley M L, Sherlock L P, Formby C. Intra- and intersubject variability in audiometric measures and loudness judgments in older listeners with normal hearing. Semin Hear. 2017;38(1):3–26. doi: 10.1055/s-0037-1598063. [DOI] [PMC free article] [PubMed] [Google Scholar]


