Abstract
Background
Atrial fibrillation (AF) affects more than 33 million individuals worldwide and increases risks of stroke, heart failure, and death. The CHARGE-AF risk score was developed to predict incident AF in three American cohorts and it was validated in two European cohorts. The CHA2DS2-VASc risk score was derived to predict risk of stroke, peripheral embolism, and pulmonary embolism in individuals with AF, but it has been increasingly used for AF risk prediction. We compared CHARGE-AF risk score versus CHA2DS2-VASc risk score for incident AF risk in a community-based cohort.
Methods and Results
We studied Framingham Heart Study participants aged 46 to 94 years without prevalent AF and with complete covariates. We predicted AF risk using Fine-Gray proportional sub-distribution hazards regression. We used the Wald χ2 statistic for model fit, C-statistic for discrimination, and Hosmer-Lemeshow (HL) χ2 statistic for calibration. We included 9722 observations (mean age 63.9 ± 10.6 years, 56% women) from 4548 unique individuals: 752 (16.5%) developed incident AF and 793 (17.4%) died. The mean CHARGE-AF score was 12.0 ± 1.2 and the sub-distribution hazard ratio (sHR) for AF per unit increment was 2.15 (95% CI, 99–131%; P < .0001). The mean CHA2DS2-VASc score was 2.0 ± 1.5 and the sHR for AF per unit increment was 1.43 (95% CI, 37%-51%; P < .0001). The CHARGE-AF model had better fit than CHA2DS2-VASc (Wald χ2 = 403 vs 209, both with 1 df), improved discrimination (C-statistic = 0.75, 95% CI, 0.73–0.76 vs C-statistic = 0.71, 95% CI, 0.69–0.73), and better calibration (HL χ2 = 5.6, P = .69 vs HL χ2 = 28.5, P < .0001).
Conclusion
The CHARGE-AF risk score performed better than the CHA2DS2-VASc risk score at predicting AF in a community-based cohort.
Atrial fibrillation (AF) is a cardiac arrhythmia, present in over 33 million individuals around the world that may have severe complications such as stroke, heart failure, dementia, and death.1–5 Multiple risk factors for AF have been identified.6 Schnabel and colleagues developed the Framingham AF risk score,7 which was later validated in two community-based cohorts: the Age Gene/Environment Susceptibility Study (AGES) and the Cardiovascular Health Study.8 A similar risk score was developed in the Atherosclerosis Risk in Communities study with comparable predictive ability.9 To increase generalizability, these studies combined their efforts in the CHARGE-AF Consortium. The CHARGE-AF investigators developed a new AF risk score derived from three community-based studies (Framingham Heart Study (FHS), Cardiovascular Health Study, and Atherosclerosis Risk in Communities Study), followed by validation in two large European community-based studies (AGES and Rotterdam Study). Derivation and validation cohorts included more than 26,000 individuals of European, European-American, and African-American ancestry, 1771 of whom developed incident AF.10 The CHARGE-AF risk score provided increased discrimination compared to the FHS AF risk score and the generalizability was wider, due to the inclusion of more diverse cohorts. Recently, the CHARGE-AF risk score was validated in >45,000 Hispanics, African-Americans, and non-Hispanic whites in an inner-city North-American population,11 and in a recalibrated version in the European population-based EPIC Norfolk study, with more than 24,000 individuals.12
The CHADS2 risk score was developed in 2001 as a tool to predict risk of stroke in individuals diagnosed with AF.13 A refined version of this score, with the acronym CHA2DS2-VASc, was validated by Lip and colleagues in 2010.14 Because of its simplicity, and better performance than CHADS2, the CHA2DS2-VASc risk score has been endorsed by guidelines to risk stratify individuals for clinical decisions regarding anticoagulation in AF.15
Recently, the CHADS2 and CHA2DS2-VASc score has been used to predict risk of AF in different clinical subgroups.16–22 However, the CHA2DS2-VASc score has not been validated to predict AF. The substantial burden of both AF and its complications motivates the development of strategies for the early detection and prevention of AF. However, prevention of the arrhythmia itself has not been systematically implemented in clinical practice thus far. To target individuals for randomized clinical trials to prevent AF, a robust risk prediction score for AF is essential. We hypothesized that the CHARGE-AF risk score would have better model performance in AF prediction in comparison with the CHA2DS2-VASc score in a community-based cohort.
Methods
Study sample
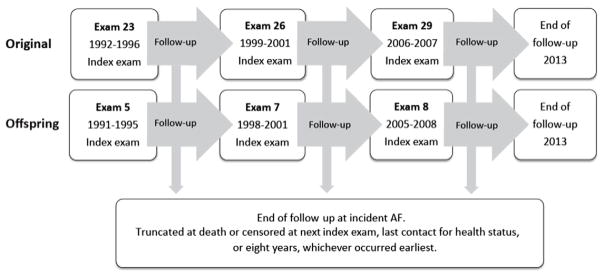
FHS was initiated in 1948 as a community-based prospective cohort with the aim to observe risk factors for cardiovascular disease.23 5209 individuals were enrolled in the Original cohort. In 1971, the Offspring cohort was enrolled, including 5,124 offspring from the Original cohort and their spouses. The diagnosis of intermittent claudication, part of the vascular disease entity of the CHA2DS2-VASc score, was not routinely collected before 1991; therefore, in the present investigation, examinations performed prior to 1991 were not included. We used a pooled-examination approach, in which we followed participants from the index examination to a study examination approximately 8 years later and subsequently pooled all follow-up windows for statistical analyses. Consequently, participants from examinations 23 (1992–1996), 26 (1999–2001), and 29 (2006–2007) from the Original cohort and examination 5 (1991–1995), 7 (1998–2001), and 8 (2005–2008) from the Offspring cohort of the FHS were eligible. Because FHS visits are scheduled at the volunteer participants’ convenience, the follow up clusters around 8 years but is not exact. We considered the six examinations the index examinations for follow-up. Follow-up continued until the participant attended her/his routinely scheduled subsequent examination or until 8 years if she/he did not attend the next scheduled examination. We used the same age-cutoff as in the derivation study for the CHARGE-AF score and excluded individuals who were <46 years or >94 years of age, who had prevalent AF, or who had missing covariates at the index examinations. All participants signed consent forms. Boston University Medical Center Institutional Review Board approved the study.
Ascertainment of AF
Ascertainment of AF was routine in the FHS. AF was defined as either atrial fibrillation or atrial flutter verified by a FHS cardiologist on an electrocardiogram obtained at a routine FHS clinic examination, obtained by an external clinician, or present in hospital records.6 AF diagnoses made outside FHS examinations were registered with their original date. Follow-up was through 2013.
Risk score covariates
The CHARGE-AF score is a continuous variable with decimals, whereas CHA2DS2-VASc is an ordinal variable that was treated as an integer in our analyses.
The CHARGE-AF risk score
Age (per 5 year increment), race, height (per 10 cm increment), weight (per 15 kg increment), systolic blood pressure (per 20 mm Hg increment), diastolic blood pressure (per 10 mm Hg increment), smoking (current vs former/never), antihypertensive medication use, diabetes, heart failure, and myocardial infarction. One calculates the CHARGE-AF risk score as follows: 0.508 × age (5 years) + 0.248 × height (10 cm) + 0.115 × weight (15kg) + 0.197 × systolic blood pressure (20 mm Hg) - 0.101 × diastolic blood pressure (10 mm Hg) + 0.359 × current smoker + 0.349 × antihypertensive medication + 0.237 × diabetes + 0.701 × congestive heart failure + 0.496 × myocardial infarction. The coefficients for each risk factor are from the derivation study for the CHARGE-AF risk score.10 We did not include race because all participants in our study were of European-American ancestry.
The CHA2DS2-VASc risk score
Age (<65, 65–74, and ≥75 years), sex, hypertension, diabetes mellitus, stroke/transient ischemic attack/thromboembolism, vascular disease, and heart failure. Each factor was assigned 1 point on the risk score, except age ≥75 years and stroke/transient ischemic attack/thromboembolism, which are assigned 2 points, resulting in a maximum score of 9 points. In a secondary analysis, women who scored 0 on all other covariates except sex category, were given the score 0, according to current clinical guidelines for stroke risk assessment, which recommends that women aged <65 years with lone AF should not receive oral anticoagulation.24
Ascertainment of risk score characteristics
Medications and smoking were determined by self-report. We used an average of two seated blood pressures measured in the FHS clinic. Hypertension was considered present if the systolic or diastolic blood pressure were ≥140 or 90 mm Hg, respectively, or if the participant answered yes to the question “Since your last exam have you taken medication for hypertension/high blood pressure?” and was on hypertension medication at the index examination. Diabetes was considered present if fasting blood glucose was ≥126 mg/dl, nonfasting glucose ≥200 mg/dl, or the participant used diabetes medications. Vascular disease was defined as prior myocardial infarction, angina pectoris, coronary insufficiency, percutaneous coronary intervention, coronary artery bypass surgery, intermittent claudication, or surgery of the lower extremity vessels. Vascular disease events, heart failure, stroke, transient ischemic attack, and thromboembolic events were determined by evaluation of medical records by a panel of 3 investigators using reported criteria.25
Statistical analysis
We present descriptive statistics using means and standard deviations for continuous variables, or number and percentages for binary variables. We calculate risk scores at each index exam. Each follow-up window ended at incident AF, at the next index exam, death, last contact for health status, or eight years, whichever occurred earliest. Follow-up windows were pooled (Figure 1).
Figure 1.
Study design. Flowchart of inclusion and follow-up of participants from the Original (top) and Offspring (middle) cohorts.
Main analyses
We estimated the cumulative incidence of AF adjusting for competing risk of death.26 To estimate risk of AF, we performed proportional sub-distribution hazards (Fine-Gray) regression separately for each risk score.27–29 Both risk scores were fitted to the model as continuous variables. We accounted for the competing risk of death using PSHREG, a SAS macro.30 With models stratified by index examinations, we used robust variance estimators to account for within-individual correlation.31 We used the Wald χ2 statistic to test the null hypothesis that the risk score was not associated with AF. We examined discrimination using the C-statistic.32
Calibration was assessed by comparing observed rates of AF within groups of observation windows for each risk score, to the predicted risk among the same follow-up windows. (1) We first divided the study sample into k groups based on the predicted probability of AF obtained from the risk prediction model we developed for each score (k = 7 [0–6+] for CHA2DS2-VASc and k = 10 [0–9] for CHARGE-AF). (2) Then we calculated the expected number of events i.e. the sum of expected probability, within each group. (1) and (2) were compared in a calibration plot. In addition, we calculated the modified Nam and D’Agostino’s Hosmer-Lemeshow (HL) χ2 statistics,33,34 accounting for death as a competing event. The statistic has k-2 degrees of freedom. A large χ2 value indicates poor calibration. The subgroups described here were exclusive to the calibration analysis.
Studies have shown that women aged <65 years with “lone” AF have low risk of stroke35–37 and thus, the clinical guidelines recommend to not treat these women with anticoagulant therapy, although they have a CHA2DS2-VASc score of 1.24 We performed a secondary analysis by assigning a score of 0 to all women <65 years of age who scored 0 on all covariates in the CHA2DS2-VASc score, except sex category. We further reran the same analyses excluding sex completely from the CHA2DS2-VASc risk score, because most studies have observed that women either have the same or lower risk of AF compared with men.
Sensitivity analyses
We performed several sensitivity analyses; (1) excluding 4925 follow-up windows (2838 individuals) that overlapped with the initial CHARGE-AF risk score study data, (2) including all ages, (3) the same analysis as in (1), including only the last observation for each individual, so that she/he could only be included in the analysis once.
To evaluate whether the predicted risks of AF were dependent on sex or age, we performed analyses of interaction, including these variables as interaction terms in our models. For these analyses, the sex category covariate was excluded from the CHA2DS2-VASc risk score. We tested both a dichotomous age cut-off of age <65 or ≥65 and age as a continuous variable.
We performed all statistical analyses using SAS software version 9.4 (SAS Institute, Cary, NC). We used a 2-sided P < .05 as our statistical significance criterion.
The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the manuscript and its final contents.
Funding
This work was supported by Boston University’s and the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. HHSN268201500001I and N01-HC-25195). Dr. Christophersen was supported by a mobility grant from the Research Council of Norway (240149/F20). Dr. Lubitz was supported by NIH K23HL114724 and a Doris Duke Charitable Foundation Clinical Scientist Development Award 2014105. Dr. Magnani was supported by Doris Duke Charitable Foundation Clinical Scientist Development Award 2015084. Dr. Ellinor was supported by grants from the National Institutes of Health (1R01HL092577, R01HL128914, 1K24HL105780), an Established Investigator Award from the American Heart Association (13EIA14220013) and the Fondation Leducq (14CVD01). Dr. Benjamin was supported by grants from the National Institutes of Health (R01HL128914; 2R01HL092577; 3R01HL092577-06S1; 1RC1HL101056).
Results
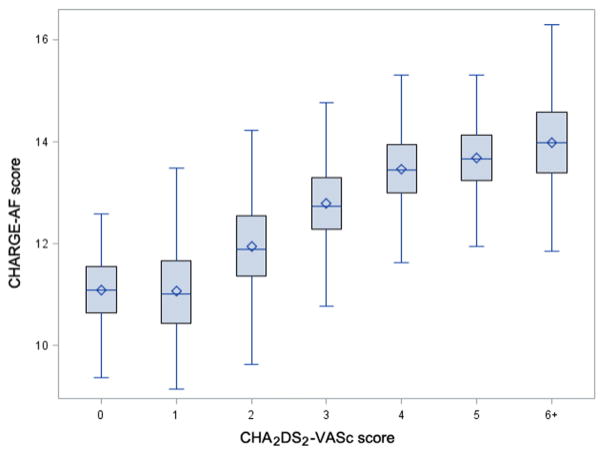
The selected index examinations included a total of 12,032 follow-up windows. Following exclusions for age (n = 900), prevalent AF (n = 735), and missing covariates (n = 675), there were 9,722 follow-up windows from 4,548 individuals in the analysis. The mean age, calculated from all observations was 63.9 ± 10.6 years and 56% were women (Table I). The mean CHARGE-AF score was 12.0 ± 1.2, and mean CHA2DS2-VASc score was 2.0 ± 1.5. Median follow-up time was 6.1 years in the Original Cohort and 7.0 years in the Offspring Cohort. Figure 2 shows the CHARGE-AF risk score broken down by CHA2DS2-VASc risk score categories. Figure S1 shows the distribution of the CHARGE-AF risk score in the study population. The two risk scores had a Spearman correlation coefficient of 0.73. We observed 752 (16.5%) occurrences of incident AF and 793 (17.4%) deaths.
Table I.
Baseline characteristics
| Characteristics | N = 9722* |
|---|---|
| Age (years) | 63.9 ± 10.6 |
| Women | 5446 (56) |
| Height (cm) | 166 ± 10 |
| Weight (kg) | 77 ± 17 |
| Current smoker | 1242 (13) |
| Systolic blood pressure (mm Hg) | 130 ± 19 |
| Diastolic blood pressure (mm Hg) | 74 ± 10 |
| Hypertension | 4964 (51) |
| Hypertension treatment | 3632 (37) |
| Current diabetes | 1032 (11) |
| Heart failure | 100 (1) |
| Stroke/Transient ischemic attack/thromboembolism | 278 (3) |
| Vascular disease | 1188 (12) |
| Myocardial infarction | 372 (4) |
| CHA2DS2-VASc risk score | 2.0 ± 1.5 |
| CHARGE-AF risk score | 12.0 ± 1.2 |
N (%) for dichotomous traits and mean ± SD for continuous traits.
There are 4548 unique individuals with 1297, 1328, and 1923 contributing 1, 2, and 3 observations, respectively.
Figure 2.
Correspondence between CHARGE-AF risk score and CHA2DS2-VASc risk score groups. Mean (diamond) and median (horizontal line) CHARGE-AF risk score for each group of the CHA2DS2-VASc, including 25% to 75% percentiles (box) and range (whiskers).
Risk model performance
Correspondence between CHARGE-AF and CHA2DS2-VASc risk groups are shown in Figure 2.
The CHARGE-AF risk score consistently performed better than the CHA2DS2-VASc risk score in predicting risk of AF, as shown by the higher Wald χ2 (403 vs 209) and higher C-statistic (0.757; 95% CI, 0.741–0.772 vs 0.712; 95% CI, 0.693–0.731) (Table II). The sub-distribution hazard ratio (sHR) per unit increase in the risk score was 2.15 for the CHARGE-AF (95% CI, 1.99–2.31; P < .0001) score vs 1.43 for the CHA2DS2-VASc risk score (95% CI, 1.37–1.51; P < .0001).
Table II.
AF prediction performance for the CHARGE-AF and the CHA2DS2-VASc risk scores
| Risk score | Wald χ2 | Sub-distribution hazard ratio* (95% CI) | P | C-statistic (95% CI) | Calibration HL χ2† |
|---|---|---|---|---|---|
| Primary analysis, n observations = 9722 | |||||
| CHARGE-AF | 403 | 2.15 (1.99, 2.31) | <.0001 | 0.757 (0.741, 0.762) | 5.6 (P = .69) |
| CHA2DS2-VASc | 209 | 1.43 (1.37, 1.51) | <.0001 | 0.712 (0.693, 0.731) | 28.5 (P < .0001) |
| CHA2DS2-VASc lone AF women | 288 | 1.45 (1.39, 1.51) | <.0001 | 0.730 (0.713, 0.747) | 35.5 (P < .0001) |
| CHA2DS2-VASc no sex | 360 | 1.56 (1.49,1.63) | <.0001 | 0.741 (0.724, 0.758) | 28.5 (P < .0001) |
| Sensitivity analysis, n observations = 4797 | |||||
| CHARGE-AF | 208 | 1.90 (1.74, 2.07) | <.0001 | 0.713 (0.693, 0.733) | 12.7 (P = .12) |
| CHA2DS2-VASc | 89 | 1.34 (1.26, 1.42) | <.0001 | 0.673 (0.652, 0.695) | 12.67 (P = .03) |
| CHA2DS2-VASc lone AF women | 125 | 1.36 (1.29, 1.43) | <.0001 | 0.684 (0.663, 0.705) | 19.8 (P = .001) |
| CHA2DS2-VASc no sex | 173 | 1.46 (1.38, 1.54) | <.0001 | 0.699 (0.678, 0.720) | 10.6 (0.06) |
Sub-distribution hazard ratios are per unit change in respective scores. HL, Hosmer-Lemeshow.
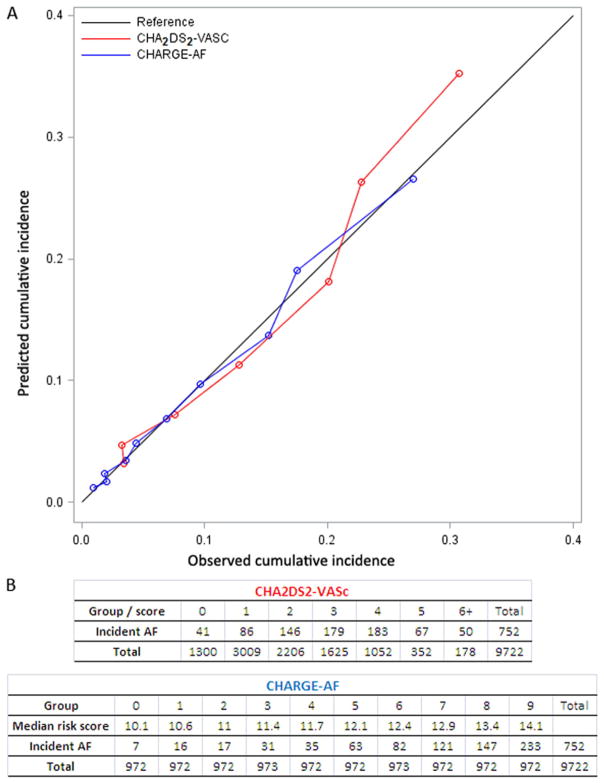
Figure 3 shows that both risk scores were well calibrated in the present study; however, the CHARGE-AF risk score displayed better calibration (HL χ2 = 5.6; P = 0.69) than CHA2DS2-VASc (HL χ2 = 28.5; P < 0.0001). The group with CHA2DS2-VASc score of 1 had lower risk of AF than the group that scored 0. In the group of individuals with a score of 1, a single participant had heart failure (0.03%), 58 vascular disease (1.9%), 68 diabetes (2.3%), 724 hypertension (24%), 296 were between 65 and 75 (9.8%) years, and 1862 were women (61.9%).
Figure 3.
Calibration curves for the CHARGE-AF and the CHA2DS2-VASc risk scores. We calculated the predicted cumulative incidence and the empirically estimated (the observed) incidence within each subgroup defined by either score. A, Comparison of the predicted values (red for CHA2DS2-VASc, blue for CHARGE-AF) and the observed cumulative incidence. The black diagonal is the reference. A shorter distance from the dots to the reference line indicates better calibration. B, The tables show the groups used for the Hosmer-Lemeshow calibration, including number of incident AF cases and total number, for each risk score. The lower rows show number of follow-up windows.
When scoring young women (<65 years) with lone AF as CHA2DS2-VASc = 0 in the model, the Wald χ2 and discrimination improved (Wald χ2 = 288; C-statistic = 0.730, 95% CI 0.713–0.747) for the CHA2DS2-VASc risk score, whereas calibration was reduced (HL χ2 = 35.5; P < .0001) (Table II, Figure S2).
Removing the sex covariate from the CHA2DS2-VASc score altogether resulted in improved discrimination (C-statistic = 0.741; 95% CI, 0.724–0.758) and reversed the association of female sex in the two groups with lowest scores. However, calibration statistics did not improve and the Wald χ2 was still inferior to the CHARGE-AF risk score (Table II, Figure S3).
Sensitivity analysis
After excluding all participants and observations that were part of the derivation study for the CHARGE-AF risk score, there were 4,797 follow-up windows in 3,820 individuals. Repeating the primary analysis, the CHARGE-AF risk score performed better at predicting risk of AF (Wald χ2 = 208; sHR, 1.90; 95% CI, 1.74–2.07; P < .0001, C-statistic = 0.713; 95% CI, 0.693–0.733) than the CHA2DS2-VASc risk score (Wald χ2 = 89; sHR, 1.34; 95% CI, 1.26–1.42; P < .0001, C-statistic = 0.673; 95% CI, 0.652–0.695) (Table II). In the model excluding individuals in the derivation study, the calibration improved slightly for the CHA2DS2-VASc risk score (HL χ2 = 12.7; df = 5; P = 0.03), whereas it was somewhat attenuated for the CHARGE-AF risk score (HL χ2 = 12.7; df = 8, P = 0.12). Table S1 shows the results when all ages were included. The number of observations increased to 10,582 and the number of incident AFs to 761, but the results were similar to the main analysis. Table S2 shows results from analyses restricted to individuals not included in the derivation study for CHARGE-AF and where each individual was only included once. The sample size was substantively reduced, to 2,726 observations; 343 incident AFs, and whereas CHARGE-AF still showed better discrimination than CHA2DS2-VASc, calibration was attenuated for both risk scores.
In models scoring young women (<65 years) with lone AF as CHA2DS2-VASc = 0 in the sensitivity analysis, the CHA2DS2-VASc risk score showed improved model fit and discrimination (Wald χ2 = 125; sHR, 1.36; 95% CI, 1.29–1.43; P < .0001, C-statistic = 0.684; 95% CI, 0.663–0.705); however, calibration was attenuated (HL χ2 = 19.8; df = 5; P = 0.001) (Figure S2, Table II).
Age and sex interactions
We found no evidence of age or sex interaction for either the CHARGE-AF risk score (categorical age: P = .94; continuous age: P = .12; sex: P = .70) or the CHA2DS2-VASc risk score (categorical age: P = .13; continuous age: P = .57; sex: P = .10). In a sex-stratified model, we saw similar results (Figure S4).
Discussion
Our results indicate that the CHARGE-AF risk score performs better than the CHA2DS2-VASc risk score to predict AF in 4,548 individuals of European descent from the FHS, with regards to both discrimination (C-statistics of 0.757 vs 0.712, respectively) and calibration (χ2 = 5.6 vs χ2 = 28.5, respectively). Reassigning young women with lone AF from a CHA2DS2-VASc of 1 to 0 or excluding sex from the model both improved discrimination for CHA2DS2-VASc, but not to the level of the CHARGE-AF score, and calibration did not improve. AF risk was overestimated in individuals with high CHA2DS2-VASc scores.
We performed a sensitivity analysis to examine whether the higher performance of the CHARGE-AF risk score was biased by the inclusion of the same observations in the current and the initial CHARGE-AF risk score study. After exclusion of all overlapping observations between the two studies, both risk scores displayed attenuated discrimination (CHARGE-AF C-statistic 0.713 vs CHA2DS2-VASc 0.673) and calibration. The lower performance in the sensitivity analysis may be explained by the decrease in power when reducing the sample size by ~50 percent. However, the CHARGE-AF risk score still performed better, suggesting that the results from the primary analysis were not heavily influenced by the inclusion of one of the derivation cohorts, the FHS, in the CHARGE-AF risk score.
To determine whether the risk scores predicted AF differentially in men and women or in different age groups, we performed interaction analyses. No evidence of interaction was identified for either sex or age, suggesting that both risk scores display uniform prediction of AF across sex and age groups. The lack of age or sex interactions was in line with the findings from the CHARGE-AF derivation study.10
Comparison with previous literature
Several alternative risk scores for predicting incident AF have been reported,8,9,38,39 but most have thus far not been replicated in other cohorts and other ethnicities. The CHARGE-AF risk score was derived in three cohorts with European and African-American ancestry and was validated in two European cohorts in the initial study. Moreover, it was recently replicated in a European community-based study, including more than 24,000 individuals, although it required recalibration,12 and in an ethnically diverse inner-city population of more than 45,000 individuals.11 Thus far, the CHARGE-AF risk score has been tested on more than 95,000 individuals across different ancestries, providing increased generalizability compared with other risk scores for AF prediction.
The CHADS2 and the CHA2DS2-VASc risk scores have been reported for prediction of new occurrence of AF40–42 and ischemic stroke,16 long-term outcomes after AF-ablation,17 arrhythmias after myocardial infarction18 and cardiac surgery,20,21,43 and rhythm outcomes after catheter ablation of AF.19 However, the CHA2DS2-VASc score has never been validated for predicting risk of AF. The CHA2DS2-VASc has potential limitations for the prediction of AF per se. CHA2DS2-VASc includes female sex, which has not been associated with increased risk of AF. On the contrary, male sex frequently has been associated with increased risk of AF.6,10 Since female sex is an integral part of the CHA2DS2-VASc, the score may misclassify women to a higher risk category. Of note, the complete removal of sex from our model improved AF discrimination for CHA2DS2-VASc, but not to the level of CHARGE-AF.
Clinical implications
AF constitutes a substantial burden for the affected individuals, who struggle with arrhythmia symptoms44,45 and serious complications, but also for the society as a whole, because of the considerable costs.46 The increase in prevalence1,47–49 and socioeconomic consequences of AF calls for measures of early detection and prevention.50,51 Prospective studies focused on new-onset AF prevention have not been reported so far; however, the ability to identify individuals with increased risk of AF will be an essential part of such a study. Although the CHARGE-AF risk score is the most validated AF risk score to date and displays comparable discrimination statistics to other AF risk scores, a C-statistic of 0.757 still holds room for improvement. Further refinement of the prediction of AF is warranted. The clinical use of the CHARGE-AF risk score is still uncertain; nonetheless, it is a good candidate for AF prediction in preventive trials and as a research tool to evaluate putative novel risk factors for AF.
Strengths and limitations
The most prominent strength of our study is the use of the comprehensive community-based FHS and a moderately large sample size. Further, the diagnosis of AF was adjudicated by cardiologists who performed thorough chart review, thus ensuring robust diagnoses. Covariates used for index characteristics and risk score calculations were routinely collected in the FHS. Another strength of the CHARGE-AF risk score is that it was developed in a community-based cohort with 7 years of follow-up. In contrast, the CHA2DS2-VASc was developed in a hospital-based cohort with only 1 year of follow-up and the proportion of women was smaller (41%).
We note several limitations to our study. First, the CHARGE-AF risk score involves more complex calculation than the CHA2DS2-VASc score, which may quickly be calculated in a hectic clinical setting. The CHARGE-AF risk score may be best suited in situations where it can be calculated automatically, for instance using a CHARGE-AF calculator when assessing risk of AF in primary care or elective secondary care, in electronic medical records, or in trial design. Second, analyses included only individuals of European American ancestry; the results are thus limited by uncertain generalizability to other ethnicities/races. Third, some diagnoses used for the CHA2DS2-VASc risk score were not collected on a regular basis in the FHS, including asymptomatic left ventricular dysfunction, peripheral embolism outside the brain, heart, eyes, and lungs, arterial thrombosis, aortic plaque, and percutaneous intervention on the abdominal aorta. Unfortunately, we cannot avoid this limitation in the present study design. However, Bekwelem and colleagues has shown that systemic embolisms outside the brain constitute 11.5% of all clinically recognized systemic embolisms, which suggests that the absence of these diagnoses may not have large impact on our results.52 Nonetheless, the lack of clinical characteristics used for calculating the CHA2DS2-VASc might have led to misclassification of the CHA2DS2-VASc risk score in some individuals. Fourth, the FHS was one of the derivation samples for the CHARGE-AF risk score; therefore, in the Framingham cohort, one might expect the CHARGE-AF risk score to predict AF better than the CHA2DS2-VASc risk score. However, the sensitivity analysis of individuals not overlapping with the sample included in the original derivation of the CHARGE-AF risk prediction model showed that it still performed better. In the evaluation of the calibration of the two risk scores using the Hosmer-Lemeshow model, the CHARGE-AF risk score has the advantage of being a continuous score that may readily be subdivided into 10 risk groups of equal numbers of observations, whereas the CHA2DS2-VASc risk score is an integer risk model, which may only be subdivided into the predefined integer scores. The integer structure of CHA2DS2-VASc may have contributed to the superior calibration statistics for the CHARGE-AF risk score. Last, we acknowledge that there may be individuals with asymptomatic AF, which may have been misclassified as referents in the present study.
Conclusion
In conclusion, both the CHARGE-AF risk score and the CHA2DS2-VASc risk score perform well in predicting risk of AF in a European-American community-based cohort; however, the CHARGE-AF score displayed better discrimination and calibration through all analyses. Albeit, the impact on clinical practice is uncertain, the CHARGE-AF prediction score may serve as a research tool for risk stratifying individuals for inclusion in primary prevention trials and evaluating novel risk factors for AF.
Supplementary Material
Appendix. Supplementary data
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ahj.2016.05.004.
Footnotes
Disclosures
Dr Ellinor is a principal investigator on a grant from Bayer HealthCare to the Broad Institute.
Emelia J. Benjamin, Ingrid E. Christophersen, Xiaoyan Yin, and Martin G. Larson have conceived the idea for this manuscript, developed the design, and interpreted the results in collaboration. All authors have contributed to drafting the manuscript, revising it for important intellectual content, and final approval of the submitted manuscript. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–47. doi: 10.1161/CIRCULATIONAHA.113.005119. [[Internet] [cited 2014 Mar 19] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4151302&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [National Heart, Lung, and Blood Institute’s Framingham Heart Study, National Institutes of Health, Mass, USA. emelia@fram.nhlbi.nih.gov] [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Rydén LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidel. Circulation. 2011;123(10):e269–367. doi: 10.1161/CIR.0b013e318214876d. [[Internet]. [cited 2015 May 7]; Available from: http://circ.ahajournals.org/content/123/10/e269] [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Benjamin EJ. Status of the epidemiology of atrial fibrillation. Med Clin North Am. 2008;92:17–40. ix. doi: 10.1016/j.mcna.2007.09.002. [Boston University, The Framingham Heart Study, 73 Mount Wayte Avenue, Framingham, MA 01702, USA. billkannel@yahoo.com] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–5. doi: 10.1161/01.CIR.0000072767.89944.6E. [Framingham Heart Study, 73 Mt Wayte Ave, Suite #2, Framingham, Mass 01702–5827, USA] [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Levy D, Vaziri SM, et al. Independent Risk Factors for Atrial Fibrillation in a Population-Based Cohort. JAMA. 1994;271(11):840–4. [[Internet]. Framingham Heart Study, MA 01701: American Medical Association; [cited 2015 Jan 15]; Available from: http://jama.jamanetwork.com.ezp-prod1.hul.harvard.edu/article.aspx?articleid=367563] [PubMed] [Google Scholar]
- 7.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–45. doi: 10.1016/S0140-6736(09)60443-8. [Framingham Heart Study, Framingham, MA, USA; Department of Medicine II, Johannes Gutenberg-University, Mainz, Germany] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithm in whites and African Americans. Arch Intern Med. 2010;170(21):1909–17. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain AM, Agarwal SK, Folsom AR, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study) Am J Cardiol. 2011;107(1):85–91. doi: 10.1016/j.amjcard.2010.08.049. [[Internet]. [cited 2015 Jun 4]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3031130&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2(2):e000102. doi: 10.1161/JAHA.112.000102. [[Internet]. [cited 2014 Jul 21]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3647274&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shulman E, Kargoli F, Aagaard P, et al. Validation of the Framingham Heart Study and CHARGE-AF Risk Scores for Atrial Fibrillation in Hispanics, African-Americans, and Non-Hispanic Whites. Am J Cardiol. 2016;117(1):76–83. doi: 10.1016/j.amjcard.2015.10.009. [[Internet]. [cited 2016 Feb 15]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/26589820] [DOI] [PubMed] [Google Scholar]
- 12.Pfister R, Brägelmann J, Michels G, et al. Performance of the CHARGE-AF risk model for incident atrial fibrillation in the EPIC Norfolk cohort. Eur J Prev Cardiol. 2015;22(7):932–9. doi: 10.1177/2047487314544045. [[Internet]. [cited 2015 Jun 15]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25059930] [DOI] [PubMed] [Google Scholar]
- 13.Gage BF, Waterman AD, Shannon W, et al. Validation of Clinical Classification Schemes for Predicting Stroke. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [[Internet]. American Medical Association; [cited 2015 Mar 30]. Available from: http://jama.jamanetwork.com/article.aspx?articleid=193912] [DOI] [PubMed] [Google Scholar]
- 14.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–72. doi: 10.1378/chest.09-1584. [University of Birmingham Centre for Cardiovascular Sciences, City Hospital, Birmingham, UK. g.y.h.lip@bham.ac.uk] [DOI] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246–80. doi: 10.1016/j.jacc.2014.03.022. [[Internet]. Journal of the American College of Cardiology; [cited 2014 Jul 9]; Available from: http://content.onlinejacc.org/article.aspx?articleid=1854230.] [DOI] [PubMed] [Google Scholar]
- 16.Zuo M-L, Liu S, Chan K-H, et al. The CHADS2 and CHA 2DS 2-VASc scores predict new occurrence of atrial fibrillation and ischemic stroke. J Interv Card Electrophysiol. 2013;37(1):47–54. doi: 10.1007/s10840-012-9776-0. [[Internet]. [cited 2015 Feb 27]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/23389054] [DOI] [PubMed] [Google Scholar]
- 17.Jacobs V, May HT, Bair TL, et al. The impact of risk score (CHADS2 versus CHA2DS2-VASc) on long-term outcomes after atrial fibrillation ablation. Heart Rhythm. 2015;12(4):681–6. doi: 10.1016/j.hrthm.2014.12.034. [Internet]. [cited 2015 May 29]; Available from: http://www.sciencedirect.com/science/article/pii/S1547527114015525. [DOI] [PubMed] [Google Scholar]
- 18.Ruwald AC, Gang U, Thomsen PEB, et al. The predictive value of CHADS2 risk score in post myocardial infarction arrhythmias - a Cardiac Arrhythmias and RIsk Stratification after Myocardial infArction (CARISMA) substudy. Int J Cardiol. 2014;173(3):441–6. doi: 10.1016/j.ijcard.2014.03.010. [[Internet]. [cited 2015 Mar 17]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24713455] [DOI] [PubMed] [Google Scholar]
- 19.Kornej J, Hindricks G, Kosiuk J, et al. Comparison of CHADS2, R2CHADS2, and CHA2DS2-VASc scores for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol. 2014;7(2):281–7. doi: 10.1161/CIRCEP.113.001182. [[Internet]. [cited 2015 Mar 17]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24610790] [DOI] [PubMed] [Google Scholar]
- 20.Sareh S, Toppen W, Mukdad L, et al. CHADS2 score predicts atrial fibrillation following cardiac surgery. J Surg Res. 2014:2–7. doi: 10.1016/j.jss.2014.02.007. [[Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24629418] [DOI] [PubMed]
- 21.Chua S-K, Shyu K-G, Lu M-J, et al. Clinical utility of CHADS2 and CHA2DS2-VASc scoring systems for predicting postoperative atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146(4):919–926. e1. doi: 10.1016/j.jtcvs.2013.03.040. [[Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23628495] [DOI] [PubMed] [Google Scholar]
- 22.Fauchier L, Clementy N, Pelade C, et al. Patients With Ischemic Stroke and Incident Atrial Fibrillation: A Nationwide Cohort Study. Stroke. 2015;46(9):2432–7. doi: 10.1161/STROKEAHA.115.010270. [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26251249. [DOI] [PubMed] [Google Scholar]
- 23.Dawber TR, Meadors GF, Moore FE. Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–81. doi: 10.2105/ajph.41.3.279. [[Internet]. [cited 2015 Mar 4]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1525365&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camm AJ, Lip GYH, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [[Internet]. Division of Clinical Sciences, St.George’s University of London, Cranmer Terrace, London SW17 0RE, United Kingdom. jcamm@sgul.ac.uk; Available from: papers2://publication/doi/10.1093/eurheartj/ehs253] [DOI] [PubMed] [Google Scholar]
- 25.Cupples LA, D’Agostino RB. Section 34: some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. In: Kannel WB, Wolf PA, Garrison RJ, editors. Framingham Heart Study: 30 Year Follow-Up. Bethesda, Md: US Department of Health and Human Services; 1987. [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [[Internet]. [cited 2015 Jun 2]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/10204198] [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [[Internet] Available from: http://www.jstor.org/stable/10.2307/2670170/npapers2://publication/uuid/11555F83-811D-4BE1-83C8-87D552B09F0D] [Google Scholar]
- 28.Dignam JJ, Zhang Q, Kocherginsky M. The use and interpretation of competing risks regression models. Clin Cancer Res. 2012;18(8):2301–8. doi: 10.1158/1078-0432.CCR-11-2097. [[Internet]. [cited 2016 Jan 17]; Available from: http://clincancerres.aacrjournals.org/content/18/8/2301.long] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolbers M, Koller MT, Stel VS, et al. Competing risks analyses: Objectives and approaches. Eur Heart J. 2014:2936–41. doi: 10.1093/eurheartj/ehu131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kohl M, Plischke M, Leffondré K, Heinze G. PSHREG: a SAS macro for proportional and nonproportional subdistribution hazards regression. Comput Methods Programs Biomed. 2015;118(2):218–33. doi: 10.1016/j.cmpb.2014.11.009. [[Internet]. [cited 2015 May 29]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25572709] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin DY, Wei LJ. The Robust Inference for the Cox Proportional Hazards Model. J Am Stat Assoc. 1989;84(408):1074–8. [Google Scholar]
- 32.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–23. doi: 10.1002/sim.1802. [[Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15211606] [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures. In: Balakrishnan N, Rao C, editors. Handbook of Statistics. Vol. 23. Amsterdam: Elsevier; 2004. pp. 1–25. [Google Scholar]
- 34.Crowson CS, Atkinson EJ, Therneau TM. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2014 doi: 10.1177/0962280213497434. [[Internet]. [cited 2016 Feb 5]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3933449&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed]
- 35.Friberg L, Benson L, Rosenqvist M, Lip GYH. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344(may30_3):e3522. doi: 10.1136/bmj.e3522. [[Internet]. [cited 2015 Dec 1]; Available from: http://www.bmj.com/content/344/bmj.e3522] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olesen JB, Fauchier L, Lane DA, et al. Risk factors for stroke and thromboembolism in relation to age among patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 2012;141(1):147–53. doi: 10.1378/chest.11-0862. [[Internet]. American College of Chest Physicians; [cited 2015 Nov 9]; Available from: http://journal.publications.chestnet.org.ezp-prod1.hul.harvard.edu/article.aspx?articleid=1149031] [DOI] [PubMed] [Google Scholar]
- 37.Olesen JB, Lip GYH, Lane DA, et al. Vascular disease and stroke risk in atrial fibrillation: a nationwide cohort study. Am J Med. 2012;125(8):826, e13–23. doi: 10.1016/j.amjmed.2011.11.024. [[Internet]. [cited 2015 Nov 5]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/22579139] [DOI] [PubMed] [Google Scholar]
- 38.Brunner KJ, Bunch TJ, Mullin CM, et al. Clinical predictors of risk for atrial fibrillation: implications for diagnosis and monitoring. Mayo Clin Proc. 2014;89(11):1498–505. doi: 10.1016/j.mayocp.2014.08.016. [[Internet]. [cited 2015 May 16]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25444486] [DOI] [PubMed] [Google Scholar]
- 39.Everett BM, Cook NR, Conen D, et al. Novel genetic markers improve measures of atrial fibrillation risk prediction. Eur Heart J. 2013;34:2243–51. doi: 10.1093/eurheartj/eht033. [[Internet]. Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA 02115, USA; [cited 2014 Mar 20]; Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3730133&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chao TF, Liu CJ, Chen SJ, et al. CHADS2 score and risk of new-onset atrial fibrillation: A nationwide cohort study in Taiwan. Int J Cardiol. 2013;168(2):1360–3. doi: 10.1016/j.ijcard.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 41.Yang Y, Zhang Z, Ng CY, et al. Meta-analysis of CHADS2 Score in Predicting Atrial Fibrillation. Am J Cardiol. 2015;116(4):554–62. doi: 10.1016/j.amjcard.2015.05.010. [[Internet] [cited 2015 Jun 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26071992] [DOI] [PubMed] [Google Scholar]
- 42.Sciacqua A, Perticone M, Tripepi G, et al. CHADS2 and CHA2DS2-VASc scores are independently associated with incident atrial fibrillation: the Catanzaro Atrial Fibrillation Project. Intern Emerg Med. 2015;10(7):815–21. doi: 10.1007/s11739-015-1243-3. [[Internet] [cited 2015 Jun 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25939338] [DOI] [PubMed] [Google Scholar]
- 43.Kashani RG, Sareh S, Genovese B, et al. Predicting postoperative atrial fibrillation using CHA2DS2-VASc scores. J Surg Res. 2015;198(2):267–72. doi: 10.1016/j.jss.2015.04.047. [[Internet] [cited 2015 Jun 15]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26004496] [DOI] [PubMed] [Google Scholar]
- 44.Rienstra M, Lubitz SA, Mahida S, et al. Symptoms and functional status of patients with atrial fibrillation: State of the art and future research opportunities. Circulation. 2012;125(23):2933–43. doi: 10.1161/CIRCULATIONAHA.111.069450. [[Internet] [cited 2015 Mar 6] Available from: http://circ.ahajournals.org.ezp-prod1.hul.harvard.edu/content/125/23/2933.long] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wynn GJ, Todd DM, Webber M, et al. The European Heart Rhythm Association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace. 2014;16(7):965–72. doi: 10.1093/europace/eut395. [[Internet] [cited 2015 Mar 11] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4070972&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim MH, Johnston SS, Chu B-C, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual outcomes. 2011;4:313–20. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 47.Haim M, Hoshen M, Reges O, et al. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non-valvular atrial fibrillation. J Am Heart Assoc. 2015;4(1):e001486. doi: 10.1161/JAHA.114.001486. [[Internet] [cited 2015 Feb 16] Available from: http://jaha.ahajournals.org/content/4/1/e001486.long] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [[Internet] [cited 2014 May 3] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16818816] [DOI] [PubMed] [Google Scholar]
- 49.Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386(9989):154–62. doi: 10.1016/S0140-6736(14)61774-8. [[Internet] [cited 2015 Jul 12] Available from: http://www.sciencedirect.com/science/article/pii/S0140673614617748] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benjamin EJ, Chen P-S, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119(4):606–18. doi: 10.1161/CIRCULATIONAHA.108.825380. [[Internet] [cited 2015 Jun 15] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2635942&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Wagoner DR, Piccini JP, Albert CM, et al. Progress toward the prevention and treatment of atrial fibrillation: A summary of the Heart Rhythm Society Research Forum on the Treatment and Prevention of Atrial Fibrillation, Washington, DC, December 9–10, 2013. Heart Rhythm. 2015;12(1):e5–29. doi: 10.1016/j.hrthm.2014.11.011. [[Internet] [cited 2015 Jun 15] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4425127&tool=pmcentrez&rendertype=abstract] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bekwelem W, Connolly SJ, Halperin JL, et al. Extracranial Systemic Embolic Events in Patients With Nonvalvular Atrial Fibrillation: Incidence, Risk Factors, and Outcomes. Circulation. 2015;132(9):796–803. doi: 10.1161/CIRCULATIONAHA.114.013243. [[Internet] [cited 2015 Nov 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/26224811] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.