Abstract
Vitamin E (α-tocopherol, VitE) was discovered in 1922 for its role in preventing embryonic mortality. We investigated the underlying mechanisms causing lethality using targeted metabolomics analyses of zebrafish VitE-deficient embryos over five days of development, which coincided with their increased morbidity and mortality. VitE deficiency resulted in peroxidation of docosahexaenoic acid (DHA), depleting DHA-containing phospholipids, especially phosphatidylcholine, which also caused choline depletion. This increased lipid peroxidation also increased NADPH oxidation, which depleted glucose by shunting it to the pentose phosphate pathway. VitE deficiency was associated with mitochondrial dysfunction with concomitant impairment of energy homeostasis. The observed morbidity and mortality outcomes could be attenuated, but not fully reversed, by glucose injection into VitE-deficient embryos at developmental day one. Thus, embryonic VitE deficiency in vertebrates leads to a metabolic reprogramming that adversely affects methyl donor status and cellular energy homeostasis with lethal outcomes.
Keywords: α-tocopherol, docosahexaenoic acid, methyl donors, mitochondria, oxygen consumption, phosphatidylcholine
Introduction
Vitamin E (α-tocopherol, VitE) was discovered in 1922 because it prevented embryonic mortality in rats [1], but the involved mechanisms remain unknown. VitE is a lipophilic antioxidant [2], protecting long-chain polyunsaturated fatty acids, especially docosahexaenoic acid (DHA) from lipid peroxidation [3,4]. Is VitE’s essentiality due to its antioxidant function? A leading theory argues VitE is an integral part of an “antioxidant network” [5], where the one-electron VitE oxidation product (generated by its scavenging peroxyl radicals) is reduced by ascorbic acid. Reduction of the ascorbyl radical utilizes other antioxidants, especially glutathione (GSH). The subsequent reduction of GSSG (the GSH oxidization product) uses endogenous reducing equivalents, primarily NADPH, to serve as the final electron-acceptor [5]. This close association between GSH and VitE is further emphasized by studies of phospholipid hydroperoxide glutathione peroxidase (GPx4), a selenium-dependent peroxidase that reduces phospholipid hydroperoxides [6]. Could dysregulation of this antioxidant network cause embryonic mortality? Maternal VitE supplementation in mice prevents GPx4 knockdown-induced lethality in offspring [7], suggesting that VitE is necessary both 1) to generate lipid hydroperoxides from peroxyl radicals (the lipid hydroperoxides then function as GPx4 substrates) and 2) in the absence of GPx4 to prevent alkoxyl radicals, which arise from the spontaneous oxidation of lipid hydroperoxides, to reinitiate lipid peroxidation.
In humans, VitE deficiency increases early miscarriage risk [8], which poses public health concerns since estimates of inadequate dietary VitE intakes exceed 80% of the global ≥14 year-old population [9]. Using our VitE-deficient zebrafish model as a tool, we undertook solving the mystery of why VitE is a necessary nutrient, especially during vertebrate embryonic development.
Materials and Methods
Study design
All experiments were performed in duplicate. All data trends and specific outcomes contained herein matched between the first and second experimental replicates. In order to maximize consistency, the results we report are all from the second set of experiments. Additional supplementary data and the complete data set is available in [10].
Materials and reagents
Reagents used for metabolomics analyses included: methanol and ultra-pure water (LC-MS grade, EMD Millipore, Gibbstown, NJ); formic acid, acetic acid (Optima LC/MS grade; Fisher Chemical, Pittsburgh, PA); and butylated hydroxytoluene (BHT, TCI America; Portland, OR), as well as zirconium oxide beads (Next Advance; Averill Park, NY). Deuterium (d) labeled internal standards DHA-d5, ARA-d8, EPA-d5, LA-d4, and 9(S)-HODE-d4 (Cayman Chemical, Ann Arbor, MI) were used for quantification of total and free fatty acids, and oxidized DHA derivatives, respectively. Sterile d6-α-tocopherol emulsion (Fresenius-Kabi, Graz, Austria) and D-(+)-glucose (Sigma Aldrich, St. Louis MO) were used for embryo microinjection rescue studies. Reagents used for bioenergetics profiling included: oligomycin (Cayman Chemicals; Ann Arbor, MI), carbonyl cyanide 4-(trifluoromethoxy) phenyl-hydrazone (FCCP), and sodium azide (Sigma-Aldrich; St. Louis, MO).
Zebrafish husbandry and diets
The Institutional Animal Care and Use Committee of Oregon State University approved this protocol (ACUP Number: 4344). Tropical 5D strain zebrafish were housed in the Sinnhuber Aquatic Research Laboratory. Adults were kept at standard laboratory conditions of 28°C on a 14-h light/10-h dark photoperiod in fish water consisting of reverse osmosis water supplemented with a commercially available salt (Instant Ocean®) to create a salinity of 600 microsiemens [11], adjusted to pH 7.4. At 55 days post-fertilization (dpf), adult zebrafish were randomly allocated to one of two diet groups, α-tocopherol deficient (E−) or α-tocopherol sufficient (E+), for the duration of the study [12]. The defined diets, which contained only fatty acids with 18 or fewer carbons and no more than 3 double bonds, were prepared with the vitamin C source as StayC (500 mg/kg, Argent Chemical Laboratories Inc., Redmond, WA) and without (E−) or with added α-tocopherol (E+, 500 mg RRR-α-tocopheryl acetate/kg diet, ADM, Decatur, IL), as described previously [12,13]. Diets were stored at −20°C until fed to the adult zebrafish.
E− and E+ embryos were obtained from adult fish fed either the E− or E+ diet, respectively, for a minimum of 80 days up to 9 months. Embryos were obtained through natural group spawning, collected, staged [11], and kept in standard embryo media (EM; as described [14]). Embryos used for biochemical analysis, described below, were euthanized prior to sampling by cold exposure (placed on ice for a minimum of 30 min.). Note that embryos are not fed. For all experiments, the E+ embryos are considered the control condition; lab embryos also were used as an additional control to monitor embryo quality (data not shown).
Vitamin E and ascorbic acid analyses
Using high-pressure liquid chromatography with electrochemical detection, diet and embryo α-tocopherol [15] and ascorbic acid [16] were determined. Measured α-tocopherol concentrations in the E− and E+ diets were 0.45 ± 0.01 and 369 ± 2 mg/kg, respectively; vitamin C was 143 ± 16 mg ascorbic acid/kg (n=3 replicate samples measured for each diet). This level of dietary vitamin C has been found to be adequate for the zebrafish [17].
Evaluation of phenotypic and developmental progress
At 24 hours post-fertilization (hpf), embryos were assessed for viability, developmental progression and spontaneous movements (earliest behavior in zebrafish), using the zebrafish acquisition and analysis program (ZAAP). ZAAP is a custom program designed to inventory, acquire, and manage zebrafish data, and was used to collect 18 developmental endpoints, as either present or absent (i.e. binary responses were recorded, described below [18]).
Developmental progression is considered perturbed if zebrafish are delayed more than 12 hours compared to control animals. Spontaneous movements are assessed over a 2 min. period and are considered perturbed if there is a lack of embryonic contractions and/or movement. At 96 and 120 hpf, larval morphology (body axis, eye, snout, jaw, otic vesicle, notochord, heart, brain, somite, fin, yolk-sac, trunk, circulation, pigment, and swim bladder) was evaluated and recorded and behavioral endpoints (motility, tactile response) were thoroughly evaluated. If the embryo was dead at either 24 or by 96–120 hpf, the non-mortality endpoints were not included in the evaluations. All images were taken using a Keyence BZ-700X microscope with a 2X objective lens under standard bright-field conditions.
Behavioral assessments
Locomotor activity [18,19] was measured in a total of n=128 embryos per VitE group using Viewpoint Zebrabox (software version 3.0, Viewpoint Life Sciences, Lyon, France). At 96 hpf, the plates containing the embryos were placed in a Viewpoint ZebraBox and embryo locomotor activity was assessed using the “tracking” setting during alternating periods of light and dark, a modification of [20]. Embryos subjected to this test typically move less during the light periods and more during dark periods, and behavioral differences can be determined by comparing distances moved during the light and/or dark periods. Locomotor activity in response to the light/dark transition was tracked during 3 min. periods of alternating light and dark for a total of 24 min. The integration time was set to 6 seconds to increase statistical power. A high definition camera (30 frames/second) tracked the total movement (swim distance, millimeters) in response to the multiple light-dark transitions.
Extraction and LC-MS/MS for metabolomic analysis
At 12 hpf, E− and E+ embryos were transferred one embryo per well into 96 well plates containing 100 μL EM per well. Following 24, 48, 72, and 120 hpf, embryos (n=15 per replicate, n=4 replicates per group) were transferred to 1.5 mL Eppendorf tubes, covered with EM, and kept on ice for 30 min. to euthanize the animals. EM was carefully removed to prevent loss of embryos and samples were stored at −80 °C overnight. To extract embryos for metabolomics analyses, solvent (300 μL 80:20 v/v methanol:water) was added, then sample extracts were homogenized with 0.5 mm zirconium oxide beads using a counter-top bullet blender for 6 min. Following 15 min. incubation on ice, the extracts were centrifuged at 4 °C at 15,000×g for 13 min. Aliquots (200 μL) of the upper layer were transferred individually to new tubes and stored at −80 °C until analysis via LC-MS/MS. To ensure the stability and repeatability of the LC–MS system, quality control (QC) samples (n=4), which were generated by pooling 10 μL aliquots from each embryo extract, were analyzed with the embryo samples.
Chromatography was performed with a Shimadzu Nexera system (Shimadzu; Columbia, MD, USA) coupled to a high-resolution hybrid quadrupole-time-of-flight mass spectrometer (TripleTOF® 5600; SCIEX; Framingham, MA, USA). Two different LC analyses using reverse phase and HILIC columns were used. In reverse phase LC, chromatographic separations were carried out using a 4.6 × 150 mm Inertsil phenyl-3 column (5 μm, GL Sciences Inc., Rolling Hills Estates, CA, USA) for positive and negative ion analyses, as we described [21]. The sample injection volume was 10 μL and the flow rate was 0.4 mL/min. The mobile phases consisted of water (A) and methanol (B), both with 0.1% formic acid. The gradient was as follows: an initial hold at 5% B for 1 min, followed by a gradient of 5–50% B in 11 min, to 100% B at 23 min, held until 35 min, then a shift to 5% B at 37 min until 50 min. The column temperature was held at 50 °C. In metabolomics HILIC LC analysis, separation was carried out using a 4.6 × 150 mm SeQuant ZIC-pHILIC (5 μm, EMD Millipore, Billerica, MA, USA). The flow rate was 0.4 mL/min and the injection volume was 10 μL. The two mobile phases consisted of 20 mM ammonium carbonate, pH 9.2 with ammonium hydroxide in water (A) and acetonitrile (B). The gradient was as follows: an initial hold at 80% B for 1 min, followed by a gradient of 80–20% B in 30 min, to 8% B at 31 min, held until 36 min, then a shift to 80% B at 37 min until 44 min. The column temperature was held at 50 °C.
Time-of-flight (TOF) mass spectrometry (MS) was operated with an acquisition time of 0.25 s and a scan range of 70–1000 Da. MS/MS acquisition was performed with collision energy set at 35 V and collision energy spread of 15 V. Each MS/MS scan had an accumulation time of 0.17 s and a range of 40–1000 Da using information-dependent acquisition (IDA). The source temperature was set at 500 °C and IonSpray voltage at 4.5 kV in positive ion mode and −4.0 kV negative ion mode, respectively.
Sample preparation, extraction and LC-MS/MS analyses of total or free DHA, EPA, ARA, and LA fatty acids and hydroxy-DHA
Analysis of total DHA, EPA, ARA, and LA were performed as described [15] with the following modifications: samples were obtained at 24, 48, 72, and 120 hpf (n=10–15 embryos per replicate, 3 replicates per group) and saponified in alcoholic KOH with 1% ascorbic acid; following cooling, the pH was adjusted to 2.5 with 12N HCl, then 2.0 mL heptane and 10 μL internal standard [DHA-d5 (1.0 μg/mL), EPA-d5 (1.0 μg/mL), ARA-d8 (20.0 μg/mL) and LA-d4 (20.0 μg/mL)] were added. Samples were mixed, the supernatant (organic layer) was removed and dried under nitrogen gas, the residue resuspended in 100 μL 80:20 v/v methanol:water with 0.5% acetic acid. Samples were stored at −80 °C until analysis by LC-MS/MS (see below).
Extracts for quantitative free fatty acid and hydroxy-DHA analyses were prepared as described for metabolomics samples, with the following modifications: extraction solvent (290 μL, 80:20 v/v methanol:water) included 50 μg/mL BHT and for quantification was combined with 10 μL per sample of internal standards containing DHA-d5 (10.0 μg/mL), EPA-d5 (5.0 μg/mL), ARA-d8 (20.0 μg/mL), LA-d4 (20.0 μg/mL), and 9(S)-HODE-d4 (2.0 μg/mL). Embryo numbers ranged from n=15–30 embryos per replicate, with n=4 replicates per group. QC samples were prepared as described for metabolomic analysis. Amounts were quantitated by relative comparison to internal standards, see below.
Chromatographic separations were carried out on 4.6 × 250 mm J’sphere ODS-H80 (4 μm, YMC Co, Kyoto, Japan) for negative ion analysis. TOF-MS and TOF-MS/MS were operated with same parameters as for metabolomics, described above. The sample injection volume was 10 μL and the flow rate was 1 mL/min. The mobile phases consisted of water (A) and acetonitrile (B), both with 0.1% acetic acid. The gradient was as follows: an initial hold at 30% B for 4 min, followed by a gradient of 30–60% B in 1 min, to 65% B at 13 min, to 80% B at 25 min, to 100% at 26 min held until 32 min, then a shift to 30% B at 33 min until 38 min. The column temperature was held at 35 °C.
Extracellular flux analyzer assay for bioenergetic profiling
Oxygen consumption rate (OCR) and proton production rate (PPR) measurements were performed using the XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA) with methods based on [22]. Dual-analyte sensor cartridges were soaked in 500 μl XF Calibrant Solution (Seahorse Bioscience, Billerica, MA) in 24-well cell-culture microplates (Seahorse Bioscience, Billerica, MA) overnight at 30°C to hydrate. Embryos were staged and placed into 20 of 24 wells on an islet microplate; the remaining four wells served as temperature control wells. Islet plate capture screens were placed over the top of the measurement area to keep the embryos in place. Preliminary experiments were performed to determine the number of embryos needed to achieve OCRs that fell within the recommended specifications of the XF24 instrument for each developmental age: four embryos per well at 24 hpf, and two embryos per well at 48 hpf. At 24 hpf, embryos (4 embryos per well; n=7 wells per VitE condition) were rinsed and placed in unbuffered EM in the XF24 islet capture plate. Briefly, we used oligomycin A to inhibit ADP phosphorylation by the mitochondrial ATP synthase, thereby initiating a decrease in OCR that approximates the fraction of total basal respiration coupled to ATP turnover. Treatment with the uncoupling/protonophore agent, FCCP, causes translocation of protons from the mitochondrial intermembrane space to the matrix, leading to an increase in the OCR that approximates “maximal respiratory capacity”. Sodium azide (NaN3) blocks the respiratory chain by inhibiting cytochrome c oxidase, leaving “non-mitochondrial respiration”. The difference in minimum OCRs measured after oligomycin A and sodium azide treatments is a metric of respiration attributed to proton leak (i.e. ion movements requiring proton motive force) across the mitochondrial inner membrane [23,24]. Titrations of each reagent were performed at 24 and 48 hpf, to determine the concentrations that produced the maximum change in respiration without inducing death within the experimental time frame. Concentrated stocks of oligomycin and FCCP were prepared in DMSO at 10 mM and 20 mM, respectively. A concentrated stock of sodium azide (5 M) was prepared in phosphate-buffered saline.
OCR and PPR were measured before and after the addition of 50 μL of 63.2 μM oligomycin A (ATP synthase inhibitor), 55.6 μL 2.5 μM FCCP (mitochondrial uncoupler), and 61.6 μL sodium azide (mitochondrial complex IV inhibitor). The same protocol was used for 48 hpf embryos (2 embryos per well; n=7 wells per VitE group) with addition of 50 μL 9.2 μM oligomycin A; then 56.0 μL 2.8 μM FCCP; then 62 μL 1.25 mM NaN3. The Seahorse protocol consisted of calibration, equilibration, 8 measurements of baseline, 8 measurements after injection of oligomycin A and FCCP, and 12 measurements after injection of NaN3. OCRs and PPRs were calculated using a modified AKOS algorithm, available with the Seahorse Bioscience software, that takes into account oxygen diffusion through the plate and atmospheric leak, in addition to the oxygen consumed by the organism [22]. Buffer capacity was calculated by monitoring pH of the media, and PPR values derived based on the calculated buffer capacity and chamber volume of each well.
Microinjection rescue studies
Embryos were microinjected using a nanoliter 2000 injector (World Precision Instruments, Sarasota, FL, USA) linked to a stereoscopic microscope. Needles for microinjection were made in a puller (Sutter Instruments, model P-97, Novato, CA, USA) using glass capillary with an internal filament (1.14 o.d.; 0.5 mm i.d.). For pilot α-tocopherol injections, embryos were injected at the 1–2 cell stage (approximately 0.5 hpf according to [11]) into the yolk-sac with a sterile vitamin E preparation made for intravenous use in humans containing d6-α-tocopherol (d6-α-T, 5.4 g/L) in an oil-water emulsion made with soybean oil and phospholipids. Embryos were injected with 4.0 nL of the d6-α-T emulsion, a quantity (20 ng, or 46 pmol) that provides an amount of α-tocopherol to the E− embryos equal to that in 0 hpf E+ embryos [15]. Glucose injections were performed as described [25]. Briefly, at 24 hpf embryos were injected (into the yolk-sac) with 4.0 nL of 2.0 M glucose solution prepared with D-glucose (Sigma-Aldrich, St Louis, MO, USA) dissolved in sterile saline solution. This dose was based on the maximum solubility of glucose in water. Control-injection conditions for both experiments consisted of saline injection with sterile saline solution (NaCl, 58 mmol/L; KCl, 0.7 mmol/L; MgSO4, 0.4 mmol/L; Ca(NO3)2, 0.6 mmol/L; Hepes, 5 mmol/L; pH 7.3). Criteria used to assess supplementation tolerance of zebrafish embryos using ZAAP were embryonic development, growth, and mortality, assessed at 24, 48, and 120 hpf.
Data processing and statistical analyses
Targeted metabolomics data processing was performed using PeakView software (SCIEX). Sample peaks for each targeted metabolite of interest were annotated using the extracted ion chromatograms (XIC) lists based on high resolution MS, MS/MS fragmentation, isotopic distribution, and retention time compared with an in-house library of 635 metabolite standards (IROA Technologies, Bolton, MA, USA). In addition to the IROA database, metabolite identities were confirmed further using the METLIN web-based metabolomics database (http://www.metlin.scripps.edu). Peak intensities for each individual metabolic feature were normalized using the corresponding mean QC sample (n=4) intensity for that feature, as described [26], to balance their differences in intensities that may have arisen due to discrepancies in the sample homogenization (sample preparation). Student’s t-tests (Excel, Microsoft) to compare the two VitE groups at each developmental time-point (24, 48, 72, and 120 hpf) were performed with statistical significance set at p < 0.05. To control for false discovery rates for these metabolomics data during follow-up analyses, study outcome significance was set at Q < 0.05, using an adaptive linear step-up procedure [27]. Subsequent statistical analyses (e.g. 2-way ANOVA with Tukey’s or Sidak’s multiple comparison tests, as recommended by the software) were performed using GraphPad Prism 6.0 software (GraphPad, La Jolla, CA).
Quantification of total and free fatty acids, and hydroxy-DHA was performed using MutliQuant Software version 3.0.2 (SCIEX). Lipid peaks were identified based on the accurate masses and retention times of each individual lipid, then quantified by integrating peak area using MultiQuant Software. Raw area counts for each lipid were normalized using area counts for the corresponding internal standard, then corrected for internal standard concentration. Statistical analyses (e.g. 2-way ANOVA with Tukey’s multiple comparison test) were performed using GraphPad Prism 6.0 software (GraphPad, La Jolla, CA).
For OCR analyses, the extracellular flux analyzer assay data was collected and processed using the XF Reader software (Seahorse Biosciences, Agilent Technologies, Santa Clara, CA) and exported using Excel 2013 (Microsoft). The baseline OCR and PPR were calculated, in addition to the ATP-linked OCR, the maximum OCR, proton leak, and mitochondrial reserve capacity, as described by [22,23]. Additional statistical analyses (e.g. 2-way ANOVA with Tukey’s multiple comparison tests) were performed using GraphPad Prism 6.0 software.
Statistical analyses for morphological and behavioral endpoints were performed using code developed in R (R Developmental Core Team 2014, http://www.R-project.org). For morphological assessments at 24 and 96 hpf, binary responses were recorded as either absent (0) or present (1) for each of the 18 endpoints. For statistical analyses of behavior, raw data files were processed using custom R scripts with methodologies based on [19]. In brief, the distance traveled by each individual animal, over each integration period, was measured and then the total area under the curve (AUC) was computed for each animal. The overall AUCs for the E− compared to the E+ embryos then were compared using a combination of percent change (minimum 30% difference from E+ embryos) and a Kolmogorov-Smirnov test (p<0.01) to determine statistical significance.
Results and Discussion
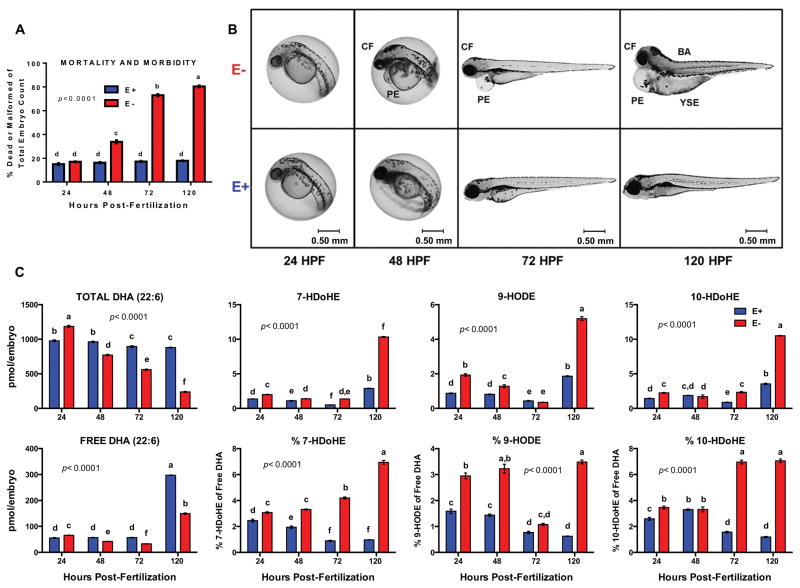
We obtained E− and E+ embryos by spawning adult 5D zebrafish fed either E− or E+ diets. E+ embryos grew normally, while E− embryos suffered >80% morbidity and mortality by 120 hpf (Fig. 1A–B). Measured α-tocopherol concentrations in living E− and E+ embryos were 1.09 ± 0.01 and 22.41 ± 0.04 pmol/embryo, respectively, at 120 hpf.
Fig. 1. VitE deficiency-induced morbidity and mortality coincides with decreased DHA and increased hydroxy-DHAs.
A. Increasing morbidity and mortality in E− embryos. B. Phenotypic deformities in E− embryos: CF=cranial-facial malformation, PE= pericardial edema, YSE= yolk-sac edema, BA= skewed body-axis. C. Relative-quantitation of free DHA and hydroxy-DHAs in E− vs. E+ embryos; area counts normalized using internal standards (n= 4 samples/group, n=15–30 embryos/sample). Percentages of each hydroxy-DHA = (hydroxy-DHA/free DHA)*100. Shown are means ± SEM; p-values from 2-way ANOVA with unique letters indicating differences (Tukey’s post-test, p<0.05).
At 48 hpf E− embryos contained decreased concentrations of DHA-containing phospholipids (DHA-PL), particularly phosphatidylcholine (PC) and phosphatidylethanolamine (PE), as well as DHA-lyso-PC [28], a major DHA transporter to the brain [29,30]. Since DHA is critical for normal neurodevelopment [29], we hypothesized that VitE deficiency causes embryonic death by depleting DHA-PC, thereby depriving the embryo of adequate DHA. We tested this hypothesis by assessing the impact of VitE status on relative quantities of total, free (unesterified) and oxidized DHA in E− vs. E+ embryos. Initially, total and free DHA were elevated in E− embryos, but by 48 hpf both were significantly lower than E+ embryos (Fig. 1C, see also Figure 1 and Tables 1 and 2 in [10]); further, the lipid peroxidation products, 7- and 10-hydroxy-DHA [31], as well as 9-HODE, were elevated in E− (Fig. 1C), ranging from 3–7% of the free DHA at 120 hpf, confirming increased DHA peroxidation.
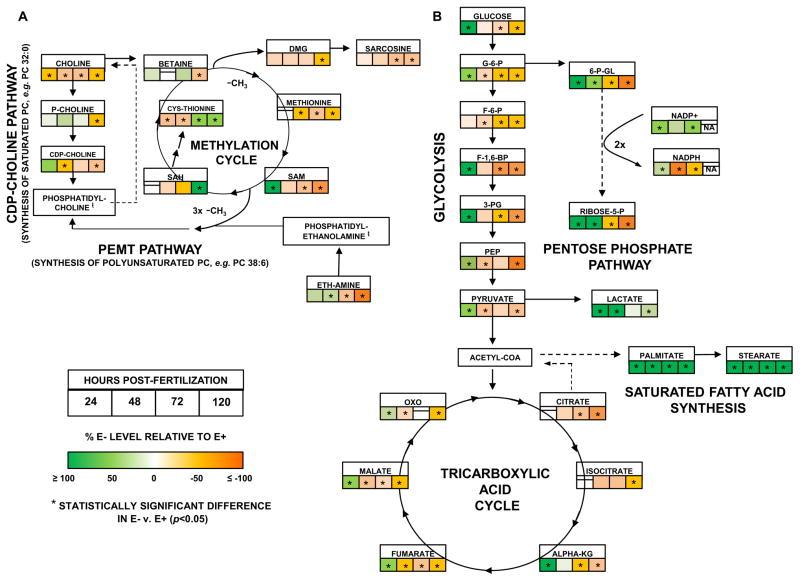
Craniofacial malformations are observed at 48 hpf in E− embryos (Fig. 1B) and in zebrafish embryos genetically manipulated to completely lack VitE [32] more severe defects are seen as early as 15–17 hpf, an embryonic stage that coincides with neurulation [11]. Since choline is a nutrient essential for neurulation in vertebrate embryos [33] and PC is a major source of choline [34], we hypothesized that the increased DHA-PC replenishment in E− embryos [28] induces a secondary choline deficiency. We found that as development progressed both choline and various choline-containing compounds in the cytidine-5-diphosphocholine (CDP-choline) pathway for PC synthesis decreased in the E− embryos (Fig. 2A; see also Figure 2, Tables 1 and 2 in [10]), consistent with our previous lipidomics-based investigations [35].
Fig. 2. Time course in E− embryos shows disrupted metabolism.
A. Choline regeneration. B. Glucose metabolism and TCA cycle. Shown are targeted metabolites (boxes (□) indicate comparisons at each time-point) with significance calculated using 2-way ANOVA (p-value = 0.05 to <0.0001, Table S3) and Sidak’s post-test (p<0.05) of normalized, log-transformed intensity values (n=4 samples/group, 15 embryos/sample, see Methods). NA = no data available. Abbreviations: A. P-Choline (phosphocholine), DMG (dimethylglycine), Eth-amine (ethanolamine), Cys-thionine (cystathionine), SAM (S-adenosyl-methionine), SAH (S-adenosyl-homocysteine), -CH3 (methyl group). B. G-6-P (glucose-6-phosphate), F-6-P (fructose-6- phosphate), F-1,6-BP (fructose-1,6-bisphosphate), 3-PG (3-phosphoglycerate), PEP (phosphoenolpyruvate), Alpha-KG (alpha-ketoglutarate), OXO (oxaloacetate), 6-P-GL (6- phosphogluconate), Ribose 5-P (ribose-5-phosphate).
PC can also be generated by the serial methylation of PE via the phosphatidylethanolamine N-methyltransferase (PEMT) pathway using the requisite methyl-donor, S-adenosylmethionine (SAM). Unlike the CDP-choline pathway that generates PC containing mono- and di-unsaturated fatty acids, the PEMT pathway generates PC containing polyunsaturated fatty acids [36] and enhances DHA transfer from mother to fetus in humans [37]. Evidence that the PEMT pathway in E− embryos caused depletion of methyl-donors is shown by the decreases at 120 hpf of SAM, methionine, and betaine, while the SAM oxidation product, S-adenosyl-homocysteine increased (Fig. 2A; see also Figure 2, Tables 1 and 2 in [10]). Thus, PC synthesis via the PEMT pathway in E− embryos became compromised due to choline and methyl-donor depletion.
VitE deficiency not only induced oxidative damage by increasing lipid peroxidation, but also depleted other antioxidants (see also Figure 3, Tables 1 and 2 in [10]). Specifically measured ascorbic acid concentrations in E− embryos were halved at 120 hpf (22.2 ± 0.3 vs. 59.8 ± 1.0 pmol/embryo in E− and E+, respectively, see also Figure 3, Tables 1 and 2 in [10]). We, therefore, hypothesized that the E− embryos have an increased need for reducing equivalents (NADPH) and that glucose would be shunted to the pentose phosphate pathway to generate NADPH [38]. In E− embryos, glucose was depleted with increases in 6-phosphogluconate and ribose-5-phosphate intermediates (Fig. 2B), while glycolytic and TCA cycle intermediates in E− embryos (although increased at 24 hpf) were decreased at subsequent time-points (Fig. 2B; see also Figure 4, Tables 1 and 2 in [10]). Moreover, NADPH was increasingly oxidized in E− embryos and NADP+/NADPH ratios increased over time (Fig. 2B; see also Figure 3, Tables 1 and 2 in [10]). This metabolic shift – aimed at ameliorating the effects of oxidative stress – occurred at the expense of energy-generating pathways, such as glycolysis and flow through the respiratory chain of tricarboxylic acid (TCA) cycle-generated reducing equivalents. Evidence for the uncoupling of the TCA cycle in E− embryos (i.e. citrate transported to cytosol for fatty acid synthesis and cytosolic reduction of pyruvate to lactate) is provided by their 1) increased levels of saturated fatty acids (palmitic and stearic, Fig. 2B, see also Figure 5, Tables 1 and 2 in [10]), 2) decreased glutamine and glutamate (see also Tables 1 and 2 in [10]), and 3) increased lactate (Fig. 2B, see also Figure 4, Tables 1 and 2 in [10]). Overall, this metabolic profile in E− embryos resembles the Warburg effect seen in cancer cells [39]. Thus, E− embryos experience dysregulation of energy metabolism, a phenomenon that is a major driver of cellular dysfunction and death as an ultimate consequence of VitE deficiency. Potentially, ferroptosis, a mechanism of programmed cell death due to increased lipid peroxidation [40,41], which is dependent on GPx4 and GSH, as well as VitE to prevent cell death [7], is responsible for the embryonic lethality. Notably, VitE has been suggested to function as a lipoxygenase inhibitor and thus preventing the generation of oxidized arachidonic and adrenic PE, which have been identified as lipid signaling molecules for ferroptosis [42]. These latter molecules were doubly and triply oxygenated, and thus beyond the technical capabilities of the approaches used for the present study, so further research is needed to assess the role of ferroptosis during VitE deficiency and embryogenesis in zebrafish. Additionally, endothelium specific Gpx4 depletion in mice showed that VitE was necessary not only for endothelial function but also mouse viability [43]. Moreover, studies in mice with a Gpx4 deletion in hematopoietic cells showed that GPx4 is essential for preventing receptor interacting protein 3 (RIP3)-dependent necroptosis in erythroid precursor cells, a process that could be ameliorated by VitE [44]. Thus, there are a number of pathways involving lipid peroxidation, GPx4 and VitE that lead to programmed cell death and emphasize the necessity for further studies to elucidate these various mechanisms.
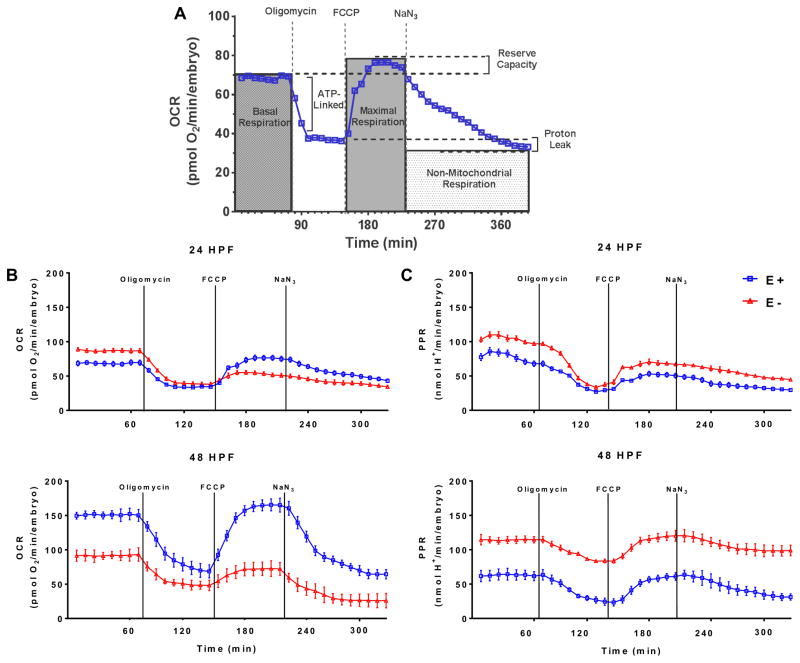
Fig. 3. Bioenergetic profiling in E− embryos.
A. Representative oxygen consumption rates (OCR) in 24 hpf E+ embryos (n=7); indicated are responses after sequential exposure to oligomycin, cyanide 4-(trifluoromethoxy) phenyl-hydrazine (FCCP) and sodium azide (NaN3). B. 25 OCR and C. Proton production rates (PPR) in E− vs. E+ embryos, showing means ± SEM, n=7 samples/group; for 24 hpf: 4 embryos/sample for 36/44 trials; for 48 hpf: 2 embryos/sample, 36/36 trials.
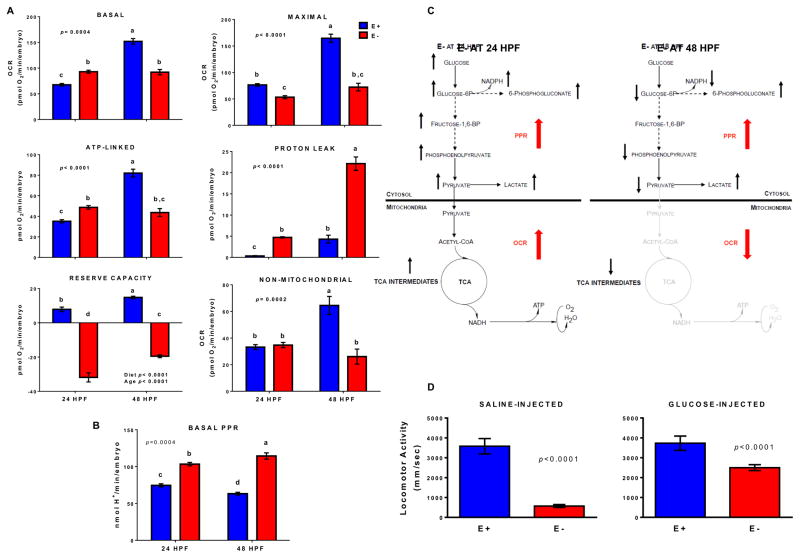
Fig. 4. Quantitation of bioenergetic profiling in E− embryos and glucose rescue.
A. OCR and B. PPR show means ± SEM (data from Fig. 3); p-value = 2-way ANOVA, Tukey’s post-test, unique letters indicate differences (p<0.05). C. Disrupted energy metabolism in E− embryos. Activity of gray pathways is reduced; red arrows denote bioenergetic rates showing metabolic switch between 24 and 48 hpf. Black arrows indicate differences in select metabolites between E− and E+ embryos (data from Fig. 2). D. E− and E+ embryos (n=128/group) injected into the yolk sac at 24 hpf with either saline or glucose were analyzed for locomotor activity at 96 hpf (mm/sec; see Methods). Glucose injection attenuated neurobehavioral abnormalities in E− embryos by ~50% (Kolmogorov–Smirnov test; p<0.0001).
To assess the degree to which energy metabolism was dysregulated during VitE deficiency, the bioenergetic profiles of E− and E+ embryos were assessed in real-time by measuring mitochondrial and non-mitochondrial respiration (Fig. 3A). At 24 hpf, E− embryos had higher basal oxygen consumption rates (OCR), but at 48 hpf their basal OCRs were decreased (Fig. 3B). At 24 and 48 hpf E− embryos had reduced levels of “maximal respiration” (Fig. 3B, 4A), demonstrating a lack of mitochondrial reserve capacity [23], and had an elevated proton production rates (PPR, Fig. 3C, 4B), concomitant with their increased lactate concentrations (Fig. 2B). Thus, E− embryos experienced a “metabolic switch” from a hyper- to a hypo-metabolic state consistent with non-energy generating glucose metabolism (i.e. pentose phosphate pathway) and TCA cycle uncoupling (i.e. citrate transport into cytosol) by 48 hpf (Fig. 2B, 4C).
We hypothesized that impairment of aerobic, mitochondrial glucose metabolism in E− embryos caused their neurobehavioral abnormalities. Morphologically normal 96 hpf E− embryos were 84% less responsive to light-dark stimuli in a locomotor assay (Fig. 4D; see also Figure 6 in [10]). To determine if increased glucose status would ameliorate the neurobehavioral defects, 24 hpf E− and E+ embryos were microinjected into the yolk sac with D-(+)-glucose in saline or with saline alone. Glucose rescued the neurological deficits in 50%, and morbidity and mortality in 31%, of E− embryos (Fig. 4D; see also Figure 7 in [10]), but they still sustained craniofacial deformities (not shown). Thus, it may be surmised that other nutrients, e.g. DHA and choline, are required by E− embryos to obtain a complete phenotypic rescue, as observed in a pilot trial employing micro-injection of a VitE-PL emulsion at 0 hpf (see Figure 6 in [10]). Deuterated polyunsaturated fatty acids, which are less susceptible to lipid peroxidation [45], and protect mitochondria from dysregulation caused by lipid peroxidation [46] could be used as rescue reagents in E− embryos to evaluate the role of the initial lipid peroxidation in embryonic lethality. Similarly, ferroptosis inhibitors, such as ferrostatin and liproxstatin [47], might be protective during VitE deficiency and would be a means to test directly the role of ferroptosis in E− embryo lethality. Clearly, further study is needed to define the molecular mechanisms by which VitE deficiency becomes lethal.
The mechanisms underlying VitE’s essentiality during neurodevelopment include its antioxidant protection of DHA and DHA-PLs, which prevents lipid peroxidation, additional antioxidant depletion, and metabolic dysregulation with secondary decreases in both choline and glucose. Additionally, specific effects on lipoxygenase enzymes may play critical roles in embryonic lethality. Such pleiotropic outcomes cause death by effectively starving the embryonic brain not only of vital nutrients, namely DHA and choline, but also of the energy needed for development. These findings acquire even greater significance upon consideration that – nearly 100 years after its discovery [1] – they provide mechanistic insights into why the vertebrate fetus requires VitE, and, as a corollary, expand the role VitE deficiency plays in human miscarriage.
Supplementary Material
Highlights.
Vitamin E deficiency depletes phosphatidylcholine, choline and methyl donors.
Increased lipid peroxidation shunts glucose to the pentose phosphate pathway.
Vitamin E deficiency causes mitochondrial dysfunction impairing energy homeostasis.
Outcomes could be attenuated by glucose injection into deficient embryos.
Vitamin E deficiency leads to a metabolic reprogramming that dysregulates cellular energy homeostasis.
Acknowledgments
The authors thank Carrie Barton, Greg Gonnerman, Andrea Knecht, Jane La Du, Scott Leonard, and Lisa Truong for providing outstanding technical assistance. Tory Hagen, Siva Kolluri and Andrew Karplus are gratefully acknowledged for reading the manuscript and providing critical advice. National Institutes of Health Grants S10RR027878 (MGT and JFS) and NIEHS P30 ES000201 (RT) supported this work. MM was supported in part by National Science Foundation Grant DGE 0965820. H-KK sabbatical support provided by The Catholic University of Korea. MGT supported in part by the Helen P Rumbel endowment to the LPI.
Abbreviations
- E+
α-tocopherol deficient
- E−
or α-tocopherol sufficient
- BHT
butylated hydroxytoluene
- FCCP
carbonyl cyanide 4-(trifluoromethoxy) phenyl-hydrazone
- CDP-choline
cytidine-5-diphosphocholine
- d
deuterium
- DHA
docosahexaenoic acid
- DHA-PL
DHA-containing phospholipids
- XIC
extracted ion chromatograms
- GSH
glutathione
- hpf
hours post-fertilization
- MS
mass spectrometry
- OCR
oxygen consumption rate
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PPR
proton production rate
- QC
quality control
- SAM
S-adenosylmethionine
- TOF
time-of-flight
- TCA
tricarboxylic acid
- VitE
vitamin E
- ZAAP
zebrafish acquisition and analysis program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 2.Burton GW, Joyce A, Ingold KU. First proof that vitamin E is major lipid-soluble, chain-breaking antioxidant in human blood plasma. Lancet. 1982;2:327. doi: 10.1016/s0140-6736(82)90293-8. [DOI] [PubMed] [Google Scholar]
- 3.Zanetti R, Catala A. Changes in n-6 and n-3 polyunsaturated fatty acids during lipid-peroxidation of mitochondria obtained from rat liver and several brain regions: effect of alpha-tocopherol. Prostaglandins Leukot Essent Fatty Acids. 2000;62:379–385. doi: 10.1054/plef.2000.0169. [DOI] [PubMed] [Google Scholar]
- 4.Wagner BA, Buettner GR, Burns CP. Free radical-mediated lipid peroxidation in cells: oxidizability is a function of cell lipid bis-allylic hydrogen content. Biochemistry. 1994;33:4449–4453. doi: 10.1021/bi00181a003. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E, Packer L, Traber MG. Antioxidants, oxidants, and redox impacts on cell function - A tribute to Helmut Sies. Arch Biochem Biophys. 2016;595:94–99. doi: 10.1016/j.abb.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Ursini F, Maiorino M, Valente M, Ferri L, Gregolin C. Purification from pig liver of a protein which protects liposomes and biomembranes from peroxidative degradation and exhibits glutathione peroxidase activity on phosphatidylcholine hydroperoxides. Biochim Biophys Acta. 1982;710:197–211. doi: 10.1016/0005-2760(82)90150-3. [DOI] [PubMed] [Google Scholar]
- 7.Carlson BA, Tobe R, Yefremova E, Tsuji PA, Hoffmann VJ, Schweizer U, Gladyshev VN, Hatfield DL, Conrad M. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 2016;9:22–31. doi: 10.1016/j.redox.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamim AA, Schulze K, Merrill RD, Kabir A, Christian P, Shaikh S, Wu L, Ali H, Labrique AB, Mehra S, Klemm RD, Rashid M, Sungpuag P, Udomkesmalee E, West KP., Jr First-trimester plasma tocopherols are associated with risk of miscarriage in rural Bangladesh. Am J Clin Nutr. 2015;101:294–301. doi: 10.3945/ajcn.114.094920. [DOI] [PubMed] [Google Scholar]
- 9.Szabolcs P, Angelika F, Roos FF, Wyss A, Eggersdorfer M, Hoffmann K, Weber P. A Systematic Review of Global Alpha-Tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. Int J Vitam Nutr Res. 2016:1–21. doi: 10.1024/0300-9831/a000281. [DOI] [PubMed] [Google Scholar]
- 10.McDougall M, Choi J, Kim H-K, Bobe G, Stevens JF, Cadenas E, Tanguay R, Traber MG. Lipid Quantitation and Metabolics Outcomes from Vitamin E Deficient and Sufficient Zebrafish Embyos from 0 to 120 Hours-Post-Fertillization. Data in Brief. 2017 doi: 10.1016/j.dib.2017.02.046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 12.Lebold KM, Jump DB, Miller GW, Wright CL, Labut EM, Barton CL, Tanguay RL, Traber MG. Vitamin E deficiency decreases long-chain PUFA in zebrafish (Danio rerio) J Nutr. 2011;141:2113–2118. doi: 10.3945/jn.111.144279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller GW, Labut EM, Lebold KM, Floeter A, Tanguay RL, Traber MG. Zebrafish (Danio rerio) fed vitamin E-deficient diets produce embryos with increased morphologic abnormalities and mortality. J Nutr Biochem. 2012;23:478–486. doi: 10.1016/j.jnutbio.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westerfield M. The Zebrafish Book; A guide for the laboratory use of zebrafish (Danio rerio) Eugene: University of Oregon Press; 2007. [Google Scholar]
- 15.Lebold KM, Kirkwood JS, Taylor AW, Choi J, Barton CL, Miller GW, La Du J, Jump DB, Stevens JF, Tanguay RL, Traber MG. Novel liquid chromatography-mass spectrometry method shows that vitamin E deficiency depletes arachidonic and docosahexaenoic acids in zebrafish (Danio rerio) embryos. Redox Biol. 2013;2:105–113. doi: 10.1016/j.redox.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381. doi: 10.1073/pnas.86.16.6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebold KM, Lohr CV, Barton CL, Miller GW, Labut EM, Tanguay RL, Traber MG. Chronic vitamin E deficiency promotes vitamin C deficiency in zebrafish leading to degenerative myopathy and impaired swimming behavior. Comp Biochem Physiol C Toxicol Pharmacol. 2013;157:382–389. doi: 10.1016/j.cbpc.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology. 2012;291:83–92. doi: 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol Sci. 2015;145:177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truong L, Saili KS, Miller JM, Hutchison JE, Tanguay RL. Persistent adult zebrafish behavioral deficits results from acute embryonic exposure to gold nanoparticles. Comp Biochem Physiol C Toxicol Pharmacol. 2012;155:269–274. doi: 10.1016/j.cbpc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkwood JS, Lebold KM, Miranda CL, Wright CL, Miller GW, Tanguay RL, Barton CL, Traber MG, Stevens JF. Vitamin C deficiency activates the purine nucleotide cycle in zebrafish. J Biol Chem. 2012;287:3833–3841. doi: 10.1074/jbc.M111.316018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stackley KD, Beeson CC, Rahn JJ, Chan SS. Bioenergetic profiling of zebrafish embryonic development. PLoS One. 2011;6:e25652. doi: 10.1371/journal.pone.0025652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, Landar A, Darley-Usmar VM. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill BG, Benavides GA, Lancaster JR, Jr, Ballinger S, Dell’Italia L, Jianhua Z, Darley-Usmar VM. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha F, Dias J, Engrola S, Gavaia P, Geurden I, Dinis MT, Panserat S. Glucose overload in yolk has little effect on the long-term modulation of carbohydrate metabolic genes in zebrafish (Danio rerio) J Exp Biol. 2014;217:1139–1149. doi: 10.1242/jeb.095463. [DOI] [PubMed] [Google Scholar]
- 26.Elie MR, Choi J, Nkrumah-Elie YM, Gonnerman GD, Stevens JF, Tanguay RL. Metabolomic analysis to define and compare the effects of PAHs and oxygenated PAHs in developing zebrafish. Environ Res. 2015;140:502–510. doi: 10.1016/j.envres.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 28.McDougall MQ, Choi J, Stevens JF, Truong L, Tanguay RL, Traber MG. Lipidomics and H2(18)O labeling techniques reveal increased remodeling of DHA-containing membrane phospholipids associated with abnormal locomotor responses in alpha-tocopherol deficient zebrafish (danio rerio) embryos. Redox Biol. 2016;8:165–174. doi: 10.1016/j.redox.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, Nguyen LN, Wenk MR, Ahmad AQ, Sreekantan-Nair A, Weedon MN, Rich P, Patton MA, Warner TT, Silver DL, Crosby AH. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47:814–817. doi: 10.1038/ng.3313. [DOI] [PubMed] [Google Scholar]
- 30.Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, Akizu N, Rosti RO, Rosti B, Scott E, Schroth J, Copeland B, Vaux KK, Cazenave-Gassiot A, Quek DQ, Wong BH, Tan BC, Wenk MR, Gunel M, Gabriel S, Chi NC, Silver DL, Gleeson JG. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47:809–813. doi: 10.1038/ng.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derogis PB, Freitas FP, Marques AS, Cunha D, Appolinario PP, de Paula F, Lourenco TC, Murgu M, Di Mascio P, Medeiros MH, Miyamoto S. The development of a specific and sensitive LC-MS-based method for the detection and quantification of hydroperoxy- and hydroxydocosahexaenoic acids as a tool for lipidomic analysis. PLoS One. 2013;8:e77561. doi: 10.1371/journal.pone.0077561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller GW, Ulatowski L, Labut EM, Lebold KM, Manor D, Atkinson J, Barton CL, Tanguay RL, Traber MG. The alpha-tocopherol transfer protein is essential for vertebrate embryogenesis. PLoS One. 2012;7:e47402. doi: 10.1371/journal.pone.0047402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Vance DE. Phosphatidylcholine and choline homeostasis. J Lipid Res. 2008;49:1187–1194. doi: 10.1194/jlr.R700019-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Choi J, Leonard SW, Kasper K, McDougall M, Stevens JF, Tanguay RL, Traber MG. Novel function of vitamin E in regulation of zebrafish (Danio rerio) brain lysophospholipids discovered using lipidomics. J Lipid Res. 2015;56:1182–1190. doi: 10.1194/jlr.M058941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pynn CJ, Henderson NG, Clark H, Koster G, Bernhard W, Postle AD. Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J Lipid Res. 2011;52:399–407. doi: 10.1194/jlr.D011916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Brenna JT, Stabler SP, Allen RH, Gregory JF, 3rd, Caudill MA. Pregnancy alters choline dynamics: results of a randomized trial using stable isotope methodology in pregnant and nonpregnant women. Am J Clin Nutr. 2013;98:1459–1467. doi: 10.3945/ajcn.113.066092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartnik BL, Lee SM, Hovda DA, Sutton RL. The fate of glucose during the period of decreased metabolism after fluid percussion injury: a 13C NMR study. J Neurotrauma. 2007;24:1079–1092. doi: 10.1089/neu.2006.0210. [DOI] [PubMed] [Google Scholar]
- 39.Devic S. Warburg Effect - a Consequence or the Cause of Carcinogenesis? J Cancer. 2016;7:817–822. doi: 10.7150/jca.14274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2016 doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuzlak S, Kaufmann T, Villunger A. Interrogating the relevance of mitochondrial apoptosis for vertebrate development and postnatal tissue homeostasis. Genes Dev. 2016;30:2133–2151. doi: 10.1101/gad.289298.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wortmann M, Schneider M, Pircher J, Hellfritsch J, Aichler M, Vegi N, Kolle P, Kuhlencordt P, Walch A, Pohl U, Bornkamm GW, Conrad M, Beck H. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ Res. 2013;113:408–417. doi: 10.1161/CIRCRESAHA.113.279984. [DOI] [PubMed] [Google Scholar]
- 44.Canli O, Alankus YB, Grootjans S, Vegi N, Hultner L, Hoppe PS, Schroeder T, Vandenabeele P, Bornkamm GW, Greten FR. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–148. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill S, Lamberson CR, Xu L, To R, Tsui HS, Shmanai VV, Bekish AV, Awad AM, Marbois BN, Cantor CR, Porter NA, Clarke CF, Shchepinov MS. Small amounts of isotope-reinforced polyunsaturated fatty acids suppress lipid autoxidation. Free Radic Biol Med. 2012;53:893–906. doi: 10.1016/j.freeradbiomed.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andreyev AY, Tsui HS, Milne GL, Shmanai VV, Bekish AV, Fomich MA, Pham MN, Nong Y, Murphy AN, Clarke CF, Shchepinov MS. Isotope-reinforced polyunsaturated fatty acids protect mitochondria from oxidative stress. Free Radic Biol Med. 2015;82:63–72. doi: 10.1016/j.freeradbiomed.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 47.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.