Afterload is an important determinant of myocardial contraction and relaxation. Afterload-dependency of relaxation has been demonstrated in the intact left ventricle (LV).1–4 Experimental animal2,3 and human4 studies demonstrate that the effects of afterload depend on its timing, with late systolic load inducing more profound impairments in LV relaxation, compared to early systolic load. In this issue of Hypertension, Gu et al5 report on a study that provides novel insights into diastolic-systolic coupling and helps us integrate and reconcile a number of previous findings.
Although the terms “myocardial afterload” and “ventricular afterload” are often used interchangeably, it is important to recognize that the relationship between LV and myocardial afterload is markedly influenced by the time-varying LV geometry during systole, which in turn affects the myocardial wall stress (MWS) for any given LV chamber pressure. LV afterload is the hydraulic load imposed by the entire systemic circulation (aortic input impedance), which depends on the pressure required to generate flow (ejection) into the proximal aorta. In contrast, myocardial afterload is determined by the MWS required to generate fiber shortening. In line with Laplace’s law of the heart, for any given LV pressure, lower MWS occurs as the ratio of LV chamber volume to LV wall volume decreases. When the MWS and LV geometry are such that LV pressure exceeds diastolic aortic pressure, LV ejection starts. Throughout ejection, the time-varying MWS (and fiber shortening) are determined by complex interactions between myocardial contractile elements, the instantaneous LV geometry and the time-varying hydraulic load imposed by the arterial tree.6 All key correlates of MWS (LV wall thickness, cavity size and pressure, which for any given flow ejected, depends on the aortic input impedance) exhibit marked variations during ejection. Therefore, time-resolving MWS during ejection (rather than using a single end-systolic estimation) is necessary to gain insights into the bidirectional dynamic interactions between the myocardium, the LV chamber properties, and the arterial tree.
Characterizing the in vivo patterns of MWS has important implications for our understanding of how the heart deals with afterload in health and disease. Previous studies have shown that, among normotensive and hypertensive adults with a normal LV ejection fraction (EF), peak MWS typically occurs in early systole.6–8 This is followed by a marked change in the relationship between LV pressure and MWS during mid-systole, which determines a lower stress for any given LV (and aortic) pressure.6 This phenomenon, which appears ideal to protect cardiomyocytes against excessive load in mid-to-late systole,6,9 depends on the dynamic reduction of LV chamber size relative to wall volume, and its magnitude is highly variable between individuals.6 In both normotensive and hypertensive individuals, a lower LV EF (even within the “normal” EF range) and a more concentric LV geometry (as seen in concentric LV remodeling or hypertrophy) are associated with a less pronounced mid-systolic shift of the pressure-stress relation, and thus a less efficient reduction in late systolic MWS for any given LV/aortic pressure.6 Therefore, although wave reflections selectively increase mid-to-late systolic LV load and MWS,6,7 the time course of LV contraction is an important determinant of the degree to which cardiomyocytes are “protected” against the effects of wave reflections in mid-to-late systole, a period of particular vulnerability to the deleterious effects of increased afterload.1–4,9
Gu et al5 studied the relationship between an index of early systolic myocardial shortening on one hand, and the subsequent course of systolic MWS or measures of diastolic function on the other, among hypertensive adults. As done in previous studies,6–8 the authors computed ejection-phase MWS using a combination of carotid tonometry (to obtain a surrogate of time-resolved ejection-phase LV pressure) and speckle-tracking 2D-echocardiography (to obtain time-resolved LV cavity and wall volumes).6–8 As an index of early systolic fiber shortening, they computed the fraction of the LV end-diastolic volume ejected prior to the first LV systolic pressure peak (which they call “first-phase ejection fraction”, EF1). The time to peak MWS (i.e., the time after which MWS first starts to decline) was called the “time to the onset of relaxation” (TOR). Measures of diastolic function included the early diastolic mitral annular tissue Doppler velocity (e′, a surrogate of relaxation) and the ratio of early diastolic mitral inflow peak velocity to e′ (E/e′, a surrogate of left atrial pressure). The authors found that a lower EF1 and a later TOR were independently associated with worse diastolic function (lower e′ and higher E/e′). Sublingual nitroglycerin had no significant effect on LV EF, but increased EF1, reduced TOR and increased e′. Interestingly, EF1 was not correlated with the characteristic impedance of the proximal aorta, which governs the pulsatile LV load during early ejection. Therefore, a lower EF1 does not appear to simply reflect an “impeded” ejection from pulsatile arterial load during early systole, but may be a marker of the contractile state of the myocardium. In support of this, the authors found that intracoronary nitroglycerin increased EF1 (relative to the overall EF), at doses that are unlikely to affect the systemic vasculature. Therefore, even in the presence of a normal EF, it appears that subtle abnormalities in contractile function can determine unfavorable patterns in time-varying MWS that induce/facilitate late systolic loading at the myocardial level.
The findings of Gu et al are in line with a previous study in the community-based Asklepios cohort, including normotensive and hypertensive middle-aged adults, in which the ratio of late/early ejection-phase MWS was independently associated with impaired LV relaxation assessed with tissue Doppler.8 However, Gu et al provide novel findings, suggesting that a lower EF1 (which presumably depends, at least partially, on intrinsic myocardial contractile function), is associated with a prolonged time course of MWS. Reduced early phase ejection also implies an impaired dynamic reduction of LV cavity size in early systole, with an impaired reconfiguration of LV geometry, thus failing to markedly reduce MWS relative to pressure in mid-to-late systole. This creates a more direct relationship between LV pressure and MWS at a time when wave reflections exert the bulk of their hemodynamic load, exposing the myocardium to the deleterious effects of afterload during a period of increased vulnerability.
The authors provide an elegant explanation for their findings, suggesting that shortening-deactivation (or lack thereof) is involved. Shortening-deactivation refers to the reduced capacity of active muscle fibers to develop force following shortening. Shortening-deactivation also increases the relaxation rate of muscle. By decreasing force at the end of shortening, and increasing the relaxation rate, shortening-deactivation appears like a “perfect” mechanism to accelerate myocardial relaxation in situations in which myocardial force development in late systole is not necessary to sustain ejection (i.e., in normal conditions in which the LV contraction and dynamic changes in its geometry quickly reduce MWS in mid-systole, as mentioned above). However, when EF1 is reduced and the pattern of LV contraction fails to protect cardiomyocytes against increased load in mid-to-late systole, only sustained force development can allow for continued fiber shortening (and LV ejection against the arterial load), tending to preserve the EF on one hand, but impairing myocardial relaxation on the other. This paradigm not only explains findings in hypertensive subjects with a preserved LV EF, but can also explain the elusive load-independence of relaxation in healthy humans with intact myocardial/LV function, and the marked non-linear influence of LV chamber function (and EF) on the load-dependence of relaxation observed in response to nitroprusside and other vasoactive interventions that modify wave reflection.1
Although the mechanism underlying the observations of Gu et al cannot be ascertained from the data, it is highly plausible that shortening-deactivation is indeed involved. However, alternative mechanisms should be considered. Stretch-activation (the counterpart of shortening-deactivation, by which an increased fiber length prior to contraction enhances shortening velocity during contraction) may also be at play via reverse causation. In the presence of increased LV diastolic stiffness, myofiber lengthening is limited for any given level of LV end-diastolic pressure. Therefore, processes that increase LV stiffness (such as myocardial fibrosis and concentric remodeling), may limit lengthening-activation, reducing early systolic contractility and EF1. Because these same processes are associated with impaired relaxation and increased filling pressures, they may represent an “epiphenomenon” contributing to the link between EF1 and diastolic dysfunction. Similarly, to the degree that the inotropic and lusitropic states of the myocardium are coupled at the biochemical level, a better EF1 could simply be a maker of better systolic (and by association, better diastolic) myocardial function. Finally, in cases of less robust myocardial tracking (an unavoidable problem with speckle tracking echocardiography), dynamic changes in cavity size may be underestimated throughout ejection. This would underestimate early systolic ejection and also overestimate late systolic cavity size (and MWS). Such correlated error may introduce (or exaggerate) relationships between EF1 and late systolic MWS, but is unlikely to explain the relationship between EF1 and diastolic function.
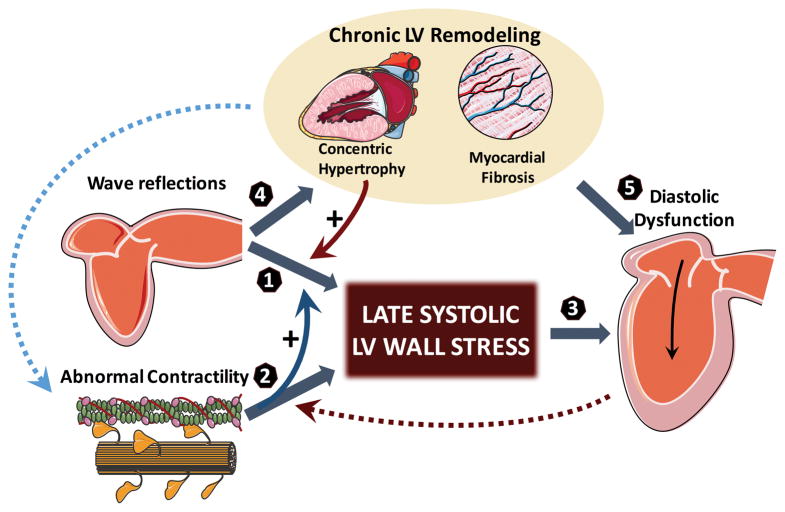
Figure 1 presents a pathophysiologic paradigm that aims to integrate our current understanding of time-varying myocardial systolic load on diastolic function in vivo. Several questions require further investigation: (1) Is the effect of wave reflections on heart failure risk9,10 explained by a “two-hit” model, by which a primary cardiomyopathic process impairs early ejection enough for the myocardium to be “exposed” to late systolic arterial load by wave reflections?; (2) Given the ill effects of late systolic arterial load on chronic LV hypertrophy and fibrosis,11,12 do wave reflections potentiate and perpetuate primary cardiomyopathic processes that impair the time course of MWS?; (3) In the presence of impaired early systolic ejection (EF1) despite preserved overall EF, could enhancing shortening-deactivation be beneficial to improve diastolic relaxation, at the expense of some impairment in late systolic ejection?; Would concomitant therapeutic reductions in wave reflection ameliorate the later problem and/or potentiate improvements in diastolic function in this setting? (4) Do these observations extend to patients with established heart failure with preserved and reduced EF?. These clinically-relevant questions can be successfully approached with human experimental studies, but only if adequate in vivo physiologic assessments are made. Gu et al should be congratulated for adopting deep phenotyping and applying clever mechanistic thinking to increase our understanding of abnormal systolic-diastolic coupling, a highly relevant problem underlying the current epidemic of congestive heart failure.
Figure 1. Working pathophysiological model linking time-varying MWS and diastolic dysfunction.
Arterial wave reflections selectively increase mid-to-late systolic LV load and MWS (1). However, the effect of wave reflections on MWS is strongly influenced by the LV contraction pattern. Normally, brisk force development and fiber shortening occur in early systole, resulting in LV ejection and a dynamic reconfiguration of LV geometry that results in a mid-systolic reduction in MWS relative to LV pressure, thus protecting the cardiomyocytes against excessive load in mid-to-late systole (a period of increased vulnerability). The phenomenon of shortening-deactivation, which decreases force development after rapid shortening, and increases the relaxation rate of muscle, is a perfect “match” to this normal physiology, because sustained myocardial force development in mid-to-late systole is unnecessary to preserve fiber shortening and LV ejection against the load imposed by wave reflections. However, in the presence of contractile abnormalities that compromise early ejection (and reduce EF1) (2), the mid-systolic dynamic geometric reconfiguration of the LV that favors a reduced MWS relative to pressure does not occur. Late systolic MWS therefore more directly correlates with LV pressure, indicating that the myocardium is less protected from late systolic arterial load induced by wave reflections, thus facilitating their ill effects (curved blue solid arrow). In these conditions, shortening-deactivation does not operate fully, such that force development continues or increases in mid-to-late systole, tending to preserve the overall EF on one hand, but impairing relaxation (3) on the other. Late systolic LV load from wave reflections also promotes chronic LV hypertrophy/fibrosis11,12 (4), which can induce diastolic dysfunction (5) and contractile abnormalities (curved blue dotted arrow) in their “own right”. Concentric LV hypertrophy/remodeling is also intrinsically associated with a blunted mid-systolic shift in the pressure-MWS relation, facilitating a myocardial loading sequence characterized by increased late systolic load (curved red solid arrow). Finally, reduced lengthening-activation from increased ventricular stiffness may impair early systolic contraction (curved red dotted arrow). For simplicity, the potential role of other factors (stored potential energy in the myocardium/restoring forces, interventricular dependence, pericardial restraint, ascending aortic stretch and
Acknowledgments
Funding information: JAC is supported by NIH grants R01-HL-121510-01A1 and R56 HL-124073-01A1.
Footnotes
Disclosures: J.A.C. has served as a consultant for Bristol-Myers Squibb, Microsoft, Merck, OPKO Healthcare, Fukuda-Denshi, and Vital Labs. He has received research grants from the National Institutes of Health, American College of Radiology Network, Fukuda-Denshi, Bristol-Myers Squibb, and Microsoft, and device loans from Atcor Medical. He is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction.
References
- 1.Eichhorn EJ, Willard JE, Alvarez L, Kim AS, Glamann DB, Risser RC, Grayburn PA. Are contraction and relaxation coupled in patients with and without congestive heart failure? Circulation. 1992;85:2132–2139. doi: 10.1161/01.cir.85.6.2132. [DOI] [PubMed] [Google Scholar]
- 2.Gillebert TC, Lew WY. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261:H805–813. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 3.Hori M, Inoue M, Kitakaze M, Tsujioka K, Ishida Y, Fukunami M, Nakajima S, Kitabatake A, Abe H. Loading sequence is a major determinant of afterload-dependent relaxation in intact canine heart. The American journal of physiology. 1985;249:H747–754. doi: 10.1152/ajpheart.1985.249.4.H747. [DOI] [PubMed] [Google Scholar]
- 4.Yano M, Kohno M, Kobayashi S, Obayashi M, Seki K, Ohkusa T, Miura T, Fujii T, Matsuzaki M. Influence of timing and magnitude of arterial wave reflection on left ventricular relaxation. Am J Physiol Heart Circ Physiol. 2001;280:H1846–1852. doi: 10.1152/ajpheart.2001.280.4.H1846. [DOI] [PubMed] [Google Scholar]
- 5.Gu H, LY, Fok H, Simpson J, Kentish JC, Shah A, Chowienczyk P. Reduced first-phase ejection fraction and sustained myocardial wall stress in hypertensive patients with diastolic dysfunction. Hypertension. 2017 doi: 10.1161/HYPERTENSIONAHA.116.08545. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, Townsend R, Ferrari VA, Wiegers SE, St John Sutton M. Time-varying myocardial stress and systolic pressure-stress relationship: Role in myocardial-arterial coupling in hypertension. Circulation. 2009;119:2798–2807. doi: 10.1161/CIRCULATIONAHA.108.829366. [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER, Asklepios I. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60:64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 8.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC, Asklepios I. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: The asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 9.Shah SJ, Wasserstrom JA. Increased arterial wave reflection magnitude: A novel form of stage b heart failure? J Am Coll Cardiol. 2012;60:2178–2181. doi: 10.1016/j.jacc.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial wave reflections and incident cardiovascular events and heart failure: Mesa (multiethnic study of atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]