Abstract
Zika virus (ZIKV) has recently emerged as an explosive pandemic associated with severe neuropathology in newborns and adults1. There are no ZIKV-specific treatments or preventatives; thus, development of a safe and effective vaccine is a high priority. Messenger RNA (mRNA) has emerged as a versatile and highly effective platform to deliver vaccine antigens and therapeutic proteins2,3. Here, we demonstrate that a single low-dose intradermal immunization with lipid nanoparticle-encapsulated nucleoside-modified mRNA (mRNA-LNP) encoding the pre-membrane and envelope (prM-E) glycoproteins of a 2013 ZIKV outbreak strain elicited potent and durable neutralizing antibody responses in mice and non-human primates. Immunization with 30 μg of nucleoside-modified ZIKV mRNA-LNPs protected mice from ZIKV challenges at 2 weeks or 5 months post-vaccination, and a single dose of 50 μg was sufficient to protect non-human primates from a challenge at 5 weeks post-vaccination. These data demonstrate that nucleoside-modified mRNA-LNPs elicit rapid and durable protective immunity and thus represent a new and promising vaccine candidate for the global fight against ZIKV.
ZIKV, first identified in 19474, is a mosquito-borne and sexually transmitted flavivirus that has recently been associated with microcephaly and other birth defects in newborns and Guillain-Barré syndrome in adults1. Effective vaccines have been approved for other closely related flaviviruses5–7, but vaccine candidates for ZIKV have only recently been developed8–12. Multiple vaccine formats have been shown to protect mice or non-human primates (NHPs) from ZIKV infection, including plasmid DNA9–12, purified inactivated virus10,11, protein subunit8, and adenovirus vectors8,10. The ideal vaccine is safe and induces protective immunity after a single immunization, regardless of prior serologic history. Of the candidate Zika vaccines described to date, only a rhesus adenovirus platform (RhAd52) has been shown to confer protection after a single immunization in NHPs; however, the efficacy of the RhAd52 vector in humans is currently undefined. Additionally, pre-existing immunity to adenovirus serotypes can limit the efficacy of such vectors13,14, and low neutralizing titers to rhesus adenoviruses, including RhAd52, have been detected in humans15.
mRNA has emerged as a promising new vaccine modality that can elicit potent immune responses (reviewed in 2,3), while avoiding the safety risks and anti-vector immunity associated with some live virus vaccines (reviewed in 16). Vaccination with mRNA offers several advantages over other vaccine platforms: (I) it is a non-integrating, non-infectious gene vector that can be readily designed to express any protein with high efficiency, (II) it has the potential for cost-effective and highly scalable manufacturing, and (III) small doses are sufficient to induce protective immune responses.
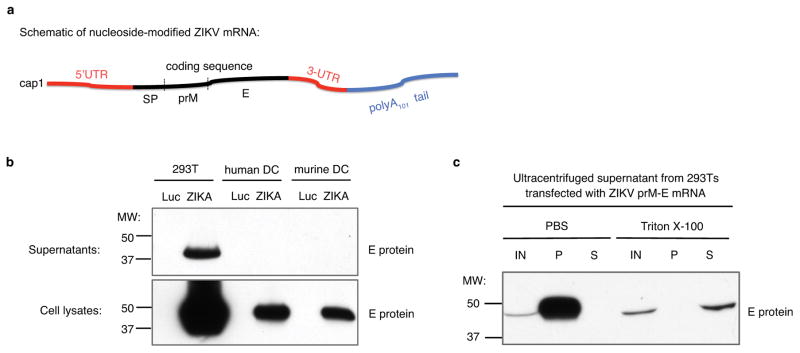
Here, we designed a novel, potent anti-ZIKV vaccine in which the prM-E glycoproteins of ZIKV H/PF/201317 are encoded by mRNA (Extended Data Fig. 1a) containing the modified nucleoside 1-methylpseudouridine (m1ψ), which prevents innate immune sensing and increases mRNA translation in vivo18. Nucleoside-modified ZIKV prM-E mRNA was formulated for vaccination in lipid nanoparticles (LNPs), which have been shown to mediate efficient and prolonged protein expression by mRNA in vivo19. Studies of ZIKV and other flaviviruses have demonstrated that co-expression of prM and E proteins is sufficient to assemble and secrete subviral particles12,20. ZIKV prM-E-encoding mRNA was first characterized by transfecting HEK 293T cells and human and murine dendritic cells (DCs). ZIKV E protein was produced by all cell types and was secreted into the supernatant of 293T cells (Extended Data Fig. 1b). We hypothesize that DCs also secrete E protein and that it is rapidly endocytosed, as has been proposed for HIV gag21. E protein in supernatant was pelleted by ultracentrifugation when incubated with PBS, but not with 0.5% Triton X-100, consistent with subviral particle production from prM-E mRNA20 (Extended Data Fig. 1c).
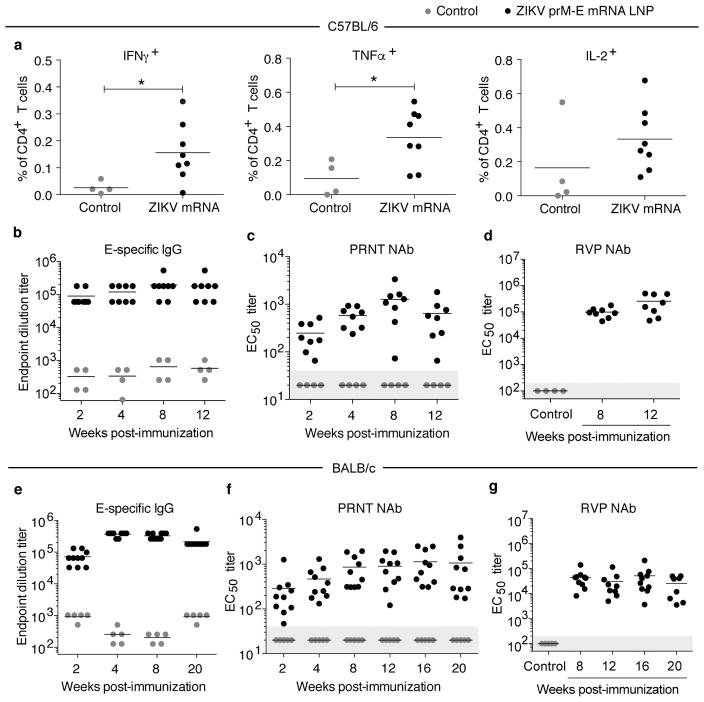
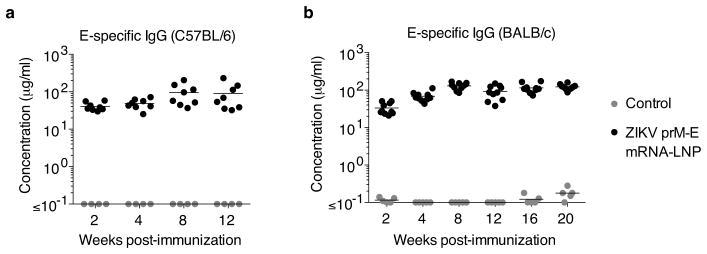
The immune response induced by the nucleoside-modified ZIKV prM-E mRNA-LNP vaccine was first evaluated in C57BL/6 mice. Animals were intradermally (i.d.) immunized with 30 μg of ZIKV prM-E mRNA-LNPs or poly(C) RNA-LNPs (negative control). No inflammation or other adverse events were observed at the sites of injection. Polyfunctional E protein-specific CD4+ T cell responses were detected based on intracellular IFN-γ, TNF-α, and IL-2 production by ZIKV E protein-stimulated splenocytes at week 2 post-vaccination (Fig. 1a and Extended Data Fig. 2). ZIKV E-specific serum IgG developed quickly in vaccinated mice and stabilized at an endpoint titer of 180,000 (~90 μg/ml) at weeks 8 to 12 (Fig. 1b and Extended Data Fig. 3a). Anti-ZIKV neutralizing antibodies (NAb) were measured using two independent assays: a standard plaque reduction neutralization test (PRNT) and a ZIKV reporter viral particle (RVP) assay12. The mean PRNT50 titer against ZIKV MR-766 peaked at ~1,300 at week 8 (Fig. 1c) and was relatively stable until week 12. The mean RVP NAb titer (EC50) against ZIKV H/PF/2013 reached ~105 at weeks 8 and 12 (Fig. 1d). The detection of higher NAb titers in the RVP assay compared to other assay formats similar to PRNT has been previously reported12. In addition, we noted that the ratio of RVP to PRNT titers was not fixed and varies with the animal model and viral isolate.
Figure 1. Nucleoside-modified ZIKV mRNA-LNP immunization elicits ZIKV-specific T helper and neutralizing antibody responses.
a–d, C57BL/6 mice were immunized i.d. with 30 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs (n=8) or control poly(C) RNA-LNPs (n=4). (a) At week 2, splenic antigen-specific CD4+ T cells were detected by intracellular cytokine staining. The antibody response was monitored by (b) ELISA, (c) PRNT using ZIKV MR-766, and (d) RVP using ZIKV H/PF/2013. e–g, BALB/c mice were immunized similarly with ZIKV mRNA-LNPs (n=10) or poly(C) RNA-LNPs (n=5) and monitored by (e) ELISA, (f) PRNT using MR-766, and (g) RVP using H/PF/2013. Points represent individual mice; horizontal lines show the mean; shaded area indicates values below the limit of detection. The controls in d and g are from the week 8 time point. Asterisk indicates p<0.05 in unpaired t-test; antibody responses in vaccine and control groups were compared at each time point by Mann-Whitney test: p<0.01 for all comparisons.
Immunogenicity of the nucleoside-modified ZIKV prM-E mRNA-LNP vaccine was next evaluated in BALB/c mice. E-specific serum IgG peaked at week 8 and remained stable between weeks 8 and 20 (endpoint titers ~200,000; 90–130 μg/ml) (Fig. 1e and Extended Data Fig. 3b). PRNT50 NAb increased to a maximum of ~1,100 at week 16 and remained stable until week 20 (Fig. 1f). The RVP NAb titer rose to 50,000 at week 8 and remained above 20,000 until week 20 (Fig. 1g).
A challenge study was conducted in BALB/c mice immunized i.d. with 30 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs or poly(C) RNA-LNPs. Mice were intravenously (i.v.) challenged at week 2 (short-term) or week 20 (long-term) post-immunization with 200 plaque-forming units (PFU) of ZIKV PRVABC59. In the short-term protection study, 8 of 9 control mice developed viremia by day 3, with a median peak of ~14,000 copies/ml. All ZIKV mRNA-immunized mice (n=9) were protected from detectable viremia (Fig. 2a). In the long-term study, all control mice (n=5) showed viremia on day 3, with a median peak of 1,200 copies/ml, while none of the ZIKV mRNA-immunized mice (n=10) had detectable viremia at days 3 or 7 (Fig. 2b). These data demonstrate that a single immunization with nucleoside-modified ZIKV prM-E mRNA-LNPs rapidly elicits durable protection from detectable viremia with a heterologous ZIKV strain in mice.
Figure 2. A single immunization of nucleoside-modified ZIKV prM-E mRNA-LNPs provides rapid and durable protection from ZIKV challenge in mice.
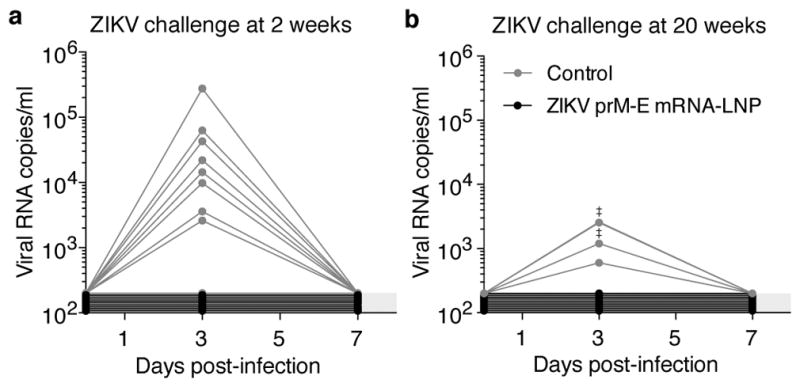
BALB/c mice immunized i.d. with 30 μg of ZIKV prM-E mRNA-LNPs or control poly(C) RNA-LNPs were challenged i.v. with 200 PFU ZIKV PRVABC59 at (a) 2 weeks (n=9 per group) or (b) 20 weeks (n=5 control mice; n=10 ZIKV mRNA-LNP mice) post-vaccination, and plasma viral loads were measured by qRT-PCR for ZIKV capsid RNA. ‡ symbol indicates two overlapping curves. Shaded area indicates values below the limit of detection (200 copies/ml), with undetectable curves staggered to show individual mice. Day 3 viremia in vaccine and control groups was compared by Mann-Whitney test: p<0.001 for both challenges.
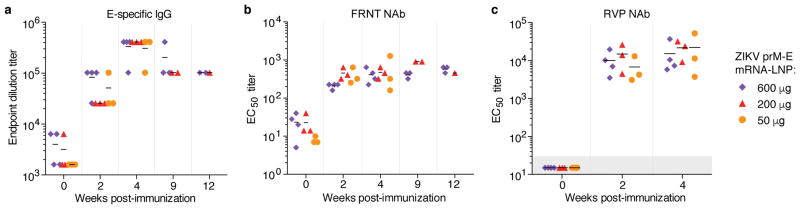
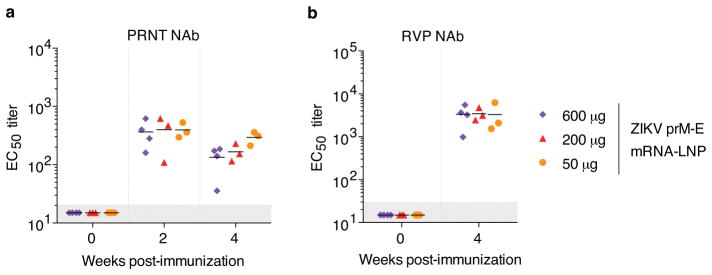
We next evaluated the efficacy of the nucleoside-modified ZIKV mRNA-LNP vaccine in rhesus macaques (Macaca mulatta), a non-human primate species that recapitulates several features of ZIKV infection in humans22. Macaques were immunized i.d. with doses of 600 μg, 200 μg or 50 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs. As in mice, no inflammation or other adverse events were observed. E-specific binding IgG and NAb were efficiently induced by all three vaccine doses, with no statistically significant differences between groups. Endpoint IgG titers rose to >300,000 in all groups at week 4 and were maintained at ≥100,000 until week 12 (Fig. 3a). PRNT50 NAb titers against MR-766 peaked at ~400 at week 2 (Extended Data Fig. 4a). To facilitate comparison of NAb titers across laboratories, the neutralization curve is shown for a human ZIKV-neutralizing mAb, A3594, in the PRNT assay (Extended Data Fig. 5). NAb titers obtained with a focus reduction neutralization test (FRNT), which has a format similar to PRNT, were stable around 400 against ZIKV MEX I-44 at weeks 2 through 12 (Fig. 3b). The RVP assay revealed NAb titers against H/PF/2013 of ~10,000 at week 2 and ~17,000 at week 4 (Fig. 3c), and a titer of ~3,000 against MR-766 at week 4 (Extended Data Fig. 4b). The neutralization of both Asian- and African-lineage viruses is consistent with a prior report demonstrating the existence of only one serotype of ZIKV23. The absence of a significant dose-dependent effect on the antibody response in any assay (Kruskal-Wallis test, p>0.05) suggests that a low dose of 50 μg (approximately 0.02 mg/kg) was sufficient, or possibly more than sufficient, to induce robust anti-ZIKV immunity in macaques.
Figure 3. Nucleoside-modified ZIKV mRNA-LNP immunization elicits potent ZIKV-specific neutralizing antibody responses in non-human primates.
Rhesus macaques were immunized with 600 μg (n=4), 200 μg (n=3), or 50 μg (n=3) of ZIKV prM-E mRNA-LNPs, and the antibody response was quantified by (a) ELISA, (b) FRNT using ZIKV MEX I-44, and (c) RVP using ZIKV H/PF/2013. Pre-challenge (weeks 0 to 4) and unchallenged animal data are shown. Points represent individual monkeys; shaded area indicates values below the limit of detection; horizontal lines indicate the mean. Immune responses in dose groups were compared by Kruskal-Wallis test: p>0.05 for all comparisons.
Rhesus macaques were challenged at week 5 by subcutaneously (s.c.) injecting 104 TCID50 of ZIKV PRVABC59 into five vaccinated animals and six control animals (Extended Data Table 1). All control animals became infected with median peak plasma viremia of 7,000 ZIKV RNA copies/ml (Fig. 4). In contrast, vaccinated macaques were highly protected from ZIKV infection. Four of five animals — including three that received the lowest dose of 50 μg and one that received the medium dose of 200 μg — had no detectable viremia (<50 copies/ml) at all time points. We detected a low and transient viral blip of 100 copies/ml at day 3 post-challenge in one animal that received the highest dose of 600 μg of ZIKV prM-E mRNA-LNPs, representing a ~99% reduction in peak viremia compared to the control animals. This animal exhibited among the lowest NAb titers in multiple assays at week 4 post-vaccination: PRNT50 of 36 to MR-766, FRNT50 of 226 to MEX I-44, and RVP titers of 986 to MR-766 and 5,812 to H/PF/2013 (Fig. 3 and Extended Data Fig. 4). The significance of a low-level viral blip in one animal, including implications for a correlate of protection, are as yet uncertain and warrant further study with a greater number of animals.
Figure 4. A single immunization of nucleoside-modified ZIKV prM-E mRNA-LNPs protects rhesus macaques from ZIKV challenge at 5 weeks post-immunization.
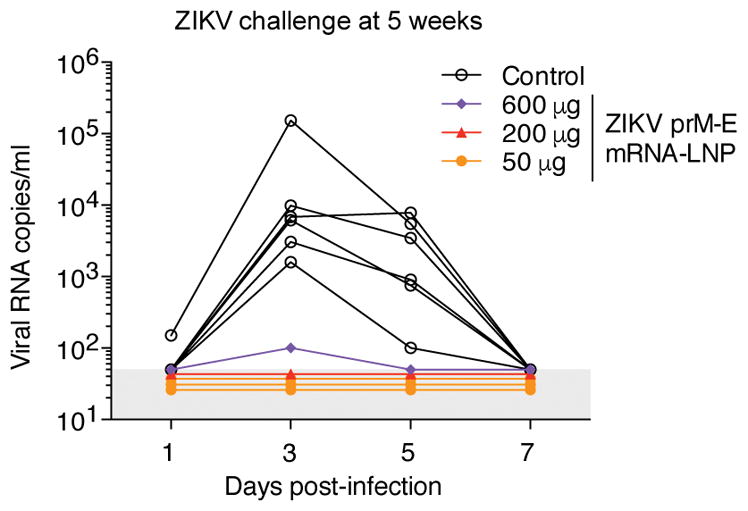
Six unvaccinated control macaques and five vaccinated macaques that received 50 μg (n=3), 200 μg (n=1), or 600 μg (n=1) of ZIKV mRNA-LNPs at week 0 were challenged s.c. with 104 TCID50 of ZIKV PRVABC59 at week 5. Viral loads were measured in plasma by qRT-PCR for ZIKV capsid RNA. Shaded area indicates values below the limit of detection (50 copies/ml), and undetectable values were staggered to show individual animals. Day 3 and 5 viremia in vaccine and control groups was compared by Mann-Whitney test: p<0.001.
In this report, we demonstrate that a single, low-dose i.d. immunization with nucleoside-modified ZIKV prM-E mRNA-LNPs is protective in both mice and rhesus macaques and elicits higher NAb responses than a single immunization of multiple recently reported ZIKV vaccine candidates, including purified inactivated virus (PIV) and plasmid DNA vaccines encoding prM-E or M-E10–12. In mice, PRNT50 NAb increased steadily over several months, rising to levels 50–100 times higher than those induced by a single immunization with PIV or DNA vaccines11,12. The ZIKV mRNA-LNP vaccine conferred complete, rapid, and durable protection in mice that was maintained for at least 5 months, and likely much longer, since NAb titers were stable. The mouse challenge studies also revealed that ZIKV PRVABC59 replicated more efficiently (p=0.02, Mann-Whitney test) in 8 week-old BALB/c mice compared to 25 week-old mice, when two identical aliquots and doses of challenge virus stock were used. A prior report has shown that ZIKV-related mortality in immune-competent mice decreases between 1 and 4 weeks of age24, but ZIKV replication in adult mice has not yet been well described.
In rhesus macaques, a single immunization with 50 μg ZIKV prM-E mRNA-LNPs induced RVP NAb titers that were 50 times higher than those induced by one immunization of 1 mg DNA vaccine and over 2 times higher than those induced by two immunizations of DNA12, as measured by the same assay in the same laboratory. ZIKV mRNA-LNP NAb titers may overlap with those elicited by one injection of PIV or RhAd52 ZIKV vaccines in macaques, although differing assay formats prevent a precise comparison. The FRNT NAb titers elicited by ZIKV mRNA-LNP in macaques were maintained at a stable level until 12 weeks post-immunization, suggesting that protection may be durable.
Future studies on the nucleoside-modified ZIKV mRNA-LNP vaccine will allow us to explore the impact of a boost, study the effect of this vaccine on other flavivirus infections, and determine efficacy in preventing fetal ZIKV infection and disease.
Ethics statement
Animals
The investigators faithfully adhered to the “Guide for the Care and Use of Laboratory Animals” by the Committee on Care of Laboratory Animal Resources Commission on Life Sciences, National Research Council. Mouse studies were conducted under protocols approved by the University of Pennsylvania (UPenn) IACUCs. Rhesus macaques (Macaca mulatta) were housed at BIOQUAL Inc. (Rockville, MD). Macaque experiments were reviewed and approved by BIOQUAL and UPenn Animal Care and Use Committees. All animals were housed and cared for according to local, state and federal policies in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility.
Human cells
Research involving human cells complied with the Declaration of Helsinki. De-identified leukapheresis cells were obtained from the UPenn Immunology Core under their Institutional Review Board (IRB) approved protocol, and were deemed exempt by the UPenn IRB.
Antibody reagents
The pan-flavivirus murine monoclonal antibody 4G2, clone D1-4G2-4-15 (EMD Millipore MAB10216) was used to detect ZIKV E protein by Western blot. The following antibodies were used for flow cytometry: anti-CD4 PerCP/Cy5.5 (Clone GK1.5, Biolegend), anti-CD3 APC-Cy7 (Clone 145-2C11, BD Biosciences), anti-CD27 PE (Clone LG.3A10, BD Biosciences), anti-TNF-α PE-Cy7 (Clone MP6-XT22, BD Biosciences), anti-IFN-γ AF700 (Clone XMG1.2, BD Biosciences), anti-IL-2 APC (Clone JES6-5H4, BD Biosciences). LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies) was used to discriminate dead cells and debris. The following antibodies were used for ELISA assays: goat anti-mouse IgG HRP (Sigma 4416), goat anti-monkey IgG HRP (Sigma 2054), and ZIKV E protein-binding mAb NR-4747 clone E19 (BEI Resources). ZIKV-neutralizing human monoclonal antibody Ab3594 was provided by the Duke University, Duke-NUS Graduate Medical School, National University of Singapore team of Charles McGee, Gregory D. Sempowski, Robert Parks, Eng Eong Ooi, Barton F. Haynes, M. Anthony Moody, Sheemei Lok, and Hua-Xin Liao.
Protein reagents
Purified recombinant ZIKV E protein (Aalto Bioreagents AZ 6312) was used in ELISAs to detect E protein-specific IgG, in Western blots as a positive control and in mouse splenocyte stimulation.
mRNA production
mRNA was produced as previously described25 using T7 RNA polymerase on linearized plasmid (pTEV-ZIKVprM-E-A101) encoding codon-optimized26 ZIKV strain H/PF/2013 (Asian lineage, French Polynesia, 2013, GenBank: KJ776791) prM-E glycoproteins. mRNA was transcribed to contain 101 nucleotide-long poly(A) tails. 1-methylpseudouridine-5′-triphosphate (TriLink) instead of UTP was used to generate modified nucleoside-containing mRNA. mRNA was capped using the m7G capping kit with 2′-O-methyltransferase to obtain cap1 and was purified by a fast protein liquid chromatography (FPLC) method, as described27. mRNA was analyzed by agarose gel electrophoresis and stored frozen at −20°C.
Cell culture
Human embryonic kidney (HEK) 293T cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 2 mM L–glutamine (Life Technologies) and 10% fetal calf serum (FCS) (HyClone) (complete medium). The 293T cell line was tested for mycoplasma contamination after receipt from ATCC and before expansion and cryopreservation. Human dendritic cells (huDCs) were generated from monocytes, as described28, and grown in RPMI 1640 medium containing 2 mM L–glutamine (Life Technologies) and 10% fetal calf serum (FCS) (HyClone) (complete medium) supplemented with 50 μg/ml recombinant human GM-CSF and 100 μg/ml recombinant human IL-4 (R&D systems). Cells were maintained by adding fresh medium containing IL-4 and GM-CSF every 3 days and used on day 7. Murine dendritic cells (muDCs) were generated from bone marrow cells obtained from the femurs of animals and grown in complete medium supplemented with 50 μg/ml murine GM-CSF (R&D systems). Cells were maintained by adding fresh medium containing murine GM-CSF every 3 days and used on day 7.
mRNA transfection
Transfection of human and murine DCs and HEK 293T cells was performed with TransIT-mRNA (Mirus Bio) according to the manufacturer instructions: mRNA (0.3 μg) was combined with TransIT-mRNA Reagent (0.34 μl) and Boost Reagent (0.22 μl) in 17 μl of serum free medium, and the complex was added to 2 × 105 cells in 183 μl complete medium. Supernatant was collected and cells were lysed for 1 hr on ice in RIPA buffer (Sigma) at 18 hr post-transfection.
Western blot analysis of E protein expression
Whole cell lysates and supernatants from ZIKV prM-E transfected cells were assayed for ZIKV E protein by non-denaturing SDS-PAGE Western blot. Samples were combined with 4× Laemmli buffer (Bio-Rad) and separated on a 4–15% precast polyacrylamide Criterion TGX gel (Bio-Rad) for 45 min at 200 V. Transfer to PVDF membrane was performed using a semi-dry apparatus (Ellard Instrumentation, Ltd.) at 10 V for 1 hr. The membrane was blocked with 5% non-fat dry milk in TBS buffer containing 0.5% Tween-20. E protein was detected using 1:10,000 4G2 ascites for 1 hr, followed by secondary goat anti-mouse IgG HRP 1:10,000 for 1 hr. Antibody incubations were performed at room temperature in blocking buffer. Blots were developed using Luminata Forte substrate (Millipore) and a Kodak X-OMAT 1000A processor.
Characterization of E protein in supernatant
Supernatant from HEK 293T cells transfected with ZIKV prM-E mRNA was tested for whether E protein could be pelleted and disrupted with detergent, consistent with subviral particles. Supernatant was incubated in PBS alone or PBS with 0.5% Triton X-100 for 1 hr on ice. Samples were then spun at 42,000 rpm for 2.5 hr in a Beckman TLA-55 rotor. The supernatant was then removed from the pellet, which was resuspended in 50 μl of PBS. Equal volumes of the input, pellet, and post-centrifugation supernatant fractions were then analyzed by Western blot, as described above.
Lipid nanoparticle (LNP) formulation of the mRNA
FPLC-purified mRNAs and polycytidylic acid (poly(C) RNA) (Sigma) were encapsulated in LNPs using a self-assembly process in which an aqueous solution of mRNA at pH 4.0 is rapidly mixed with a solution of lipids dissolved in ethanol29. LNPs used in this study were similar in composition to those described previously29,30, which contain an ionizable cationic lipid (proprietary to Acuitas)/phosphatidylcholine/cholesterol/PEG-lipid (50:10:38.5:1.5 mol/mol) and were encapsulated at an RNA to total lipid ratio of ~0.05 (wt/wt). They had a diameter of ~80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS instrument (Malvern Instruments Ltd, Malvern, UK). RNA-LNP formulations were stored at −80°C at a concentration of RNA of ~1 μg/μl.
Administration of LNPs to mice and rhesus monkeys
Mice
Female BALB/c and C57BL/6 mice aged 8 weeks were purchased from Charles River Laboratories. mRNA-LNPs were diluted in PBS and injected into animals intradermally with a 3/10cc 29½G insulin syringe (BD Biosciences). Four sites of injection (30 μl each) over the lower back were used.
Monkeys
Ketamine anesthetized animals were shaved on their back and injected with mRNA-LNPs diluted in PBS. Ten sites of injection (60 μl each) were used. Animals of similar age and weight were randomly designated to dose groups.
Blood collection from mice and rhesus macaques
Mice
Blood was collected from the orbital sinus under isoflurane anesthesia. Blood was centrifuged for 10 min at 13,000 rpm and the serum was stored at −20°C and used for ELISA and virus neutralization assays. EDTA-plasma was collected to isolate RNA for qRT-PCR analysis.
Monkeys
Blood was collected by femoral venipuncture under ketamine anesthesia, and serum and EDTA-plasma were collected and stored at −80°C for ELISA, neutralization analysis, and to isolate RNA for qRT-PCR.
Stimulation and staining of splenocytes
Single cell suspensions from spleens were made in complete medium. Splenocytes were washed once in PBS and resuspended in complete medium at 2 × 107 cells/ml. 2 × 106 cells (100 μl) per sample were stimulated for 6 hr at 37°C using 2 μg/ml purified recombinant ZIKV E protein. Golgi Plug (brefeldin A, BD Biosciences) and Golgi Stop (monensin, BD Biosciences) were diluted 1:100 and 1:143 in complete medium, respectively, and 20 μl from both diluted reagents were added to each sample to inhibit the secretion of intracellular cytokines after 1 h. An unstimulated sample for each animal was included. PMA (10 ng/ml)-ionomycin (250 ng/ml) (Sigma) stimulated samples were used as positive controls.
After stimulation, cells were washed in PBS and stained using the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies) and then surface stained for CD4 and CD27. Antibodies were incubated with cells for 30 min at RT. Following surface staining, cells were washed in FACS buffer and fixed using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Following fixation, the cells were washed in the appropriate perm buffer and incubated with antibodies against CD3, TNF-α, IFN-γ and IL-2 for 1 hr at RT. Following staining, the cells were washed with the appropriate perm buffer, fixed (PBS containing 1% paraformaldehyde) and stored at 4°C until analysis.
Flow cytometry
Splenocytes were analyzed on a modified LSR II flow cytometer (BD Biosciences). One hundred thousand events were collected per specimen. After the gates for each function were created, the Boolean gate platform was used to create the full array of possible combinations, equating to seven response patterns when testing three functions. Data were expressed by subtracting the percentages from the unstimulated stained cells from the E protein stimulated stained cells.
Enzyme-linked immunosorbent assays (ELISA) for ZIKV E-specific IgG
Immulon 4HXB ELISA plates were coated with 6 μg/ml purified recombinant ZIKV E protein in 0.1 M sodium bicarbonate buffer overnight at 4°C. The plate was blocked with 2% BSA in PBS for 1 hr, and washed three times with wash buffer (PBS with 0.05% Tween-20). Mouse or rhesus macaque sera were diluted in blocking buffer and incubated on the plate for 1 hr at room temperature, followed by four washes. Secondary antibody HRP conjugate was diluted 1:10,000 in blocking buffer and incubated on the plate for 1 hr, followed by four washes. TMB substrate (KPL) was applied to the plate and the reaction was stopped with 2 N sulfuric acid. The absorbance was measured at 450 nm using an MRX Revelation microplate reader. ZIKV E-specific IgG was expressed in two ways: as an endpoint dilution titer, defined as the highest reciprocal dilution of serum to give an OD greater than the sum of the background OD plus 0.01 units; and as an estimate of the absolute IgG concentration, which was based on the murine mAb NR-4747 as a standard (applicable only to mouse samples). All samples were run at least in technical duplicates.
ZIKV MR-766 plaque reduction neutralization tests (PRNT)
ZIKV strain MR-766 (African lineage, Uganda, 1947, GenBank: AY632535) (UTMB Arbovirus Reference Collection) was produced in Vero cells (ATCC CCL-81) and 50 plaque forming units were incubated with increasing dilutions of heat-inactivated sera in serum-free DMEM (Corning) medium for 1 hr at 37°C. The virus/serum mixture (200 μl) was added to a confluent monolayer of Vero cells in 6-well format and incubated for 1.5 hr at 37°C with intermittent rocking. Then, 3 ml of overlay, containing a final concentration of 0.5% methylcellulose (4,000 centipoise) (Sigma), 1X DMEM (Gibco), 16 mM HEPES, 0.56% sodium bicarbonate, 1.6X GlutaMAX (Gibco), 1X penicillin/streptomycin (Corning), and 4 μg/ml amphotericin B (Gibco), was added to each well, and plates were incubated for 5 days at 37°C in 5% CO2. The overlay was aspirated and cells were fixed and stained with 0.5% crystal violet (Sigma) in 25% methanol, 75% deionized water. Wells were rinsed with deionized water to visualize plaques. Neutralization titers (EC50) were determined by plotting a line through the linear portion of the curve that crossed 50% inhibition and calculating the reciprocal dilution of sera required for 50% neutralization of infection. EC50 titers below the limit of detection are reported as half of the limit of detection.
ZIKV MEX I-44 focus reduction neutralization tests (FRNT)
ZIKV MEX I-44 (Asian lineage, Mexico, 2016, GenBank: KX856011) stocks were generated via propagation in Vero 76 cells (ATCC CRL-1587) and harvested as clarified cell culture lysate/supernatant. Stock titers were quantified via standard focus forming assay. FRNT was performed by combining a standard dose of ZIKV with two-fold serial dilutions of heat-inactivated serum for one hour at 37°C. Virus-serum mixtures (100 μl) were then inoculated onto Vero 76 monolayers, incubated at 37°C for 1 hr and overlayed with an Avicel (FMC Biopolymer)-containing growth medium. After 3 days of incubation, plates were formalin-fixed, permeabilized, blocked, and stained via sequential incubation with biotin-conjugated 4G2 mAb (ATCC HB-112), streptavidin-HRP (BD Biosciences) and TrueBlue Peroxidase Substrate (KPL). Virus input was verified in parallel (acceptable range: 20–60 foci). FRNT50 (EC50 titers) are reported as the highest reciprocal dilution giving a focus count ≤ the 50% neutralization cutoff, and the geometric mean was computed for technical duplicates.
Reporter virus particle (RVP) production
Pseudo-infectious RVPs were produced by complementation of a GFP-expressing WNV sub-genomic replicon23,31 with a plasmid encoding the viral structural proteins (capsid-prM-E). Briefly, ZIKV MR-766 and ZIKV H/PF/2013 RVPs were produced via co-transfection of HEK-293T cells with the structural gene and replicon plasmids (3:1 ratio by mass) using Lipofectamine 3000 per the manufacturer’s protocol (Invitrogen). Transfected cells were incubated at 30°C and RVP-containing supernatants harvested on days 3–6. Stocks were passed through a 0.2 μm filter and aliquots stored at −80°C until use. Stock titers were determined by infecting Raji-DCSIGNR cells with serial dilutions of filtered RVP supernatants. GFP-positive cells were assessed by flow cytometry at 48 hr post-infection and RVP titers calculated.
RVP neutralization assay
Previously titered RVPs were diluted to ensure antibody excess at informative points on the dose-response curves and incubated with serial dilutions of mouse or macaque sera for 1 hr at 37°C to allow for steady-state binding. Raji-DCSIGNR cells were then infected with antibody-RVP complexes in duplicate technical replicates. Infections were carried out at 37°C and GFP-positive infected cells detected by flow cytometry 24–48 hr later. Neutralization results were analyzed by non-linear regression to estimate the reciprocal dilution of sera required for half-maximal neutralization of infection (EC50 titer) (Prism 6, GraphPad). The initial dilution of sera (based on the final volume of RVPs, cells, and sera) was set as the limit of confidence of the assay. Titers for which non-linear regression was predicted to be below this threshold were reported as a titer half the limit of confidence. Individual EC50 titers are reported as the geometric mean of technical replicates.
Preparation of challenge ZIKV virus
Mice
Challenge ZIKV strain PRVABC59 (Asian lineage, Puerto Rico, 2015, GenBank: KU501215) (BEI Resources NR-50240) was grown in Vero CCL81 cells. A T175 flask of cells at 75–90% confluency was inoculated with an MOI of 0.01 ZIKV in 10 ml of serum-free DMEM medium. The flask was incubated at 37°C, 5% CO2 for 1.5 hr with intermittent gentle rocking, then warmed media was added to a final concentration of 1.5% FBS, 1X GlutaMAX (Gibco) and 1X penicillin/streptomycin (Corning) in a final volume of 25 ml. The flask was incubated for 4 days or until cytopathic effects were visible. Then supernatant was collected and ultra-centrifuged at 20,000 rpm for 1 hr at 4°C in a Sorvall SureSpin 630 rotor. The supernatant was removed and the pellet was resuspended in 1 ml of serum-free DMEM, aliquoted, and stored at −80°C. Before challenge, virus was thawed and diluted in PBS to 2,000 PFU/ml.
Monkeys
Challenge ZIKV strain PRVABC59 was grown in Vero 76 CRL-1587 cells. T150 flasks of cells at 80–85% confluency were used for propagation. Infection was performed with 100 μl stock virus diluted in 4 ml of fresh L-15 media (Gibco) supplemented with 10% fetal bovine serum (Gibco), 10% tryptose phosphate broth (Sigma Aldrich), 1X penicillin/streptomycin (Gibco) and L-glutamine (Gibco) and adsorbed for 1 hr at room temperature with gentle agitation every 15 min. Each flask received 7 ml of fresh L-15 media after adsorption and was incubated for 4 days at 37°C. Cellular debris was removed by centrifugation at 1,200 rpm for 5 min at 4°C in an Eppendorf A-4-62 rotor. Virus stocks were aliquoted and stored at −80°C.
Zika virus challenge in mice and rhesus macaques
Mice
Two or 20 weeks after vaccination, mice were bled and then challenged intravenously with 200 PFU of ZIKV-PR (PRVABC59) in 100 μl of PBS. Blood was collected 3 and 7 days post-challenge to determine viral loads (ZIKV RNA copies/ml) in plasma.
Monkeys
Macaques were anesthetized with ketamine and injected subcutaneously in the hind thigh with 104 TCID50 of ZIKV-PR in a volume of 1 ml in PBS. Blood was collected 1, 3, 5, and 7 days post-challenge to determine viral loads (ZIKV RNA copies/ml) in plasma.
Viral load quantification (qRT-PCR)
Using blinded samples, RNA was isolated from 200 μl (macaque) or 50 μl (mouse) plasma using the QIAamp MinElute Virus spin kit (Qiagen). Extracted RNA was used for amplification using the SensiFAST Probe Lo-ROX One-Step Kit (Bioline BIO-78005) on a 7500 Real-Time PCR system (Applied Biosystems). Primers and probe were designed to amplify a conserved region of the capsid gene from ZIKV BeH815744, as follows: Fwd: 5′GGAAAAAAGAGGCTATGGAAATAATAAAG; Rev: 5′CTCCTTCCTAGCATTGATTATTCTCA; probe: 5′AGTTCAAGAAAGATCTGGCTG.
Primers and probe were used at a final concentration of 2 μM, and the following program was run: 48°C for 30 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 1 min at 60°C. Assay sensitivity was 50 copies/ml for macaque and 200 copies/ml for mouse samples.
Statistical analysis
GraphPad Prism 5.0f was used to perform Mann-Whitney and Kruskal-Wallis (with Dunn’s correction) tests to compare immune responses in vaccinated and control mice and in different dose groups of macaques, respectively. SPICE 5.35 and Microsoft Excel software was used to perform Student’s t tests to compare T cell responses in vaccinated and control mice.
Data availability
All data and full plasmid sequences are available by request of the corresponding author.
Extended Data
Extended Data Figure 1. Design and characterization of ZIKV prM-E mRNA.
(a) The ZIKV mRNA encodes the signal peptide (SP) from MHC class II and prM and E glycoproteins from ZIKV H/PF/2013. (b) mRNA was transfected into 293T cells (n=3), human DC (n=3), or murine DC (n=2). E protein expression in cell lysate and supernatant was probed by Western blot, using firefly luciferase-encoding mRNA-transfected cells as a negative control. (c) ZIKV mRNA supernatant from transfected 293T cells was characterized by ultracentrifugation in the presence and absence of 0.5% Triton X-100, followed by Western blot of input (IN), pellet (P), and final supernatant (S) fractions (n=3).
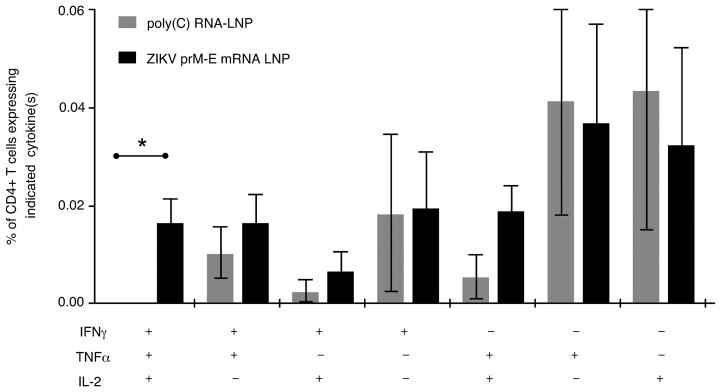
Extended Data Figure 2. Nucleoside-modified ZIKV mRNA-LNP immunization elicits polyfunctional ZIKV E-specific CD4+ T cell responses.
C57BL/6 mice were immunized with 30 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs (n=8) or control poly(C) RNA-LNPs (n=4). At week 2, antigen-specific CD4+ T cells were detected by intracellular cytokine staining. Bar graph shows mean frequencies of combinations of cytokines produced by CD4+ T cells. Error bars indicate the SEM, and asterisk indicates a significant difference (p<0.05) by Student’s t-test.
Extended Data Figure 3. ZIKV E-specific IgG concentration in mice.
Sera from (a) C57BL/6 mice (n=4 control; n=8 ZIKV mRNA-LNP) or (b) BALB/c mice (n=5 control; n=10 ZIKV mRNA-LNP) were assayed by ELISA, and estimates of ZIKV E-specific IgG concentrations were calculated using murine mAb NR-4747 as a standard. Points represent individual mice; horizontal lines indicate the mean. Responses in vaccine and control groups were compared at each time point by Mann-Whitney test: p<0.01 for all comparisons.
Extended Data Figure 4. Neutralizing antibody responses against ZIKV MR-766 in macaques immunized with ZIKV prM-E mRNA-LNPs.
Sera from immunized macaques were evaluated for neutralization of ZIKV MR-766 using (a) the PRNT assay or (b) the RVP assay at the indicated time points. Shaded area indicates values below the limit of detection and horizontal bars indicate the mean. Immune responses in dose groups were compared by Kruskal-Wallis test: p>0.05 for all comparisons.
Extended Data Figure 5. Neutralization curve for a human anti-ZIKV neutralizing mAb.
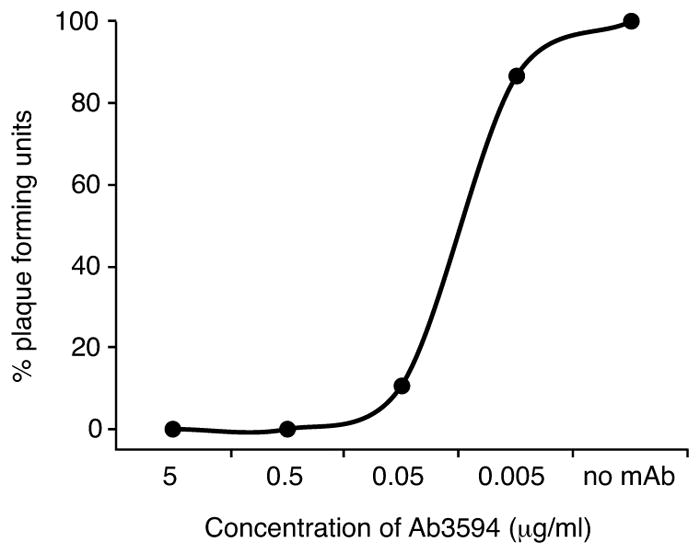
ZIKV MR-766 was neutralized by Ab3594, a human ZIKV-neutralizing monoclonal antibody, as a positive control in the PRNT assay. Shown is a representative curve (n=4). Mean EC50 = 0.026 μg/ml, SD=5.4.
Extended Data Table 1. Characteristics of rhesus macaques in vaccination and challenge experiments.
Asterisk indicates the animals that were challenged with ZIKV.
| Immunization | Group | ID | Weight (kg) | Sex | DOB |
|---|---|---|---|---|---|
| ZIKV prM-E mRNA-LNP, 600 μg | 1 | 6858 | 3.3 | M | 5/19/14 |
| 150250 | 3.05 | F | 3/22/15 | ||
| 150793 | 2.55 | M | 4/1/15 | ||
| 150796* | 2.75 | F | 4/13/15 | ||
|
| |||||
| ZIKV prM-E mRNA-LNP, 200 μg | 2 | 150251 | 2.95 | M | 3/22/15 |
| 150795 | 2.55 | M | 4/12/15 | ||
| 150798* | 2.35 | F | 4/26/15 | ||
|
| |||||
| ZIKV prM-E mRNA-LNP, 50 μg | 3 | 6857* | 3.15 | F | 6/6/14 |
| 150252* | 2.15 | F | 3/25/15 | ||
| 150794* | 2.75 | M | 4/5/15 | ||
|
| |||||
| Challenge control group (unimmunized) | 4 | 6143* | 7.85 | F | 7/3/09 |
| 6154* | 8.95 | F | 4/6/10 | ||
| 6076* | 7.6 | M | 10/20/11 | ||
| 6150* | 11.55 | M | 1/26/10 | ||
| 6211 * | 8.65 | M | 5/2/10 | ||
| 6157* | 10.15 | F | 4/3/10 | ||
Acknowledgments
We thank Robert Tesh at the UTMB World Reference Center for Emerging Viruses and Arboviruses for providing ZIKV stocks. We gratefully acknowledge the technical or administrative support of Michael Bertrand, Leslee Arwood, Christopher Sample, Maggie J. Barr, Callie Vivian, Thaddeus Gurley and M. Anthony Moody, Eng Eong Ooi, Sheemei Lok, Hua-Xin Liao, Sita Awasthi, Lauren Hook, and Farida Shaheen (UPenn CFAR). The Virology Unit of the Duke Regional Biocontainment Laboratory (Duke Human Vaccine Institute) received support from the NIH, NIAID (UC6-AI058607). Supported by the NIH, NIAID Duke Center for HIV/AIDS Vaccine Immunology AI100645. DV received funding from the Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University. R.S.P., C.R.D., K.A.D., T.C.P., and B.S.G. were funded by the intramural program of the National Institute of Allergy and Infectious Diseases to the Division of Intramural Research and the Vaccine Research Center. S.E.H. received funding through U19AI057229. M.J.H. received funding from NIH T32 AI007632-14. D.W. received the following funding: NIAID/NIH R01-AI050484, R01-AI124429 and R01-AI084860, Takeda Pharmaceuticals, New Frontier Science.
Footnotes
Author Contributions
N.P., M.J. Hogan, B.F.H, T.C.P., B.S.G, M.G.L., and D.W. designed the studies and evaluated data. N.P. and H.M. conducted mouse studies. M.J. Hogan performed virologic and immunologic assays for mouse and NHP studies. D.W., M.J. Hogan, N.P., and K.K. developed the mRNA construct and platform. E.W. and S.E.H. provided virologic technical assistance and reagents. V.H. provided molecular assay assistance. C.E.M., G.D.S., R.S.P., C.R.D., K.A.D., and T.C.P. conducted NAb assays. B.L.M., Y.K.T., T.D.M., and M.J. Hope developed and provided LNPs. Y.H., D.V., and S.H. provided NHP challenge stock. H.A., J.G., and M.W. conducted viral load analysis. M.G.L., L.L.S., R.M.S., W.W., and A.G. carried out the NHP immunization and challenge trial. M.J.B., J.Y., L.M., M.A.M., and S. Santra, H.B., S. Sahu, and M.L. processed NHP samples and conducted assays for NHP studies. N.P., M.J. Hogan, and D.W. wrote the paper with all co-authors.
Author Information
In accordance with the University of Pennsylvania policies and procedures and our ethical obligations as researchers, we report that Norbert Pardi, Michael J. Hogan, Katalin Karikó and Drew Weissman are named on patents that describe the use of nucleoside-modified mRNA as a platform to deliver therapeutic proteins and vaccines. We have disclosed those interests fully to the University of Pennsylvania, and we have in place an approved plan for managing any potential conflicts arising from licensing of our patents.
References
- 1.Pierson TC, Graham BS. Zika Virus: Immunity and Vaccine Development. Cell. 2016;167:625–631. doi: 10.1016/j.cell.2016.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissman D. mRNA transcript therapy. Expert Rev Vaccines. 2015;14:265–281. doi: 10.1586/14760584.2015.973859. [DOI] [PubMed] [Google Scholar]
- 3.Sahin U, Kariko K, Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 4.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 5.Beck AS, Barrett AD. Current status and future prospects of yellow fever vaccines. Expert Rev Vaccines. 2015;14:1479–1492. doi: 10.1586/14760584.2015.1083430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarmer J, et al. Variation of the specificity of the human antibody responses after tick-borne encephalitis virus infection and vaccination. J Virol. 2014;88:13845–13857. doi: 10.1128/JVI.02086-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol. 2016;14:45–54. doi: 10.1038/nrmicro.2015.2. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, et al. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muthumani K, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. npj Vaccines. 2016;1:1–11. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbink P, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science. 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larocca RA, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowd KA, et al. Rapid development of a DNA vaccine for Zika virus. Science. 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ledgerwood JE, et al. A replication defective recombinant Ad5 vaccine expressing Ebola virus GP is safe and immunogenic in healthy adults. Vaccine. 2010;29:304–313. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Sumida SM, et al. Neutralizing antibodies and CD8+ T lymphocytes both contribute to immunity to adenovirus serotype 5 vaccine vectors. J Virol. 2004;78:2666–2673. doi: 10.1128/JVI.78.6.2666-2673.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbink P, et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol. 2015;89:1512–1522. doi: 10.1128/JVI.02950-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minor PD. Live attenuated vaccines: Historical successes and current challenges. Virology. 2015;479–480:379–392. doi: 10.1016/j.virol.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Baronti C, et al. Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc. 2014;2 doi: 10.1128/genomeA.00500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andries O, et al. N(1)-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J Control Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 19.Pardi N, et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang PG, et al. Efficient assembly and secretion of recombinant subviral particles of the four dengue serotypes using native prM and E proteins. PLoS One. 2009;4:e8325. doi: 10.1371/journal.pone.0008325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weissman D, et al. HIV gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J Immunol. 2000;165:4710–4717. doi: 10.4049/jimmunol.165.8.4710. [DOI] [PubMed] [Google Scholar]
- 22.Dudley DM, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowd KA, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Cell Rep. 2016;16:1485–1491. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazear HM, et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pardi N, Muramatsu H, Weissman D, Kariko K. In vitro transcription of long RNA containing modified nucleosides. Methods Mol Biol. 2013;969:29–42. doi: 10.1007/978-1-62703-260-5_2. [DOI] [PubMed] [Google Scholar]
- 26.Thess A, et al. Sequence-engineered mRNA Without Chemical Nucleoside Modifications Enables an Effective Protein Therapy in Large Animals. Mol Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weissman D, Pardi N, Muramatsu H, Kariko K. HPLC purification of in vitro transcribed long RNA. Methods Mol Biol. 2013;969:43–54. doi: 10.1007/978-1-62703-260-5_3. [DOI] [PubMed] [Google Scholar]
- 28.Kariko K, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maier MA, et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayaraman M, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson TC, et al. A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology. 2006;346:53–65. doi: 10.1016/j.virol.2005.10.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and full plasmid sequences are available by request of the corresponding author.