Abstract
Background
Increased oxidative stress leads to loss of glutathione (GSH). We have reported lower cerebral GSH in patients with secondary progressive multiple sclerosis (SPMS), indicating the involvement of oxidative stress in MS pathophysiology.
Objective
This study expanded upon our earlier work by examining longitudinal changes in cerebral GSH in patients with SPMS in relation to their clinical status.
Methods
Thirteen patients with SPMS (EDSS=4.0–6.5; MS duration=21.2±8.7 years) and 12 controls were studied over 3–5 years. GSH mapping was acquired from frontal and parietal regions using a multiple quantum chemical shift imaging technique at 3T. Clinical assessments of the patient’s disability included EDSS, gait, motor strength, ataxia, tremor, brainstem function and vision changes.
Results
Brain GSH concentrations in patients were lower than those in controls for both baseline and 3–5 year follow-ups. Longitudinal GSH changes of patients were associated with their neurologist’s blinded appraisal of their clinical progression. Patients judged to have worsening clinical status had significantly greater declines in frontal GSH concentrations than those with stable clinical status.
Conclusion
GSH provides a distinct measure associated with the disease progression in SPMS, possibly due to its dynamic alignment with pathogenic processes of MS related to oxidative stress.
Keywords: Glutathione (GSH), secondary progressive multiple sclerosis (SPMS), oxidative stress, neurodegeneration, human brain, magnetic resonance spectroscopy, chemical shift imaging
INTRODUCTION
For the majority of patients with multiple sclerosis (MS), the clinical course eventually changes from the relapsing-remitting MS (RRMS) to secondary progressive MS (SPMS) typified by continuous decline in neurological status without distinct relapses1. Magnetic resonance (MR) imaging has contributed to the notion that this conversion in the clinical presentation of MS may signify a shift in underlying pathology, from one dominated by recurrent inflammatory processes to neurodegeneration stemming from other pathological mechanisms such as glutamate excitotoxity and oxidative stress2–5.
Increased oxidative stress has been suggested in the brains of patients with SPMS6 using a noninvasive measure of cerebral glutathione (GSH) obtained through an innovative selective multiple quantum chemical shift imaging (CSI) technique at 3 T7, 8. GSH, a major endogenous antioxidant, plays a predominant role in protecting cells against oxidative damage by reactive oxygen species generated in the CNS, which are known to cause cellular damage, impaired cell function and eventual cell death9–11. During this protective process of detoxifying reactive oxygen species, GSH is either consumed during the formation of GSH-S-conjugates by GSH-S-transferases or converted to its oxidized form (GSSG) by GSH peroxidase. Thus, lower GSH concentrations serve as a marker of oxidative stress, indicating increased vulnerability to neurodegeneration11–15.
Our previous study reported significantly lower cerebral GSH concentrations in patients with SPMS compared with age- and sex-matched healthy controls. Lower cerebral GSH has also been reported in a small sample of MS patients with unspecified disease subtypes16. In our previous study, cerebral GSH concentrations were positively related to patients’ age at diagnosis, even when adjusted for differences in age. Both disease duration and disability on the Expanded Disability Status Scale (EDSS)17 tended to be negatively related to GSH concentrations, although these correlations were not significant. The correlations between GSH concentrations and disability scores were attenuated by a restriction in the variability of the EDSS scores; all but four of the 17 SPMS patients had ratings of either 6.0 or 6.5. The disease progression of SPMS varies significantly among individuals, thus longitudinal follow-ups of patients will exhibit variability in disease status and the changes in GSH concentrations over the disease course might align with changes in their clinical status.
In this study, we enrolled the same patients with SPMS and controls from our initial study to evaluate their cerebral GSH concentrations with follow-up scans 3–5 years later. The purpose of this study was 1) to determine whether the previously observed differences in GSH concentrations between patients and controls persisted over this extended period, and 2) to examine the relationship between changes in patients’ clinical status and oxidative stress using a longitudinal study design.
METHODS
Participants
At least 3 years following our initial MR scans (Time 1) of 17 patients with SPMS and 17 healthy controls, follow-up scans (Time 2) were performed on 13 patients (10 females, 3 males) and 12 controls (11 females, 1 male), who were available and willing to volunteer. The interval between the scans ranged from 3.0 to 5.1 years (3.7 ± 0.3, mean ± SD). Throughout the intervening period, all of these patients continued to be under the care of the same neurologist in the MS Clinic. Despite the loss of 4 patients and 5 controls, the two groups remained closely similar in age (patients: 54.2 ± 8.6; controls: 54.6 ± 5.7) and in years of education (patients: 15.8 ± 1.5; controls: 15.8 ± 1.6). Patients’ median disability score remained the same as the initial study (median EDSS = 6.0; range: 4.0 – 6.5). Their duration of disease at Time 2 ranged from 10 to 39 years (21.2 ± 8.7). Healthy controls had no changes in their health status during the intervening period and remained free of disorders or conditions that could have possible neurological impact.
MR protocol
All MR scan protocols and set-ups for this longitudinal study were identical to our initial study6 performed on a 3 T MR system (Allegra, Siemens, Erlangen, Germany) using a custom-built partial volume transmit/receive coil, a quadrature radiofrequency (RF) helmet coil18. The participants lay supine in the MR scanner and a series of MR scans were performed identical to their initial MR scans at Time 1: 1) three-plane scout MR images to locate the volume of interest (VOI), a 3-cm axial slab positioned above the corpus callosum including the frontal to parietal regions; 2) localized automated shimming to optimize static magnetic field homogeneity in the CSI volume by adjusting all first- and second-order shim currents based on measured field maps; 3) conventional CSI for creatine measurement (slice thickness = 2 cm, matrix size = 16 × 16, FOV = 20 × 20 cm2, VOI = 10 × 12 cm2, TE/TR = 30/2000 ms, and number of averages = 2); and 4) the selective multiple quantum CSI of GSH acquired from the selected CSI slice (slice thickness = 3 cm, matrix size = 8 × 8, FOV = 20 × 20 cm2, TE/TR = 115/1500 ms, spectral width=2 kHz, and number of averages = 12). The CSI of GSH is based on a two-echo scheme, allowing simultaneous acquisition of the creatine signal, which serves as an internal concentration reference. The mean GSH/creatine ratio was calculated from the 5 × 5 × 3 cm3 area located in the fronto-parietal region along with the anterior (2.5 × 5 × 3 cm3) and posterior (2.5 × 5 × 3 cm3) halves along the horizontal axis of this volume. Although the two halves do not correspond perfectly with the frontal and parietal regions, it is still reasonable and convenient to refer to the concentrations in these halves as “frontal” and “parietal.” Creatine concentrations were quantified using the LCModel analysis package19 and an external concentration reference method20 that incorporates RF coil loading and the brain tissue volume21. Creatine concentrations in the fronto-parietal regions were obtained from the 5 × 5 × 2 cm3 area located in the same fronto-parietal regions for GSH measurements.
Statistical analysis
The design of this study conformed to a 2 (Group) × 2 (Time) repeated measures ANOVA. The main effects being tested were whether there were overall differences in GSH concentrations between the patients and controls (Group) or over the course of the intervening period (Time). The interaction term tested whether changes in GSH concentrations over the intervening period differed between patients and controls. Independent sample t-tests were also performed to compare GSH concentrations between patients and controls at each time.
GSH values of two control subjects at Time 1 (4% of entire data) were unavailable due to subjects’ motion during the scans. The missing values of these two subjects were substituted through multiple imputation using the automated, fully conditional specification procedure available in SPSS (version 22). Missing values were estimated on the basis of their age, sex, and existing frontal, parietal, and fronto-parietal GSH values at Time 1 and Time 2. Ten imputations were performed for each missing value and the resulting estimates were then averaged to represent the subject’s score on the variable.
Clinical assessment of patients with SPMS
The neurologist’s clinical assessment included the examination of patients’ records and medical information such as the diagnosis and history of MS, medications, past medical history, demographics, MS subtypes by the Lublin-Reingold criteria, previous EDSS ratings, and evidence of recent worsening. EDSS17, 22 was assessed to evaluate patients’ current level of disability. Other assessments of patients included evaluations of gait, motor strength, ataxia, tremor, brainstem abnormalities, and vision changes during their longitudinal follow-up visits of 3–5 years.
RESULTS
Longitudinal assessment of GSH concentration changes
GSH concentrations for patients and controls at the baseline scan (Time 1) and the longitudinal follow-up scan (Time 2) are summarized in Table 1. A 2 × 2 (Group × Time) repeated-measures ANOVA performed on GSH concentrations in the frontal region showed significant main effects for Group (F = 19.99, df = 1, 23, p < 0.001, eta2 = 0.47) and Time (F = 6.41, df = 1, 23, p = 0.02, eta2 = 0.22) with no significant interaction (F<1). The analysis of GSH concentrations in the fronto-parietal region showed similar outcomes for Group (F = 8.77, df = 1, 23, p = 0.007, eta2 = 0.28) and Time (F = 6.41, df = 1, 23, p = 0.02, eta2 = 0.22) with no significant interaction (F<1). The analysis of GSH concentrations in the parietal region resulted in no significant main effects for Group (F = 2.36, df = 1, 23, p = 0.14, eta2 = 0.09) or Time (F = 3.51, df = 1, 23, p = 0.07, eta2 = 0.13) and no significant interaction (F<1). The significant main effects for Time were due to lower frontal and fronto-parietal GSH concentrations when averaged across both patients and controls at Time 2 than at Time 1. The significant main effects for Group were due to lower frontal and fronto-parietal GSH concentrations in patients than controls when averaged across the two time points. Independent sample t tests comparing patients and controls at each time point showed that patients had significantly lower GSH in frontal and fronto-parietal regions at both time points compared with controls (Table 1). The non-significant interactions indicate that the disparities between patients and controls in GSH concentrations were similar at each time point.
Table 1.
Longitudinal assessment of GSH concentrations in the brain of patients with SPMS
| Brain region | Time | GSH (µmol/g) in SPMS (n = 13) (mean ± SD) |
GSH (µmol/g) in controls (n = 12) (mean ± SD) |
t (df = 23) |
p |
|---|---|---|---|---|---|
| Fronto- parietal |
1 | 1.06 ± 0.14 | 1.19 ± 0.15 | 2.25 | 0.03 |
| 2 | 0.98 ± 0.14 | 1.09 ± 0.08 | 2.25 | 0.03 | |
| Frontal | 1 | 1.02 ± 0.14 | 1.23 ± 0.17 | 3.39 | 0.003 |
| 2 | 0.96 ± 0.15 | 1.10 ± 0.07 | 3.09 | 0.005 | |
| Parietal | 1 | 1.07 ± 0.14 | 1.14 ± 0.15 | 1.13 | 0.27 |
| 2 | 1.01 ± 0.15 | 1.09 ± 0.12 | 1.41 | 0.17 |
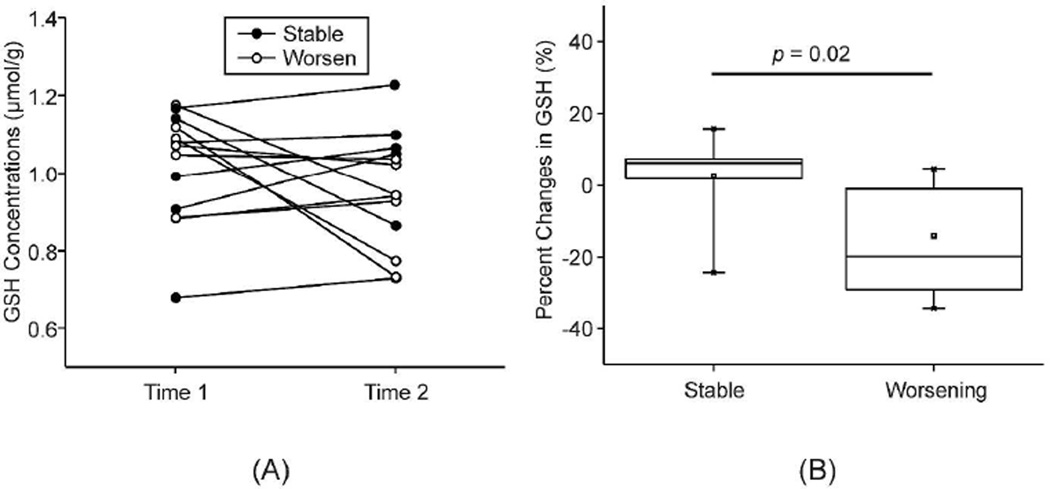
The average EDSS for patients with SPMS remained the same between Time 1 and Time 2 (median = 6.0). However, the individual disability ratings between the two time points showed that the clinical condition of some patients had declined while others had remained the same or were slightly improved by a half-step. Therefore, the neurologist who had followed all the patients was asked to designate patients as having either “stable” or “worsening” clinical status during the course of this 3–5 year interval. The neurologist’s blinded appraisal of patients’ clinical progression, as either stable or worsening, was based solely on a review of the patient’s medical records, and the neurologist was unaware of the outcome of MR scans including GSH concentrations. Seven of the patients were designated as “stable” and six as “worsening” in their clinical status during the intervening period. The percentage changes in each patient’s GSH concentrations from Time 1 to Time 2 were compared for the stable and worsening subgroups of patients using a nonparametric statistical test (Mann-Whitney U test). The percentage change in frontal GSH was significantly different between stable and worsening subgroups (z = 2.29, p = 0.02), while those in fronto-parietal and parietal GSH were not significantly different between the two subgroups (p = 0.1, and p = 0.6). The worsening subgroup showed a decrease of frontal GSH by 14%, in contrast to the stable subgroup showing a slight increase of frontal GSH by 2.8% from Time 1 to Time 2. Creatine concentrations, which were independently quantified using LCModel and used as a concentration reference for GSH, were not different between patients and controls at both Time 1 and Time 2 (p > 0.09). Furthermore, creatine concentrations did not differ between Time 1 and Time 2 in both patients and controls (p > 0.7).
DISCUSSION
GSH concentrations in the frontal and fronto-parietal regions of patients with SPMS remained lower compared with those of controls 3–5 years after the initial scans. However, the differences between patients and controls were not appreciably different at Time 2 (effect size: frontal GSH: d = 1.24; fronto-parietal GSH: d = 0.90) than at Time 1 (frontal GSH: d = 1.36; fronto-parietal GSH: d = 0.90). Thus, the interactions between Group and Time were not significant. Furthermore, GSH concentrations at Time 2 were not highly correlated with those at Time 1 in patients (frontal GSH: r = 0.29; fronto-parietal GSH: r = 0.42; both p’s > 0.15). These findings suggest the dynamic nature of GSH measures due to the reversible reactions that depend on the current status of oxidative stress, in contrast to measures such as brain volume or N-acetylaspartate (NAA), which presumably reflect cumulative neurodegeneration through neuronal loss.
The question remains whether changes in GSH concentrations are aligned with changes in patients’ clinical status over time. The present study provided only limited information regarding this matter because only modest changes occurred in patients’ disability levels, i.e., the change on EDSS was between 0 (n=4) and ±0.5 (n=5) for 9 of the 13 patients. Previous longitudinal studies have shown that at higher levels of disability, the EDSS is not very sensitive to changes in a patient’s disability over time23. Thus, patients could remain at the same EDSS for long periods of time in spite of the deterioration of their clinical condition. Furthermore, minor changes in factors such as gait, motor strength, ataxia, tremor, brainstem abnormalities, and vision upon which the EDSS is based cannot be easily quantified. Instead of relying exclusively on the EDSS, we sought further evidence of clinical status changes based on the neurologist’s evaluation of the patient performed during multiple routine neurological examinations over the course of the intervening period; on the basis of this evidence, the neurologist categorized the sample into subgroups of patients with worsening or stable clinical status. When the patients’ clinical status was compared with their GSH concentrations, those with worsening clinical status had a greater decline in frontal GSH concentrations than those with stable clinical status. In fact, clinically stable patients showed no changes or even slight increases in GSH concentrations from baseline. This suggests an involvement of oxidative stress in disease progression of MS and the possibility that cerebral antioxidants may play an important role in disease activity and treatment. Whereas these results are interesting, they may be considered preliminary due to the small sample size. Replication of this study with a larger sample of patients is necessary to properly establish the relationship.
Our results indicate that the frontal region may be more sensitive to MS pathology linked to oxidative stress as frontal GSH showed the most changes in patients with worsening clinical status compared to those with stable clinical status or controls than parietal GSH. The reason why frontal GSH concentrations are more affected than parietal GSH is unclear. A possible explanation to this observation is tissue composition differences in the frontal and parietal VOIs with gray matter fractions of 52% and 47%, respectively. Together with the previous reports of selective alteration of GSH in gray matter in MS16, the differences in tissue composition could in part explain the observed regional differences of GSH alterations. One limitation of the current study is the use of creatine as an internal concentration reference as creatine concentrations might be altered in pathological conditions including MS24–27. However, quantified creatine concentrations were not different at both time points (Time 1 and Time 2), which supports the validity of the use of creatine as a concentration reference. Therefore, it is not likely that GSH differences and changes observed in this study are due to potential changes in creatine concentrations.
Although significant technical advances have been reported in detecting neurochemicals using MR spectroscopy techniques, in vivo measures of neurochemicals that are presumably aligned with specific pathogeneses or disease mechanisms are as yet few in number. In particular, the capability of examining the role of oxidative stress in MS afforded by in vivo assessment of GSH in the living human brain is still new. The work described here may provide useful ideas as to how this capability should be exploited in future clinical studies to gain better understanding of disease activity and progression, and to evaluate the efficacy of treatments and interventions linked to altering ongoing oxidative stress. Further studies may help to determine whether this technique can be used to track disease activity.
CONCLUSIONS
In this study, the longitudinal assessment of cerebral GSH in patients with SPMS is demonstrated through the 3–5 year follow-up measures using a specially designed selective multiple quantum CSI technique. The longitudinal follow-up scans revealed that GSH concentrations remain markedly lower in the brains of SPMS patients compared with healthy controls. However, the degree of changes in GSH concentrations varied among patients and were correlated with changes of their clinical status. Further study is needed to evaluate longitudinal changes of GSH in patients at a larger scale and to correlate these with changes in clinical and functional status in patients with different subtypes of MS. The direct in vivo evaluation of ongoing oxidative stress in the human brain could lead to better understanding of the disease pathophysiology and progression, and to new developments of treatment strategies and interventions linked to the disease mechanisms.
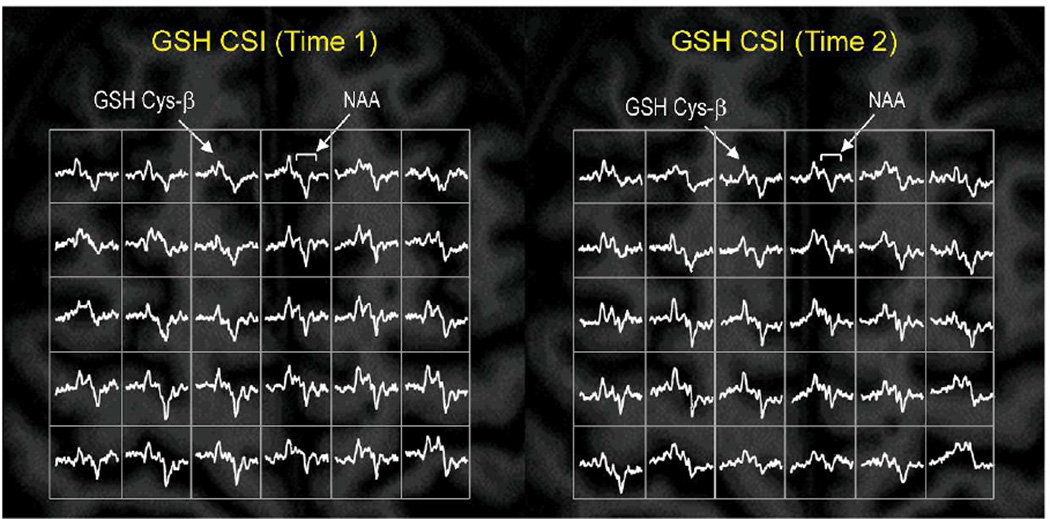
Figure 1. Longitudinal mapping of GSH in the brain of a patient with SPMS.
Partial views of the in vivo mapping of GSH show consistent detection of GSH signals in the brain of a patient with SPMS at the initial scan (Time 1, left) and at the follow-up scan (Time 2, right) at 3 T. The data were acquired from the same patient who was clinically stable during the study period of 43 months. The GSH CSI acquisition parameters of Time 1 and Time 2 were identical: slice thickness = 3 cm, matrix size = 8 × 8, FOV = 20 × 20 cm2, TE/TR = 115/1500 ms, spectral width = 2 kHz, and number of averages = 12). GSH CSI data were overlaid on the corresponding anatomical MR images from the middle of the CSI slab. Nominal spatial resolution of the CSI was 1.25 × 1.25 × 3 cm3 after 1 × zero padding.
Figure 2. Longitudinal change in brain GSH and clinical status of patients with SPMS.
(A) GSH concentrations in the frontal region at Time 1 and Time 2 are shown for each patient distinguished according to stable or worsening clinical status. (B) Box plot for the percentage change in frontal GSH concentrations is depicted for patients with stable (n=7) versus worsening (n=6) clinical status over the 3–5 year interval between Time 1 and Time 2, based on the neurologists’ blinded appraisal. The percentage changes of stable and worsening subgroups were compared using a nonparametric statistical test (Mann-Whitney U test).
Acknowledgments
This study was supported in part by the National Multiple Sclerosis Society (SGL) and a grant from the NIH (R03AG022193) (IYC). The Hoglund Brain Imaging Center is supported by grants from the NIH (UL1TR000001) and the Hoglund Family Foundation.
Appendix
| Hardware | |
|---|---|
| Field strength | 3 |
| Manufacturer | Siemens |
| Model | Allegra |
| Coil type (e.g. head, surface) |
Helmet coil |
| Number of coil channels | 1 |
| Acquisition sequence | ||
|---|---|---|
| Type (e.g. FLAIR, DIR, DTI, fMRI) |
Multiple quantum filtered CSI sequence for GSH |
|
| Acquisition time | 19 min | |
| Orientation | Axial | |
| Alignment (e.g. anterior commissure/poster commissure line) |
Axial above ventricle | |
| Voxel size | Nominal voxel size = 1.25 × 1.25 × 3 cm3after 1× zero padding. |
|
| TR | 1500 ms | |
| TE | 115 ms | |
| TI | ||
| Flip angle | 90 | |
| NEX | 12 | |
| Field of view | 20 × 20 cm2 | |
| Matrix size | 8 × 8 | |
| Parallel imaging | Yes | No |
| If used, parallel imaging method: (e.g. SENSE, GRAPPA) |
||
| Cardiac gating | Yes | No |
| If used, cardiac gating method: (e.g. PPU or ECG) |
||
| Contrast enhancement | Yes | No |
| If used, provide name of contrast agent, dose and timing of scan post-contrast administration |
||
| Other parameters: | ||
| Acquisition sequence | ||
|---|---|---|
| Type (e.g. FLAIR, DIR, DTI, fMRI) |
PRESS Localized CSI | |
| Acquisition time | 7 min | |
| Orientation | Axial | |
| Alignment (e.g. anterior commissure/poster commissure line) |
Axial above ventricle | |
| Voxel size | Nominal voxel size = 0.625 × 0.625 × 2 cm3after 1× zero padding. |
|
| TR | 2000 ms | |
| TE | 30 ms | |
| TI | ||
| Flip angle | 90 | |
| NEX | 2, Weighted averaging | |
| Field of view | 20 × 20 cm2 | |
| Matrix size | 16 × 16 | |
| Parallel imaging | Yes | No |
| If used, parallel imaging method: (e.g. SENSE, GRAPPA) |
||
| Cardiac gating | Yes | No |
| If used, cardiac gating method: (e.g. PPU or ECG) |
||
| Contrast enhancement | Yes | No |
| If used, provide name of contrast agent, dose and timing of scan post-contrast administration |
||
| Other parameters: | ||
| Image analysis methods and outputs | |
|---|---|
| Lesions | |
| Type (e.g. Gd-enhancing, T2-hyperintense, T1-hypointense) |
GSH quantification |
| Analysis method | Internal reference method using simultaneously acquired creatine signals |
| Analysis software | In-house software written in IDL |
| Output measure (e.g. count or volume [ml]) |
µmol/g tissue |
| Tissue volumes | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord) |
|
| Analysis method | |
| Analysis software | |
| Output measure (e.g. absolute tissue volume in ml, tissue volume as a fraction of intracranial volume, percentage change in tissue volumes) |
|
| Tissue measures (e.g. MTR, DTI, T1-RT, T2-RT, T2*, T2’, 1H-MRS, perfusion, Na) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) |
|
| Analysis method | |
| Analysis software | |
| Output measure | |
| Other MRI measures (e.g. functional MRI) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) |
|
| Analysis method | |
| Analysis software | |
| Output measure | |
Other analysis details:
| Image analysis methods and outputs | |
|---|---|
| Lesions | |
| Type (e.g. Gd-enhancing, T2-hyperintense, T1-hypointense) |
Creatine quantification |
| Analysis method | LCModel analysis with an external reference method and partial tissue volume fraction correction |
| Analysis software | LCModel and in-house software written in Matlab |
| Output measure (e.g. count or volume [ml]) |
µmol/g tissue in an institutional unit |
| Tissue volumes | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord) |
|
| Analysis method | |
| Analysis software | |
| Output measure (e.g. absolute tissue volume in ml, tissue volume as a fraction of intracranial volume, percentage change in tissue volumes) |
|
| Tissue measures (e.g. MTR, DTI, T1-RT, T2-RT, T2*, T2’, 1H-MRS, perfusion, Na) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) |
|
| Analysis method | |
| Analysis software | |
| Output measure | |
| Other MRI measures (e.g. functional MRI) | |
| Type (e.g. whole brain, grey matter, white matter, spinal cord, normal-appearing grey matter or white matter) |
|
| Analysis method | |
| Analysis software | |
| Output measure | |
Footnotes
A preliminary account of this work has been presented at the meeting of ECTRIMS in Lyon, France, on October 10–13, 2012.
REFERENCES
- 1.Burks J, Bigley GK, Hill H. Multiple Sclerosis Disease Courses. In: Lin VW, Cardenas DD, Cutter NC, editors. Spinal Cord Medicine: Principles and Practice. New York: Demos Medical Publishing; 2003. [Google Scholar]
- 2.Bakshi R, Thompson AJ, Rocca MA, Pelletier D, Dousset V, Barkhof F, et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008;7(7):615–625. doi: 10.1016/S1474-4422(08)70137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippi M, Agosta F. Magnetic resonance techniques to quantify tissue damage, tissue repair, and functional cortical reorganization in multiple sclerosis. Prog Brain Res. 2009;175:465–482. doi: 10.1016/S0079-6123(09)17531-3. [DOI] [PubMed] [Google Scholar]
- 4.Filippi M, Rocca MA, De Stefano N, Enzinger C, Fisher E, Horsfield MA, et al. Magnetic resonance techniques in multiple sclerosis: the present and the future. Arch Neurol. 2011;68(12):1514–1520. doi: 10.1001/archneurol.2011.914. [DOI] [PubMed] [Google Scholar]
- 5.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi I-Y, Lee S-P, Denney DR, Lynch SG. Lower Levels of Glutathione (GSH) in the Brains of Secondary Progressive Multiple Sclerosis Patients Measured by 1H Magnetic Resonance Chemical Shift Imaging at 3 T. Mult Scler. 2011;17(3):289–296. doi: 10.1177/1352458510384010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi I-Y. Regional distribution of glutathione in the human brain in vivo. J Neurochem. 2003;87(Suppl. 1):161. [Google Scholar]
- 8.Choi IY, Lee P. Doubly selective multiple quantum chemical shift imaging and T1 relaxation time measurement of glutathione (GSH) in the human brain in vivo. NMR Biomed. 2013;26(1):28–34. doi: 10.1002/nbm.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 10.Bains JS, Shaw CA. Oxidativestress and neurological diseases: Is glutathione depletion a common factor? In: Shaw CA, editor. Glutathione in the nervous system. Washington, DC: Taylor & Francis; 1998. pp. 355–384. [Google Scholar]
- 11.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18(9):685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cutler RG. Human longevity and aging: possible role of reactive oxygen species. Ann N Y Acad Sci. 1991;621:1–28. doi: 10.1111/j.1749-6632.1991.tb16965.x. [DOI] [PubMed] [Google Scholar]
- 13.Harman D. Role of free radicals in aging and disease. Ann N Y Acad Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 14.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38(3):357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 15.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson's disease. Biochem Pharmacol. 2002;64(5–6):1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 16.Srinivasan R, Ratiney H, Hammond-Rosenbluth KE, Pelletier D, Nelson SJ. MR spectroscopic imaging of glutathione in the white and gray matter at 7 T with an application to multiple sclerosis. Magn Reson Imaging. 2010;29:163–170. doi: 10.1016/j.mri.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 18.Choi I-Y, Lee S-P, Garwood M, Ugurbil K, Merkle H. Simple partial volume transceive coils for in vivo 1H MR studies at high magnetic fields. Concepts in Magnetic Resonance Part B: Magnetic Resonance Engineering. 2007;31B(2):71–85. [Google Scholar]
- 19.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 20.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 21.Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, et al. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage. 2002;16(1):49–60. doi: 10.1006/nimg.2002.1057. [DOI] [PubMed] [Google Scholar]
- 22.Kurtzke JF. Historical and clinical perspectives of the expanded disability status scale. Neuroepidemiology. 2008;31(1):1–9. doi: 10.1159/000136645. [DOI] [PubMed] [Google Scholar]
- 23.Daumer M, Neuhaus A, Herbert J, Ebers G. Prognosis of the individual course of disease: the elements of time, heterogeneity and precision. J Neurol Sci. 2009;287(Suppl 1):S50–S55. doi: 10.1016/S0022-510X(09)71301-2. [DOI] [PubMed] [Google Scholar]
- 24.Kirov II, Tal A, Babb JS, Herbert J, Gonen O. Serial proton MR spectroscopy of gray and white matter in relapsing-remitting MS. Neurology. 2013;80(1):39–46. doi: 10.1212/WNL.0b013e31827b1a8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattingen E, Magerkurth J, Pilatus U, Hubers A, Wahl M, Ziemann U. Combined (1)H and (31)P spectroscopy provides new insights into the pathobiochemistry of brain damage in multiple sclerosis. NMR Biomed. 2011;24(5):536–546. doi: 10.1002/nbm.1621. [DOI] [PubMed] [Google Scholar]
- 26.Aboul-Enein F, Krssak M, Hoftberger R, Prayer D, Kristoferitsch W. Reduced NAA-levels in the NAWM of patients with MS is a feature of progression. A study with quantitative magnetic resonance spectroscopy at 3 Tesla. PLoS One. 2010;5(7):e11625. doi: 10.1371/journal.pone.0011625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vrenken H, Barkhof F, Uitdehaag BM, Castelijns JA, Polman CH, Pouwels PJ. MR spectroscopic evidence for glial increase but not for neuro-axonal damage in MS normal-appearing white matter. Magn Reson Med. 2005;53(2):256–266. doi: 10.1002/mrm.20366. [DOI] [PubMed] [Google Scholar]