Abstract
Numerous findings demonstrate that there is a strong association between maternal health during pregnancy and cardiovascular disease in adult offspring. The purpose of the present study was to test whether maternal gestational hypertension modulates brain renin-angiotensin-aldosterone system (RAAS) and proinflammatory cytokines that sensitizes angiotensin II-elicited hypertensive response in adult offspring. Also, the role of renal nerves and the RAAS in the sensitization process was investigated. RT-PCR analyses of structures of the lamina terminalis and paraventricular nucleus indicated upregulation of mRNA expression of several RAAS components and proinflammatory cytokines in 10-week old male offspring of hypertensive dams. Most of these increases were significantly inhibited by either renal denervation performed at 8 weeks of age or treatment with an angiotensin converting enzyme inhibitor, captopril, in drinking water starting at weaning. When tested beginning at 10 weeks of age, a pressor dose of angiotensin II resulted in enhanced upregulation of mRNA expression of RAAS components and proinflammatory cytokines in the lamina terminalis and paraventricular nucleus and an augmented pressor response in male offspring of hypertensive dams. The augmented blood pressure change and most of the increases in gene expression in the offspring were abolished by either renal denervation or by captopril. The results suggest that maternal hypertension during pregnancy enhances pressor responses to angiotensin II through overactivity of renal nerves and the RAAS in male offspring, and that upregulation of the brain RAAS and proinflammatory cytokines in these offspring may contribute to maternal gestational hypertension-induced sensitization of the hypertensive response to angiotensin II.
Keywords: Maternal hypertension, Sensitization of hypertension, Renal nerves, Renin-angiotensin-aldosterone system, Inflammation
Introduction
Preeclampsia and gestational hypertension are common conditions affecting 5% to 7% of all pregnancies.1 Multiple case-control and cohort studies have demonstrated that offspring of hypertensive pregnancies have higher blood pressure (BP) in childhood and adolescence and are at increased risk for developing adult hypertension.2–7 Studies investigating the mechanisms that contribute to the development of hypertension in the offspring of mothers with preeclampsia and gestational hypertension found that male adolescents had increased aldosterone levels, a trend for increased circulating renin activity8 and increased sympathetic activity before and during isometric exercise.9 These results suggest the renin-angiotensin-aldosterone system (RAAS) and sympathetic nerve activity (SNA) are involved in the development of higher BP in the offspring of mothers with high BP during pregnancy.
Animal experiments have shown that uteroplacental insufficiency, protein restriction, chronic secondary hypertension or glucocorticoid treatment during pregnancy leads to hypertension in the offspring.10 Consistent with the RAAS and SNA playing a role in mediating the fetal programming of hypertension, several studies have demonstrated that both captopril, an angiotensin converting enzyme (ACE1) inhibitor, and losartan, an angiotensin (ANG) II receptor blocker, administered in the drinking water reduced increased BP in the offspring of dams fed low protein diet,11,12 and of spontaneously hypertensive rats, 13 while renal denervation (RD) abolished hypertension in the offspring from diabetic rats14 or from pregnant rats with reduced uterine perfusion.15,16 Moreover, Mizuno and colleagues demonstrated that the RAAS contributes to the enhancement of the renal sympathetic and pressor responses to physical stress in male offspring exposed to maternal protein restriction.17,18 Recent studies also indicate that activation of inflammatory pathways is present early in the kidney of offspring from diabetic dams,19 and that early life NF-κB dyshomeostasis in conduit arteries induced by prenatal inflammatory exposure plays a role in the development of hypertension through triggering RAAS overactivity.20 Most of these previous studies have focused on the roles of the peripheral RAAS and SNA in the development of hypertension in prenatally programmed hypertensive rats. Whether the RAAS, proinflammatory cytokines or SNA in the central nervous system (CNS) is affected during the prenatal period to predispose the offspring to higher BP is not clear.
Recently, a link between the peripheral RAAS activated in hypertension and the CNS as a source of neurohumoral drive has been identified. In this context, structures associated with the lamina terminalis (LT) and hypothalamic paraventricular nucleus (PVN) have emerged as sites that sense and process information related to humoral signals generated peripherally in response to challenges leading to augmented sympathetic drive and hypertension.21,22 An earlier study from Pladys et al. demonstrated that intracerebroventricular injection of an ACE inhibitor or an AT1R antagonist significantly reduced BP of fetal protein-restricted offspring. Expression of the ANG II receptor type 1 (AT1R) in the subfornical organ (SFO) and the vascular organ of the lamina terminalis (OVLT) was increased in these offspring.23 These data implicate the brain RAAS in hypertension associated with antenatal nutrient deprivation.
It has been shown that the risk to the offspring is graded and greatest in those whose mothers had more severe hypertensive signs, such as early onset hypertension or preeclampsia.24 This phenomenon was further confirmed in a recent study from Staley et al. who compared the BP of offspring from mothers with hypertension present at the start of pregnancy with those from mothers with pregnancy-induced hypertension or preeclampsia. These investigators found that a higher BP in early pregnancy was associated with higher BP during childhood in the offspring.6
In previous studies in adult animals, we used an Induction-Delay-Expression experimental design to study sensitization of the hypertensive response. In these studies, during Induction rats were exposed over a relatively short period of time to various types of challenges that did not produce by themselves any sustained effect on BP. Then following a period of Delay, a sensitized hypertensive response to a slow-pressor dose of ANG II was observed in these rats when challenged during a period of Expression.25–28 The present studies addressed the question of the permanence of sensitization of the hypertensive response and associated CNS changes in expression of RAAS components and proinflammatory cytokines. We did this by inducing sensitization of the offspring during the prenatal period by subjecting pregnant dams to ANG II-elicited hypertension. Expression of the sensitized hypertensive response was studied when the offspring were adult while changes in the mRNA expression of components of the RAAS and of proinflammatory cytokines were primarily studied in the offspring at the time of weaning and as adults. We also determined whether RD or blockade of the RAAS reversed the sensitization and whether these effects were related to changes in the RAAS and proinflammatory cytokines in forebrain cardiovascular regulatory nuclei associated with the LT and PVN.
METHODS
Twenty-eight female and twenty-eight male rats (Sprague-Dawley, 10-week old, Harlan) were used for breeding. The dams were chronically instrumented with telemetry probes (TA11PA-C40; DSI) through the femoral artery for continuous monitoring of mean arterial pressure (MAP) and heart rate (HR). After baseline MAP and HR recordings were made, female dams were given vehicle (saline) or ANG II (sc, 250 ng/kg/min, model 2004, 4 weeks, Alzet) throughout mating and pregnancy. The offspring were weighed and counted at birth and male offspring were used in all experiments. Each experimental group was composed of individual subjects that were randomly selected from different litters.
At three weeks of age, male offspring from normotensive (NT) or hypertensive (HT) dams were euthanized and had their brains collected for analyses of mRNA expression of the RAAS components and proinflammatory cytokines (n=5 per group). Likewise, 10-week old offspring from NT or HT dams and offspring with RD performed at 8 weeks of age as previously described 29 or application of ACE inhibitor in the drinking water beginning at the time of weaning were also used to determine the changes in RAAS components and proinflammatory cytokines in brain (n=5 per group). The structures lying along the lamina terminalis [LT, i.e., the SFO, median preoptic nucleus (MnPO), OVLT] and the PVN were used for these analyses.
In separate functional experiments, at 10 weeks of age, male offspring of both NT dams and HT dams were used to determine whether maternal hypertension during pregnancy sensitized the hypertensive response to slow pressor doses of ANG II (120 ng/kg/min, model 2002; 2 weeks, Alzet) and to evaluate the effects of either RD or ACE inhibition on sensitization. The RD experiments included four groups: 1) offspring of NT dam with sham surgery plus ANG II (NT dam-offspring/sham+ANG II, n=6); 2) offspring of HT dam with sham surgery plus ANG II (HT dam-offspring/sham+ANG II, n=6); 3) offspring of NT dam with RD plus ANG II (NT dam-offspring/RD+ANG II, n=6); 4) offspring of HT dam with RD plus ANG II (HT dam-offspring/RD+ANG II, n=6). The ACE inhibition experiments were comprised of four groups: 1 and 2) offspring of NT or HT dams were given tap water alone (vehicle) and ANG II infusion at 10 weeks of age (NT dam-offspring/vehicle+ANG II, HT dam-offspring/vehicle+ANG II, n=6 per group); 3 and 4) offspring of NT or HT dams were given an ACE1 inhibitor, captopril (Cap, 0.5 mg/ml), in the drinking water continuously until baseline recording began. Then ANG II infusion was started at 10 weeks of age (NT dam-offspring/Cap+ANG II, HT dam-offspring/Cap+ANG II, n=6 per group). Upon completion of the studies, the offspring were anesthetized and brains and kidneys were harvested. The kidneys were assayed for tissue norepinephrine (NE) content; and the brains were analyzed for mRNA expression of the RAAS components and of proinflammatory cytokines in the LT and PVN.
All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Iowa Animal Care and Use Committee.
Data Analysis
MAP and HR are presented as mean daily values. Differences for MAP and HR were calculated for each animal based on the mean of the 5-day baseline subtracted from the mean of the final 5 days of ANG II treatment. Two-way ANOVA for the experimental groups was then conducted on daily MAP, HR or the means of calculated differences. After establishing a significant ANOVA, post-hoc analyses were performed with Tukey multiple comparison tests between pairs of mean changes. One-way ANOVAs and post-hoc Tukey analyses were used to analyze the differences in NE content, mRNA or protein expression of the RAAS components and proinflammatory cytokines in the LT and PVN. All data are expressed as means ± SE. Statistical significance was set at P < 0.05.
Additional Methods: Please see the online-only Data Supplement.
RESULTS
Effects of hypertension during gestation on the dams and neonates
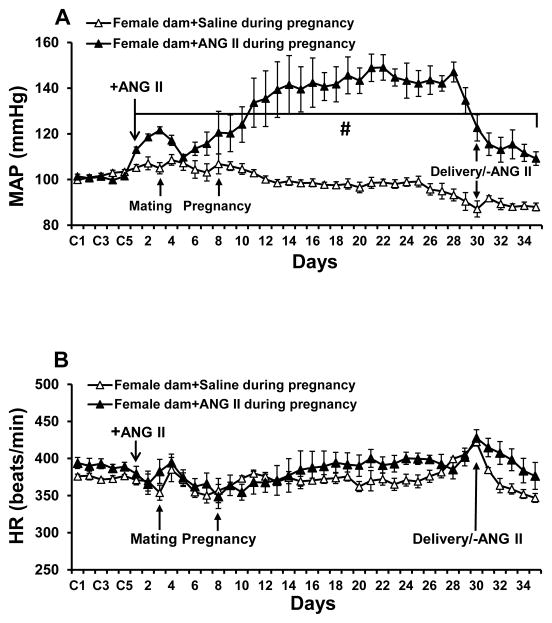
There were no differences in baseline BP and HR between NT and HT dams. During pregnancy, BP was significantly increased in dams receiving ANG II infusions when compared to female treated with saline, but there were no differences in HR (Fig. 1A and 1B). ANG II-induced hypertension did not impair reproduction and all females conceived. Fourteen hypertensive dams produced total 147 pups including 71 males and 76 females while fourteen normotensive dams produced a total of 166 pups including 77 males and 89 females. Although there was a tendency for the normotensive dams to have more pups than the hypertensive dams, there were no significant differences in litter sizes (10.7±1.4 pups vs. 11.8±0.6 pups, p>0.05) or birthweights of the pups (5.98±0.44 g/pup vs. 5.96±0.36 g/pup, p>0.05) from HT dams and NT dams, respectively.
Figure 1.
Changes in mean arterial pressure (MAP, Fig. 1A) and heart rate (HR, Fig. 1B) in normotensive (saline) and hypertensive [angiotensin (ANG) II, 250 ng/kg/min, sc] dams from beginning of baseline recording to recovery from delivery (n=6/group; +ANG II, beginning of ANG II infusion, -ANG II, stop of ANG II infusion; # p<0.05 vs. saline-treated dams).
mRNA and protein expression of RAAS components and proinflammatory cytokines in the brain of young and adult offspring without ANG II treatment
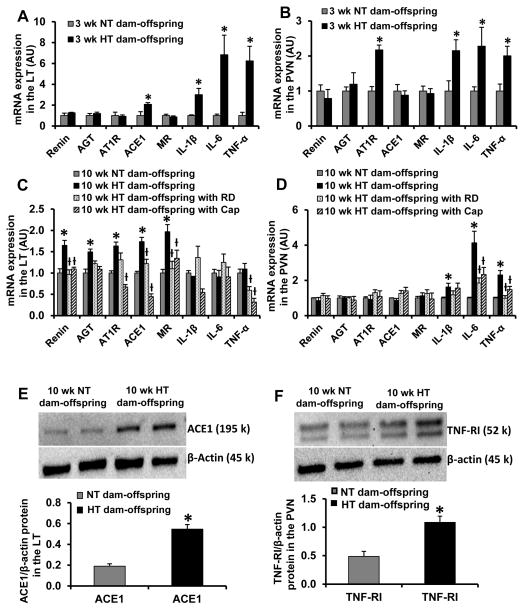
In brain tissues collected from pups at 3 weeks of age, offspring from HT dams exhibited increased mRNA expression of RAAS components (ACE1 in the LT and AT1R in the PVN) and the proinflammatory cytokines (i.e. TNF-α, IL-6, IL-1β in both the LT and PVN) when compared with offspring of NT dams (p<0.05, Fig. 2A and 2B). In contrast, in brain tissues collected at 10 weeks of age, the offspring of HT dams showed upregulation of mRNA expression of the RAAS components (renin, AGT, AT1R, ACE1 and MR) only in the LT and of the proinflammatory cytokines (i.e. TNF-α, IL-6, IL-1β) only in the PVN (p>0.05, Fig. 2C and 2D), which were significantly inhibited by either RD or captopril treatment. Western blotting analysis confirmed the upregulated effects of maternal hypertension during pregnancy on genomic expression in the adult offspring by determining the protein expression for one of RAAS components (ACE1) or for one of components of TNF-α proinflammatory pathway (TNF-α receptor I). The increased protein expression of ACE1in the LT and TNF-α receptor I in the PVN was evident (p<0.05, Fig. 2E and 2F).
Figure 2.
Quantitative comparison of the mRNA expression of renin-angiotensin system components and proinflammatory cytokines in the lamina terminalis (LT) and paraventricular nucleus (PVN) in male offspring of normotensive (NT) and hypertensive (HT) dams at 3 weeks of age (Fig 2A and 2B) and at 10 weeks of age before and after renal denervation (RD) and captopril (Cap) treatment (Fig. 2C and 2D). Fig. 2E and 2F show representative Western blots and quantitative comparison of protein levels for ACE1 in the LT (Fig 2E) and TNF-α receptor I (TNF-RI) in the PVN (Fig 2F) in male offspring of NT and HT dams at 10 weeks of age. Values are corrected by β-actin and expressed as mean ± SEM. (n=5/group; * p<0.05 vs NT-dam offspring; ƚ p<0.05 vs. HT-dam offspring without RD or Cap treatment).
Effects of maternal hypertension on the hypertensive response of adult offspring and effects of renal denervation on sensitization of ANG II-induced hypertension
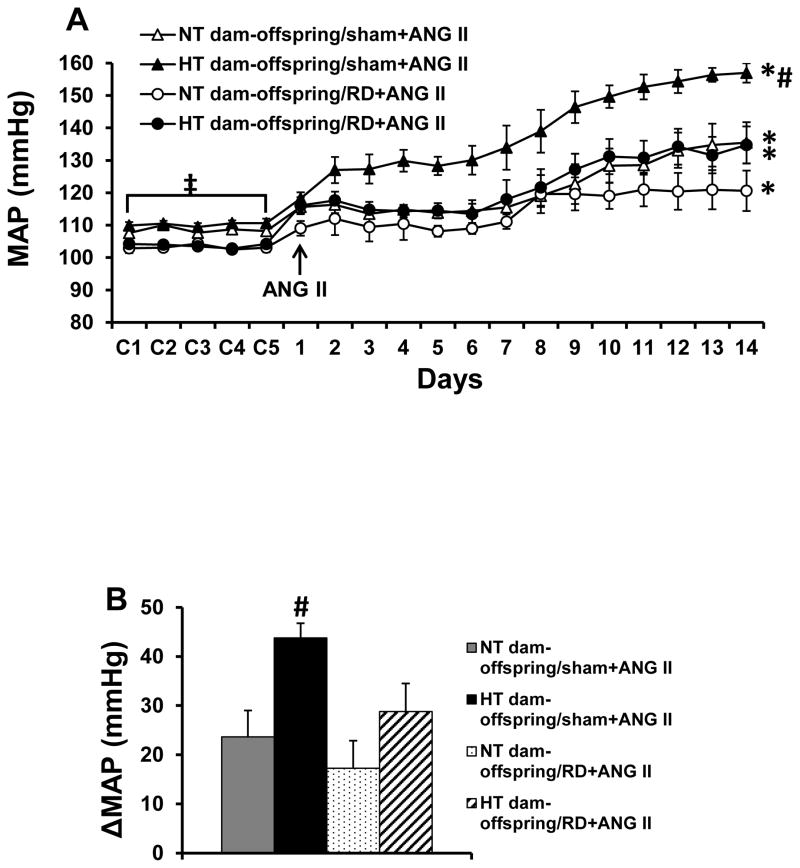
At 10 weeks of age (baseline), there were no significant differences in MAP (108.6±1.6 vs. 110.2±1.9 mmHg) and HR (345.7±8.9 vs. 342.8±10.5 beats/min) between RD sham offspring of NT and HT dams. However, during infusion of the slow pressor dose of ANG II, the male offspring of HT dams showed a significantly enhanced hypertensive response (43.7±3.1 mmHg) compared with the male offspring of NT dams (23.6±5.4 mmHg, p<0.05, Fig. 3A and 3B).
Figure 3.
Pressor effects (Fig. 3A and 3B) induced by angiotensin (ANG) II in adult offspring with or without renal denervation (RD) from normotensive (NT) and hypertensive (HT) dams. The enhanced pressor effect in offspring of HT dams was attenuated by bilateral renal denervation (RD). (n=6/group; * p<0.05 vs baseline; # p<0.05 vs RD offspring of HT dams and all offspring of NT dams; ‡ p<0.05 vs. offspring with RD).
RD significantly reduced baseline MAP in both offspring of NT (108.6±1.6 to 103.2±1.2 mmHg, p<0.05, Fig. 3A) and HT dams (110.2±1.9 to 103.7±0.6 mmHg, p<0.05, Fig. 3A), but did not alter baseline HR. Bilateral RD significantly reduced the ANG II-elicited pressor response including daily MAP and the means of difference score for MAP, in the offspring of the HT dams (28.7±4.7 mmHg, p<0.05, Fig. 3A and 3B). In RD offspring of NT dams, a declining trend in daily MAP of the final 5 days of ANG II treatment was noticed when compared with sham offspring of NT dams. However, RD did not result in a significant reduction in the ANG II-induced pressor response because of a lower baseline BP produced by RD (the means of difference score for MAP, 17.2±5.5 mmHg, p>0.05, Fig. 3A and 3B).
Effect of renal denervation on ANG II-induced mRNA expression of RAS components and proinflammatory cytokines in the brain
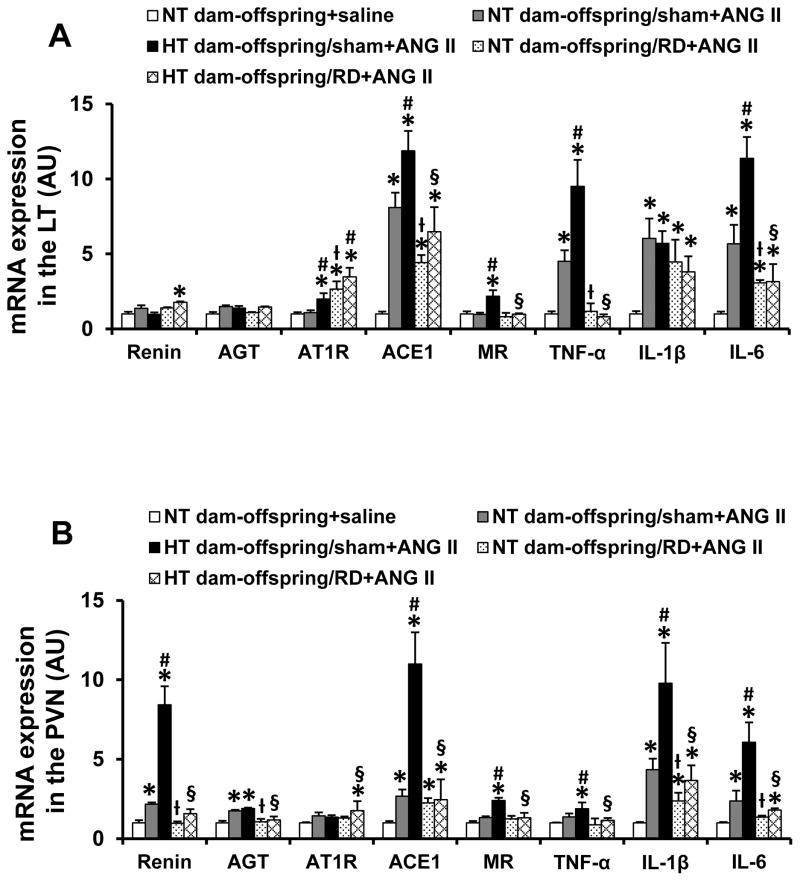
In LT tissues, the slow-pressor ANG II infusion resulted in a significant increase in mRNA expression of ACE1 and proinflammatory cytokines (i.e. TNF-α, IL-6, IL-1β) in the offspring of NT dams when compared with the saline group (p<0.05, Fig. 4A). The mRNA expression of renin, AGT, AT1R and MR was not higher after ANG II (p>0.05). Compared with the NT dam offspring treated with ANG II, the offspring of HT dams exhibited enhanced expression of AT1R, MR, ACE1, TNF-α and IL-6 (p<0.05), but not IL-1β (p>0.05) after ANG II treatment (Fig. 4A). RD significantly attenuated the increased mRNA expression of ACE1, MR, TNF-α and IL-6 (p<0.05), but AT1R and IL-1β expression remained high in both offspring of NT and HT dams following slow-pressor ANG II administration (p>0.05). Even mRNA expression of AT1R was upregulated in RD offspring of NT dams when compared with RD sham offspring of NT dams (p<0.05, Fig. 4A).
Figure 4.
Quantitative comparison of the mRNA expression of renin-angiotensin-aldosterone system components and proinflammatory cytokines in the lamina terminalis (LT, Fig 4A) and paraventricular nucleus (PVN, Fig 4B) of adult offspring receiving sham or renal denervation (RD) after angiotensin (ANG) II administration. (n=6/group; * p<0.05 vs. NT dam-offspring+saline; # p<0.05 HT dam-offspring +ANG II vs. NT dam-offspring+ANG II; ƚ p<0.05 RD NT dam-offspring+ANG II vs. sham NT dam-offspring+ANG II; § p<0.05 RD HT dam-offspring+ANG II vs. sham HT dam-offspring +ANG II).
In PVN tissues, ANG II infusion elicited a significant increase in the mRNA expression of renin, AGT, ACE1, IL-6 and IL-1β in the offspring of NT dams when compared with the saline group (p<0.05, Fig. 4B). The mRNA expression of MR and TNF-α was not higher after ANG II (p>0.05). The offspring of HT dams showed enhanced mRNA expression of renin, ACE1, MR, TNF-α, IL-1β and IL-6 after ANG II treatment (p<0.05, Fig. 4B). RD significantly attenuated increased gene expression produced by the slow-pressor ANG II infusion (p<0.05, Fig. 4B) in offspring of both NT and HT dams.
Effect of captopril treatment on prenatal gestational hypertension-induced sensitization of ANG II hypertension
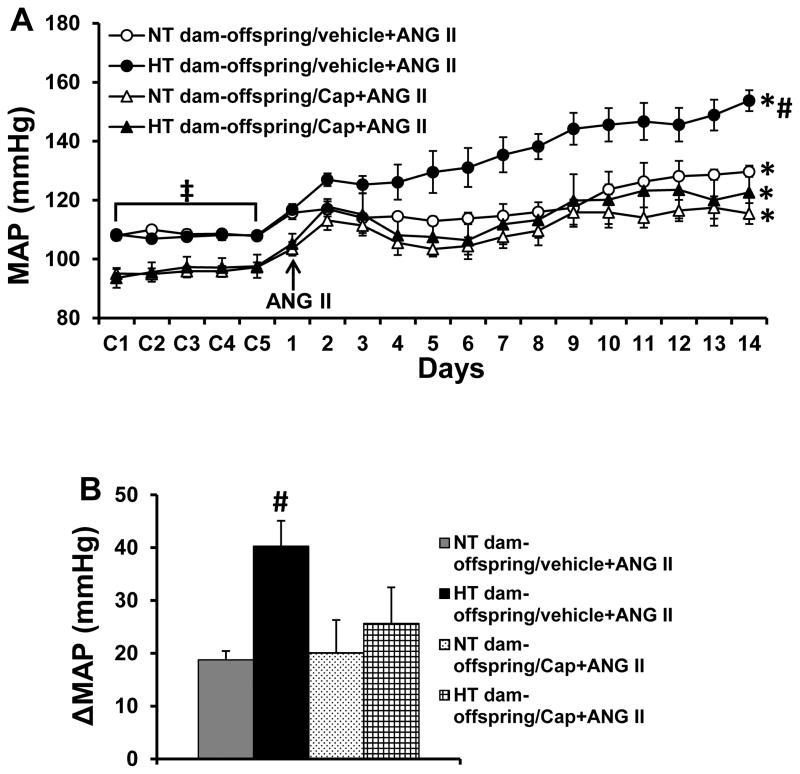
Captopril administered in the drinking water after weaning significantly reduced baseline MAP in both offspring of NT (108.6±1.4 to 95.8±1.7 mmHg, p<0.05, Fig. 5A) and HT dams (107.8±1.1 to 96.5±3.4 mmHg, p<0.05, Fig. 5A), but had no effect on basal HR. Furthermore, the captopril treatment significantly attenuated the enhanced hypertensive response produced by the slow-pressor dose of ANG II as compared to animals without captopril treatment in the offspring of HT dams (40.3±4.8 vs. 25.6±5.8 mmHg, p<0.05, Fig. 5A and 5B). In captopril-treated offspring from NT dams, a declining trend in daily MAP of the final 5 days of ANG II treatment was evident when compared with the non-captopril treated offspring of NT dams. However, the alteration in the ANG II-induced pressor response produced by captopril treatment was not significant (the means of difference score for MAP, 18.7±1.7 vs. 20.1±3.6 mmHg, p>0.05, Fig. 5A and 5B).
Figure 5.
Pressor effects (Fig. 5A and 5B) induced by angiotensin (ANG) II in adult offspring with or without ACE1 inhibitor, captopril (Cap), treatment from normotensive (NT) and hypertensive (HT) dams. The enhanced pressor effect in offspring of HT dams was attenuated by pretreatment with Cap. (n=6/group; * p<0.05 vs baseline; # p<0.05 vs Cap-treated offspring of HT dams and all offspring of NT dams; ‡ p<0.05 vs. offspring treated with Cap).
Effect of captopril treatment on ANG II-induced mRNA expression of RAS components and proinflammatory cytokines in the brain
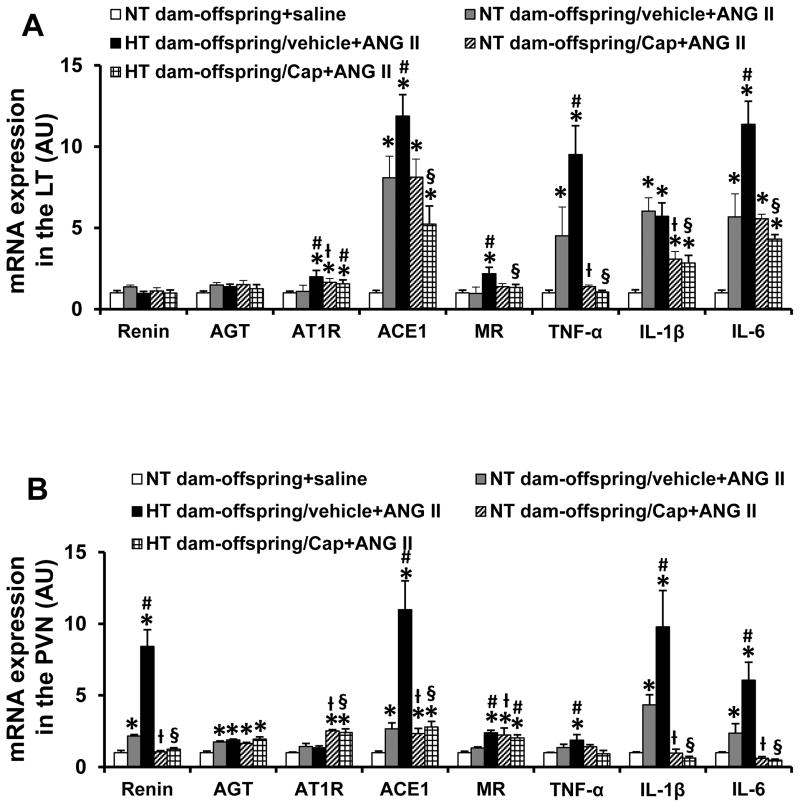
In LT tissues, captopril treatment significantly attenuated ANG II-induced increases in mRNA expression of TNF-α and IL-1β (p<0.05, Fig. 6A), but not ACE1 and IL-6 (p>0.05) in offspring of NT dams. mRNA expression of AT1R was also upregulated in the captopril-treated offspring of NT dams. In contrast, the enhanced mRNA expression of ACE1, MR, TNF-α, IL-1β and IL-6 after ANG II were significantly reduced by captopril treatment in the offspring of HT dams (p<0.05, Fig. 6A).
Figure 6.
Quantitative comparison of mRNA expression of renin-angiotensin-aldosterone system components and proinflammatory cytokines in the lamina terminalis (LT, Fig 6A) and paraventricular nucleus (PVN, Fig 6B) of adult offspring with or without captopril (Cap) after saline or angiotensin (ANG) II administration. (n=6/group; * p<0.05 vs. NT dam-offspring+saline; # p<0.05, HT dam-offspring +ANG II vs. NT dam-offspring+ANG II; ƚ or § p<0.05 Cap treated NT- or HT-dam offspring+ANG II vs. non-Cap treated NT or HT-dam offspring +ANG II, respectively).
In PVN tissues, the slow-pressor ANG II infusion elicited enhanced increases in the mRNA expression of renin, ACE1, MR, TNF-α, IL-6 and IL-1β in the offspring of HT dams when compared with those in the offspring of NT dams (p<0.05, Fig. 6B). Captopril treatment reversed most of the increased expression in both offspring of NT and HT dams.
Surprisingly, in the offspring of NT dams, captopril treatment did not attenuate the increased mRNA expression of AGT in the PVN, but upregulated mRNA expression of MR in the PVN and mRNA expression of AT1R in both the LT and PVN after delivery of the slow-pressor dose of ANG II. In contrast, the increased mRNA expression of AGT and MR in the PVN and AT1R in the LT were not altered, and mRNA expression of AT1R in the PVN was upregulated in the offspring of HT dams (Fig. 6A and 6B).
DISCUSSION
The major findings of the present study are: 1) The offspring of HT dams exhibited normal BP at 10 weeks of age, but had a significantly enhanced hypertensive response to a slow-pressor dose of ANG II; 2) Prior to challenging adult offspring with a slow-pressor dose of ANG II, the ten-week old offspring of HT dams showed upregulated expression of both RAAS components and proinflammatory cytokines in the LT and PVN when compared with the offspring of NT dams; 3) Either RD or captopril treatment blocked sensitization of the hypertensive response and reversed the changes in RAAS and proinflammatory cytokine mRNA expression of the offspring of HT dams. Taken together, these results indicate that maternal hypertension during the prenatal period produces long lasting sensitization of the hypertensive response, perhaps lasting a lifetime, and suggest that these alterations are mediated by changes in CNS factors located in the neural network contributing to the regulation of BP.
The predisposition of the offspring of HT dams to display an enhanced hypertensive response appears to be very similar to sensitization of this response produced by prior challenges that we have found in adult animals.25–28 In these previous studies, we used an Induction-Delay-Expression experimental design to investigate whether peripheral or central pretreatment with non-pressor doses of ANG II, aldosterone, leptin or TNF-α, or feeding of high dietary fat sensitized the hypertensive response to a slow-pressor dose of ANG II. We found that these mild physiological or dietary challenges upregulated mRNA expression of several components of the RAAS and proinflammatory cytokines in the brain, whereas central inhibition of AT1-R, MR and inflammation reversed the changes in gene expression and prevented the sensitization of ANG II-elicited hypertension. The results suggest that the sensitization of hypertension depends on the functional integrity of the brain RAAS and proinflammatory cytokines.25–28 In the present studies, Induction during the prenatal period was sufficient to sensitize the hypertensive response observed during Expression after an intervening 10-week postnatal Delay. Also, at ten weeks of age, before administering a pressor challenge, the adult offspring showed evidence of long-term neurochemical changes in brain regions implicated in the control of BP. The present experiments demonstrate that reprogramming of the mechanisms controlling BP during a sensitive prenatal period produces long lasting phenotypic changes in the CNS and increased responsiveness to a hypertensinogenic challenge.
Increased basal BP has been shown in childhood and adolescence of preeclamptic or hypertensive mothers,2–7 or in the offspring of rat dams with diabetes,19 protein restriction,11 and uteroplacental insufficiency16 during pregnancy. Alterations of the RAAS and proinflammatory cytokines in peripheral tissues and plasma have been implicated as important mediators in the fetal programming of increased BP. 30 Washburn et al. found increased plasma aldosterone levels in male adolescents born to mothers with preeclampsia.8 In animal experiments, the offspring from dams with protein restriction,11,12 diabetes19 or inflammation20 showed significant increases in intrarenal RAAS components such as angiotensinogen and ANG II, renal and arterial proinflammatory cytokines and pulmonary and plasma ACE activity, which were associated with higher BP in these offspring. However, not all studies involving treatments increasing the BP of pregnant dams have reported increased basal BP in the offspring. For example, there was no increase in basal BP in adult male offspring from dams with aldosterone-induced31 or two-kidney-1kidney-wrapped hypertension32 during pregnancy. The absence of changes in resting BP in these models is similar to the finding of a normal basal BP seen in the present study.
It has been shown that hypertension is associated with exaggerated sympathetic activity which is due to an imbalance between inhibitory and excitatory mechanisms within specific areas in the CNS including the LT and PVN, two forebrain regions involved in regulation of BP and sympathetic activity.22 Based on previous evidence, it is reasonable to hypothesize that elevated activity of RAAS components and proinflammatory cytokines and a synergistic interaction between the RAAS and proinflammatory cytokines in these forebrain nuclei play a pivotal role in the process of sensitization of sympathetic nerve activity and the hypertensive response.25–28,33 In the present study, we found an up-regulation of mRNA expression of several RAAS components and proinflammatory cytokines in both LT and PVN at 3 weeks age, whereas at 10 weeks age, upregulation of RAAS components was evident only in the LT and upregulation of proinflammatory cytokines was seen only in the PVN. It is unclear why the pattern of expression of message and protein of these RAAS components and proinflammatory cytokines changes over the ontogenetic period studied, but considering the dynamic developmental changes that occur in progressing from early childhood through puberty into adulthood, such effects might be expected. The important point to recognize is that exposure to hypertension during pregnancy results in a distinct brain phenotype which might predispose offspring to develop high BP in response to challenges or pressor stimuli that come into play later in life. As expected, we did find that the gestational hypertension induced by a high dose of ANG II sensitized the ANG II-induced hypertension in male offspring resulting in an augmented increase in BP.
Increased renal SNA and overactivity of RAAS and proinflammatory cytokines have been implicated as causal mechanisms in prenatal programming of hypertension. RD or chronic blockade of the RAAS and inflammation has been demonstrated to reduce elevated BP through restoration of renal and arterial function in offspring of dams after different types of insult to the mother during pregnancy.11,12,14–16,20 Mizuno et al. demonstrated that the RAAS plays a significant role not only in the generation of increased basal BP but also in the development of the enhanced renal sympathetic and pressor responses to physical stress in prenatally programmed adult hypertensive rats.17,18 Based on such observations, we investigated whether disrupting the integrity of the renal nerves or of the RAAS would alter the maintenance of sensitization of ANG II-induced hypertension in offspring of HT dams. The results of these experiments showed that either bilateral RD or captopril treatment in the offspring of HT dams reduced the sensitized hypertensive response to a level seen in the offspring of NT dams.
The protective effects of both RD and blockade of the RAAS were accompanied by attenuation of the enhanced expression of LT and PVN RAAS components and proinflammatory cytokines induced by either prenatal gestational hypertension or ANG II treatment. The results indicate that RD and ACE1 inhibition appear to have similar effects and that the renal nerves and the RAAS affect neural activity of cardiovascular nuclei that have been implicated the sensitization of ANG II-induced hypertension.22 The central changes observed in our study are consistent with previous work showing that maternal dietary protein restriction induced an increase in brain AT1R expression in the offspring and central blockade of ACE or AT1R abolished increased BP.23 Also, the present findings are reinforced by a recent study showing that RD ameliorated heart failure through down-regulation of the brain RAAS and markers of inflammation in rats.34
In contrast to the consistency of RD and ACE1 inhibitor abrogating the sensitizing effects on BP, the alterations of mRNA expression found in the LT and PVN after 2 weeks of infusion of a slow-pressor dose of ANG II did not provide a consistent pattern of change. For example, at the end of Expression, the AT1R was significantly increased in the LT in the RD offspring from NT dams and in the PVN of the RD offspring from HT dams. Also there was the increased expression of IL-1β in the LT that was not reduced by RD in both types of offspring (Fig. 4). Likewise, captopril treatment did not attenuate the increased mRNA expression of several RAAS components, and some of these RAAS components were actually upregulated (Fig. 6). At present there is no clear explanation for these changes except to speculate that the increased level of message and proteins in key brain nuclei may reflect compensatory mechanisms necessary for maintaining viable BP following RD or captopril treatment.
There are some potential limitations to our study. 1) Age may serve as an important factor determining the impact of the development of adverse cardiovascular function in offspring of dams after prenatal programming.15,31,32 We only examined basal BP and HR at 10–12 weeks of age in the offspring of HT dams and saw no difference from those of offspring of NT dams. Studying BP in older offspring (e.g. 6–12 months of age) may show differences in basal BP and warrants further investigation. 2) The elevated maternal RAAS activity has been demonstrated to influence development of renal sympathetic nerves and contribute to programming of adult hypertension.35 Besides this, alterations in gene expression and the delayed evolution of hypertension seems to be linked to the epigenetic changes induced during pregnancy.10 Epigenetic mechanisms involved in altering brain RAAS and proinflammatory cytokines of offspring in the ANG II maternal hypertensive programming model also need to be investigated in the future. 3) We have considered the effects of maternal ANG II-induced hypertension per se, increased circulating ANG II, ANG II-induced cytokines or combination of these factors to produce sensitization of the hypertensive response due to factors operating while the pup is in utero.36 However, since the pups were raised to the time of weaning by their natural mothers, it cannot be ruled out that maintained changes in the mother’s physiology or behavior may also have played a role in the process of sensitization. Future studies employing cross-fostering of pups need to be conducted to determine if there are potential effects of mothering on sensitization of the hypertensive response.
Perspectives
The present study demonstrated the influence of prenatal hypertensive programming on BP in adult offspring. Such programming was associated with altered gene expression of the RAAS and proinflammatory cytokines in the CNS that may, at least in part, explain the increased sensitization of ANG II-induced hypertension. The protective effects of RD and RAAS blockade on the sensitization of ANG II-induced hypertension in these offspring suggest that maternal hypertension-induced sensitization of male offspring can be reversed by interventions delivered between the time of weaning and testing for the expression of the hypertensive response. This new model of sensitization opens up many possibilities to investigate interventions that will reverse presumed epigenetic and other molecular changes that mediate nervous system plasticity and the long-term maintenance of sensitization.
Supplementary Material
Novelty and Significance.
What is New?
These studies demonstrate that maternal hypertension during pregnancy sensitized the hypertensive response to angiotensin (ANG) II in adult male offspring through upregulation of message for components of the brain renin-angiotensin-aldosterone system (RAAS) and proinflammatory cytokines. Either earlier renal denervation (RD) or prior RAAS inhibition prevents the prenatal gestational hypertension-elicited sensitization of ANG II hypertension.
What is Relevant?
The demonstration that maternal hypertension during pregnancy facilitated mRNA expression of the central RAAS components and of proinflammatory cytokines in the offspring indicates that the neurohumoral systems are likely to play an important role in the pathogenesis of prenatal programming of hypertension. Also, of importance is the findings that either RD or postnatal administered ACE inhibitor reversed the effects of fetal programming of the sensitized hypertensive response.
Summary
The study indicates that prenatal gestational hypertension results in reprogramming of mechanisms involved in the control of BP and that these changes are maintained for a long period time in the offspring. Overactivity of renal sympathetic nerve and RAAS as well as altered mRNA expression of RAAS and proinflammatory cytokines within components of a forebrain cardiovascular control network are associated with the sensitizing process.
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL-14388 (AKJ) and HL-98207 (AKJ)
Footnotes
Disclosures: None
References
- 1.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. doi: 10.1161/HYPERTENSIONAHA.109.130765. [DOI] [PubMed] [Google Scholar]
- 2.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, Kylintireas I, Contractor H, Singhal A, Lucas A, Neubauer S, Kharbanda R, Alp N, Kelly B, Leeson P. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–165. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 3.Himmelmann A, Svensson A, Hansson L. Five-year follow-up of blood pressure and left ventricular mass in children with different maternal histories of hypertension: the Hypertension in Pregnancy Offspring Study. J Hypertens. 1994;12:89–95. [PubMed] [Google Scholar]
- 4.Himmelmann A, Svensson A, Hansson L. Relation of maternal blood pressure during pregnancy to birth weight and blood pressure in children. The Hypertension in Pregnancy Offspring Study. J Intern Med. 1994;235:347–352. doi: 10.1111/j.1365-2796.1994.tb01085.x. [DOI] [PubMed] [Google Scholar]
- 5.Tenhola S, Rahiala E, Halonen P, Vanninen E, Voutilainen R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr Res. 2006;59:320–324. doi: 10.1203/01.pdr.0000196734.54473.e3. [DOI] [PubMed] [Google Scholar]
- 6.Staley JR, Bradley J, Silverwood RJ, Howe LD, Tilling K, Lawlor DA, Macdonald-Wallis C. Associations of blood pressure in pregnancy with offspring blood pressure trajectories during childhood and adolescence: findings from a prospective study. J Am Heart Assoc. 2015;4:e001422. doi: 10.1161/JAHA.114.001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser A, Nelson SM, Macdonald-Wallis C, Sattar N, Lawlor DA. Hypertensive disorders of pregnancy and cardiometabolic health in adolescent offspring. Hypertension. 2013;62:614–620. doi: 10.1161/HYPERTENSIONAHA.113.01513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washburn LK, Brosnihan KB, Chappell MC, Diz DI, Gwathmey TM, Nixon PA, Russell GB, Snively BM, O’Shea TM. The renin-angiotensin-aldosterone system in adolescent offspring born prematurely to mothers with preeclampsia. J Renin Angiotensin Aldosterone Syst. 2015;16:529–538. doi: 10.1177/1470320314526940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopes HF, Consolim-Colombo FM, Barreto-Filho JA, Riccio GM, Negrão CE, Krieger EM. Increased sympathetic activity in normotensive offspring of malignant hypertensive parents compared to offspring of normotensive parents. Braz J Med Biol Res. 2008;41:849–853. doi: 10.1590/s0100-879x2008005000042. [DOI] [PubMed] [Google Scholar]
- 10.Liang M, Cowley AW, Jr, Mattson DL, Kotchen TA, Liu Y. Epigenomics of hypertension. Semin Nephrol. 2013;33:392–399. doi: 10.1016/j.semnephrol.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 12.Mansuri A, Elmaghrabi A, Legan SK, Gattineni J, Baum M. Transient Exposure of Enalapril Normalizes Prenatal Programming of Hypertension and Urinary Angiotensinogen Excretion. PLoS One. 2015;10:e0146183. doi: 10.1371/journal.pone.0146183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu JN, Berecek KH. Prevention of genetic hypertension by early treatment of spontaneously hypertensive rats with the angiotensin converting enzyme inhibitor captopril. Hypertension. 1993;22:139–146. doi: 10.1161/01.hyp.22.2.139. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues AF, de Lima IL, Bergamaschi CT, Campos RR, Hirata AE, Schoorlemmer GH, Gomes GN. Increased renal sympathetic nerve activity leads to hypertension and renal dysfunction in offspring from diabetic mothers. Am J Physiol Renal Physiol. 2013;304:F189–197. doi: 10.1152/ajprenal.00241.2012. [DOI] [PubMed] [Google Scholar]
- 15.Intapad S, Tull FL, Brown AD, Dasinger JH, Ojeda NB, Fahling JM, Alexander BT. Renal denervation abolishes the age-dependent increase in blood pressure in female intrauterine growth-restricted rats at 12 months of age. Hypertension. 2013;61:828–834. doi: 10.1161/HYPERTENSIONAHA.111.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alexander BT, Hendon AE, Ferril G, Dwyer TM. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension. 2005;45:754–758. doi: 10.1161/01.HYP.0000153319.20340.2a. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno M, Siddique K, Baum M, Smith SA. Prenatal programming of hypertension induces sympathetic overactivity in response to physical stress. Hypertension. 2013;61:180–186. doi: 10.1161/HYPERTENSIONAHA.112.199356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizuno M, Lozano G, Siddique K, Baum M, Smith SA. Enalapril attenuates the exaggerated sympathetic response to physical stress in prenatally programmed hypertensive rats. Hypertension. 2014;63:324–329. doi: 10.1161/HYPERTENSIONAHA.113.02330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Correa-Costa M, Landgraf MA, Cavanal MF, Semedo P, Vieira DA, De Marco DT, Hirata AE, Câmara NO, Gil FZ. Inflammatory milieu as an early marker of kidney injury in offspring rats from diabetic mothers. Eur J Pharmacol. 2012;689:233–240. doi: 10.1016/j.ejphar.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 20.Deng Y, Deng Y, He X, et al. Prenatal inflammation-induced NF-κB dyshomeostasis contributes to renin-angiotensin system over-activity resulting in prenatally programmed hypertension in offspring. Sci Rep. 2016;6:21692. doi: 10.1038/srep21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–86. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 22.Johnson AK, Zhang Z, Clayton SC, Beltz TG, Hurley SW, Thunhorst RL, Xue B. The roles of sensitization and neuroplasticity in the long-term regulation of blood pressure and hypertension. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1309–1325. doi: 10.1152/ajpregu.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pladys P, Lahaie I, Cambonie G, Thibault G, Lê NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 24.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009;40:1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 25.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (Ang II)-initiated hypertension: induction of sensitization by prior Ang II treatment. Hypertension. 2012;59:459–466. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue B, Zhang Z, Roncari CF, Guo F, Johnson AK. Aldosterone acting through the central nervous system sensitizes angiotensin II-induced hypertension. Hypertension. 2012;60:1023–1030. doi: 10.1161/HYPERTENSIONAHA.112.196576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xue B, Thunhorst RL, Yu Y, Guo F, Beltz TG, Felder RB, Johnson AK. Central Renin-Angiotensin System Activation and Inflammation Induced by High-Fat Diet Sensitize Angiotensin II-Elicited Hypertension. Hypertension. 2016;67:163–170. doi: 10.1161/HYPERTENSIONAHA.115.06263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue B, Yu Y, Zhang Z, Guo F, Beltz TG, Thunhorst RL, Felder RB, Johnson AK. Leptin Mediates High-Fat Diet Sensitization of Angiotensin II-Elicited Hypertension by Upregulating the Brain Renin-Angiotensin System and Inflammation. Hypertension. 2016;67:970–976. doi: 10.1161/HYPERTENSIONAHA.115.06736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension. 2013;61:806–811. doi: 10.1161/HYPERTENSIONAHA.111.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasinger JH, Davis GK, Newsome AD, Alexander BT. Developmental Programming of Hypertension: Physiological Mechanisms. Hypertension. 2016;68:826–831. doi: 10.1161/HYPERTENSIONAHA.116.06603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Sanchez EP, Gomez-Sanchez CE. Maternal hypertension and progeny blood pressure: role of aldosterone and 11beta-HSD. Hypertension. 1999;33:1369–1373. doi: 10.1161/01.hyp.33.6.1369. [DOI] [PubMed] [Google Scholar]
- 32.Denton KM, Flower RL, Stevenson KM, Anderson WP. Adult rabbit offspring of mothers with secondary hypertension have increased blood pressure. Hypertension. 2003;41:634–639. doi: 10.1161/01.HYP.0000052949.85257.8E. [DOI] [PubMed] [Google Scholar]
- 33.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, Francis J. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li JD, Cheng AY, Huo YL, Fan J, Zhang YP, Fang ZQ, Peng W, Sun HS, Zhang JS, Wang HP, Xue BJ. Bilateral renal denervation ameliorates isoproterenol-induced heart failure through downregulation of the brain renin-angiotensin system and inflammation in rat. Oxidative Medicine and Cellular Longevity. 2016 doi: 10.1155/2016/3562634. ID 3562634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maduwegedera D, Kett MM, Flower RL, Lambert GW, Bertram JF, Wintour EM, Denton KM. Sex differences in postnatal growth and renal development in offspring of rabbit mothers with chronic secondary hypertension. Am J Physiol Regul Integr Comp Physiol. 2007;292:R706–714. doi: 10.1152/ajpregu.00458.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hsiao EY, Patterson PH. Placental regulation of maternal-fetal interactions and brain development. Dev Neurobiol. 2012;72:1317–1326. doi: 10.1002/dneu.22045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.