Abstract
IMPORTANCE
Preclinical models and studies in the metastatic and neoadjuvant settings suggest that single nucleotide polymorphisms in FCGR3A and FCGR2A may be associated with differential response to trastuzumab in the treatment of ERBB2/HER2–positive breast cancer, by modulating antibody-dependent cell-mediated cytotoxic effects.
OBJECTIVE
To evaluate the effect of FCGR2A and FCGR3A polymorphisms on trastuzumab efficacy in the adjuvant treatment of ERBB2/HER2–positive breast cancer.
DESIGN, SETTING, AND PARTICIPANTS
This is a retrospective analysis of patients enrolled in the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial, a phase 3 cooperative group study conducted between 2000 and 2005. The NSABP B-31 trial randomized 2119 women with surgically resected node-positive, ERBB2/HER2–positive breast cancer to treatment with doxorubicin and cyclophosphamide followed by paclitaxel or the same regimen with the addition of 1 year of weekly trastuzumab. Patients were accrued at cooperative group sites across the United States and Canada. This analysis was performed between 2013 and 2016.
INTERVENTIONS
Doxorubicin and cyclophosphamide followed by paclitaxel or the same regimen with the addition of 1 year of weekly trastuzumab.
MAIN OUTCOMES AND MEASURES
Disease-free survival.
RESULTS
The genotyped cohort (N = 1251) resembled the entire B-31 cohort based on clinical variables and the degree of benefit from trastuzumab. Median follow-up time was 8.2 years in the genotyped samples. The disease-free survival probability at 3, 5, and 8 years was 74% (95%CI, 71%–79%), 66%(95%CI, 62%–71%), and 58%(95%CI, 54%–63%) in patients who received ACT and 86%(95%CI, 83%–89%), 82%(95%CI, 79%–85%), and 78%(95%CI, 74%–81%) in patients who received ACTH. Addition of trastuzumab significantly improved patient outcome (hazard ratio [HR], 0.46; 95%CI, 0.37–0.57; P < .001). The expected trend for interaction between polymorphisms and trastuzumab was observed for both genes, but only FCGR3A-158 polymorphism reached statistical significance for interaction (P < .001). As hypothesized, patients with genotypes FCB3A-158V/V or FCB3A-158V/F received greater benefit from trastuzumab (HR, 0.31; 95%CI, 0.22–0.43; P < .001) than patients who were homozygous for the low-affinity allele (HR, 0.71; 95%CI, 0.51–1.01; P = .05).
CONCLUSIONS AND RELEVANCE
The FCGR3A-158 polymorphism is predictive of trastuzumab efficacy in this cohort of patients with early ERBB2/HER2–positive breast cancer. Patients who are homozygous for phenylalanine at this position represent a considerable proportion of the population and, in contrast to previously reported analyses from similarly designed trials, our results indicate that trastuzumab may be less efficacious in these patients.
TRIAL REGISTRATION
clinicaltrials.gov Identifier for NSABP B-31: NCT00004067
Clinical trial National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31, analyzed jointly with the North Central Cancer Treatment Group (NCCTG) N9831, established the benefit of adding trastuzumab, a monoclonal antibody targeting the ERBB2/HER2 protein, to standard chemotherapy in patients with early stage ERBB2/HER2–positive breast cancer. However, only about one-third to one-half of patients benefit from this therapy in advanced or adjuvant settings, respectively.1,2 Although treatment is generally well tolerated, its high cost, associated cardiotoxic effects, and the availability of promising alternatives warrant an attempt to identify patients who will not benefit.
Although many mechanisms have been attributed to the success of trastuzumab, the original patent described 2 primary potential mechanisms. First, by binding to ERBB2/HER2 on the cancer cell membrane, it prevents ERBB2/HER2 dimerization, blocks downstream signaling, and subsequently blocks proliferation. Second, it triggers the host immune system to attack and kill trastuzumab-bound tumor cells. This immune response, known as antibody-dependent cell-mediated cytotoxic effect (ADCC), is initiated when the FCγ receptor on natural killer cells (NK) binds to the Fc portion of trastuzumab. As a result, NK cells release factors including IFN-γ, perforins, and granzymes, which cause tumor cell death via apoptosis. A similar process, known as antibody-dependent cellular phagocytosis (ADCP), is initiated when the FCγ receptor on a macrophage binds to trastuzumab and results in tumor cell death by phagocytosis.
Early studies in ERBB2/HER2–positive breast cancer cell lines demonstrated potent ADCC caused by trastuzumab in vitro.3 Experiments in mice have demonstrated reduced trastuzumab efficacy if the Fc fragment is removed from the antibody or if the mouse is deficient in FCγ receptors.4,5 In addition, an increase in the infiltration of lymphoid cells into tumor samples was observed after trastuzumab treatment compared with paired pretreatment samples.6 Collectively, these findings support a strong role of ADCC or ADCP in the mechanism of trastuzumab efficacy.
Additional preclinical studies have demonstrated an association of single-nucleotide polymorphisms in the genes encoding FCγ receptors with the strength of the immune response. Many studies have focused on position 158 of FCGR3A, which encodes a valine or phenylalanine, and position 131 of FCGR2A, which encodes a histidine or arginine (reviewed in Mellor et al).7 In vitro ADCC assays demonstrated greater trastuzumab-mediated ADCC with the FCGR3A-158 valine (V)/V genotype and a trend for association with the FCGR2A-131 histadine (H)/H genotype.8 Analyses of paired pretreatment and posttreatment peripheral blood mononuclear cells (PBMCs) demonstrated more differential changes in gene expression from patients with the V/V or H/H genotypes than did patients with phenylalanine (F)/F or arginine (R)/R genotypes, indicating a difference in the molecular response to trastuzumab according to genotype.9 These observations are consistent with the observation that the FCγRIIA-131H and FCγRIIIA-158V have a higher affinity to immunoglobin (IgG) than the proteins encoded by alternate alleles.10 Cells bearing the high-affinity alleles mediate ADCC more effectively.7
Clinical studies using therapeutic monoclonal antibodies such as rituximab for the treatment of lymphoma and cetuximab for the treatment of colon cancer have shown association of the FCGR3A and FCGR2A genotypes with patient outcomes.11–15 However, clinical studies examining the association of these single-nucleotide polymorphisms with trastuzumab benefit for patients with breast cancer are not clear. Tamura and colleagues16 demonstrated improved response rates to trastuzumab in patients with a FCGR2A-131 H/H genotype in the neoadjuvant setting and improved objective response rate in the metastatic setting. Musolino and colleagues8 observed improved objective response rate and progression-free survival for patients with the FCGR3A-158 V/V genotype.8 However, while conducting our study, 2 large studies of patients enrolled in the NCCTG-N983117 and BCIRG-00618 clinical trials found no association of these loci with trastuzumab efficacy in the adjuvant treatment of ERBB2/HER2–positive breast cancer.
To explore this discrepancy, we retrospectively examined the FCGR3A-158 and FCGR2A-131 genotypes in all available pretreatment blood specimens from NSABPB-31. Our prespecified primary objective was to determine whether patients with breast cancer with FCGR3A-158 V/V or FCGR3A 158 V/F received greater benefit from trastuzumab than patients with the FCGR3A F/F genotype.
Methods
Patient Cohort
Detailed patient characteristics, eligibility criteria, adverse events, and clinical trial results have previously been reported.2,19,20 The NSABPB-31 trial randomly assigned women with surgically resected node-positive ERBB2/HER2–positive breast cancer to treatment with doxorubicin and cyclophosphamide followed by paclitaxel (ACT) or the same regimen with the addition of 1 year of weekly trastuzumab (ACTH). Patients were accrued between February 2000 and April 2005 at cooperative group sites across the United States and Canada. Treatment assignments were balanced according to nodal status, planned hormonal therapy, type of surgery, intended radiotherapy, and institution, with the use of a biased-coin minimization algorithm. Additional inclusion requirements for the current study include availability of clinical follow-up, appropriate informed consent, and availability of pretreatment blood specimens. Informed consent forms were approved by a local human investigations committee in accordance with an assurance filed with and approved by the US Department of Health and Human Services to permit use of banked tissue and blood samples. These trials were approved by local human investigations committees or institutional review boards in accordance with assurances filed with and approved by the Department of Health and Human Services. Written informed consent was obtained from participants in each trial.
Genotyping
Whole blood was collected before treatment and stored at −80° C. Participant DNA was prepared from 100 μL of whole blood using the Mag-Bind Blood DNA HDQ kit (Omega Biotek) and quantified with picogreen (ThermoFisher). Genotyping of rs1801274 (FCGR2A-131 R/H) and rs396991 (FCGR3A-158 V/F) was performed using iPLEX Pro chemistry and mass spectrometry (Agena) according to the manufacturer’s instructions. Nested polymerase chain reaction was used for FCGR3A-158 to achieve specificity against its homologue FCGR3B. Genotypes were determined with Typer software using default settings after autoclustering (Agena). Two-thirds of the rs396991 assays were performed in duplicate. Bacterial artificial chromosome clones RP11-5K23 (FCGR3A), RP11-100D4 (FCGR3B), and an equimolar mixture of the 2 were used as controls to ensure the rs396991 primers specifically amplified the 3A homologue. Detailed reaction conditions and primer sequences are provided in eMethods in the Supplement. All assays were performed blinded to clinical outcome using deidentified specimens.
Statistical Methods
The use of patient blood specimens and a detailed statistical analysis plan were approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI) before starting the current investigation. The primary end point for survival analyses was disease-free survival. Disease-free survival events included local, regional, and distant recurrence; contralateral breast cancer; a second primary cancer; or death from any cause. Follow-up included events recorded before June 30, 2012. In 2005, during a planned joint analysis with NCCTG-N9831, the early-stopping boundary was reached and patients on the ACT arm were offered trastuzumab. Patients who crossed over to the trastuzumab arm were censored at the time of crossover. The treatment benefit and prognostic effect for each genotype was first investigated using the Kaplan-Meier method and treatment arms were compared using log-rank tests. As the primary aim, FCGR3A genotype by treatment interaction was tested using proportional hazard regression models. For purposes of the interaction tests, the high-affinity genotypes were combined (FCGR3A-158 V/V or V/F vs. F/F). Similar analyses were performed for FCGR2A as a secondary objective. Survival analyses provided here were adjusted for estrogen receptor (ER) (positive or negative), progesterone receptor (PR) (positive or negative), age (years), tumor size (cm), and nodal status (1–3, 4–9, or ≥10 positive lymph nodes). Univariable models are reported in the Supplement or are clearly indicated as such. Missing values in the covariates resulted in the exclusion of 8 cases for multivariable analyses of FCGR3A and 7 cases for FCGR2A. The Fisher exact test was used to test deviation from Hardy Weinberg Equilibrium and to test the association of genotype with clinical characteristics. Linkage disequilibrium was investigated in the subset of patients with results for both assays. All statistical tests were 2-sided and performed in R.
Results
Patient Characteristics
Among 2006 eligible patients with follow-up and informed consent, pretreatment blood specimens were available for 1253 and DNA was successfully prepared for 1251. Details regarding the reasons for sample loss are shown in eFigure 1 in the Supplement. Clinical and pathological characteristics were well balanced in the treatment arms of the study population and closely resembled the entire B-31 cohort (eTable 1 and eTable 2 in the Supplement). Median follow-up time was 8.2 years in the genotyped samples. The disease-free survival probability at 3, 5, and 8 years was 74%(95%CI, 71%–79%), 66%(95%CI, 62%–71%), and 58% (95% CI, 54%–63%) in patients who received ACT and 86% (95% CI, 83%–89%), 82% (95% CI, 79%–85%), and 78% (95% CI, 74%–81%) in patients who received ACTH. Addition of trastuzumab significantly improved patient outcome (hazard ratio [HR], 0.46; 95% CI, 0.37–0.57; P < .001).
Genotyping Results
Of 883 assays performed in duplicate, 3 discordances were observed (0.3%) for FCGR3A-158 (rs396991). These 3 observations were excluded from further analysis. Approximately 2% of FCGR2A-131 and 7% of FCGR3A-158 assays failed default genotyping quality controls in the Typer software, yielding 21 and 87 missing values, respectively. Some of the FCGR3A reactions excluded by the software may actually represent heterozygous patients with 3 germline copies of the gene.21 However, these wells are indistinguishable from wells with contamination or poor reaction conditions and their expected frequency of 2.5%limits our power to detect meaningful clinical significance. For these reasons, any sample that failed quality control was eliminated. Thirteen samples failed both assays, suggesting poor-quality DNA. The patients genotyped showed 25% H/H, 49% H/R, and 26% R/R alleles for FCGR2A-131 and 46%F/F, 42% F/V, and 12% V/V for FCGR3A-158. Both loci conform to the Hardy Weinberg Equilibrium in our data. In 1156 specimens with both assays successful, linkage disequilibrium analysis indicated the FCGR3A and FCGR2A genes are strongly linked (D′ = 0.30; P < .001) as expected from their close genomic proximity and previous studies.18
FCGR Polymorphisms and Clinical Characteristics
FCGR3A-158 single-nucleotide polymorphisms were not associated with nodal status, ER, PR, tumor size, or race (eTable 3 in the Supplement). However, patients with the homozygous V/V genotype tended to be older (P < .001). The mean age was 52 years for V/V patients and 49 to 50 years for the other genotypes. FCGR2A-131 was not associated with nodal status, ER, PR, tumor size, or age. However, it was weakly associated with race (eTable 4 in the Supplement).
FCGR3A Polymorphism and Trastuzumab Efficacy
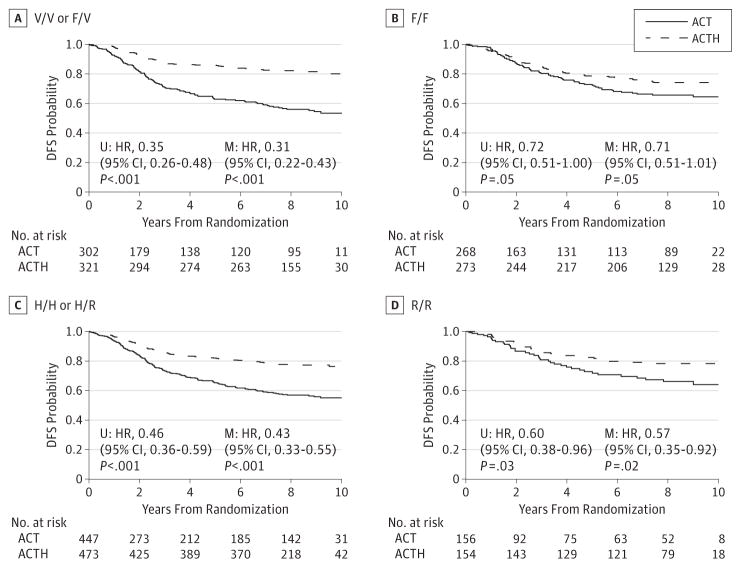
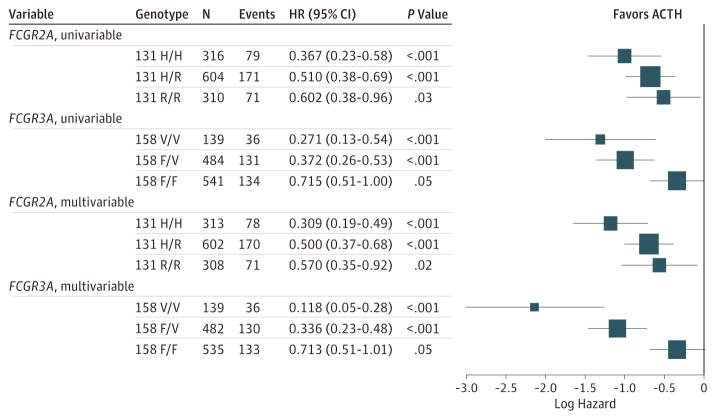
Patients with genotypes that included the higher-affinity alleles FCGR3A-158 V/V or V/F received greater benefit from trastuzumab (HR, 0.31; 95% CI, 0.22–0.43; P < .001) than patients who were homozygous for the low-affinity allele (HR,0.71; 95% CI,0.51–1.01; P = .05) (Figure 1A and B). Genotype by treatment-interaction test indicates an association between FCGR3A-158 and benefit from trastuzumab (P < .001). In an exploratory analysis, the FCGR3A-158, high-affinity V/V homozygous patients received the most benefit (HR, 0.12; 95% CI, 0.05–0.28; P < .001), heterozygous F/V patients received intermediate benefit (HR, 0.34; 95%CI, 0.23–0.48; P < .001), and homozygous low-affinity F/F patients received the least benefit (HR, 0.71; 95% CI, 0.51–1.01; P = .05) (Figure 2). Similar results were observed using invariable models.
Figure 1. Treatment Effect According to FCGR Polymorphism.
ACT indicates doxorubicin and cyclophosphamide followed by paclitaxel; ACTH, doxorubicin and cyclophosphamide followed by paclitaxel with the addition of 1 year of weekly trastuzumab; DFS, disease-free survival; HR, hazard ratio; M, multivariable; U, univariable.
Figure 2. Cox Models Examining Treatment Affect According to Genotype.
The box size is proportional to the precision. ACTH, doxorubicin and cyclophosphamide followed by paclitaxel with the addition of 1 year of weekly trastuzumab; HR, hazard ratio.
FCGR2A Polymorphism and Trastuzumab Efficacy
The FCGR2A polymorphism demonstrated the trends expected from preclinical models (Figure 2). FCGR2A-131, high-affinity H/H homozygous patients received the most benefit (HR, 0.31; 95%CI, 0.19–0.49; P < .001), heterozygous H/R patients received intermediate benefit (HR, 0.50; 95% CI, 0.37–0.68; P < .001), and homozygous low-affinity R/R patients received the least benefit (HR, 0.57; 95%CI, 0.35–0.92; P = .02) (Figure 2). However, treatment interaction tests indicate no difference in trastuzumab efficacy according to FCGR2A-131 (P = .24 for H/H or H/R vs. R/R).
FCGR Polymorphism and Prognosis
In an exploratory analysis in the ACT arm, patients with FCGR3A-158 V/For V/V had worse prognosis than patients with F/F (HR, 1.57; 95% CI, 1.15–2.14; P = .005) (eFigure 2 in the Supplement). On the other hand, in the ACTH arm, patients with FCGR3A-158 V/F or V/V had better prognosis than patients with F/F (HR, 0.68; 95% CI, 0.48–0.96; P = .03). Univariable models demonstrated similar results. FCGR2A-131 genotypes showed no evidence of prognosis in either arm.
Combined Genotypes
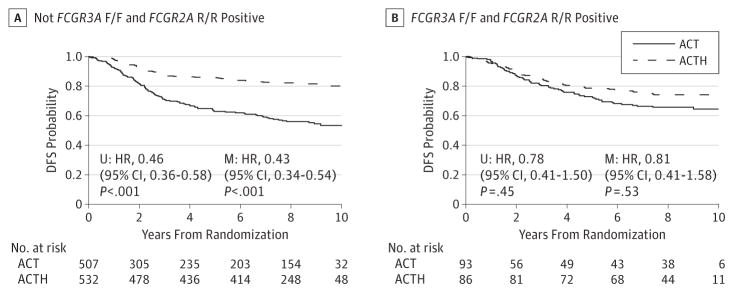
Additional exploratory analyses revealed approximately 15%of the population are homozygous for both of the low-affinity alleles (FCGR3A-158 F/F and FCGR2A-131 R/R; n = 179) (Figure 3). Trastuzumab appears less effective in these patients (HR,0.81; 95%CI,0.41–1.58; P = .53). However, treatment interaction tests of this population were not significant (P = .06 for F/F and R/R vs others).
Figure 3. Combined Genotypes.
ACT indicates doxorubicin and cyclophosphamide followed by paclitaxel; ACTH, doxorubicin and cyclophosphamide followed by paclitaxel with the addition of 1 year of weekly trastuzumab; DFS, disease-free survival; HR, hazard ratio; M, multivariable; U, univariable.
Discussion
Analysis of FCGR3A-158 polymorphism in NSABP B-31 demonstrated differential trastuzumab benefit predicted by preclinical models, studies of other antibodies, and smaller studies of trastuzumab in the metastatic and neoadjuvant settings.7 Treatment interaction tests indicate polymorphisms at this position are associated with variability in the degree of benefit from adjuvant trastuzumab. Patients with the 158 F/F genotype have a better prognosis when treated with ACT and received less relative benefit (HR, 0.71) from the addition of trastuzumab, where as patients with 158 F/V or V/V have a worse prognosis with ACT and receive significantly more relative benefit from trastuzumab (HR, 0.31). These results indicate that ADCC may play a substantial component in the efficacy of trastuzumab for the treatment of breast cancer in the adjuvant setting; ADCC also activates tumor–antigen-specific cellular immunity via intercellular crosstalk among NK and dendritic cells, which may also enhance the efficacy of anti-ERBB2/HER2 therapy.22,23
We also observed that the FCGR2A-131 polymorphism showed the expected trend for trastuzumab benefit but treatment interaction tests indicated no evidence of differential trastuzumab treatment effect according to FCGR2A genotypes. Perhaps this may be owing to the lack of expression of FCγRIIa on NK cells because NK cells are thought to be the main effectors of ADCC; although cytotoxic effects mediated through other effector cells via FCγRIIa have been reported.24 Our observation that the significant differential benefit from trastuzumab was limited to the FCGR3A-158 polymorphism and was not seen in the FCGR2A-131 single-nucleotide polymorphisms may also be owing to the fact that FCγRIIIa-158V was found to bind to IgG1 immune complexes at low concentrations but did not bind to FCγRIIIa-158F. Conversely, no binding differences were detected between FCγRIIa-131H and 131R at low IgG1 concentrations.25
Our results differ from recent reports from the NCCTG-N983117 and BCIRG-006 trials,18 which found no association of FCGR3A-V158 and benefit from trastuzumab. These trials also examined the addition of trastuzumab to ACT in the adjuvant treatment of ERBB2/HER2–positive breast cancer and differed from NSABP B-31 primarily in the timing of the treatments. Both studies had sample sizes remarkably similar to our own and demonstrated substantial benefits from the addition of trastuzumab to chemotherapy. The methodologies used were rigorous: BCIRG-006 measured each single-nucleotide polymorphisms in quadruplicate (duplicate measurements on 2 platforms) and NCCTG-N9831 had 100% concordance in one-third of the population measured in duplicate. However, both of the previous FCGR studies suffered from sampling bias. In the BCIRG-006 study, the 1286 patients who were genotyped did not show significant benefit from trastuzumab (HR, 0.84; P = .19) and the authors acknowledged that they cannot exclude a contribution of FCGR polymorphisms to the outcome of trastuzumab-treated patients due to the sampling bias. FCGR3A-158 also deviated from the Hardy Weinberg Equilibrium in the BCIRG-006 study. Blood for genotyping was collected from NCCTG-N9831 patients after the trial closed to accrual, leading to differences from the study population and the intent-to-treat population. The NCCTG-N9831–genotyped cohort included a greater proportion of trastuzumab-treated patients, less nodal disease, smaller tumors, a greater number of hormone receptor–positive tumors, and patients who were slightly older than the entire NCCTG-N9831 cohort. As a result, the genotyped patients had a substantially better disease-free survival than the entire NCCTG-N9831 cohort. For both studies, these biases resulted in a loss of events and as a result reduced power to detect genotype-treatment interaction.
In our exploratory analysis, the observation that the FCGR3A low-affinity allele was associated with a better prognosis than the high-affinity allele in the ACT arm was unexpected. Chemotherapy has been shown to induce an immune response and possibly the differential binding affinity of FCγRIIIa-158V and FCγRIIIa-158F proteins to intrinsic antibodies may result in differential benefit from chemotherapy. Polymorphisms in other immune molecules such as TLR426 and P2RX727 have been reported as candidate biomarkers predicting progression-free survival associated with chemotherapy-elicited cell death in breast cancer. The observed direction for prognosis is in the opposite direction that might be expected based on the binding affinities of the 2 alleles and might represent a chance finding. However, the considerably diverse expression of this receptor on a number of different immune cells (macrophages, γΔT cells, and many others) taken together with a wide variety of functions that often vary depending on the local microenvironment suggests that clinical outcomes can be very difficult to predict.
We have shown that patients with breast cancer with 1 or 2 of the FCGR3A-158V alleles receive greater benefit from trastuzumab than patients who are homozygous for the FCGR3A-158F allele. Although multivariable analysis showed that patients with the F/F genotype had a slightly higher residual risk, this difference was not remarkable. Thus, genotyping does not seem to be sufficient for selection of patients for additional treatment.
Currently, many therapies, including new anti-ERBB2/HER2 monoclonal antibodies or pan-tyrosine kinase inhibitors are being used in addition to trastuzumab to treat patients with breast cancer in the metastatic and neoadjuvant settings. These agents also engage the immune system and boost trastuzumab-induced ADCC.28–30 Recent investigation of additional immune checkpoint inhibitors to anti-ERBB2/HER2 therapies in patients with ERBB2/HER2–positive metastatic breast cancer is based on the hypothesis that a substantial component of trastuzumab benefit is mediated through ADCC and cross-priming of antigen-specific cytotoxic T cells,22,23,31 which could enable the inhibition of immune checkpoints to improve the long-term efficacy of anti-ERBB2/HER2 therapy. However, carefully designed clinical trials would be needed to determine if additional agents enhance trastuzumab activity in specific genotypes.
Studies on NK cell mediated therapies suggest that the Fc region on monoclonal antibodies such as trastuzumab could be re-engineered to augment the affinity for a variety of inhibitory and activating FCGR allelotypes.10,32,33 Single amino acid changes to the antibody at the binding site can strongly influence the binding strength. This notion also requires additional clinical studies.
Conclusions
This study supports the hypothesis that ADCC activity plays an important role in the efficacy of trastuzumab. However, a great number of different molecules are involved in determining ADCC activity, therefore it may be useful to collect peripheral blood monocytes from patients in future clinical trials so that functional ADCC activity can be assessed and associated with treatment efficacy of trastuzumab and other therapeutic monoclonal antibodies.34
Supplementary Material
Key Points.
Question
Do polymorphisms in FCγ receptors affect trastuzumab efficacy?
Findings
In this secondary analysis of 1251 patients with early-stage ERBB2/HER2–positive breast cancer enrolled in a randomized clinical trial, patients homozygous for FCGR3A-158 F benefited less from the addition of trastuzumab to chemotherapy than did patients with F/V or V/V genotypes.
Meaning
Significant association between polymorphisms of FCGR3A and trastuzumab benefit suggests that antibody-dependent cell-mediated cytotoxic effect plays a role in determining the efficacy of trastuzumab in the adjuvant treatment of ERBB2/HER2–positive breast cancer.
Acknowledgments
Funding/Support: This work was supported by National Cancer Institute, Department of Health and Human Services, Public Health Service (grants U10-CA-180868, U10-CA-180822, and UG1CA-189867); by a grant from the Pennsylvania State Department of Health; and by Genentech, Inc.
Role of the Funder/Sponsor: The funder/sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Mamounas is a consultant for the Genentech/Roche speakers board. Dr Swain has received honoraria, uncompensated steering committees, and research grants to her institution from Genentech/Roche. Dr Geyer is a consultant for Genentech. No other conflicts are reported.
Additional Contributions: We thank Teresa L. Bradley, PhD; Ethan Barry, BA; and Joyce Mull, MPM, for regulatory affairs; Barbara Harkins, RN, MN; and Frances Fonzi, MPM, for protocol development; Barbara C. Good, PhD, and Wendy L. Rea, BA, for manuscript editing and submission; and Christine I. Rudock for graphics. We also thank NSABP members who contributed tissue blocks as well as patients enrolled in the study. None of the additional contributors have been compensated beyond their usual salary.
Additional Information: The Pennsylvania State Department of Health specifically disclaims responsibility for any analysis, interpretations, or conclusions of this study.
Author Contributions: Dr Pogue-Geile, Mr Gavin, and Dr Song had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Gavin, Lipchik, Johnson, Rastogi, Mamounas, Swain, Jeong, Costantino, Wolmark, Paik, Pogue-Geile.
Acquisition, analysis, or interpretation of data: Gavin, Song, Kim, Lipchik, Bandos, Finnigan, Fehrenbacher, Wickerham, Geyer, Costantino, Paik, Pogue-Geile.
Drafting of the manuscript: Gavin, Kim, Lipchik, Rastogi, Geyer, Paik, Pogue-Geile.
Critical revision of the manuscript for important intellectual content: Song, Kim, Lipchik, Johnson, Bandos, Finnigan, Rastogi, Fehrenbacher, Mamounas, Swain, Wickerham, Geyer, Jeong, Costantino, Wolmark, Paik, Pogue-Geile.
Statistical analysis: Gavin, Song, Bandos, Paik.
Obtained funding: Pogue-Geile.
Administrative, technical, or material support: Gavin, Kim, Lipchik, Johnson, Bandos, Finnigan, Wickerham, Costantino, Wolmark, Paik, Pogue-Geile.
Study supervision: Kim, Rastogi, Geyer, Jeong, Costantino, Paik, Pogue-Geile.
References
- 1.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20(3):719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27(10):1533–1541. doi: 10.1016/s0301-472x(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 4.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 5.Barok M, Isola J, Pályi-Krekk Z, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol Cancer Ther. 2007;6(7):2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 6.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10(17):5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 7.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6:1. doi: 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu C, Mogushi K, Morioka MS, et al. Fc-Gamma receptor polymorphism and gene expression of peripheral blood mononuclear cells in patients with HER2-positive metastatic breast cancer receiving single-agent trastuzumab. Breast Cancer. 2016;23(4):624–632. doi: 10.1007/s12282-015-0614-y. [DOI] [PubMed] [Google Scholar]
- 10.Shields RL, Namenuk AK, Hong K, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276(9):6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 11.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Edberg JC, Redecha PB, et al. A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. J Clin Invest. 1997;100(5):1059–1070. doi: 10.1172/JCI119616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25(24):3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 14.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of FcgammaRIIa-FcgammaRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 15.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Shimizu C, Hojo T, et al. FcγR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22(6):1302–1307. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 17.Norton N, Olson RM, Pegram M, et al. Association studies of Fcγ receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol Res. 2014;2(10):962–969. doi: 10.1158/2326-6066.CIR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurvitz SA, Betting DJ, Stern HM, et al. Analysis of Fcγ receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res. 2012;18(12):3478–3486. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 21.Asano K, Matsumoto T, Umeno J, et al. Impact of allele copy number of polymorphisms in FCGR3A and FCGR3B genes on susceptibility to ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2061–2068. doi: 10.1097/MIB.0b013e318298118e. [DOI] [PubMed] [Google Scholar]
- 22.Lee SC, Srivastava RM, López-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50(2–3):248–254. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bianchini G, Gianni L. The immune system and response to ERBB2/HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–e68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 24.Gillis C, Gouel-Chéron A, Jönsson F, Bruhns P. Contribution of human FcγRs to disease with evidence from human polymorphisms and transgenic animal studies. Front Immunol. 2014;5:254. doi: 10.3389/fimmu.2014.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruhns P, Iannascoli B, England P, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113(16):3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 26.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13(9):1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 27.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15(10):1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi K, Mimura K, Izawa S, et al. Lapatinib acts on gastric cancer through both antiproliferative function and augmentation of trastuzumab-mediated antibody-dependent cellular cytotoxicity. Gastric Cancer. 2013;16(4):571–580. doi: 10.1007/s10120-012-0219-5. [DOI] [PubMed] [Google Scholar]
- 29.Mimura K, Kono K, Maruyama T, et al. Lapatinib inhibits receptor phosphorylation and cell growth and enhances antibody-dependent cellular cytotoxicity of EGFR- and ERBB2/HER2-overexpressing esophageal cancer cell lines. Int J Cancer. 2011;129(10):2408–2416. doi: 10.1002/ijc.25896. [DOI] [PubMed] [Google Scholar]
- 30.El-Sahwi K, Bellone S, Cocco E, et al. In vitro activity of pertuzumab in combination with trastuzumab in uterine serous papillary adenocarcinoma. Br J Cancer. 2010;102(1):134–143. doi: 10.1038/sj.bjc.6605448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcγRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28(3):285–295. doi: 10.1016/j.ccell.2015.08.004. Erratum in: Cancer Cell, 2015, 28(4), 543. [DOI] [PubMed] [Google Scholar]
- 32.Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs. 2010;2(2):181–189. doi: 10.4161/mabs.2.2.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67(18):8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 34.Tada M, Ishii-Watabe A, Suzuki T, Kawasaki N. Development of a cell-based assay measuring the activation of FcγRIIa for the characterization of therapeutic monoclonal antibodies. PLoS One. 2014;9(4):e95787. doi: 10.1371/journal.pone.0095787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.