Abstract
Compared to HIV-infected persons who do not inject drugs (non-IDU), persons who inject drugs (PWID) experience disparities in linking to medical care, initiating antiretroviral therapy (ART) and achieving viral suppression. There has been little attention to changes in these disparities over time. We estimated the proportion of PWID and non-IDU retained in care, on ART, and virally suppressed each year from 2001–2012 in the Johns Hopkins HIV Clinical Cohort (JHHCC). We defined active clinic patients as those who had ≥1 clinical visit, CD4 cell count, or viral load between July 1 of the prior year, and June 30 of the analysis year. Within a calendar year, retention was defined as ≥2 clinical visits or HIV-related laboratory measurements >90 days; ART use was defined as ≥1 ART prescription active ≥30 days; and viral suppression was defined as ≥1 HIV viral load <400 copies/mL. While PWID were less likely to be retained in earlier years, the gaps in retention closed around 2010. After 2003–2004, PWID and non-IDU retained in care had similar probability of receiving a prescription for ART and PWID and non-IDU on ART had similar probability of viral suppression.
Keywords: Antiretroviral therapy, HIV care continuum, Injection drug use, Viral load suppression
Introduction
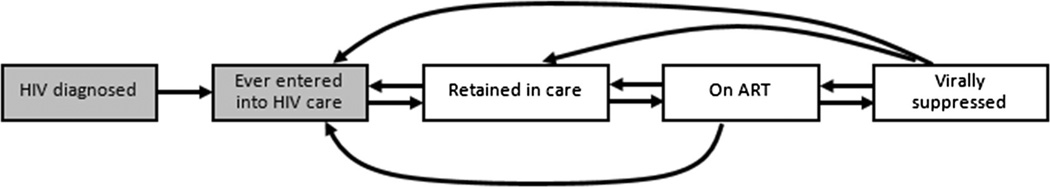
Given the reduced morbidity, mortality and infectiousness associated with viral suppression on antiretroviral therapy (ART), achieving viral suppression is the ultimate goal for HIV-infected individuals, HIV providers and public health practitioners [1–3]. Achieving viral suppression requires successful transition through several steps, including HIV-diagnosis, entry into medical care, retention in care, and prescription of ART. This framework for measuring progress towards viral suppression in a population has been dubbed the HIV care continuum [4, 5]. There are multiple versions of the care continuum but one simple depiction is available in Fig. 1. Examining attrition from one stage to the next may give clues about potential targets for population interventions to improve HIV outcomes [6, 7]. Examining the distribution of an HIV-infected cohort across the continuum stages over time is a means for monitoring progress towards improved public health systems for people living with HIV [8, 9]. Within a single clinic (or health system), the HIV care continuum framework can be used as a metric by which the clinic (one entity within the larger public health system) can monitor changes in clinical practices over time.
Fig. 1.
Schematic depiction of the HIV care continuum, where the proportion in each stage (boxes) over time is of interest and arrows represent patient movement through the continuum; this analysis focuses on the three unshaded boxes on the right
Injection drug use (IDU) is associated with poorer HIV-related outcomes and faster progression of HIV disease [10–12]. Specifically, prior research has suggested that persons who inject drugs (PWID) experience greater delays in linking to HIV medical care, are less likely to be retained in care, are less likely to be prescribed ART and are less likely to be virally suppressed once on ART [6, 13–15]. However, few prior studies have considered the association between IDU and progression through multiple stages of the HIV care continuum in the same cohort [16]. Furthermore, as ART regimens have improved (perhaps alleviating some concerns about initiating a patient with prior IDU on ART) and clinical care models have evolved, we are not aware of any studies that have examined how progression through the HIV care continuum has changed over time for PWID versus non-IDU.
Herein, we present estimates of the proportion of PWID and non-IDU retained, started on ART and virally suppressed by calendar year (unshaded boxes in Fig. 1), after enrollment into care in a large, urban HIV clinic. In secondary analyses, we examine different markers of retention and their association with IDU and calendar time.
Methods
Study Population
The Johns Hopkins HIV Clinical Cohort (JHHCC) consists of all HIV-infected persons age 18 years or older who enroll in HIV care at Johns Hopkins outpatient HIV clinic and consent to share their data (approximately 95 % of persons who enroll into continuity care). For this study, we included all HIV-infected persons who enrolled in the JHHCC since the cohort inception in 1995 through June 30, 2012, who had at least one clinical visit in the outpatient HIV clinic or elsewhere on the Johns Hopkins medical campus (the Johns Hopkins Hospital and associated ambulatory clinics) or at least one CD4 cell count or HIV viral load measurement between July 1, 2000 and June 30, 2012. We did not employ any exclusion criteria.
Patient characteristics including sex, race, age, HIV acquisition risk factors, prior AIDS diagnosis, and prior use of any antiretroviral drugs were ascertained through conversations between patient and physician at enrollment. Collection of these data were standard (collected on all patients) but not standardized (asked in the exact same manner of all patients), and like all self-report data are subject to recall bias and social desirability bias. Patients who reported IDU as a probable source of their HIV infection when they enrolled into care were classified as PWID. Baseline laboratory values were defined as those measured closest to the date of study enrollment, within a window 6 months prior to and 1 month after enrollment. Patients enrolling in the JHHCC may be new to HIV care or may be transferring care from another HIV provider. Patients were classified as having AIDS at baseline if the date of their first AIDS diagnosis preceded their enrollment date. Patients were classified as ART-naïve if they had not initiated ART prior to their enrollment date. Collection of data on patients in the JHHCC, and this analysis of that data, were approved by the Johns Hopkins Hospital institutional review board.
The cohort of HIV-infected patients on which each calendar year-specific estimate of retention, ART use and viral suppression was based, varied. Because the JHHCC is a clinical cohort, patients may transfer to another clinic and not notify the JHHCC; for these patients, failure to return to the JHU clinic is not the same as failure to be retained in HIV care. Therefore, to estimate the probability of retention in a given year, we need to restrict the study sample to the subset of patients ever enrolled in the JHHCC with some indication that they are still actively enrolled in care at JHU. We defined patients as “active” in a calendar year if they had at least one clinical visit (within the HIV clinic or in any other setting within the Johns Hopkins Medical system), CD4 cell count, or HIV viral load between July 1 of the prior year and June 30 of the present year. CD4 cell count and HIV viral loads conducted anywhere within the Johns Hopkins Medical system or at one of two large commercial laboratories in Baltimore are captured by the JHHCC and are utilized in this analysis.
Outcomes Measurement
The primary definition of retention we used was attendance at ≥2 clinical visits or HIV-specific laboratory measurements (CD4 cell count or HIV viral load) >90 days apart between January 1 and December 31 of the calendar year. This definition of retention is similar to the Health Resources and Services Administration (HRSA) HIV/AIDS Bureau (HAB) metric for retention in place during the bulk of our study period, and guidelines for clinical practice that advised patients be seen approximately every 3–4 months for viral load monitoring [17, 18]. Because HIV-infected patients in the JHHCC may receive HIV-specific care outside of the HIV clinic but in the institution (e.g., by their gynecologist), we counted any visit within the Johns Hopkins Hospital medical campus (other than an emergency room visit) as a clinical visit. We classified patients as prescribed ART if they had at least one ART prescription spanning ≥30 days during the calendar period. ART prescriptions are those recorded in the medical record (and due to the nature of the Johns Hopkins medical record system sent to the pharmacy); we do not have information on whether prescriptions were picked up. We classified patients as virally suppressed if they had ≥1 HIV viral load <400 copies/mL during the calendar year. Deaths were obtained from clinic sources and regular matches against the Social Security Death Index.
Definitions for retention in care, ART use and viral suppression (Table 1) were chosen to be highly sensitive for membership in each stage of the HIV care cascade. More specific definitions for membership in each stage of the HIV care cascade are possible. For example, we could have defined viral suppression as having the most recent HIV viral load measurement <400 copies/mL or all HIV viral load measurements in the calendar year <400 copies/mL. A threshold of <400 copies/mL was used rather than a more stringent criterion such as <50 copies/mL since the calendar time frame of our analysis substantially covered years when <400 copies/mL was the clinical standard for defining viral suppression. As a result of our highly sensitive, but potentially less specific definitions, our results should be interpreted as an upper bound for estimates of retention in care, ART use and viral suppression. Due to the nature of the data (clinical cohort) we are unable to estimate the rate of entry to care after HIV diagnosis (Fig. 1).
Table 1.
HIV care continuum definitions for the current analyses
| “Active” patients | Those with a clinic visit, CD4 cell count or viral load in July 1 of prior year to June 30 of present year |
| Retained patients | Among active patients, those with ≥2 clinic visits, CD4 cell counts or viral loads (or any combination of those) >90 days apart between January 1 and December 31 of present year |
| Proportion retained | # Retained patients/# active patients |
| Patients using ART | Among retained patients, those with ≥1 ART prescription for ≥30 days between January 1 and December 31 of present year |
| Proportion using ART | # Patients using ART/# active patients |
| Patients virally suppressed |
Among patients using ART, those with ≥1 HIV viral load <400 copies/mL between January 1 and December 31 of present year |
| Proportion virally suppressed |
# Patients virally suppressed/# active patients |
Analysis
We calculated crude proportions of patients retained, on ART and virally suppressed as the proportion of active patients who met the definition of retention, the proportion of patients who met the definition of retention who also met the definition for being on ART, and the proportion of patients who met the definition for being on ART who also met the definition for viral suppression, respectively. That is, persons classified as being virally suppressed were, by definition, a subset of those on ART. Those on ART were, in turn, a subset of those retained, and those retained were a subset of active patients. Persons who died in a calendar year were included in the numerator and denominator of any calculations if they also met the definition the numerator or denominator prior to death (i.e., persons were not excluded from estimates if they died).
To quantitatively evaluate the association between IDU and retention, ART use and viral suppression, and to determine whether that association changed over time, we fit inverse-probability weighted log-binomial regression models for: (1) retention in care among active patients; (2) ART use among those retained; and (3) viral suppression among those on ART. Dependent variables in these models were: IDU, indicator variables for calendar year (to allow retention, ART use and viral suppression to vary over time), and the product of IDU and calendar year indicator variables (to be able to detect differences in the risk ratio for retention, ART use and viral suppression associated with IDU to vary over time). Because patients could contribute multiple records to the analysis (one for each year in which they were an active patient) we used generalized estimating equations (GEE) with an exchangeable correlation matrix to control for correlated observations. We report the relative risk of retention, ART use and viral suppression comparing PWID to non-IDU by year, standardized to the marginal distribution of: age, sex, race, male-to-male sexual contact (MSM) acquisition risk, heterosexual acquisition risk, and ART use, history of AIDS diagnosis, and values of CD4 cell count and HIV viral load at enrollment into the cohort. Standardization was accomplished using inverse probability weights [19]. Weighted estimates control for differences in demographic or baseline clinical characteristics among PWID and non-IDU who enrolled, so that any disparities in continuum outcomes are not due to these other patient characteristics. We estimated the weights using predicted probabilities of IDU acquisition risk from a logistic regression model for IDU, with all of the standardization variables as predictors. To improve model fit by allowing for a non-linear relationship between continuous variables and the probability of reporting IDU acquisition risk, we modeled all continuous variables using restricted quadratic splines with knots at the 5th, 35th, 65th, and 95th percentiles [20]. We used robust standard errors when calculating 95 % confidence intervals for all estimates.
Secondary Analyses
A number of different definitions of retention in HIV care have been employed in the literature [21, 22], and HIV clinical care guidelines continue to change (specifically, by increasing the acceptable interval between visits for patients who are well-established on ART and virally suppressed) [18, 23]. These changing guidelines may affect the sensitivity of the current HRSA definition of retention in care [24]. Therefore, we examined the impact of several other retention definitions. In addition to (1) our primary definition of retention (≥2 clinical visits >90 days apart), we also considered the following definitions of retention: (2) ≥2 HIV-related laboratory measurements (CD4 cell count or HIV viral load) >90 days apart; and (3) ≥2 routine, general health-related laboratory measurements (hemoglobin or sodium measurements) >90 days apart. The second definition of retention is one primarily used by HIV surveillance programs [25]. The third definition of retention measures any engagement in medical care within the Johns Hopkins system. We examined trends in retention over time, stratified by IDU, according to of these three definitions. We also compared subsequent HIV care continuum outcomes (probability of being on ART or virally suppressed), stratified by IDU, for person-years meeting each of these three definitions (by person-years we mean that patients contributed records for each year in which they were active in the clinic, each of which could be independently classified as retained, on ART or virally suppressed). We conducted all analyses in SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
Study Sample
There were 4602 persons enrolled in continuity care in the JHHCC with at least one clinical visit, CD4 cell count or HIV viral load between July 1, 2000 and June 30, 2012. Of those, the majority were men, black, and heterosexual. The median age at enrollment was 40 years [interquartile range (IQR) 34, 47]. Most enrolled patients had some indication of having been in care elsewhere previously: 52 % had a history of antiretroviral use prior to enrollment and 27 % had an AIDS diagnosis that preceded enrollment. Median CD4 cell count at baseline was 282 (IQR: 105, 480) and median log10 viral load was 4.3 (IQR 3.0, 5.0). Thirty-six percent were classified as PWID based on HIV acquisition risk (Table 2).
Table 2.
Baseline characteristics of 4602 HIV-infected persons ever enrolled in the Johns Hopkins Clinical Cohort who had at least one clinical visit, CD4 cell count or HIV viral load July 1, 2000–June 30, 2012, stratified by self-report of injection drug use as their likely route of HIV acquisition
| N | PWID 1659 |
Non-IDU 2943 |
Total 4602 |
|---|---|---|---|
| Male sexa | 1104 (67 %) | 1922 (65 %) | 3026 (66 %) |
| Ageb | 42 (37, 48) | 39 (32, 46) | 40 (34, 47) |
| Race | |||
| Black | 1365 (82 %) | 2106 (72 %) | 3471 (75 %) |
| White | 273 (16 %) | 702 (24 %) | 975 (21 %) |
| Other | 21 (1 %) | 135 (5 %) | 156 (3 %) |
| HIV acquisition risk | |||
| MSM | 142 (9 %) | 1077 (37 %) | 1219 (26 %) |
| Heterosexual | 756 (46 %) | 1640 (56 %)s | 2396 (52 %) |
| History of any antiretroviral use |
865 (52 %) | 1512 (51 %) | 2377 (52 %) |
| AIDS | 465 (28 %) | 767 (26 %) | 1232 (27 %) |
| CD4 cell count (cells/µL)b |
285 (115, 486) | 280 (98, 477) | 282 (105, 480) |
| <50 | 232 (14 %) | 494 (17 %) | 726 (16 %) |
| 50–199 cells/µL | 366 (22 %) | 609 (21 %) | 975 (21 %) |
| 200–349 | 371 (22 %) | 587 (20 %) | 958 (21 %) |
| ≥350 cells/µL | 636 (38 %) | 1153 (39 %) | 1789 (39 %) |
| Missing | 54 | 100 | 154 |
| Viral load (HIV RNA log10 copies/mL)b |
4.3 (3.0, 4.9) | 4.3 (2.9, 5.0) | 4.3 (3.0, 5.0) |
| Missing | 194 | 286 | 480 |
HIV human immunodeficiency virus, PWID persons who inject drugs, IDU injection drug use, MSM men who have sex with men, ART antiretroviral therapy, AIDS acquired immune deficiency syndrome
N (%) unless otherwise specified
Median (IQR)
Each year, from 2001 to 2012, between 670 and 824 PWID and between 1145 and 1604 non-IDU were active clinic patients (defined as having had a visit or an HIV laboratory measurement in the last 6 months of the prior year, or the first 6 months of the current year). The number of active PWID declined very slightly over time, while the number of active non-IDU increased over time.
Retention
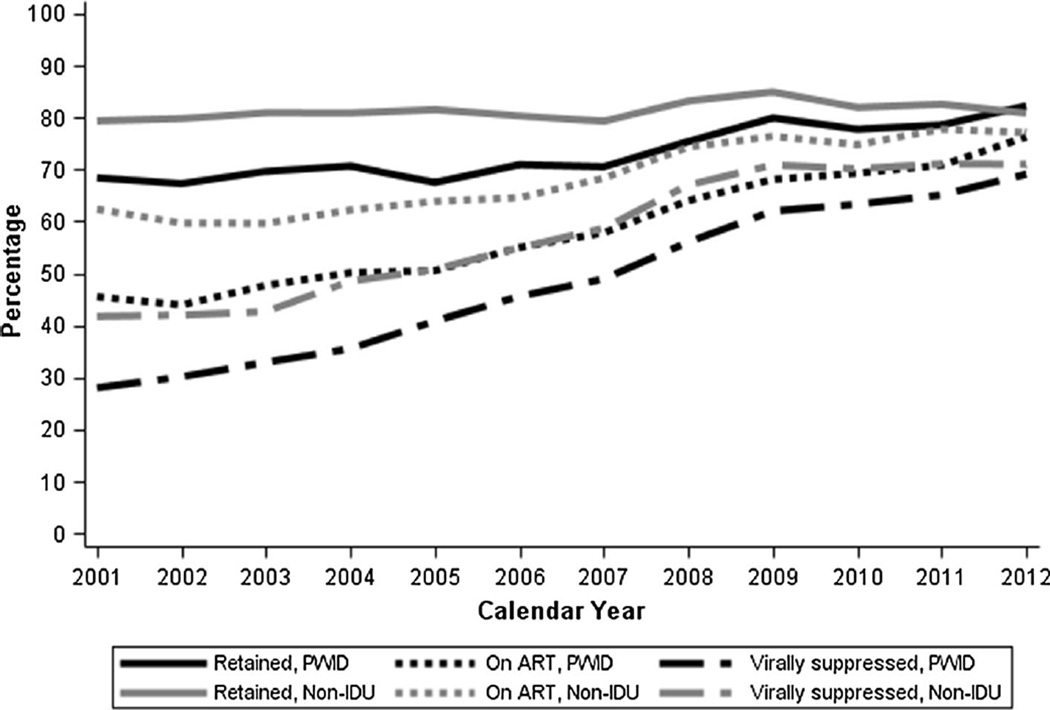
The proportion of active clinic patients who had ≥2 clinical visits in a given calendar year (retained) increased over time for PWID, with the sharpest increase coming from 2007 (70.5 % retained) to 2008 (75.4 % retained) (Table 3). The proportion of non-IDU retained was approximately constant over time, and was higher than the proportion of PWID in all years except 2012. The result of these trends was that disparities in retention between PWID and non-IDU disappeared over time. Indeed, after standardizing on baseline demographics and clinical status, PWID had a statistically significantly lower probability of being retained in care in all calendar years until 2010 (Table 4).
Table 3.
Crude number and proportion of HIV-infected persons in the Johns Hopkins HIV Clinical Cohort retained, on ART, and virally suppressed, stratified by calendar year and history of injection drug use
| PWID | Non-IDU | |||||||
|---|---|---|---|---|---|---|---|---|
| Active patientsa N |
Retainedb N (%) |
On ARTc N (%) |
Virally suppressedd N (%) |
Active patientsa N |
Retainedb N (%) |
On ARTc N (%) |
Virally suppressedd N (%) |
|
| 2001 | 756 | 517 (68.4 %) | 344 (45.5 %) | 212 (28.0 %) | 1145 | 909 (79.4 %) | 714 (62.4 %) | 478 (41.7 %) |
| 2002 | 822 | 553 (67.3 %) | 361 (43.9 %) | 248 (30.2 %) | 1253 | 1000 (79.8 %) | 748 (59.7 %) | 526 (42.0 %) |
| 2003 | 824 | 574 (69.7 %) | 393 (47.7 %) | 271 (32.9 %) | 1311 | 1061 (80.9 %) | 782 (59.6 %) | 559 (42.6 %) |
| 2004 | 794 | 561 (70.7 %) | 398 (50.1 %) | 282 (35.5 %) | 1344 | 1087 (80.9 %) | 836 (62.2 %) | 652 (48.5 %) |
| 2005 | 754 | 509 (67.5 %) | 381 (50.5 %) | 308 (40.8 %) | 1345 | 1097 (81.6 %) | 859 (63.9 %) | 683 (50.8 %) |
| 2006 | 702 | 498 (70.9 %) | 386 (55.0 %) | 320 (45.6 %) | 1347 | 1082 (80.3 %) | 870 (64.6 %) | 740 (54.9 %) |
| 2007 | 709 | 500 (70.5 %) | 410 (57.8 %) | 347 (48.9 %) | 1412 | 1120 (79.3 %) | 965 (68.3 %) | 829 (58.7 %) |
| 2008 | 703 | 530 (75.4 %) | 450 (64.0 %) | 394 (56.0 %) | 1422 | 1183 (83.2 %) | 1057 (74.3 %) | 953 (67.0 %) |
| 2009 | 708 | 566 (79.9 %) | 482 (68.1 %) | 439 (62.0 %) | 1480 | 1257 (84.9 %) | 1131 (76.4 %) | 1049 (70.9 %) |
| 2010 | 720 | 560 (77.8 %) | 499 (69.3 %) | 456 (63.3 %) | 1547 | 1268 (82.0 %) | 1157 (74.8 %) | 1085 (70.1 %) |
| 2011 | 696 | 547 (78.6 %) | 493 (70.8 %) | 453 (65.1 %) | 1584 | 1308 (82.6 %) | 1232 (77.8 %) | 1126 (71.1 %) |
| 2012 | 670 | 551 (82.2 %) | 511 (76.3 %) | 463 (69.1 %) | 1604 | 1297 (80.9 %) | 1237 (77.1 %) | 1139 (71.0 %) |
PWID persons who inject drugs, IDU injection drug use, self-reported transmission risk
“Active” patient defined as having had ≥1 clinical visit, CD4 cell count, or HIV viral load measurement between July 1 of the prior year and June 30 of the calendar year of interest
Retained in care defined as attending ≥2 clinical visits in the Johns Hopkins Medical system >90 days apart between January 1 and December 31 of the calendar year, conditional on being an active patient
On ART defined as having ≥1 antiretroviral prescription for ≥30 days, conditional on being retained in care
Viral suppression defined as having ≥HIV viral load measurement <400 copies/mL, conditional on being on ART
Table 4.
Risk ratio (95 % confidence interval) for association between IDU and membership within each stage of the HIV care continuum, conditional on being an active patient (for retained) or membership in preceding stage (for on ART and virally suppressed), by calendar year
| Crude | Standardizeda | |||||
|---|---|---|---|---|---|---|
| Retainedb | On ARTc | Virally suppressedd | Retainedb | On ARTc | Virally suppressedd | |
| 2001 | 0.85 (0.80, 0.90) | 0.83 (0.78, 0.90) | 0.89 (0.81, 0.99) | 0.84 (0.77, 0.92) | 0.88 (0.79, 0.97) | 0.94 (0.81, 1.09) |
| 2002 | 0.83 (0.78, 0.87) | 0.86 (0.81, 0.93) | 0.97 (0.90, 1.06) | 0.86 (0.79, 0.93) | 0.88 (0.78, 0.98) | 1.05 (0.94, 1.18) |
| 2003 | 0.84 (0.80, 0.89) | 0.90 (0.84, 0.96) | 0.95 (0.88, 1.03) | 0.86 (0.79, 0.93) | 0.87 (0.77,0.98) | 0.90 (0.76, 1.05) |
| 2004 | 0.85 (0.81, 0.90) | 0.91 (0.85, 0.96) | 0.90 (0.84, 0.97) | 0.91 (0.85, 0.98) | 0.90 (0.81, 1.00) | 0.85 (0.73, 0.98) |
| 2005 | 0.80 (0.76, 0.85) | 0.93 (0.87, 0.98) | 1.01 (0.95, 1.07) | 0.82 (0.75, 0.89) | 0.89 (0.80, 0.99) | 1.01 (0.91, 1.12) |
| 2006 | 0.86 (0.81, 0.91) | 0.95 (0.90, 1.00) | 0.97 (0.92, 1.02) | 0.86 (0.78, 0.94) | 0.98 (0.89, 1.08) | 0.90 (0.78, 1.04) |
| 2007 | 0.86 (0.81, 0.91) | 0.94 (0.89, 0.98) | 0.98 (0.93, 1.03) | 0.82 (0.74, 0.92) | 0.98 (0.92,1.05) | 1.02 (0.96, 1.09) |
| 2008 | 0.89 (0.84, 0.93) | 0.94 (0.91, 0.98) | 0.96 (0.92, 1.00) | 0.89 (0.82, 0.97) | 0.96 (0.90, 1.02) | 0.96 (0.90, 1.03) |
| 2009 | 0.93 (0.89, 0.98) | 0.93 (0.90, 0.97) | 0.97 (0.94, 1.00) | 0.93 (0.87, 0.99) | 0.95 (0.89, 1.02) | 0.99 (0.95, 1.03) |
| 2010 | 0.94 (0.89, 0.99) | 0.96 (0.93, 0.99) | 0.96 (0.93, 0.99) | 0.95 (0.88,1.03) | 0.95 (0.87, 1.03) | 0.99 (0.96, 1.02) |
| 2011 | 0.94 (0.89, 0.98) | 0.95 (0.92, 0.98) | 0.99 (0.95, 1.02) | 0.93 (0.85, 1.03) | 0.94 (0.89, 1.00) | 0.99 (0.95, 1.03) |
| 2012 | 1.01 (0.96, 1.06) | 0.96 (0.93, 0.98) | 0.97 (0.93, 1.00) | 0.99 (0.92, 1.07) | 0.94 (0.88, 1.01) | 0.98 (0.94, 1.03) |
HIV human immunodeficiency virus, PWID persons who inject drugs, IDU injection drug use, MSM men who have sex with men, ART antiretroviral therapy, AIDS acquired immune deficiency syndrome
Standardized by sex, age, race, MSM and heterosexual transmission risk, and history of ART, AIDS, CD4 cell count and HIV viral load at enrollment
Retained in care defined as attending ≥2 clinical visits in the Johns Hopkins Medical system >90 days apart between January 1 and December 31 of the calendar year, conditional on being an active patient
On ART defined as having ≥1 antiretroviral prescription for ≥30 days, conditional on being retained in care
Viral suppression defined as having ≥HIV viral load measurement <400 copies/mL, conditional on being on ART
ART Use
The proportion of active PWID who were on ART increased over time; increases were gradual and began almost immediately from 2002–2003. In contrast, the proportion of active non-IDU who were on ART was fairly constant until 2007, increased until 2009, and remained approximately constant again through the end of the study period in 2012. Given PWID had a lower probability of being retained than non-IDU, it is perhaps not surprising that across all years, active PWID also had a lower probability of ART use (Supplemental Table 1). Yet, among persons who were retained in care, standardizing on baseline demographics and clinical status, PWID and non-IDU had similar probabilities of ART use [relative risk of ART use in 2012 was 0.94, 95 % confidence interval (CI) 0.88, 1.01] (Table 4).
Viral Suppression
Increases in viral suppression followed a pattern similar to ART use, increasing gradually and consistently over the 12-year period covered by this study (Fig. 2). The proportion of active non-IDU who were virally suppressed was consistently higher than the proportion of PWID who were suppressed, but the difference in proportion suppressed decreased over time. By 2012, 69.1 % of active PWID were virally suppressed while 71.0 % of non-IDU were virally suppressed (Table 3). Among persons who were on ART, PWID and non-IDU had similar probabilities of being virally suppressed. For example, in 2012, the standardized relative risk of viral suppression comparing PWID to non-IDU who were on ART was 0.98 (95 % CI 0.94, 1.03) (Table 4).
Fig. 2.
Proportion of HIV-infected persons in the Johns Hopkins HIV Clinical Cohort expected to be in care in each calendar year who were retained, on ART and virally suppressed, stratified by history of injection drug use, 2001–2012. ART antiretroviral therapy, IDU injection drug use, PWID persons who inject drug
Supplemental Analyses: Defining Retention
We contrasted retention as defined by ≥2 clinical visits >90 days apart, with retention as defined by ≥2 HIV laboratory measurements >90 days apart and retention as defined by ≥2 general laboratory measurements >90 days apart. Inference about patterns of retention over time for PWID and non-IDU were approximately the same regardless of the definition of retention used. Retention defined by clinical visits was generally lower than retention defined by HIV laboratory measurements. Among PWID, retention defined by HIV laboratory measurements was generally lower than retention defined by general laboratory measurements. Among non-IDU, retention estimates were generally equal or slightly higher when retention was defined by HIV laboratory measurements or general laboratory measurements (Supplemental Table 2). The probability of ART use and viral suppression was highest in person-years in which patients were classified as retained by both clinical visits and HIV laboratory measurements (86.2 and 75.1 %, respectively), and lowest in person-years in which patients were not classified as retained by either definition (1.4 and 0.8 %, respectively), and was similar for PWID and non-IDU (Table 5). Contrasting person-years in which patients were classified as retained by one measure but not both, ART use and viral suppression was generally most prevalent when patients were retained by HIV laboratory measurements and not clinical visits (75.6 and 60.2 %, respectively, compared to 71.5 and 43.5 %). Upon knowing whether a patient was retained as defined by visits and as defined by HIV laboratory measurements, knowing whether he or she was retained by general laboratory measurements did not appreciably change estimates of the probability of ART use or viral suppression (Supplemental Table 3).
Table 5.
Comparison of person-years (PY) according to their classification as “retained” based on three different definitions of retention in care, stratified by IDU
| Retained by visits Retained by HIV labs |
Yes | No | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| PWID | ||||
| Person-years | 4374 | 541 | 1498 | 2445 |
| PY (%) on ART | 3643 (83.3 %) | 343 (63.4 %) | 1096 (73.2 %) | 26 (1.1 %) |
| PY (%) virally suppressed | 3120 (71.3 %) | 196 (36.2 %) | 866 (57.8 %) | 11 (0.4 %) |
| Non-IDU | ||||
| Person-years | 10,222 | 961 | 2416 | 3195 |
| PY (%) on ART | 8943 (87.5 %) | 731 (76.1 %) | 1863 (77.1 %) | 51 (1.6 %) |
| PY (%) virally suppressed | 7839 (76.7 %) | 457 (47.6 %) | 1489 (61.6 %) | 34 (1.1 %) |
IDU injection drug use, self-reported HIV transmission risk, PWID persons who inject drugs, PY person-year
Discussion
We used the HIV care continuum framework to measure engagement in HIV clinical care among PWID and non-IDU over time in a single, large, urban HIV clinic. Probability of retention in care, ART use and viral suppression all increased over time. In nearly all calendar years, the probability of retention in care, ART use or viral suppression was lower among PWID compared to non-IDU. However, disparities between PWID and non-IDU were no longer statistically significant after 2010, and were nearly mitigated by 2012. When we conditioned on engagement in the prior continuum stage, after around 2003–2004, among people retained in care, PWID and non-IDU had similar probabilities of having had a prescription for ART and among people on ART, PWID and non-IDU had approximately the same probability of having at least one undetectable viral load measurement.
Prior studies have reported on poorer retention in care, lower probability of ART use, and lower probability of viral suppression among PWID compared to non-IDU [6, 13–15], but we believe this study to be one of the first to consider all three outcomes together, over time. By including calendar time in our analysis, we have been able to document improvements in retention among PWID such that the probability of retention in recent years was statistically indistinguishable comparing PWID and non-IDU. There are multiple interventions that have been shown to increase engagement in care [26–28] and the Johns Hopkins HIV Clinic has put a priority on retention. By considering all three outcomes together, we were able to determine that the lower probability of viral suppression seen among PWID may primarily be a function of ART use.
Another strength of our analysis was the consideration of several different definitions of retention. Although HRSA recommends measuring retention using visit attendance, public health surveillance data is forced to rely on laboratory-based measures of retention [29]. We did not consider the other main retention measure: missed clinical visits. The definitions of retention are not perfectly correlated, as seen in our study and as reported previously [21, 22]. Our results indicate that considering both retention measures provides additional information about patients’ level of engagement in care, beyond the information provided in a single measure alone.
As mentioned in the methods section, the definitions of retention, ART use and viral suppression were highly sensitive, but not particularly specific. This may have implications for our finding that PWID who were on ART were not less likely to be virally suppressed compared to non-IDU. In a previous examination comparing PWID and non-IDU in the JHHCC, it was found that PWID spent more time after enrollment on ART but not virally suppressed [30]. In this study, PWID who had at least one viral load measure <400 copies/mL (and classified as virally suppressed) may have been more likely to have other viral load measures during the calendar year ≥400 copies/mL than non-IDU. If we had, instead, used a stricter definition of viral suppression, such as “all viral load measurements during the calendar year <400 copies/mL,” we may have inappropriately classified persons as not suppressed if they were unsuppressed at the beginning of the year, but initiated ART during the calendar year and had all subsequent viral load measurements <400 copies/mL. Reconciling these issues requires shifting estimation of the HIV care continuum from purely cross-sectional to be more longitudinal [30].
While our definitions of retention, ART use and viral suppression were highly sensitive, we did exclude patients from being classified as on ART if they were not retained or from being classified as virally suppressed if they were not on ART (Supplemental Table 4). Including these people as on ART or virally suppressed, respectively, would necessarily increase our estimates of the proportion of patients meeting those definitions, but not the observed patterns. Furthermore, patients on ART but not retained are less likely to be stable on ART throughout the year, and patients who are virally suppressed but not on ART are less likely to be stably virally suppressed throughout the year. Thus estimates of ART use and viral suppression that included these patients are likely to be misleadingly optimistic.
A limitation of our analysis is that IDU was measured only as self-reported history of IDU at enrollment into HIV clinical care. There is the possibility that due to social desirability bias, new patients would under-report history of IDU. However, physicians in the Johns Hopkins HIV clinic are non-judgmental in their interactions with patients while ascertaining HIV acquisition risk and the prevalence of reported IDU was high in this cohort. As such, we believe under-reporting of IDU in this clinical cohort to be similar to or less than other clinical cohorts. History of IDU is not equivalent to ongoing drug use (and non-IDU can use illicit drugs) so our results do not correspond to the association between current drug use and retention, ART use or viral suppression. Further investigations into current illicit drug use could provide insights into the observed association between history of IDU and ART use. However, for measuring and monitoring disparities in progression through the HIV care continuum, history of IDU is measured consistently across nearly all HIV cohorts, which allows for comparability between studies. Under-ascertainment of IDU would likely bias our estimates of disparities comparing PWID and non- IDU toward the null (since PWID incorrectly classified as non-IDU would make the non-IDU group look more like the PWID).
In conclusion, we have documented improvements in HIV clinical engagement for PWID and the diminishment of disparities in retention, ART use and viral suppression between PWID and non-IDU. Reductions in disparities appear to be primarily driven by increases in retention and ART use (rather than viral suppression while on ART). Retention is not a simple concept to measure, and understanding the many different ways that patients engage with the healthcare system may help identify barriers to care and opportunities for increasing retention and subsequent engagement for both PWID and non-IDU.
Supplementary Material
Acknowledgments
Funding This work was supported by NIH Grants U01 DA036935 and P30 AI094189.
CRL has received a speaker honorarium from Gilead Sciences, Inc. for work unrelated to this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10461-016-1585-5) contains supplementary material, which is available to authorized users.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individuals included in this study.
Conflict of Interest No other authors have conflicts of interest to report.
References
- 1.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group ISS, Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin infect dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenberg AE, Hader SL, Masur H, Young AT, Skillicorn J, Dieffenbach CW. Fighting HIV/AIDS in Washington, D.C. Health Aff. 2009;28(6):1677–1687. doi: 10.1377/hlthaff.28.6.1677. [DOI] [PubMed] [Google Scholar]
- 6.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 7.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 8.Toren KG, Buskin SE, Dombrowski JC, Cassels SL, Golden MR. Time from HIV diagnosis to viral load suppression, 2007–2013. Sex Transm Dis. 2016;43(1):34–40. doi: 10.1097/OLQ.0000000000000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosyk B, Montaner JS, Colley G, Lima VD, Chan K, Heath K, et al. The cascade of HIV care in British Columbia, Canadam, 1996–2011: a population-based retrospective cohort study. Lancet Infect Dis. 2014;14(1):40–49. doi: 10.1016/S1473-3099(13)70254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucas GM, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidemiol. 2006;163(5):412–420. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 11.Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15(9):1115–1123. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35(1):46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, et al. Retention among North American HIV-infected persons in clinical care, 2000–2008. J Acquir Immune Defic Syndr. 2013;62(3):356–362. doi: 10.1097/QAI.0b013e31827f578a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanna DB, Buchacz K, Gebo KA, Hessol NA, Horberg MA, Jacobson LP, et al. Trends and disparities in antiretroviral therapy initiation and virologic suppression among newly treatment-eligible HIV-infected individuals in North America, 2001–2009. Clin Infect Dis. 2013;56(8):1174–1182. doi: 10.1093/cid/cit003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gebo KA, Fleishman JA, Conviser R, Reilly ED, Korthuis PT, Moore RD, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA intern med. 2013;173(14):1337–1344. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 17.Aberg JA, Gallant JE, Anderson J, Oleske JM, Libman H, Currier JS, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39(5):609–629. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 18.Aberg JA, Kaplan JE, Libman H, Emmanuel P, Anderson JR, Stone VE, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 19.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 20.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mugavero MJ, Westfall AO, Zinski A, Davila J, Drainoni ML, Gardner LI, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–580. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aberg JA, Gallant JE, Ghanem KG, Emmanuel P, Zingman BS, Horberg MA. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(1):1–10. doi: 10.1093/cid/cit757. [DOI] [PubMed] [Google Scholar]
- 24.Valdiserri RO, Forsyth AD, Yakovchenko V, Koh HK. Measuring what matters: development of standard HIV core indicators across the U.S. Department of Health and Human Services. Public Health Rep. 2013;128(5):354–359. doi: 10.1177/003335491312800504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lesko CR, Sampson LA, Miller WC, Clymore J, Leone PA, Swygard H, et al. Measuring the HIV care continuum using public health surveillance data in the United States. J Acquir Immune Defic Syndr. 2015;70(5):489–494. doi: 10.1097/QAI.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabral HJ, Tobias C, Rajabiun S, Sohler N, Cunningham C, Wong M, et al. Outreach program contacts: do they increase the likelihood of engagement and retention in HIV primary care for hard-to-reach patients? AIDS Patient Care and STDs. 2007;21(Suppl 1):S59–S67. doi: 10.1089/apc.2007.9986. [DOI] [PubMed] [Google Scholar]
- 27.Hightow-Weidman LB, Jones K, Wohl AR, Futterman D, Outlaw A, Phillips G, 2nd, et al. Early linkage and retention in care: findings from the outreach, linkage, and retention in care initiative among young men of color who have sex with men. AIDS Patient Care and STDs. 2011;25(Suppl 1):S31–S38. doi: 10.1089/apc.2011.9878. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an international association of physicians in AIDS care panel. Ann Intern Med. 2012 doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Institute of Medicine (U.S.) Committee on the Ryan White CARE Act: Data for Resource Allocation Planning and Evaluation. Measuring what matters: allocation, planning, and quality assessment for the Ryan White Care Act. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 30.Lesko CR, Edwards JK, Moore RD, Lau B. A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of injection drug use. AIDS. 2016;30(14):2227–2234. doi: 10.1097/QAD.0000000000001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.