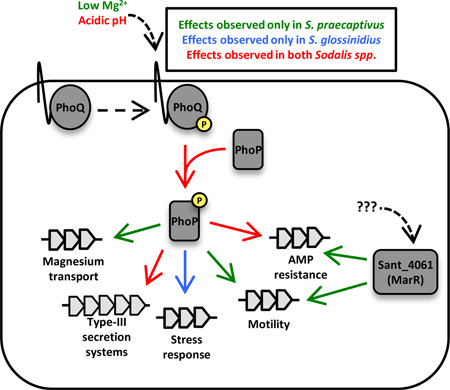
Summary
Many bacteria utilize two-component systems consisting of a sensor kinase and a transcriptional response regulator to detect environmental signals and modulate gene expression for adaptation. The response regulator PhoP and its cognate sensor kinase PhoQ compose a two-component system known for its role in responding to low levels of Mg2+, Ca2+, pH, and to the presence of antimicrobial peptides and activating the expression of genes involved in adaptation to host association. Compared to their free-living relatives, mutualistic insect symbiotic bacteria inhabit a static environment where the requirement for sensory functions is expected to be relaxed. The insect symbiont, Sodalis glossinidius, requires PhoP to resist killing by host derived antimicrobial peptides. However, the S. glossinidius PhoQ was found to be insensitive to Mg2+, Ca2+, and pH. Here we show that Sodalis praecaptivus, a close non host-associated relative of S. glossinidius, utilizes a magnesium sensing PhoP-PhoQ and an uncharacterized MarR-like transcriptional regulator (Sant_4061) to control antimicrobial peptide resistance in vitro. While the inactivation of phoP, phoQ or Sant_4061 completely retards the growth of S. praecaptivus in the presence of an antimicrobial peptide in vitro, inactivation of both phoP and Sant_4061 is necessary to abrogate growth of this bacterium in an insect host.
Short abstract
Transition to a static lifestyle reduces the requirement for environmental sensing and differential regulation. In this study we examine the functions of two regulatory circuits that Sodalis-allied symbionts use to control cellular adaptations leading to antimicrobial peptide resistance.
Introduction
Bacteria must sense the presence or absence of signals in their environment in order to generate appropriate cellular responses. Two component systems are widely used to sense environmental signals and respond by facilitating transcriptional changes in accordance with environmental signal input (Stock et al., 2000). Such systems generally consist of a membrane bound histidine kinase that senses environmental stimuli such as ions or nutrients. In the absence of ligand, the sensor kinase autophosphorylates and transfers its phosphate to a cognate transcriptional response regulator. Most response regulators are capable of freely diffusing through the bacterial cytoplasm and modulating gene expression, usually through interaction with gene promoters (Krell et al., 2010). The ability of a bacterium to sense and modulate transcription provides an adaptive advantage in a dynamic environment where the flexibility provided by a sensory response capability substantially increases the efficiency of resource allocation.
Mutualistic symbionts of insects inhabit a relatively static environment, often residing in specialized host tissues and undergoing strict vertical transmission (Wernegreen, 2002). Following the transition to symbiotic life, a large fraction of the symbiont genome evolves under relaxed selection and is inactivated and lost, leaving only a minimal suite of genes that is required to survive on the resources provided by the host and to fulfill symbiotic functions (Andersson and Kurland, 1998; Dale and Moran, 2006; O’Fallon, 2008). Consequently, long established symbionts maintain the smallest genomes characterized to date among bacteria, with many representatives having genome sizes of less than 200 kbp (Nakabachi et al., 2006; McCutcheon and von Dohlen, 2011; Bennett and Moran, 2013). Many genes involved in regulatory functions are lost early in the process of genome degeneration following the onset of symbiosis (Clayton et al., 2012), presumably because their functions are of limited value (or perhaps deleterious) in a static lifestyle (Moran et al., 2005; Shigenobu et al., 2000; Gil et al., 2003). However, it is clear that the functions of some regulators are maintained at least for a period of time following the onset of symbiosis (Toh et al., 2006; Pontes et al., 2008; Pontes et al., 2011; Clayton et al., 2012).
The PhoP-PhoQ system of the tsetse fly symbiont, Sodalis glossinidius, is one example of a regulatory system that maintains an essential function following the transition to symbiosis. This two-component system is known to control genes involved in nutrient acquisition and virulence in a wide range of facultative pathogenic microbes (Llama-Palacios et al., 2005; Bozue et al., 2011; Gellatly et al., 2012). The functions of PhoQ have been well characterized in Salmonella enterica and Escherichia coli, in which it acts as a sensor of Mg2+ (Perez et al., 2009; Miyashiro and Goulian, 2007), Ca2+ (García Véscovi et al., 1996;), pH (Prost et al., 2007) and antimicrobial peptides (Bader et al., 2005; Prost and Miller, 2008). At high Mg2+ concentrations (10 mM), PhoP is dephosphorylated, whereas at low concentrations of Mg2+ (10 µM), PhoP is phosphorylated by PhoQ. The latter condition drives an increase in expression of genes involved in Mg2+transport (Groisman, 2001), virulence, and resistance to low pH and antimicrobial peptides (AMPs) (Groisman and Mouslim, 2006; Mitrophanov et al., 2008). AMP resistance is facilitated by the modification of LPS by increasing its charge, disrupting electrostatic forces within the LPS and thereby preventing damage caused by host derived AMPs (Guaní-Guerra et al., 2010).
Previous work on the PhoP/PhoQ system of S. glossinidius showed that it is required for the bacteria to survive in its insect host by mediating the expression of genes involved in resistance to host AMPs (Pontes et al., 2011). However, S. glossinidius PhoQ lacks the ability to sense Mg2+, pH and antimicrobial peptides (Pontes et al., 2011). It was hypothesized that an ancestral precursor of the S. glossinidius PhoQ protein likely had a functional Mg2+sensing capability but that relaxed selection on the sensory capability of PhoQ resulted in the loss of this capability in the symbiotic lifestyle (Pontes et al., 2011). This was predicted to be a consequence of mutations in the metal binding “acidic patch” of the PhoQ protein, which prevent the binding of divalent cations that result in dephosphorylation of PhoP.
The recent discovery of a close free-living relative of S. glossinidius, designated S. praecaptivus (Clayton et al., 2012), provides an opportunity to examine changes in the functionality of PhoP-PhoQ following transition to obligate symbiosis. Several Sodalis-allied symbionts, including S. glossinidius, have evolved independently from an S. praecaptivus-like ancestor, as evidenced by comparative genomic analyses showing that the symbiont genomes are subsets of this free-living relative (Clayton et al., 2012). In the current study we identified two regulatory circuits in S. praecaptivus, PhoP-PhoQ and a previously uncharacterized MarR-like transcriptional regulator (Sant_4061), that are required to facilitate antimicrobial peptide resistance in vitro. Transcriptomic analyses show that the PhoP-PhoQ systems of S. praecaptivus and S. glossinidius differ in their sensory capabilities and responses. Surprisingly, a mutant S. praecaptivus strain lacking PhoQ showed no deficiency in its ability to maintain infection in an insect host, implying that PhoP alone is sufficient to maintain AMP resistance in vivo. Furthermore, while mutant strains lacking either PhoP or Sant_4061 maintained infection at reduced bacterial densities, a mutant strain lacking both PhoP and Sant_4061 was completely avirulent. These results suggest that there is a synergistic interplay between the PhoP-PhoQ and Sant_4061 regulatory circuits that facilitates AMP resistance and survival in vivo.
Results
Identification of AMP resistance determinants in S. praecaptivus
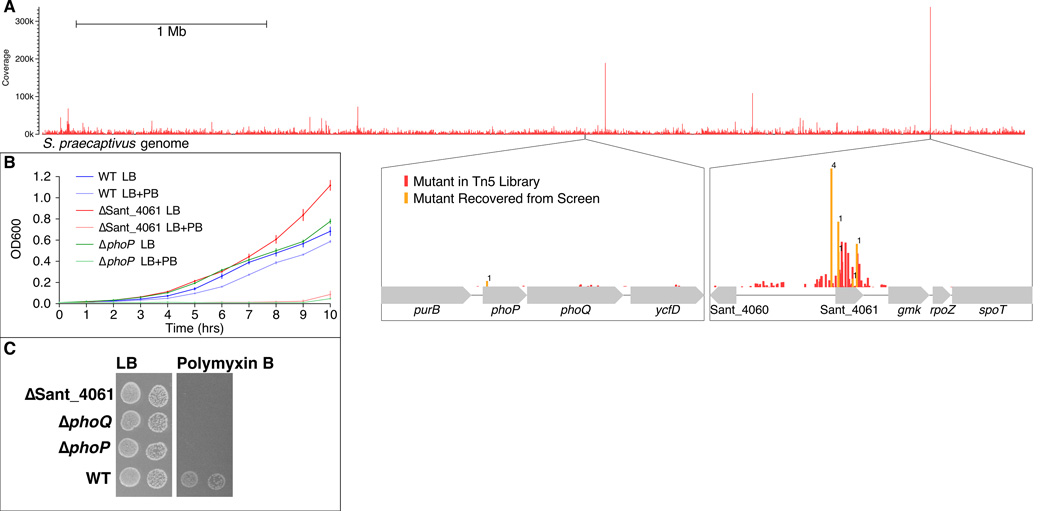
The results of our in vivo assays suggested that AMP resistance genes might be subject to control by a PhoP-independent mechanism in S. praecaptivus. In order to identify genes involved in facilitating resistance to AMPs, we screened a library of > 34,000 S. praecaptivus transposon Tn5 mutants by replica plating mutants on non-selective media, and media containing polymyxin B, and identified a total of nine polymyxin B sensitive mutants. The Tn5 insertion sites within these mutants were then identified using an inverse PCR and sequencing approach. While PhoP is known to regulate genes involved in LPS modifications that mediate resistance to AMPs (Pontes et al., 2011; Mitrophanov et al., 2008), we were surprised to find that only one of our Tn5 mutants had a disrupted phoP allele. The remaining eight Tn5 mutants all contained insertions in the promoter region or coding sequence of a gene designated Sant_4061 in the S. praecaptivus genome annotation (Figure 1, panel A).
Figure 1.
Distribution and sequence coverage of Tn5 mutants in the S. praecaptivus genome are depicted as a histogram (A, Upper). Note that mutations affecting Sant_4061 are the most abundant in the library, following growth in LB media. The zoomed in regions of the histogram (A, Lower) reveal the distribution and abundance of Tn5 mutants in the genomic regions encoding phoPQ and Sant_4061 (in red), with the mutants recovered in the screen for polymyxin B sensitivity highlighted in orange, with the number of recovered mutants shown above each column. Growth curves for WT S. praecaptivus and Sant_4061 and phoP mutants grown in either LB or LB + Polymyxin B (PB) for 10 hours (B). Standard errors are shown above and below the average of three replicates at each time point. ΔSant_4061, ΔphoQ, and ΔphoP are unable to grow on LB supplemented with polymyxin B (C).
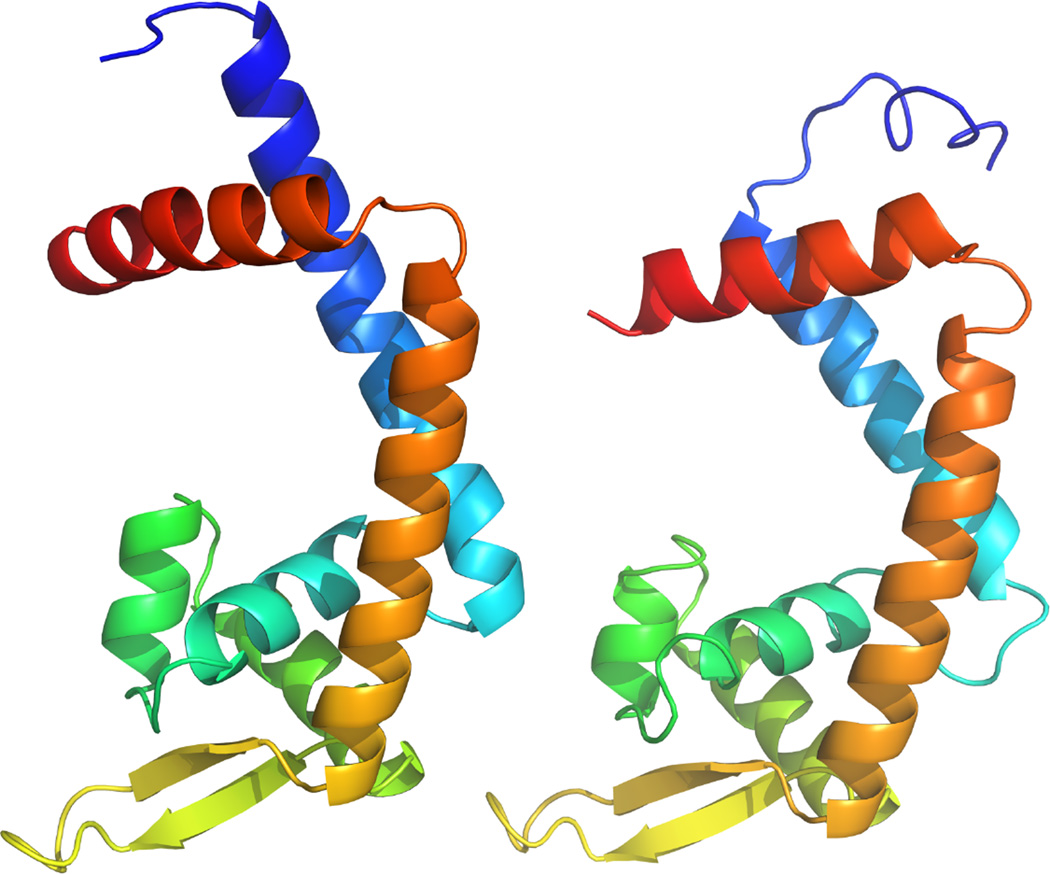
According to domain (Finn et al., 2014) and structural prediction analyses (Zhang, 2008), Sant_4061 is a member of the MarR (multiple antibiotic resistance regulator) family of transcriptional regulators (Figure 2). Members of this family are typically repressors that control the expression of genes involved in host immune resistance (Spory et al., 2002; Stapleton et al., 2002; Shi et al., 2004), antibiotic resistance (Poole et al., 1996), the oxidative stress response (Sukchawalit et al., 2001) and catabolism of aromatic compounds (Providenti and Wyndham, 2001; for a review see Wilkinson and Grove, 2006). The abundance of Sant_4061 mutants in our screen prompted us to check the representation of these mutants in our Tn5 library. High-throughput sequence analysis of the Tn5 library shows that the Sant_4061 mutants were highly represented in the library prior to the polymyxin B plate-based selection experiment (Figure 1, panel A). This suggests that the inactivation of Sant_4061, or disruption of its promoter sequence, provides an adaptive advantage for growth in LB medium. This was confirmed by growth assays in LB media in which an independently generated ΔSant_4061 mutant demonstrated a 39% increase in fitness over the wild type (WT) strain (Figure 1, panel B).
Figure 2.
Structural prediction of Sant_4061 (left) and MarR (E. coli; right). Structures are colored from N to C termini in red to blue respectively. The DNA-binding helix-turn-helix domain is located at the bottom of each predicted structure.
Following identification of Sant_4061 and PhoP in our screen, we sought to determine if independently generated mutant strains lacking PhoP, PhoQ or Sant_4061 were sensitive to polymyxin B (Figure 1, panel C). Notably, all three mutant strains were sensitive to this AMP. Furthermore, resistance to polymyxin B was restored in mutant strains lacking PhoP and Sant_4061 following complementation with intact alleles in trans (Figures S1 and S2 respectively).
Phylogenetic analysis of phoQ and 16S rDNA
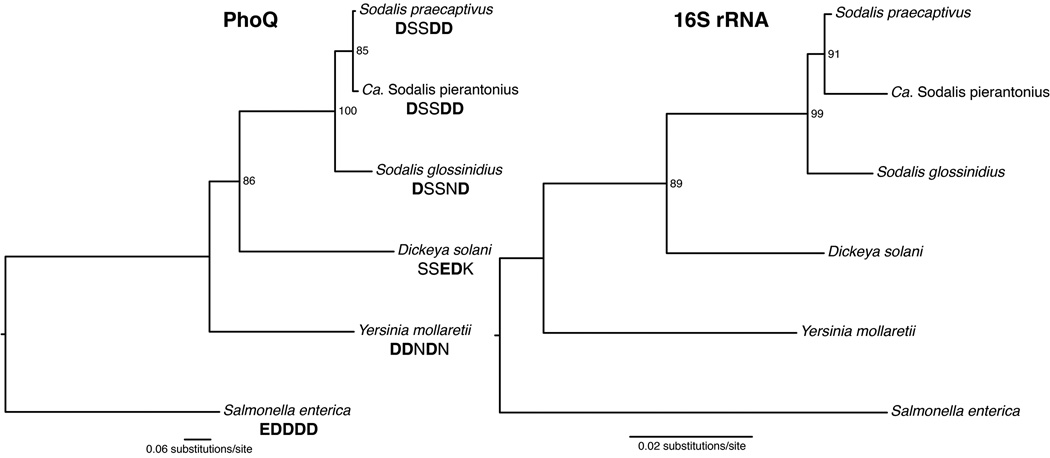
In S. enterica, PhoQ functions as a sensor of divalent cations by coordinating the binding of these metals using various conserved acidic residues throughout the protein (Cho et al., 2006; Prost et al., 2008). Inspection of these acidic residues revealed a single region where acidic residues had been lost. This region in particular (located between Glu148 and Asp152 in S. enterica), is composed of 5 tandem acidic residues, which are positioned in the periplasmic domain of PhoQ. This "acidic patch" is best characterized for its role in coordinating the binding the divalent cations including, among others, Mg2+ (Chamnongpol et al., 2003). The change of three of these acidic residues to non-acidic residues in S. glossinidius was anticipated to contribute to its loss of sensitivity to divalent cations (Pontes et al., 2011). In order to determine how the PhoQ sensor of S. praecaptivus compares with S. glossinidius and other Enterobacteriaceae, we performed a phylogenetic analysis of the nucleotide sequence of PhoQ and 16S rDNA in other related bacteria. The phylogenetic tree shows that PhoQ of S. praecaptivus is most closely related to other members of the Sodalis-allied clade of insect symbionts (Figure 3). We also performed an amino acid alignment of PhoQ and assessed the content of its "acidic patch". We found that S. praecaptivus maintains an additional acidic residue in this region that is absent in S. glossinidius (Figure 3), which could explain any increased sensitivity to PhoQ activating divalent cations (Figure 4).
Figure 3.
Phylogeny of S. praecaptivus and related Sodalis-allied endosymbionts and free-living bacteria based on maximum likelihood analyses of the phoQ coding sequence (1.45 kbp) and 16S rRNA (1.46 kbp). Sequences below strain names on the phoQ phylogeny show the amino acids sequences of the Mg2+-binding site within PhoQ, with acidic residues highlighted in bold. The numbers adjacent to nodes indicate maximum likelihood bootstrap values shown for nodes with bootstrap support > 80%.
Figure 4.
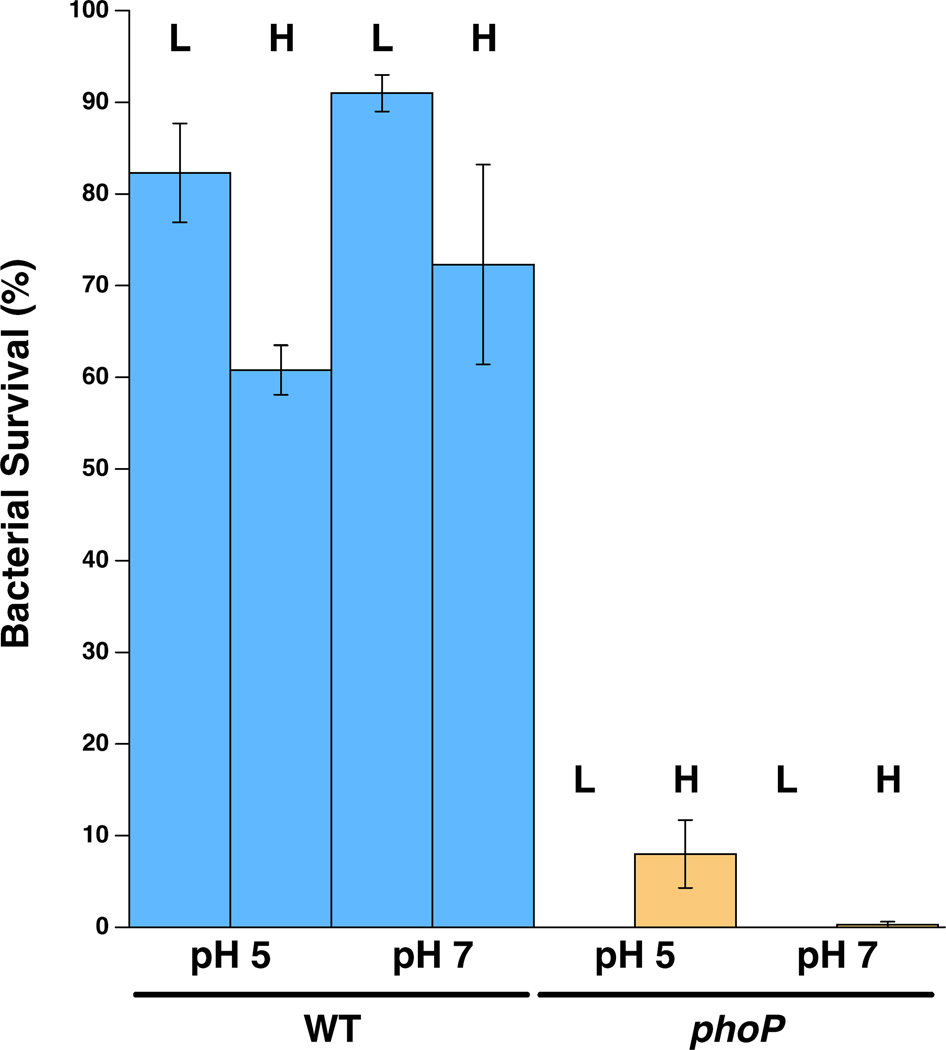
Survival rates of WT and phoP mutant S. praecaptivus following 3 hours of growth in high (H: 10 mM) or low (L: 10 µM) Mg2+ and Ca2+ at pH 5 or 7 followed by a one-hour challenge with polymyxin B (50 µg/mL). Error bars represent standard errors.
Antimicrobial peptide resistance
In order to assess the functionality of the PhoP-PhoQ system in S. praecaptivus, we assayed the abilities of WT and phoP strains to resist the killing effect of the AMP, polymyxin B, in vitro (Figure 4). Under all conditions tested, the phoP mutant strain was highly susceptible to polymyxin B, indicating that the PhoP-PhoQ system plays an important role in mediating resistance to AMPs in this bacterium, similar to what was observed previously in S. glossinidius (Pontes et al., 2011). Wild type S. praecaptivus also showed a small but significant increase in polymyxin B sensitivity when grown in media containing high levels of Mg2+ and Ca2+, similar to what was observed previously in S. glossinidius. This effect was observed in media at both pH 5 and pH 7, although pH did not significantly affect bacterial survival following challenge. It should be noted that AMPs (such as polymyxin B) are known to be capable of displacing Mg2+ cations from PhoQ (Bader et al. 2005), potentially reducing the effects of the different cation concentrations used in our experiment.
Transcriptomic analyses
In facultative pathogens, PhoP is activated in low Mg2+, low pH, and in the presence of antimicrobial peptides (Prost and Miller, 2008) where it responds by activating genes involved in resistance to AMPs, type III secretion, and magnesium transport (Prost and Miller, 2008). Based on qPCR analysis of select PhoP-regulated genes and assays of AMP resistance, it was previously demonstrated that the S. glossinidius PhoQ is insensitive to changes in the levels of Mg2+ (Pontes et al. 2011). This results in the constitutive PhoP-mediated activation of genes involved in AMP resistance and type III secretion such that a phoP mutant is unable to survive following microinjection into an insect host (Pontes et al. 2011). In order to determine the genome-wide regulatory capability of PhoP and to understand changes that have been mediated in its regulatory network in the transition to symbiosis, we performed transcriptomic analysis of WT and phoP mutants of S. praecaptivus and S. glossinidius following growth in media containing high (10 mM) or low (10 µM) Mg2+ and Ca2+ at pH 7 or pH 5. The resulting data was then analyzed in pairwise conditional comparisons to delineate PhoP-dependent and PhoP-independent transcriptional changes that take place as a consequence of changes in the concentrations of Mg2+/Ca2+ and pH.
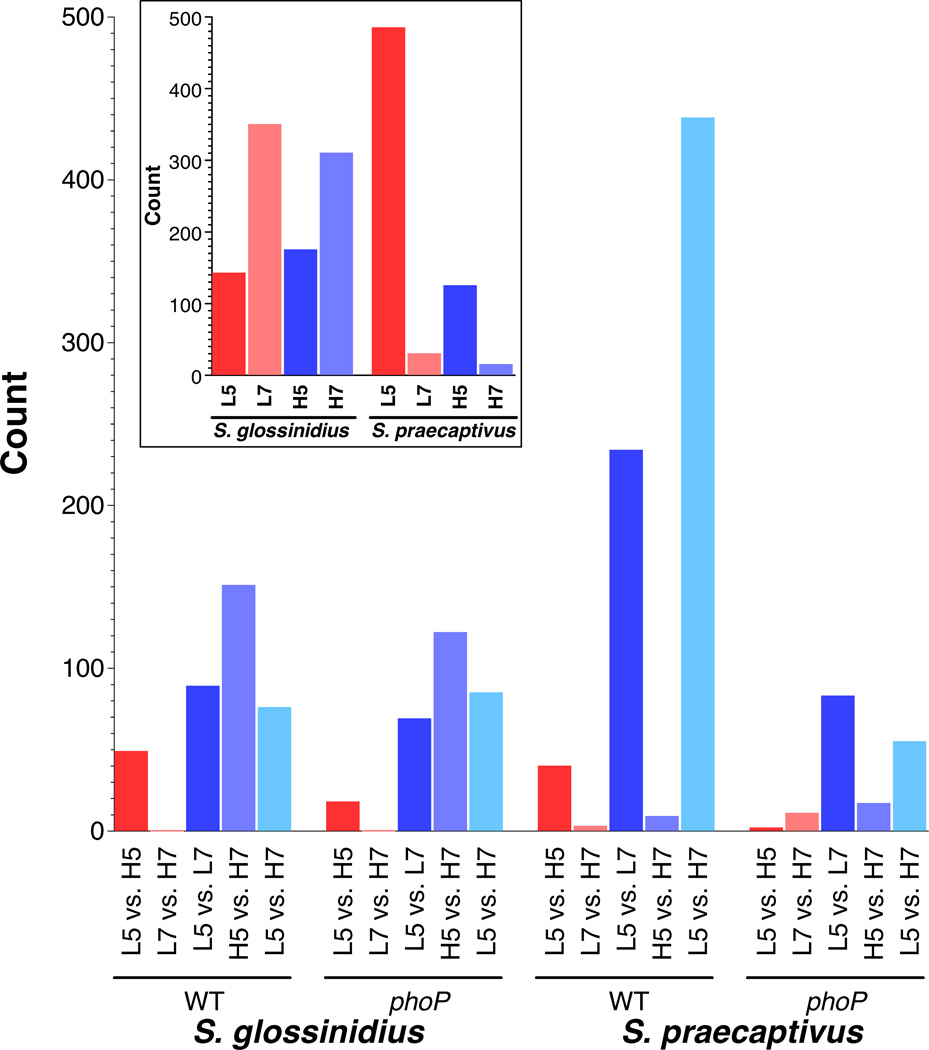
Significant differences in gene expression were observed in all pairwise comparisons and the complete datasets are presented in supplementary file S3. Figure 5 shows the numbers of genes undergoing a log2fold change in expression > 2 in response to a change in cation concentration, pH or bacterial genotype. The most striking observation is that both S. glossinidius and S. praecaptivus have similar numbers of genes whose expression changes in accordance with the concentration of Mg2+/Ca2+ in the culture media (Figure 5), despite the fact that S. glossinidius has lost over half of its gene inventory following the transition to symbiosis. This effect is most apparent at pH 5 and almost exclusively represents genes that are up regulated in low Mg2+/Ca2+ conditions, which is consistent with the known functionality of the PhoP-PhoQ system (Prost and Miller, 2008). In this context, it is notable that this effect is only observed in the pairwise comparisons conducted on data derived from WT strains, which strongly suggests that it is a consequence of PhoP-mediated signaling in both bacterial species.
Figure 5.
The number of genes exhibiting significant expression changes (adjusted p-value < 0.05) and log2fold changes > 2 or < −2 after growth in low (L; 10 µM) or high (H; 10 mM) Mg2+/Ca2+ at pH 5 or pH 7. Pairwise comparisons of WT or a phoP mutant are shown below, while comparisons of WT and a phoP mutant grown in the same conditions are shown in the inset box.
In S. praecaptivus we observed many PhoP-dependent transcriptional changes occurring as a consequence of a change in pH at a low cation concentration. However, the pH-mediated changes observed in S. glossinidius occurred at both low and high cation concentrations and are largely PhoP-independent, based on the fact that similar numbers of genes show significant changes in expression in both the WT and phoP mutant strains. Pairwise analyses of gene expression changes between WT and phoP mutants grown in the same conditions (Figure 5; inset) shows that the S. glossinidius PhoP-PhoQ system modulates the expression of similar numbers of genes under both low and high Mg2+/Ca2+ concentrations, with increased numbers of genes showing changes in expression at pH 7. This is in contrast to S. praecaptivus, in which most PhoP-mediated changes were observed to occur in low pH with a low level of Mg2+/Ca2+ (Figure 5). The S. praecaptivus data is consistent with the canonical role of PhoP-PhoQ modulating expression of genes under conditions of low pH and low levels of Mg2+/Ca2+, whereas the data relating to S. glossinidius indicates that its PhoP-PhoQ system has a modified function that lacks sensitivity towards these conditions.
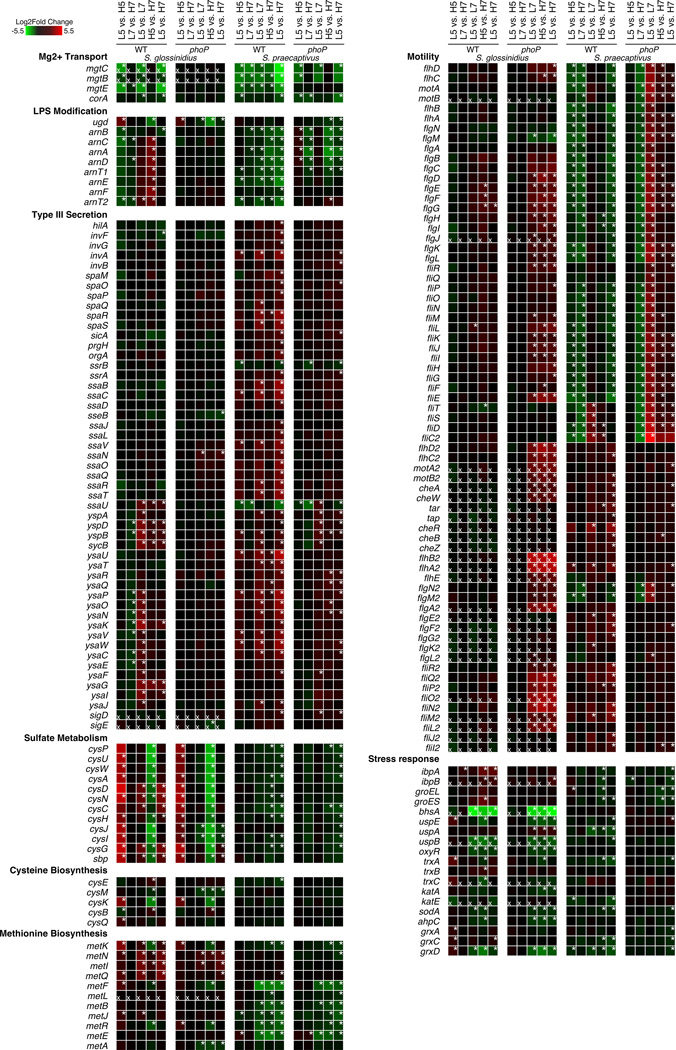
In order to further understand the role of PhoP-PhoQ in S. praecaptivus and S. glossinidius, we manually inspected the transcriptomic data to identify changes that impact known PhoP-regulated functions and genes sharing similar functions (Figure 6). This analysis revealed that genes involved in Mg2+ transport (mgtCB) are highly expressed in WT S. glossinidius at low pH, but that this expression is reduced in the presence of high concentrations of Mg2+/Ca2+. However, it is notable that both the mgtC and mgtB genes in S. glossinidius are pseudogenes that are disrupted by mutations, indicating that the role of PhoP in driving their expression is a relic of a bygone lifestyle, as described previously (Pontes et al. 2011). This is supported by the fact that the intact mgtC and mgtB Mg2+ transport genes in WT S. praecaptivus display significant activation by PhoP under conditions of low pH and low Mg2+/Ca2+. However, S. praecaptivus exhibits a strong pH dependent ability to significantly change the expression of mgtC and mgtB in the absence of PhoP, suggesting that there are additional regulatory mechanism(s) controlling expression of these genes. In support of this prediction, the mgtCB operon of S. enterica is known to undergo increased expression in response to acid pH (Smith et al., 1998).
Figure 6.
Heatmaps depicting log2fold changes in expression of genes involved in adaptation to changes in Mg2+/Ca2+ concentrations and pH as well as other pathways containing large numbers of genes showing significant differences in expression. Conditions being compared in each column are shown at the top. Log2fold differences are colored as an increase (red) or decrease (green) in expression in the second condition relative to the first. Conditions being compared are abbreviated above each column, L5 = 10 µM Mg2+/Ca2+ pH 5, H5 = 10 mM Mg2+/Ca2+ pH 5 etc. Genes are grouped by broad functional categories that exhibited significant changes in our data. Statistically significant log2fold changes (adjusted p-value < 0.05) are marked with a white asterisk in the top right corner of the box. Pseudogenes are marked by a white "x" in the lower left corner of the box.
Genes involved in LPS modification (and AMP resistance) in S. glossinidius, aside from ugd (pmrE) also depend on PhoP for their activation under conditions of low pH and low levels of Mg2+/Ca2+. However, it is interesting that many of these genes also exhibit a significant increase in expression at high Mg2+/Ca2+ levels at pH 7. This represents a novel adaptation in S. glossinidius, which is not observed in S. praecaptivus, in which the majority of LPS modification genes are most strongly activated by low pH.
Analysis of type III secretion genes in S. glossinidius shows that a large fraction of genes in the ysa-island are responsive to a change in pH in a PhoP-dependent manner. However the type III secretion system genes of S. praecaptivus seem to exhibit a much broader response to PhoP, and changes in expression are responsive to both pH and the levels of Mg2+/Ca2+. Similarly, genes involved in flagellar motility are generally more responsive to PhoP-mediated activation in S. praecaptivus, whereas changes in the expression of these genes in S. glossinidius seem to be enhanced in the absence of PhoP.
Interestingly, our analysis reveals that S. glossinidius, but not S. praecaptivus, shows significant upregulation of genes involved in sulfate transport and cysteine biosynthesis in 10 mM Mg2+/Ca2+ at pH 5 which is independent of PhoP. CysP, CysU, CysW, and CysA together make up a sulfate/thiosulfate permease known as SulT (Aguilar-Barajas et al., 2011). Once inside the cell, CysD, CysN, CysC, CysH, CysJ, and CysI convert sulfate into hydrogen sulfide, which can then be added to O-acetyl-L-serine by CysK to produce L-cysteine (Aguilar-Barajas et al., 2011). The expression of this system appears to represent a unique adaptation in S. glossinidius, possibly to enhance cysteine biosynthesis for its host, or to mediate increased resistance to oxidative stress (MacLeod et al., 2007) via glutathione production, which is dependent on L-cysteine. Curiously, MetK is also upregulated in S. glossinidius under the same conditions as the cysteine biosynthesis genes discussed above, although the remaining methionine biosynthesis genes are not (Figure 4). MetK catalyzes the synthesis of S-adenosoylmethionine, which can also act as an antioxidant (Cavallaro et al., 2010). We also inspected genes involved in a more general stress response to determine if they are also upregulated in any of our transcriptomic data. To this end, we observed that many other genes involved in stress response in S. glossinidius exhibit significant upregulation in response to pH, relative to S. praecaptivus, albeit in a largely PhoP-independent manner (Figure 6).
Insect infection assays involving phoP, phoQ and Sant_4061 mutants
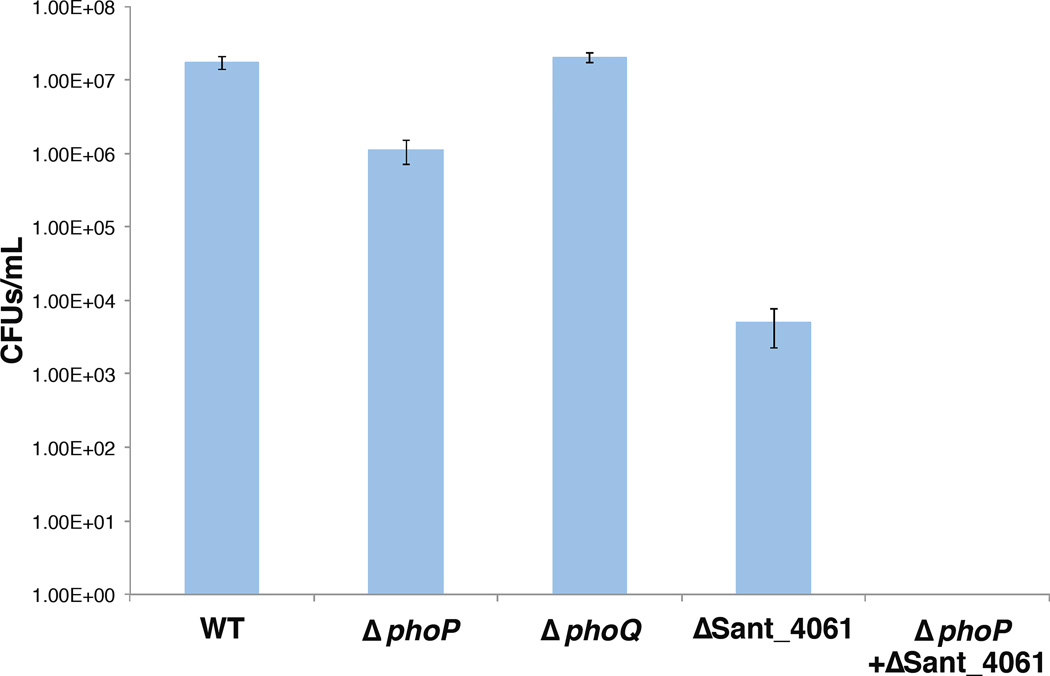
The insect immune system combats invading pathogenic microbes by producing and secreting AMPs (Lemaitre and Hoffmann, 2007). Some recently derived insect symbionts such as S. glossinidius are known to inhabit multiple host tissues, including the haemolymph (Cheng and Aksoy, 1999), where they encounter host AMPs. The maize weevil Sitophilus zeamais maintains a symbiont that is a very close relative of S. praecaptivus and S. glossinidius known as Sitophilus zeamais primary endosymbiont (SZPE; Heddi et al., 1999). Unlike S. glossinidius, SZPE resides in a specialized host tissue known as the bacteriome, which contains a specialized set of immune factors that control the size of the symbiont population (Anselme et al., 2008). It was also demonstrated that the weevil host could produce an immune challenge to SZPE cells when microinjected into the haemolymph (Anselme et al., 2008). Thus, we chose to use S. zeamais for an in vivo immune challenge to assess the abilities of WT, ΔphoP, ΔphoQ and ΔSant_4061 mutant strains of S. praecaptivus to maintain infection following intrathoracic microinjection. The results (Figure 7) show that the phoQ null mutant strain demonstrates no significant reduction in its ability to maintain infection in vivo in the insect host. Furthermore, strains lacking PhoP or Sant_4061 also retained the capability to infect the weevil, albeit at a reduced bacterial density. Indeed, only a ΔphoP ΔSant_4061 double mutant was incapable of establishing an infection in S. zeamais following intrathoracic microinjection. This provides a stark contrast to the results obtained from the in vitro studies, which clearly show that PhoP, PhoQ and Sant_4061 are required to facilitate polymyxin B resistance.
Figure 7.
Survival of S. praecaptivus following 14-day incubation post microinjection in maize weevils.
Discussion
In this study we sought to identify the determinants of AMP resistance in S. praecaptivus, which is a close free-living relative of the Sodalis-allied insect endosymbionts. In a previous study focusing on the tsetse fly symbiont, S. glossinidius, it was shown that PhoP is required for AMP resistance in vitro and for the ability of this bacterium to infect its insect host (Pontes et al., 2011). It was also hypothesized that the ability of PhoQ to sense divalent cations had been attenuated in S. glossinidius in transition to the obligate static lifestyle of symbiosis. This was based on the notion that the S. glossinidius PhoQ is not responsive to changes in the levels of divalent cations, and that a Mg2+-binding patch in the PhoQ sensor protein is lacking in acidic amino acid residues that are known to participate in cation binding (Pontes et al., 2011). Analysis of the genome sequence of S. praecaptivus shows that it has not undergone any of the characteristic changes associated with genome degeneration in symbiotic life. Experimental comparisons between S. praecaptivus and S. glossinidius therefore provide an opportunity to characterize the molecular changes that accompany the transition from a free-living to symbiotic state.
Analysis of the PhoQ sensor protein in S. praecaptivus reveals that it maintains one additional acidic amino acid residue in its sensory domain in comparison to its counterpart in S. glossinidius. This occurs as a result of a G > A transition that changes an aspartic acid residue at position 151 to an asparagine. Based on an alignment of PhoQ homologues from related Enterobacteriaceae that includes the weevil symbiont Candidatus Sodalis pierantonius, this mutation occurred only in the lineage leading to S. glossinidius, which is consistent with the notion that it is an adaptive mutation that has modulated the functionality of PhoQ in the S. glossinidius-tsetse fly symbiosis to ensure constitutive expression of genes facilitating AMP resistance.
Based on assays of AMP resistance in vitro, the PhoQ homolog of S. praecaptivus is capable of sensing divalent cations under both acidic and neutral conditions. Transcriptomic analyses confirm this finding, indicating that the S. praecaptivus PhoQ is adept at sensing conditions of low pH and low levels of Mg2+/Ca2+, which is typical of PhoQ function in a wide range of bacteria. However, the transcriptomic analyses focusing on S. glossinidius suggest that its PhoP-PhoQ system lacks the ability to differentiate between different conditions. It is interesting to note that the S. glossinidius PhoP-PhoQ system still seems to play a role in driving the expression of large numbers of genes (Figure 5, inset), however this effect is greatest at pH 7 regardless of Mg2+/Ca2+ concentration, supporting the observation that the PhoP-PhoQ system has undergone modifications that limit its ability to respond to canonical signals and instead results in a constitutive expression profile of PhoP-regulated genes in this organism. Moreover, it should be noted that the PhoQ of S. glossinidius can respond to changes in divalent cation concentrations, as we observed in the transcriptomic profile of the pseudogenes mgtC and mgtB, which are activated in low levels of divalent cations, but only at pH 5. This requirement of low pH for the expression of mgtCB may be a consequence of the loss of acidic residues in the Mg2+ sensing "acidic patch" of PhoQ, whose Mg2+-binding capability might be enhanced at lower pH. Alternatively, the activation of the mgtCB operon at low pH could also be a more widely conserved characteristic among Enterobacteriaceae in general.
We also observed unique PhoP-independent responses to changes in Mg2+/Ca2+ in S. glossinidius, including the activation of pathways involved in cysteine and S-andenosylmethionine production. The activation of these pathways in S. glossinidius may reflect a requirement to either limit oxidative stress in the bacteria or to increase production of cysteine for its insect host. Other pathways involved in the stress response were also activated uniquely in S. glossinidius, however neither the cysteine biosynthesis nor the stress response pathways exhibit a dependence on PhoP for their expression, identifying these as additional novel adaptations that likely result from obligate host association. The PhoP-PhoQ system is known to influence the expression of both type III secretion and motility genes in S. enterica (Adams et al., 2001; Beuzón et al., 2001), and we observed a larger number of these genes in S. praecaptivus that displayed dependence on PhoP for their expression. The small number of type III secretion and motility genes influenced by PhoP in S. glossinidius likely reflects a regulatory decoupling of PhoP from the regulation of these genes in the symbiosis.
The static host environment encountered by S. glossinidius is anticipated to have driven modifications in PhoP-PhoQ such that the system constitutively activates the expression of AMP resistance genes. In this study, we determined that the expression of PhoP-regulated genes in S. praecaptivus could be modulated by either pH or changes in Mg2+/Ca2+ concentration. However, while an S. praecaptivus phoP mutant was found to be sensitive to AMPs in vitro, it was still capable of maintaining infection in an insect host (in the presence of AMPs), unlike its S. glossinidius counterpart. In addition, an S. praecaptivus phoQ mutant showed no significant difference relative to the wild type strain in its ability to maintain infection in an insect host, suggesting that there is no requirement for signaling between PhoQ and PhoP to mediate AMP resistance in the insect host.
Our work also revealed that PhoP is not the only transcriptional regulator involved in driving the expression of genes involved in AMP resistance in S. praecaptivus. By screening a Tn5 mutant library for AMP sensitive mutants we identified a novel regulator (Sant_4061) that is also needed for AMP resistance in vitro. This protein is a member of the MarR-family of transcriptional regulators that are found in a wide range of bacteria and respond to myriad environmental and intracellular signals to modulate gene expression. Consequently, while strains lacking phoP, phoQ, or Sant_4061 were capable of maintaining infections in the weevil (albeit at reduced levels in the case of phoP or Sant_4061 mutants), a ΔphoP ΔSant_4061 double mutant strain of S. praecaptivus was found to be completely incapable of maintaining infection. These results suggest that there is a complex interplay between the actions of the PhoP-PhoQ and Sant_4061 regulatory circuits in facilitating bacterial survival in vivo. While our results show that S. praecaptivus is capable of maintaining infection in an insect host, it should be noted that this bacterium was isolated from a human wound [Clayton et al., 2012]. In addition, based on the presence of Sodalis-allied symbionts in plant feeding insects, it has been predicted that S. praecaptivus is also capable of infecting plants [Clayton et al., 2012; Husnik and McCutcheon, 2016]. Thus, it seems likely that the mechanisms of AMP resistance utilized by S. praecaptivus have evolved to accommodate a variety of physiological conditions and signals. Interestingly, orthologs of Sant_4061 are maintained in S. glossinidius and other Sodalis-allied symbionts with a high level of sequence conservation, based on BLAST analysis. However, a phoP mutant strain of S. glossinidius was found to be completely defective in vivo (Pontes et al., 2011) suggesting that Sant_4061 is no longer a key determinant of AMP resistance in this symbiont.
The transition from a free-living lifestyle to obligate host association is accompanied by the loss of genes that provide an adaptive advantage in the facultative lifestyle but are no longer required in symbiosis. This often includes genes encoding sensory and regulatory functions that have little adaptive value in a lifestyle in which the environment remains relatively static. While PhoP-PhoQ is still required to drive the expression of important symbiotic determinants (including AMP resistance) in the insect symbiont S. glossinidius, it has clearly undergone changes in its sensory and regulatory capabilities. In addition, a second regulatory system that serves as an alternative means to activate AMP resistance in S. praecaptivus, appears to have lost those functions in the transition to obligate symbiosis in S. glossinidius. Together, these results illustrate how changes in lifestyle can lead to the modification of regulatory circuits whilst retaining the adaptive advantages of the functions that they control.
Experimental Procedures
Bacterial strains and culture conditions
Sodalis praecaptivus str. HS1 was grown at 30°C in LB or at 25°C in a defined medium composed of 6 g/L casamino acids, 4 g/L glucose, 0.2 g/L KCl, 7.0 g/L NaCl, 0.12 g/L NaHCO3, 0.18 g/L, 0.18 g/L NaH2PO3, 10 mg/mL of thiamine, 10 mM or 10 mM of CaCl2, 10 µM or 10 mM MgCl2, and pH 5 or pH 7. When indicated antibiotics were added at the following concentrations unless otherwise indicated: 50 µg mL−1 polymyxin B, 40 µg mL−1 spectinomycin, 30 µg mL−1 chloramphenicol, 50 µg mL−1 streptomycin, 10 µg mL−1 gentamicin or 30 µg mL−1 kanamycin. Sodalis glossinidius was maintained at 25°C in the semi-defined liquid Mitsuhashi-Maramorosch (MM) medium as described previously (Dale and Maudlin, 1999) or in the same defined media described above.
Transposon Tn5 mutagenesis
WT S. praecaptivus was mutagenized by electroporation with the transposon Tn5 using the EZ-Tn5 method (Epicentre) as follows: 4 mL cultures were grown in LB at 30°C overnight. 25 mL of fresh LB was then added and cultures were grown until the OD600 reached 0.4. Cultures were then placed on ice for 10 minutes followed by centrifugation at 6,700 × g for 10 minutes at 4°C. Cell pellets were then resuspended in 20 mL of ice-cold nuclease free water. Centrifugation was repeated and cells were washed with 10 mL of ice-cold nuclease free water. Centrifugation was repeated and the supernatant was discarded. Cell pellets were resuspended in the residual liquid and placed on ice for 10 minutes. 80 µl aliquots of cells were then mixed with 1 µl of transposon and incubated on ice for 10 minutes. The entire volume was transferred to a chilled 1 mm electroporation cuvette and pulsed with 1.6 kV using an Eppendorf electroporator 2510. Cells were resuspended in 1 mL LB and allowed to recover for 3 hours shaking (225 rpm) at 30°C. Following recovery, cells were spread on LB agar plates containing kanamycin. Mutagenesis was repeated until ~23,000 mutants were obtained. All Tn5 mutants were then combined in LB + kanamycin in a single 50 mL culture and grown overnight at 30°C with shaking (225 rpm). DNA was isolated from a 1 mL aliquot of overnight culture using the DNeasy Blood and Tissue Kit (Qiagen). 1 mL aliquots of the remaining culture were then mixed with 225 µl of sterile 80% glycerol and archived at −80°C for subsequent experiments.
Screening Tn5 mutants
100 µl of the Tn5 mutant library was diluted in LB to 10−7 and plated on LB kanamycin. 10,000 mutants were screened by replica printing onto plates containing LB + kanamycin and LB + kanamycin and polymyxin B. Colonies that grew on LB + kanamycin and did not grow on polymyxin B were characterized by inverse PCR to identify the site of Tn5 insertion.
Inverse PCR to identify transposon insertions
Individual Tn5 mutants were grown overnight in 4 mL cultures of LB with kanamycin at 30°C with shaking (225 rpm). 1 mL aliquots of each mutant were then mixed with 225 µl of sterile 80% glycerol and stored at −80°C. DNA from 1.5 mL of each culture was prepared using CTAB as described previously (Wilson, 2001). 500 ng of DNA was digested using the restriction enzyme HpyCH4IV (NEB) followed by heat inactivation according to the manufacturer’s recommendations. 1 µl of digested DNA was then ligated in a 10 µl reaction using T4 DNA Ligase (Thermo Fisher) according to the manufacturer’s recommendations. 1 µl of ligated template was then used in a 50 µl PCR reaction using 2X PCR Master Mix (Thermo Fisher) and the following primers (F: GGGTGTTATGAGCCATATTCAACGGG, R: CATCGATGATGGTTGAGATGTG). Thermocycling conditions were as follows: 95°C for 5 minutes following by 35 cycles of 95°C for 30 seconds, 49°C for 30 seconds, 72°C for 40 seconds followed by 1 cycle of 72°C for 5 minutes. PCR products were visualized in a 1% agarose gel stained with ethidium bromide and purified using AMPure XP magnetic beads (Beckman Coulter) according to the manufacturer’s recommendations. Cleaned PCR products were submitted with primer F for Sanger sequencing at the University of Utah sequencing core facility. Chromatograms were analyzed using Geneious version 8.1.7 (Kearse et al., 2012).
High throughput sequencing of transposon mutants
DNA from the entire pool of Tn5 mutants was submitted for library construction and 50 bp single-end sequencing at the Huntsman Cancer Institute sequencing core at the University of Utah. Library construction was performed as described previously (Subashchandrabose et al., 2013). Briefly, DNA is sheared and sequencing adapters are ligated. Enrichment of transposon-chromosome junctions is performed by PCR using primers that anneal to the 3’ end of the transposon and the sequencing adapter. Sequencing is then performed on the HiSeq 2500 using a primer targeting the end of the transposon. Sequence reads were filtered using NGSQCToolkit (Patel and Jain, 2012) using optional settings -l 70 -s 20. High quality reads were separated and trimmed using the "separate reads by barcode" function in Geneious version 8.1.7 (Kearse et al., 2012) to include only reads beginning with the last six bases of the Tn5 transposon. This tool also removes the six bases of transposon sequence from each filtered read. Reads were then mapped to the S. praecaptivus genome using the Geneious mapper with optional settings "minimum overlap identity" 100 % and "maximum mismatches per read" 0 %. Alignment files in .sam format were exported from Geneious with the “export padded CIGARs” option enabled. Because the Tn5 transposon duplicates a nine base insertion sequence the alignment file was curated to include only the first or last nine bases of forward or reverse sequence respectively using custom perl scripts. Reads were then counted and visualized according to the first base of the insertion site.
Growth curves of S. praecaptivus
S. praecaptivus strains were grown overnight in 4 mL cultures of LB shaking (225 rpm) at 30° C. The following morning each culture was diluted to an OD600 = 0.01 in fresh LB or LB + polymyxin B. Cultures were incubated with shaking (225 rpm) at 30° C. OD600 measurements were recorded every hour for 10 hrs. Each strain was grown in triplicate and the average OD600. Error bars show standard error of the mean.
Comparison of predicted structures of Sant_4061 and MarR
Amino acid sequences from Sant_4061 and MarR from Escherichia coli str. K-12 substr. MG1655 were submitted for structural prediction using the I-TASSER online submission tool (Zhang, 2008). Protein structures were aligned using the PyMOL Molecular Graphics System, Version 1.7.4 (Schrödinger, LLC). Following alignment each structure was manually placed side-by-side for visual comparison. Sequences are available from GenBank under the following accession numbers: Sant_4061 (CP006569.1), E. coli marR (NC_000913.2).
Phylogenetic analysis
Sequence alignments were generated using MUSCLE (Edgar, 2004) for the phoQ and 16S rRNA genes from S. praecaptivus, Ca. S. pierantonius, S. glossinidius, Dickeya solani, Yersinia mollaretii, Salmonella enterica. Alleles used in this study were retrieved from GenBank under the following accession numbers: S. praecaptivus phoQ and 16S (CP006569.1), Ca. S. pierantonius phoQ and 16S (CP006568.1), S. glossinidius phoQ (AP008232.1) and 16S (NR_074525), Dickeya solani phoQ (AMWE01000003.1) and 16S (NZ_AMWE01000004.1), Yersinia mollaretii phoQ (NZ_CQDS01000001.1) and 16S (NZ_CQDS01000018.1), Salmonella enterica phoQ and 16S (NC_003197.1). PhyML (Guindon et al., 2010) was then used to construct phylogenetic trees using the HKY85 (Hasegawa et al. 1985) model of sequence evolution with 25 random starting trees and 100 bootstrap replicates. Sequences of the putative Mg2+-binding region were added below each strain name on the PhoQ tree.
Construction of phoP and phoQ knockouts
A plasmid construct consisting of approximately 500 bp of homologous sequence up and downstream of phoP with an internal kanamycin cassette was commercially produced (Genscript). Plasmid template was used for PCR amplification of the knockout construct. PCR products were gel purified to remove residual plasmid template using the QIAquick Gel Extraction Kit (Qiagen). A construct consisting of approximately 200 bp of homologous sequence up and downstream of phoQ with an internal spectinomycin cassette was amplified and joined together using PCR as described previously (Shevchuk et al., 2004) using Phusion polymerase (Thermo Fisher). PCR products were then cleaned used AMPure XP magnetic beads (Beckman Coulter) according to the manufacturers recommendations.
Transformation of plasmid and knockout constructs
WT S. praecaptivus was transformed with pKD3 containing the lambda red recombination genes and transformants were selected on LB agar plates containing appropriate antibiotics. Subsequent gene knockouts were generated as follows: cultures were grown in 25 mL of LB until the OD600 reached 0.4. Arabinose was added (0.5%) to induce expression of lambda red genes followed by an additional 30 mins of growth at 30°C. Cultures were placed on ice for 10 minutes and centrifuged at 6,700 × g for 10 minutes at 4°C and washed twice with ice-cold nuclease free water. Following washes, the pellet was resuspended in the residual liquid and 80 µl aliquots of cells were transferred to chilled 1.5 mL tubes. 25–100 ng of DNA was added and cells were incubated on ice for 10 minutes. The entire volume was transferred to a chilled 1 mm electroporation cuvette and pulsed with 1.6 kV using an Eppendorf electroporator 2510. Cells were resuspended in 1 mL of LB and allowed to recover for 3 hours shaking (225 rpm) at 30°C. Following recovery, cells were plated on LB agar plates containing the appropriate antibiotic.
Complementation of phoP
The WT phoP allele was amplified by PCR using the following primers: BamHI_phoP_comp_F1; ATGCGAGTTCTGGTAATAG and HindIII_phoP_comp_R1; AGAACTACGCAGGCTGCAGC. PCR products were cleaned using AMPure XP magnetic beads (Beckman Coulter) according to the manufacturers recommendations. PCR product and plasmid pKH66 were digested using BamHI and HindIII FastDigest enzymes (Thermo Fisher). Plasmid was digested by combining 200 ng of plasmid DNA with 1 µl of HindIII enzyme and 1 µl of a 1:140 fold dilution of BamHI enzyme in a 20µl reaction. PCR product was digested by combining 1 µg of plasmid DNA with 1 µl of both HindIII and BamHI enzymes in a 20µl reaction. Both plasmid and PCR digestions were incubated at 37°C for 10 min and 80°C for 5 min. Digested DNA was purified using AMPure XP magnetic beads (Beckman Coulter) according to the manufacturers recommendations and quantified using a NanoDrop Lite (Thermo Fisher). Digested insert and vector were ligated in a 2:1 ratio in a 20 µl reaction using 1 µl of T4 DNA ligase (Thermo Fisher) according to the manufacturers recommendations to produce the complementation plasmid pKH66/phoP+. This plasmid was transformed into the ΔphoP mutant using the aforementioned protocol and transformants were plated on LB plates containing streptomycin and spectinomycin. Transformants were then screened for resistance to polymyxin B.
Complementation of Sant_4061
Plasmid pSE526 was made from pCM66 (Marx & Lidstrom, 2001) by replacing the region between bases 3771–7486, containing the traA and kanamycin resistance genes, with a gentamycin resistance gene by recombineering in E. coli CD31(Datsenko & Wanner, 2000). The Sant_4061+ locus was PCR amplified using the following primers: #1277; GTAAAACGACGGCCAGTGAATTCCGACGACAGGATATCAGGGG and #1278; CAGCTATGACCATGATTACGCCAAGCTTAAAGTAATGCTGTCCGTGCG. Insertion of the Sant_4061+ gene into pSE526 was performed using homologous recombination as described previously (Li & Elledge, 2007). The plasmid and PCR product were transformed together into TOP10 E. coli (Thermo Fisher) according to the manufacturers recommendations. Transformants were selected in L broth containing gentamicin.
Antimicrobial peptide resistance assay
Assays were performed using a modified version of a previously described method (Groisman et al., 1992). Briefly, 1 mL aliquots from 4 mL overnight cultures of S. praecaptivus WT and ΔphoP grown in LB were washed twice in 1 mL of 0.85% NaCl. After washing the aliquots were inoculated in 4 mL of defined media containing 10 µM or 10 mM Mg2+ and Ca2+ at pH 5 or pH 7 and grown for 3 hours at 30°C with shaking (225 rpm). Cultures were then washed twice and resuspended in 1 mL 0.85% NaCl. 100 µl of each culture was diluted and plated on LB agar to determine the initial number of colony forming units (CFUs) in the culture. A negative control without AMP or Polymyxin B was then added to the remaining cells and incubated at room temperature for 1 hour. 100 µl of each culture was again diluted and plated on LB agar. CFUs mL−1 were determined from each condition tested and standard errors were calculated.
RNA isolation
WT and ΔphoP S. praecaptivus were grown in 20 mL of LB overnight and washed twice with 10 mL of 0.85% NaCl and diluted to an OD600 = 0.01 in 20 mL of defined media. WT and ΔphoP S. glossinidius were grown in stationary 20 mL cultures of MM until the OD600 reached 0.13. Cultures were then placed in an orbital shaker and grown until the OD600 was between 0.3–0.4 then cultures were washed 2× with 10 mL of 0.85% NaCl and diluted to and OD600 = 0.01 in 20 mL of defined media. Both S. praecaptivus and S. glossinidius were then grown at 25°C shaking (225 rpm) for 8 hours. Total nucleic acid content was extracted from each culture as described previously (Sung et al., 2003). Following nucleic acid extraction, RNA was purified using the PureLink RNA mini kit (Ambion) according to the manufacturer’s recommendations. To avoid biases in transcriptomic analyses, we excluded any additional rRNA removal steps in our sample preparation. Samples were submitted for library preparation according to the manufacturer’s recommendations. Samples were multiplexed in a single HiSeq 2500 lane and sequenced using 50 bp single-end sequencing at the University of Utah sequencing core facility. All experiments were performed in triplicate.
Comparative transcriptomics
Sequence reads were filtered using NGSQCToolkit (Patel and Jain, 2012) using optional settings -l 70 -s 20. Filtered reads were then mapped to the appropriate reference genome using bowtie2 (Langmead and Salzberg, 2012) using the --very-sensitive preset. Counts of reads mapping to each gene were generating using HTSeq (Anders et al., 2015) by running htseq-count using the options -s no -a 0 -m intersection-nonempty -t gene. WT and ΔphoP replicate counts files from each condition were combined using custom perl scripts. Combined counts files were then normalized and compared using DESeq2 (Love et al., 2014). Significant differences in expression were identified as having adjusted p-values less than 0.05.
Reannotation of S. glossinidius
Prior to the discovery of S. praecaptivus, the annotation of recently established Sodalis-allied symbiont genomes was complicated by large numbers of pseudogenes and insertion sequence elements present in these genomes. Due to this fact, we sought to improve the sensitivity of transcriptomic data from S. glossinidius by reannotating its genome using the manually annotated genome of S. praecaptivus as a guide. This was accomplished using the "transfer annotations" feature built into Geneious version 8.1.7 (Kearse et al., 2012) requiring 75% sequency identity for an annotation to be transferred. This annotation of S. glossinidius was then manually inspected for errors and any unannotated region larger than 100 bp was inspected for coding sequences using blastx (Camacho et al., 2009). The reannotated genome of S. glossinidius is available as supplementary file S4.
Weevil microinjection and screening
Aposymbiotic Sitophilus zeamais weevils were generated by diluting 100 µl of a 10 mg/ml solution of rifampicin dissolved in N,N-Dimethylformamide (DMF) into 2.9 ml of nuclease free water and mixing this solution with 100 g of organic whole yellow corn (Purcell Mountain Farms). After the corn had dried for 16 hours approximately 100 weevils were added and allowed to oviposit in the corn for one week at 25°C and 62% relative humidity. 5th instar larvae were confirmed to be lacking bacteriome organs (aposymbiotic) by dissection. Newly emerged adults were microinjected by dipping the tips of capillary needles in overnight cultures of cells grown in LB and inserting the tip of the needle on the underside of the insect on the left or right side of the insect, between the middle and hind legs. Insects were maintained on fresh corn following microinjection for 14 days. Following incubation, 10 surviving weevils from each group were surface sterilized in a 10% bleach solution for 5 minutes and dried. Insects were homogenized in 100 µl of sterile nuclease-free water and serially diluted and plated on LB. Growth was assessed following a 24 hour incubation at 30° C.
Supplementary Material
Table 1.
Bacterial strains used in this study
| Strain | Genotype (Source) |
|---|---|
| S. praecaptivus | WT (Clayton et al., 2012) |
| S. praecaptivus | phoP::kan (this study) |
| S. praecaptivus | phoP::spc (this study) |
| S. praecaptivus | phoQ::spc (this study) |
| S. praecaptivus | Sant_4061::gen (this study) |
| S. praecaptivus | phoP::kan+Sant_4061::gen (this study) |
| S. praecaptivus | phoQ::S. glossinidius phoQ (this study) |
| S. glossinidius | WT (Pontes et al., 2011) |
| S. glossinidius | phoP::cat (Pontes et al., 2011) |
Acknowledgments
This work was funded by the National Institutes of Health (Grant No. 1R01AI095736) awarded to C.D. We thank Kelly T. Hughes for providing plasmids used for complementation experiments.
Footnotes
The authors declare no financial conflict of interest.
References
- Adams P, Fowler R, Kinsella N, Howell G, Farris M, Coote P, O’Connor CD. Proteomic detection of PhoPQ- and acid-mediated repression of Salmonella motility. Proteomics. 2001;1:597–607. doi: 10.1002/1615-9861(200104)1:4<597::AID-PROT597>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Aguilar-Barajas E, Díaz-Pérez C, Ramírez-Díaz MI, Riveros-Rosas H, Cervantes C. Bacterial transport of sulfate, molybdate, and related oxyanions. Biometals. 2011;24:687–707. doi: 10.1007/s10534-011-9421-x. [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson SG, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. doi: 10.1016/s0966-842x(98)01312-2. [DOI] [PubMed] [Google Scholar]
- Anselme C, Pérez-Brocal V, Vallier A, Vincent-Monegat C, Charif D, Latorre A, et al. Identification of the weevil immune genes and their expression in the bacteriome tissue. BMC Biol. 2008;6:43. doi: 10.1186/1741-7007-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader MW, Sanowar S, Daley ME, Schneider AR, Cho U, Xu W, et al. Recognition of antimicrobial peptides by a bacterial sensor kinase. Cell. 2005;122:461–472. doi: 10.1016/j.cell.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Bennett GM, Moran NA. Small, smaller, smallest: the origins and evolution of ancient dual symbioses in a Phloem-feeding insect. Genome Biol Evol. 2013;5:1675–1688. doi: 10.1093/gbe/evt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozue J, Mou S, Moody KL, Cote CK, Trevino S, Fritz D, Worsham P. The role of the phoPQ operon in the pathogenesis of the fully virulent CO92 strain of Yersinia pestis and the IP32953 strain of Yersinia pseudotuberculosis . Microb Pathog. 2011;50:314–321. doi: 10.1016/j.micpath.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Beuzón CR, Unsworth KE, Holden DW. In vivo genetic analysis indicates that PhoP-PhoQ and the Salmonella pathogenicity island 2 type III secretion system contribute independently to Salmonella enterica serovar Typhimurium virulence. Infect Immun. 2001;69:7254–7261. doi: 10.1128/IAI.69.12.7254-7261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, Madden TL. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro RA, Fuso A, Nicolia V, Scarpa S. S-adenosylmethionine prevents oxidative stress and modulates glutathione metabolism in TgCRND8 mice fed a B-vitamin deficient diet. J Alzheimers Dis. 2010;20:997–1002. doi: 10.3233/JAD-2010-091666. [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Cromie M, Groisman EA. Mg2+ sensing by the Mg2+ sensor PhoQ of Salmonella enterica . J Mol Biol. 2003;325:795–807. doi: 10.1016/s0022-2836(02)01268-8. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Aksoy S. Tissue tropism, transmission and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol. 1999;8:125–132. doi: 10.1046/j.1365-2583.1999.810125.x. [DOI] [PubMed] [Google Scholar]
- Cho US, Bader MW, Amaya MF, Daley ME, Klevit RE, Miller SI, Xu W. Metal bridges between the PhoQ sensor domain and the membrane regulate transmembrane signaling. J Mol Biol. 2006;356:1193–1206. doi: 10.1016/j.jmb.2005.12.032. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Oakeson KF, Gutin M, Pontes A, Dunn DM, von Niederhausern AC, et al. A novel human-infection-derived bacterium provides insights into the evolutionary origins of mutualistic insect-bacterial symbioses. PLoS Genet. 2012;8:e1002990. doi: 10.1371/journal.pgen.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale C, Maudlin I. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans . Int J Syst Bacteriol. 1999;49:267–275. doi: 10.1099/00207713-49-1-267. [DOI] [PubMed] [Google Scholar]
- Dale C, Moran NA. Molecular interactions between bacterial symbionts and their hosts. Cell. 2006;126:453–465. doi: 10.1016/j.cell.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accu- racy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Bateman A, Clements J, Coggill P, Eberhardt RY, Eddy SR, et al. Pfam: the protein families database. Nucleic Acids Res. 2014;42:D222–D230. doi: 10.1093/nar/gkt1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Véscovi E, Soncini FC, Groisman EA. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Gellatly SL, Needham B, Madera L, Trent MS, Hancock RE. The Pseudomonas aeruginosa PhoP-PhoQ two-component regulatory system is induced upon interaction with epithelial cells and controls cytotoxicity and inflammation. Infect Immun. 2012;80:3122–3131. doi: 10.1128/IAI.00382-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil R, Silva FJ, Zientz E, Delmotte F, González-Candelas F, Latorre A, et al. The genome sequence of Blochmannia floridanus: comparative analysis of reduced genomes. Proc Natl Acad Sci U S A. 2003;100:9388–9393. doi: 10.1073/pnas.1533499100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA. The pleiotropic two-component regulatory system PhoP- PhoQ. J Bacteriol. 2001;183:1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Heffron F, Solomon F. Molecular genetic analysis of the Escherichia coli phoP locus. J Bacteriol. 1992;174:486–491. doi: 10.1128/jb.174.2.486-491.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol. 2006;4:705–709. doi: 10.1038/nrmicro1478. [DOI] [PubMed] [Google Scholar]
- Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol. 2010;135:1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. New algorithms and methods to estimate maxi- mum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Kishino H, Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Husnik F, McCutcheon JP. Repeated replacement of an intrabacterial symbiont in the tripartite nested mealybug symbiosis. Proc Natl Acad Sci U S A. 2016;113:E5416–E5424. doi: 10.1073/pnas.1603910113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddi A, Grenier AM, Khatchadourian C, Charles H, Nardon P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc Natl Acad Sci U S A. 1999;96:6814–6819. doi: 10.1073/pnas.96.12.6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krell T, Lacal J, Busch A, Silva-Jiménez H, Guazzaroni ME, Ramos JL. Bacterial sensor kinases: diversity in the recognition of environmental signals. Annu Rev Microbiol. 2010;64:539–559. doi: 10.1146/annurev.micro.112408.134054. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster . Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Li MZ, Elledge SJ. Harnessing homologous recombination in vitro to generate recombinant DNA via SLIC. Nat Methods. 2007;4:251–256. doi: 10.1038/nmeth1010. [DOI] [PubMed] [Google Scholar]
- Llama-Palacios A, López-Solanilla E, Rodríguez-Palenzuela P. Role of the PhoP-PhoQ system in the virulence of Erwinia chrysanthemi strain 3937: involvement in sensitivity to plant antimicrobial peptides, survival at acid pH, and regulation of pectolytic enzymes. J Bacteriol. 2005;187:2157–2162. doi: 10.1128/JB.187.6.2157-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod ET, Maudlin I, Darby AC, Welburn SC. Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology. 2007;134:827–831. doi: 10.1017/S0031182007002247. [DOI] [PubMed] [Google Scholar]
- Marx CJ, Lidstrom ME. Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiology. 2001;147:2065–2075. doi: 10.1099/00221287-147-8-2065. [DOI] [PubMed] [Google Scholar]
- McCutcheon JP, von Dohlen CD. An interdependent metabolic patchwork in the nested symbiosis of mealybugs. Curr Biol. 2011;21:1366–1372. doi: 10.1016/j.cub.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrophanov AY, Jewett MW, Hadley TJ, Groisman EA. Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet. 2008;4:e1000233. doi: 10.1371/journal.pgen.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashiro T, Goulian M. Stimulus-dependent differential regulation in the Escherichia coli PhoQ PhoP system. Proc Natl Acad Sci U S A. 2007;104:16305–16310. doi: 10.1073/pnas.0700025104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran NA, Dunbar HE, Wilcox JL. Regulation of transcription in a reduced bacterial genome: nutrient-provisioning genes of the obligate symbiont Buchnera aphidicola . J Bacteriol. 2005;187:4229–4237. doi: 10.1128/JB.187.12.4229-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabachi A, Yamashita A, Toh H, Ishikawa H, Dunbar HE, Moran NA, Hattori M. The 160-kilobase genome of the bacterial endosymbiont Carsonella. Science. 2006;314:267. doi: 10.1126/science.1134196. [DOI] [PubMed] [Google Scholar]
- O’Fallon B. Population structure, levels of selection, and the evolution of intracellular symbionts. Evolution. 2008;62:361–373. doi: 10.1111/j.1558-5646.2007.00289.x. [DOI] [PubMed] [Google Scholar]
- Patel RK, Jain M. NGS QC Toolkit: a toolkit for quality control of next generation sequencing data. PLoS One. 2012;7:e30619. doi: 10.1371/journal.pone.0030619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez JC, Shin D, Zwir I, Latifi T, Hadley TJ, Groisman EA. Evolution of a bacterial regulon controlling virulence and Mg(2+) homeostasis. PLoS Genet. 2009;5:e1000428. doi: 10.1371/journal.pgen.1000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MH, Babst M, Lochhead R, Oakeson K, Smith K, Dale C. Quorum sensing primes the oxidative stress response in the insect endosymbiont, Sodalis glossinidius. PLoS One. 2008;3:e3541. doi: 10.1371/journal.pone.0003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes MH, Smith KL, De Vooght L, Van Den Abbeele J, Dale C. Attenuation of the sensing capabilities of PhoQ in transition to obligate insect-bacterial association. PLoS Genet. 2011;7:e1002349. doi: 10.1371/journal.pgen.1002349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K, Tetro K, Zhao Q, Neshat S, Heinrichs DE, Bianco N. Expression of the multidrug resistance operon mexA-mexB-oprM in Pseudomonas aeruginosa, mexR encodes a regulator of operon expression. Antimicrob Agents Chemother. 1996;40:2021–2028. doi: 10.1128/aac.40.9.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Le Sage V, Bader MW, Le Moual H, Klevit RE, Miller SI. Activation of the bacterial sensor kinase PhoQ by acidic pH. Mol Cell. 2007;26:165–174. doi: 10.1016/j.molcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Prost LR, Miller SI. The Salmonellae PhoQ sensor: mechanisms of detection of phagosome signals. Cell Microbiol. 2008;10:576–582. doi: 10.1111/j.1462-5822.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- Prost LR, Daley ME, Bader MW, Klevit RE, Miller SI. The PhoQ histidine kinases of Salmonella and Pseudomonas spp. are structurally and functionally different: evidence that pH and antimicrobial peptide sensing contribute to mammalian pathogenesis. Mol Microbiol. 2008;69:503–519. doi: 10.1111/j.1365-2958.2008.06303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Providenti MA, Wyndham RC. Identification and functional characterization of CbaR, a MarR-like modulator of the cbaABC-encoded chlorobenzoate catabolism pathway. Appl Environ Microbiol. 2001;67:3530–3541. doi: 10.1128/AEM.67.8.3530-3541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchuk NA, Bryksin AV, Nusinovich YA, Cabello FC, Sutherland M, Ladisch S. Construction of long DNA molecules using long PCR-based fusion of several fragments simultaneously. Nucleic Acids Res. 2004;32:e19. doi: 10.1093/nar/gnh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Latifi T, Cromie MJ, Groisman EA. Transcriptional control of the antimicrobial peptide resistance ugtL gene by the Salmonella PhoP and SlyA regulatory proteins. J Biol Chem. 2004;279:38618–38625. doi: 10.1074/jbc.M406149200. [DOI] [PubMed] [Google Scholar]
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407:81–86. doi: 10.1038/35024074. [DOI] [PubMed] [Google Scholar]
- Smith RL, Kaczmarek MT, Kucharski LM, Maguire ME. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology. 1998;144:1835–1843. doi: 10.1099/00221287-144-7-1835. [DOI] [PubMed] [Google Scholar]
- Spory A, Bosserhoff A, von Rhein C, Goebel W, Ludwig A. Differential regulation of multiple proteins of Escherichia coli and Salmonella enterica serovar Typhimurium by the transcriptional regulator SlyA. J Bacteriol. 2002;184:3549–3559. doi: 10.1128/JB.184.13.3549-3559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton MR, Norte VA, Read RC, Green J. Interaction of the Salmonella typhimurium transcription and virulence factor SlyA with target DNA and identification of members of the SlyA regulon. J Biol Chem. 2002;277:17630–17637. doi: 10.1074/jbc.M110178200. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Subashchandrabose S, Smith SN, Spurbeck RR, Kole MM, Mobley HL. Genome-wide detection of fitness genes in uropathogenic Escherichia coli during systemic infection. PLoS Pathog. 2013;9:e1003788. doi: 10.1371/journal.ppat.1003788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukchawalit R, Loprasert S, Atichartpongkul S, Mongkolsuk S. Complex regulation of the organic hydroperoxide resistance gene (ohr) from Xanthomonas involves OhrR, a novel organic peroxide-inducible negative regulator, and posttranscriptional modifications. J Bacteriol. 2001;183:4405–4412. doi: 10.1128/JB.183.15.4405-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung K, Khan SA, Nawaz MS, Khan AA. A simple and efficient Triton X-100 boiling and chloroform extraction method of RNA isolation from Gram-positive and Gram-negative bacteria. FEMS Microbiol Lett. 2003;229:97–101. doi: 10.1016/S0378-1097(03)00791-2. [DOI] [PubMed] [Google Scholar]
- Toh H, Weiss BL, Perkin SA, Yamashita A, Oshima K, Hattori M, Aksoy S. Massive genome erosion and functional adaptations provide insights into the symbiotic lifestyle of Sodalis glossinidius in the tsetse host. Genome Res. 2006;16:149–156. doi: 10.1101/gr.4106106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernegreen JJ. Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet. 2002;3:850–861. doi: 10.1038/nrg931. [DOI] [PubMed] [Google Scholar]
- Wilkinson SP, Grove A. Ligand-responsive transcriptional regulation by members of the MarR family of winged helix proteins. Curr Issues Mol Biol. 2006;8:51–62. [PubMed] [Google Scholar]
- Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb0204s56. Chapter 2: Unit 2.4. [DOI] [PubMed] [Google Scholar]
- Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.