Abstract
Cognitive decline in chronic diabetic patients is a less investigated topic. Diabetes and obesity are among the modifiable risk factors for Alzheimer’s disease (AD), the most common form of dementia. Studies have identified several overlapping neurodegenerative mechanisms, including oxidative stress, mitochondrial dysfunction, and inflammation that are observed in these disorders. Advanced glycation end products generated by chronic hyperglycemia and their receptor RAGE provide critical links between diabetes and AD. Peripheral inflammation observed in obesity leads to insulin resistance and type 2 diabetes. Although the brain is an immune-previleged organ, cross-talks between peripheral and central inflammation have been reported. Damage to the blood brain barrier (BBB) as seen with aging can lead to infiltration of immune cells into the brain, leading to the exacerbation of central inflammation.
Neuroinflammation, which has emerged as an important cause of cognitive dysfunction, could provide a central mechanism for aging-associated ailments. To further add to these injuries, adult neurogenesis that provides neuronal plasticity is also impaired in the diabetic brain. This review discusses these molecular mechanisms that link obesity, diabetes and AD.
Keywords: Obesity, diabetes, Alzheimer’s disease, advanced glycation end products, oxidative stress, mitochondria, inflammation
1. Introduction
The prevalence of diabetes worldwide has been projected to be 366 million by 2030 according to the World Health organization [1]. The management of typical complications of diabetes, including cardiovascular diseases, peripheral neuropathy, retinopathy, and nephropathy has improved significantly in recent decades because of improved glycemic control and additional treatment strategies that target diabetic complications. Therefore, the life span of diabetic patients has considerably lengthened. Research indicates that the older diabetic population is more susceptible to aging-associated cognitive decline than aged persons without diabetes. Although pharmacological interventions have improved their survival from life-threatening complications, silent neurodegenerative pathways over decades contribute to cognitive decline with aging and compromise the quality of life. The main focus of diabetes management is glycemic control. However, other diabetes-associated factors that are not downstream of chronic hyperglycemia may affect the brain gradually over time, and the molecular events that are known to precede cognitive decline go unnoticed for decades until it becomes clinically apparent. Increasing evidence suggest that obesity and diabetes, combined with aging, contribute to a person’s susceptibility to Alzheimer’s disease (AD) (Figure 1). The purpose of this review article is to highlight the common factors relating to obesity, T2D, and AD, all of which involve the overlapping pathways of oxidative stress/mitochondrial dysfunction, and inflammation.
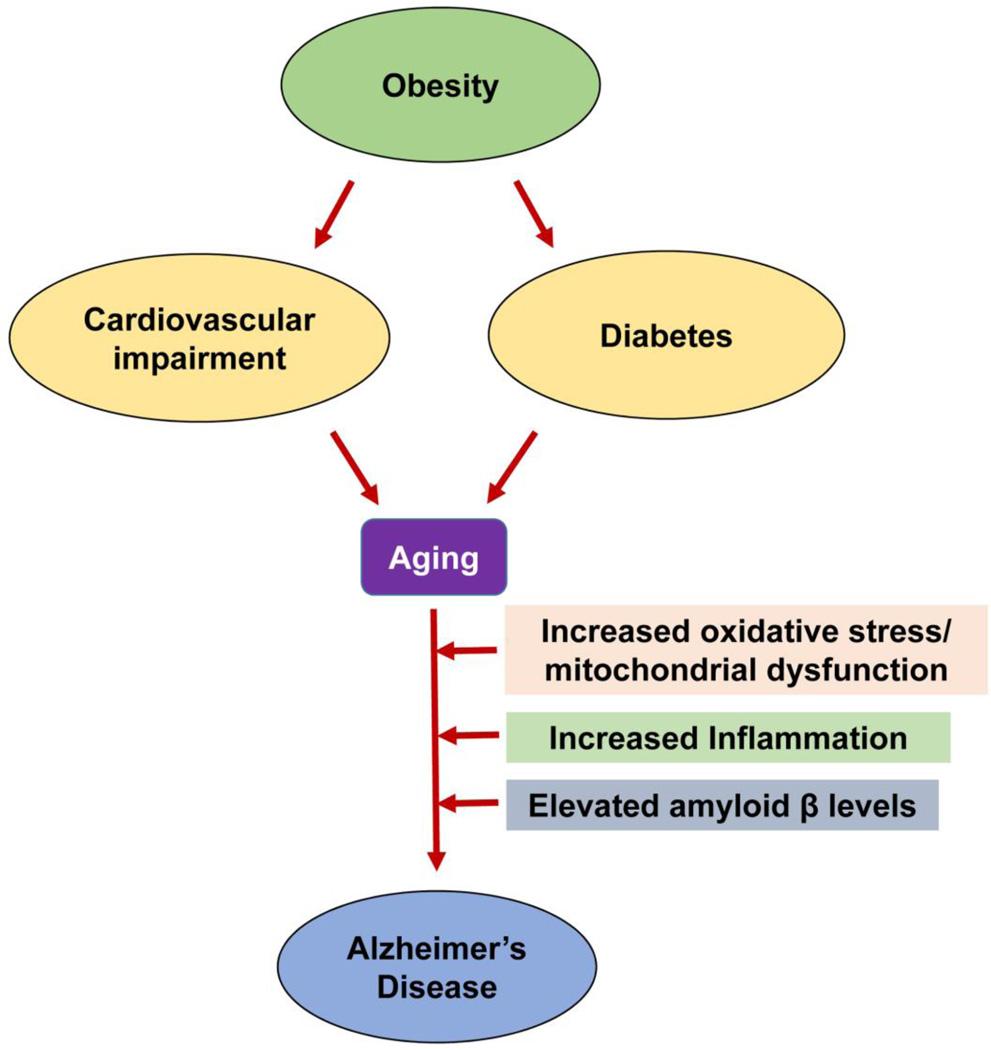
Figure 1. Common pathways of aging-associated disorders.
The risk of Alzheimer’s disease (AD) in elderly individuals is increased by other aging-associated comorbidities including obesity, diabetes and cardiovascular impairment. Oxidative stress, mitochondrial dysfunction and chronic inflammation observed in these conditions are also some of the important causes of AD.
2. Obesity and dementia
The prevalence of overweight and obesity is increasing at an alarming rate throughout the world. The number of overweight and obese adults is projected to be 1.35 billion and 573 million respectively by 2030 [2]. Obesity has been shown to increase the risk of dementia, independent of T2D. A longitudinal study of 6,583 individuals was conducted by measuring the participants’ sagittal abdominal diameter [3]. Individuals with the largest diameter were determined to have nearly a three-fold risk of developing dementia, when compared with those with the smallest diameter [3]. Another study observed that larger waist-hip ratio is associated with decreased hippocampal volume [4]. The link between obesity in mid-life and the future risk of dementia has been reported by several studies [5–9]. Xu et al. reported that overweight persons (BMI >25–30) at midlife and obese individuals (BMI >30) at midlife developed dementia at a mean odds ratio of 1.71 and 3.88, respectively [10]. A linkage cohort study found that the risk ratio for dementia was significantly high in obese subjects aged 30–39, and it steadily decreased in subjects whose obesity decreased as they aged [11]. A cohort projection model based on an Australian population showed that dementia in old age could be lowered by 10% in 2050 if midlife obesity was decreased by 20% [12]. Although these reports suggest that obesity in mid-life can lead to cognitive dysfunction in later life, a recent study has questioned the link between obesity and the risk of dementia [13]. This retrospective cohort study observed that underweight in middle age carries an increased risk of dementia in later life. The link between obesity and dementia needs to be investigated further because of these conflicting reports. Furthermore, weight loss in the elderly population is a risk factor for morbidity and mortality and therefore cannot be an interventional strategy. Nevertheless, chronic low-grade inflammation associated with mid-life obesity provides a mechanistic link to progressive cognitive decline by cross-talk with central inflammation. This angle will be discussed in details in a later section.
3. High-fat diet and the brain
Consumption of a high-fat diet is a major cause of overweight and obesity. Chronically elevated levels of circulating free fatty acids have been found to cause many deleterious effects, including low-grade inflammation, which plays an important role in insulin resistance. Studies examining inflammatory pathways in obesity have focused on macrophages of the periphery [14, 15]. These studies found that inflammatory events are common in diabetes and obesity. Although esterified fatty acids pass through the blood brain barrier (BBB) at limited capacity [16], positron emission tomography has confirmed fatty acid uptake by the brain [17]. The brain’s uptake and subsequent accumulation of fatty acids have been observed in subjects with a metabolic syndrome that is reversible by weight reduction [17]. A high-fat diabetogenic diet promotes AD pathogenesis, in contrast to a diet with docosahexaenoic acid (DHA), which has protective effects against AD [18]. The mechanism involved appears to be through the activation of the immune system because the saturated fatty acids act through the toll-like receptor 4 (TLR4) protein that detects lipopolysaccharides. TLR4 activation leads to the generation of cytokines in astrocytes [19]. Saturated fatty acids induce an inflammatory response through TLR4 in the hypothalamus [20]. This study also showed that the loss of function of TLR4 and its pharmacological inhibition protects diet-induced obesity.
The protein tau, in elevated levels, has been found in diabetic and AD mice, independent of the peripheral metabolic status, indicating a molecular link between these diseases [21]. Free fatty acids have been shown to stimulate the assembly of both amyloid and tau filaments in vitro [22]. These studies strongly suggest that saturated fatty acids could directly influence glial activation in the brain by crossing the BBB. This could be one of the mechanisms by which obesity causes cognitive dysfunction.
4. Cognitive decline in diabetes
Diabetes management has generally focused on the traditional complications, including diabetic retinopathy, peripheral neuropathy, nephropathy, and cardiovascular diseases. However, in recent years, there have been several reports suggesting that chronic diabetes does affect brain functions. The following are some important studies that have reported the association between cognitive dysfunction and diabetes. Higher levels of glycosylated hemoglobin are associated with lower scores of cognitive function, measured by multiple tests [23]. The Hisayama study observed that glucose intolerance is associated with all-cause dementia [24]. The Edinburgh type 2 diabetes study reported that participants with diabetic retinopathy also performed poorly in cognitive function tests [25]. Adult changes in the Thought study [26] has shown that higher average blood glucose levels in the preceding 5 years correlates with an increased risk of dementia among participants with and without diabetes. Greater cognitive decline over a 12-year period is observed in diabetic patients when compared to non-diabetic subjects, as shown by decreases in the speed of information processing and in word recall, according to the Maastricht Aging Study [27]. An overview of prospective observational studies shows that diabetes increases the odds of cognitive decline by 1.2 to 1.7 fold [28]. The risk of developing MCI and dementia has been correlated with glycosylated hemoglobin levels in postmenopausal osteoporotic women without diabetes [29]. Even acute hyperglycemia has been shown to affect cognitive function and mood state in a group of type 2 diabetic patients [30]. Changes in glycemic control and even the trajectories of glycemic control correlate with cognitive performance [31, 32]. However, aggressive glucose management in the elderly is not practical. The American Geriatrics Society does not recommend the lowering of A1c below 8% because of the complications associated with hypoglycemic episodes [33]. Therefore, glycemic control as an interventional strategy to prevent cognitive decline needs to be initiated at an earlier stage.
Multiple factors have been suggested to play a role in cognitive decline of diabetic patients. For example, insulin resistance has been shown to be associated with AD-like decreases in cerebral glucose metabolism even in prediabetic individuals with normal cognitive function, [34], and MRI studies have revealed chronic hyperglycemia-mediated hippocampal dysfunction [17]. In addition to hyperglycemia, inflammatory mediators, rheological factors, and defects in hypothalamic-pituitary-adrenal axis are likely to play important roles in cognitive dysfunction [35]. Wang et al. observed elevated histone deacetylases class IIa in the brains of diabetic subjects [36], leading to the hypothesis of an epigenetic mechanism of cognitive decline in aged obese patients diabetes.
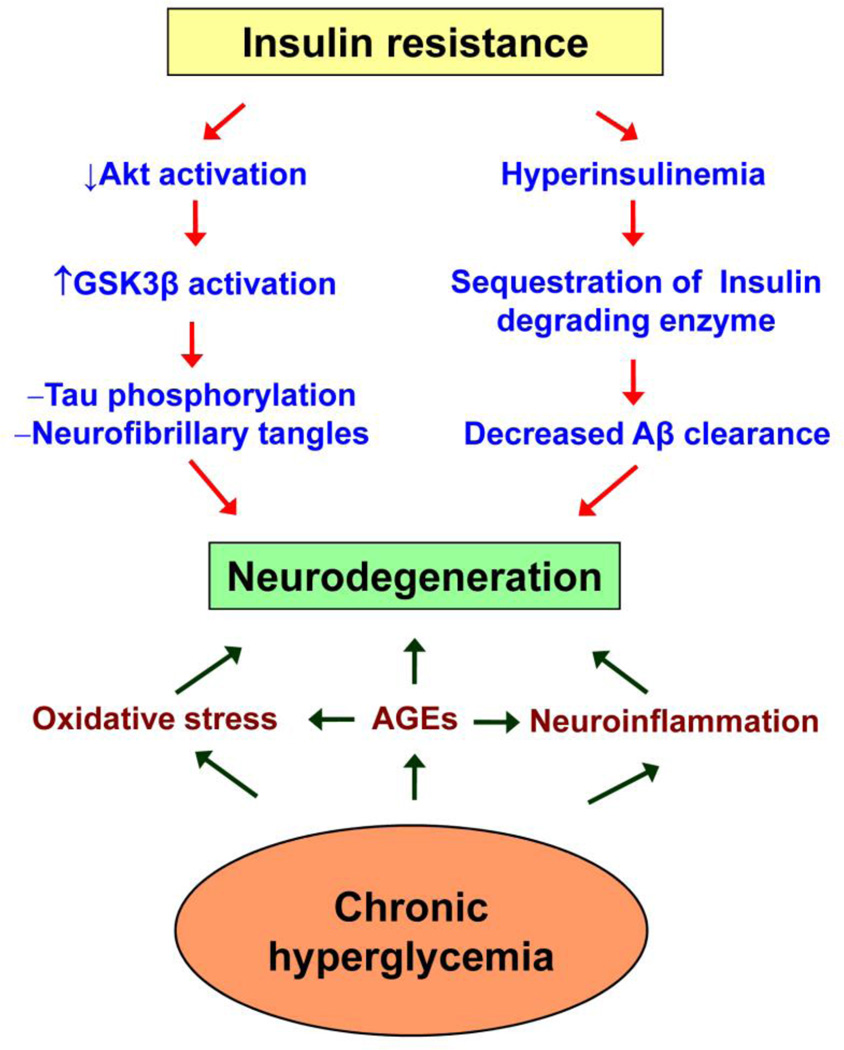
The following are some of the pathways in the diabetic brain that resemble early events in AD (Figure 2). (i) Insulin resistance leads to decreased activation of Akt, a protein that plays a key role in multiple cellular processes, such as glucose metabolism and the inhibition of GSK3β, one of the kinases that phosphorylate tau. Therefore, during insulin resistance, increased GSK3β activation may likely lead to hyperphosphorylation of tau, an important component of neurofibrillary tangles found in the brains of persons with AD. (ii) Insulin-degrading enzyme (IDE) degrades insulin as well as Aβ peptide. Therefore, hyperinsulinemia sequesters IDE away from Aβ, facilitating its accumulation [37, 38]. (iii) Protein misfolding, oxidative stress, and inflammation are some of the common pathways observed in brain tissues from patients with AD and with diabetes [39, 40]. (iv) Advanced glycation end products (AGE), an important cause of diabetic complications, has been found accumulated in the brain.
Figure 2. Pathways of neurodegeneration in the diabetic brain.
Neurodegeneration in Alzheimer’s disease is caused by the formation of neurofibrillary tangles and the deposition of extracellular β amyloid plaques. Both pathways are facilitated by insulin resistance, the major cause of type 2 diabetes. Chronic hyperglycemia-generated advanced glycation end products (AGEs), oxidative stress and neuroinflammation are also important causes of neurodegeneration, thus providing critical links between diabetes and AD.
5. Neurogenesis in the diabetic brain
A decrease in the hippocampal size in older diabetic patients has been reported following MRI studies (Neurology 2014). Age-dependent cortical and hippocampal atrophies are also observed in diabetes (db/db) mice [41]. These changes could have resulted from decreased neurogenesis and elevated neuronal death. The dentate gyrus of the hippocampus and the subventricular zone are two important areas in which new neuronal cells are generated throughout adult life [42]. Neurogenesis consists of the proliferation of neuroprogenitor cells (NPCs) and their differentiation into neurons, astrocytes, and oligodendrocytes. Impaired neurogenesis has been reported in animal models of diabetes and obesity. For example, decreased survival of NPCs has also been observed in the Goto-Kakizaki rat, a genetic model for type 2 diabetes [43]. Bromodeoxyuridine- BrdU labeling of newly generated NPCs decreases in the dentate gyrus of spontaneous, nonobese diabetic mice, a model for type 1 diabetes [44]. Impaired NPC proliferation is associated with decreased brain derived neurotrophic factor (BDNF) expression and elevated glucocorticoid levels in spontaneous as well as Streptozotocin-induced diabetic mice [45]. Similar to diabetic rats, high-fat diet-induced impairment of NPCs has been attributed to the activation of the NF-kB pathway in the mouse hypothalamus [46]. In a recent study, we have reported that when NPCs are differentiated in the presence of cytokines including IL-1β, TNF-α, and IL-6, the levels of neuronal markers decrease significantly and the NPCs differentiate into a glial phenotype [47]. Findings from this study suggest that elevated circulating cytokines in diabetes can potentially interfere with neurogenesis in the brain. These studies have advanced our understanding of diabetes and its links to neurogenesis.
6. Advanced glycation end products (AGEs) and RAGE signaling
Glucolipotoxicity resulting from chronic hyperglycemia and dyslipidemia plays a central role in the onset of diabetic complications. AGEs generated by hyperglycemia have been identified as a crucial link between diabetes and AD [48–54]. Elevated levels of AGE in the circulation and in the brain have been associated with cognitive dysfunction in patients with AD [55]. Accumulation of the AGEs, pentosidine, and glyceraldehydes-derived pyridinium (GLAP) has been observed in the brain of diabetic rats [51]. Pentosidine and GLAP also induce the expression of BACE1, a key enzyme in the generation of Aβ by the activation of NF-kB. AGEs contribute to AD by supporting fibrillary tangles and amyloid plaques formations, which are hallmarks of AD, in addition to increasing the cytotoxicity of Aβ. Microglia synthesize and secrete AGE-albumin, which is increased by Aβ [56]. AGEs induce the expression of its receptor, RAGE, which is also a putative receptor for Aβ [49, 57]. The levels of RAGE have been shown to be increased in several cell types in the AD brain. For example, RAGE-immunoreactive microglia are elevated in the human postmortem AD brain [58]. Aβ also increases the expression of RAGE in cultured microglia. RAGE expression is elevated in neurons and astrocytes of 3XTg AD mouse brain [59]. Colocalization of RAGE with intracellular Aβ and Tau is also observed. The presence of AGE and RAGE has been reported in the astrocytes of AD post-mortem brain [60]. Another study reported increased levels of microvascular RAGE in human AD brain, which correlated with the severity of AD pathology [61]. Streptozotocin-induced diabetes in AD transgenic mice further enhances the levels of RAGE in the brain, and the senile plaque formation is accelerated [62]. Microglia-specific overexpression of RAGE in transgenic AD mice enhances the production of proinflammatory cytokines along with accelerated cognitive decline [63]. Neuron-specific overexpression of a dominant negative RAGE, on the other hand, results in the preservation of cognitive function along with decreased neuropathological changes in the AD mice [64]. An inhibitor of RAGE is able to decrease microglial activation and Aβ production in the AD transgenic mice [65]. Although a clinical trial of a RAGE inhibitor was not successful due to its adverse side effects [66], the therapeutic potential of RAGE inhibitors in AD cannot be ignored [67]. Soluble form of RAGE (sRAGE) acts as a decoy for RAGE and provides a counter-regulatory mechanism. Selvin et al. have reported that the low levels of sRAGE are associated with diabetes risk and mortality [68]. Decreased levels of sRAGE have also been observed in AD patients [69].
In addition to hyperglycemia-generated AGE formation, diet also can be a source of AGEs. Recent studies have suggested that increased consumption of AGEs derived from high-fat and dry-heat processed foods can elevate the accumulation of AGEs in the brains of diabetes patients and obese individuals [70–72] and can contribute to circulating AGEs [72]. Feeding mice a diet rich in methyl glyoxal, an AGE, results in the development of a metabolic syndrome and deposits of AGEs, a decrease in SIRT1, and cognitive decline [54]. Thus, the accumulation of AGEs in the brain is a common neurodegenerative mechanism linking diabetes and AD.
7. Mitochondrial dysfunction and inflammasome formation
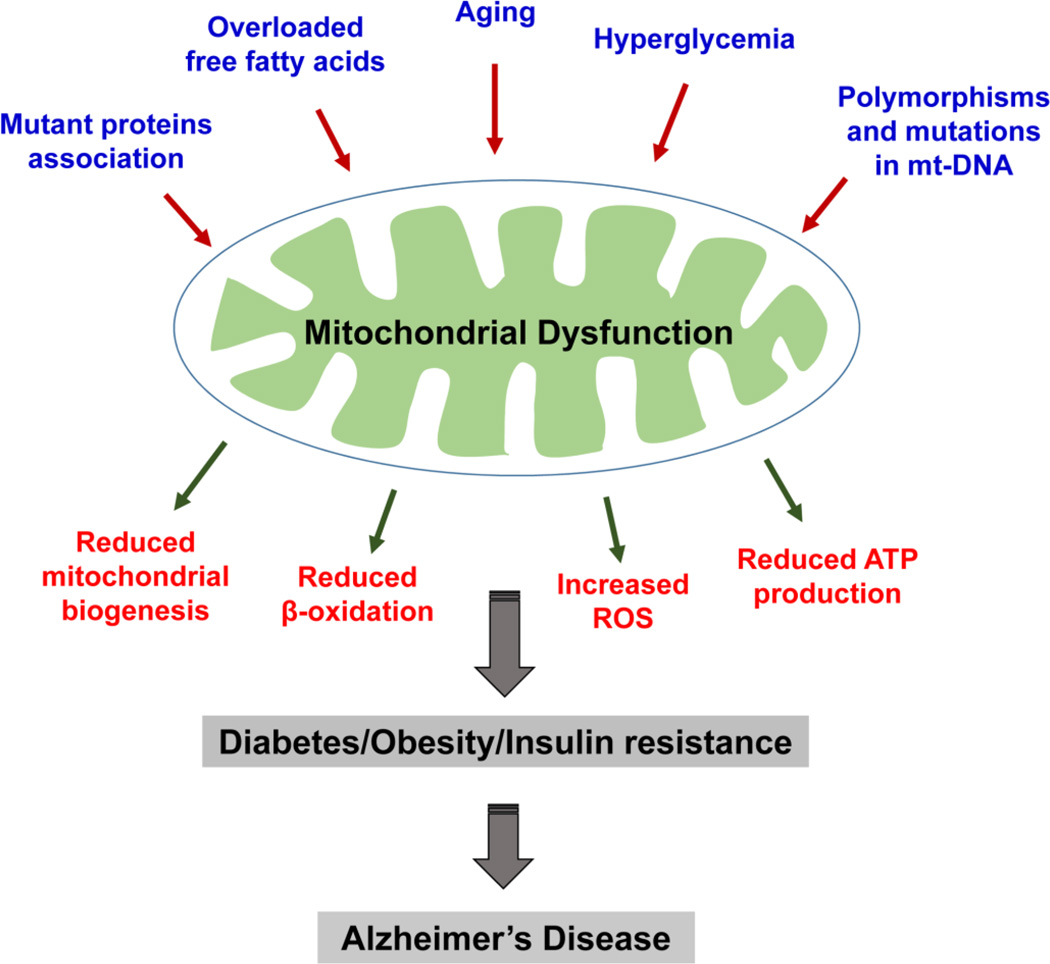
Mitochondrial dysfunction is another critical link between obesity, diabetes, and AD. Oxidative stress and mitochondrial dysfunction have been extensively reported in patients with AD, diabetes, and obesity as well as in rodent models of all these conditions (Figure 3). For example, impairment of the respiratory chain has been observed in mitochondria isolated from sucrose-fed AD transgenic mice [73].
Figure 3. Causes and consequences of mitochondrial dysfunction.
Multiple factors are known to cause mitochondrial dysfunction. These factors include aging, increased free fatty acids, hyperglycemia, polymorphisms and DNA mutations in mitochondrial genome and mutant protein(s) association with mitochondria. Dysfunctional mitochondria produce reduced ATP, decreased biogenesis, impaired β-oxidation and increased reactive oxygen species. These events may contribute to diabetes, obesity and insulin resistance ultimately causing Alzheimer’s disease in elderly individuals.
Mitochondrial injury is also an important trigger for inflammasome formation [74–76]. Inflammasome, a multiprotein cytosolic complex, is generated in response to infection, cellular damage, and metabolic dysregulation [77]. Its formation leads to the activation of caspase-1 and to the proteolytic cleavage and secretion of the cytokines IL-1β and IL-18 [78] (Figure 4). While the inflammatory pathway protects the brain from infection in patients, the formation of sterile inflammasomes in response to cellular stress can cause neuronal injury [79]. Oxidized mitochondrial DNA released into the cytosol induces the formation of the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome [80]. It is during NLRP3 inflammation that autophagy has been found to be essential for restoring mitochondrial dynamics [81]. Inflammasome formation is also caused by mitochondrial destabilization, which in turn is triggered by calcium signaling [59]. Glucolipotoxicity in T2D is known to cause mitochondrial injury [82]. The study of inflammasome formation in obese patients and in T2D patients has focused mainly on macrophages [14, 15, 83]. Microglia, the brain-resident macrophages, are also known to induce inflammasome formation in other disease states [84]. The possibility that the activation of microglia in the diabetic brain may contribute to inflammatory injury has not been fully investigated, although the microglia activation pathway in the hypothalamus has been documented in diabetes [85]. Stress signals that induce inflammasome formation include chemokines and damage-associated molecular patterns, such as the high mobility group proteins HMGB1 and ATP [86–88]. For the therapeutic targeting of inflammasome formation induced by mitochondrial injury, sirtuins appear to show promise [89, 90].
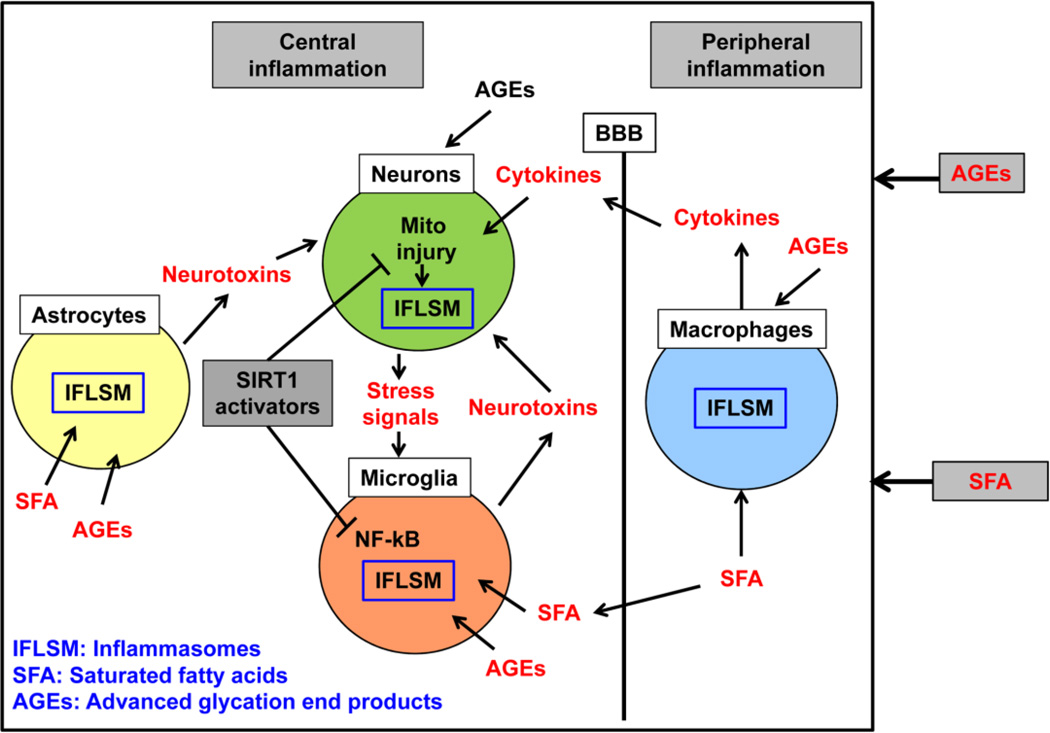
Figure 4. Neuroinflammation in the Diabetic brain.
Brain inflammation in diabetes is considered to result from peripheral inflammation. Cytokines produced by macrophages can pass through the blood brain barrier (BBB). However the less understood area is the potential direct effects of saturated fatty acids (SFA) and advanced glycation end products (AGEs) on neurons astrocytes and microglia, the resident macrophages of the brain. Increased uptake of fatty acids by the brain in metabolic syndrome has been reported. Neurotoxins generated by astroglia cause injuries to neurons. Stress signals released by dying neurons can further activate the glial cells, thus leading to a vicious cycle. Activators of SIRT1 have two beneficial therapeutic actions, namely, increasing neuronal mitochondrial biogenesis and anti-inflammatory action in glial cells.
The silent information regulator (SIRT) genes (sirtuins) comprise a highly conserved family of proteins that use NAD+ as a co-substrate to catalyze the deacetylation and/or the mono-ADP ribosylation of target proteins. SIRT1 deacetylates histone, which includes non-histone transcriptional and co-transcriptional factors that regulate glucose homeostasis, fat oxidation, and inflammation of the brain [91]. Nicotinamide riboside, a precursor of NAD+ and an activator of SIRT1, has two beneficial actions. It increases neuronal mitochondrial biogenesis and anti-inflammatory action in glial cells [91–93]. Further research is needed to understand the beneficial effects of nicotinamide riboside in the brain.
8. Peripheral and central inflammation connection
Neuroinflammation has emerged as a key player in the pathogenesis of AD [94]. Markers of neuroinflammation have been observed in the brain of AD models [95, 96]. Microglia, the resident macrophages of the brain, are chronically activated in the vicinity of amyloid plaques. Although they reduce Aβ burden by phagocytosis, uncontrolled chronic inflammation leads to the release of neurotoxic factors including inflammatory mediators and reactive oxygen species by glial cells, resulting in exacerbation of the AD pathology. Although the central nervous system was previously considered immune-privileged, this view has been challenged because of the bidirectional cross-talk between peripheral and central inflammation [97]. Chronic low-grade inflammation that causes aging-associated morbidity and mortality has been referred to as ‘inflammaging’ [98]. Systemic inflammation is also an important cause of insulin resistance and the pathogenesis of type 2 diabetes [99]. Microglial response to peripheral inflammation is generally adaptive in nature. Following systemic challenge with IL-1β and TNFα in mice, induction of cytokines and chemokines in hippocampus is observed [100]. Another study reported that stimulation of the immune system in mice leads to AD-like brain pathology including deposition of APP and its proteolytic fragments and altered Tau phosphorylation [101]. A meta-analysis of forty studies in which peripheral blood cytokine concentrations reveal that AD is accompanied by higher peripheral concentrations of IL-6, TNF-α, IL-1β, TGF-β, IL-12, and IL-8 [102]. Activation of circulating peripheral immune cells is observed in patients with early stages of AD [103]. Several studies have also reported the correlation between peripheral inflammation and cognitive dysfunction. For example, peripheral inflammatory markers have been observed in patients with MCI and AD [104]. Meta-analyses of previous studies reveal that peripheral inflammatory markers correlate with the risk of dementia [105, 106]. Framingham study reported that higher peripheral levels of IL-1β and TNF-α may be markers for the risk of AD [107]. PBMCs isolated from MCI patients produce more IL-6 and IL-8 following the stimulation by phytohemagglutinin (PHA) compared to healthy elderly controls [108]. Post-operative cognitive dysfunction correlates with peripheral inflammatory markers [109]. Proinflammatory cytokines have been shown to pass through BBB [110–112]. The aging process acts as a priming stimulus for microglia and they respond to peripheral inflammation with greater severity and duration [113]. Peripheral inflammation has been shown to trigger brain-specific inflammatory responses by gene expression profiling [114]. Central inflammation is also likely to be exacerbated with damage to the BBB, followed by the entry of immune cells into the brain. It is not clear if peripheral inflammation leads to central inflammation or vice versa. Further research is needed to understand the molecular links among diabetes, obesity and AD in relation to inflammation.
9. Damage to the blood brain barrier (BBB)
The BBB consists of human brain microvascular endothelial cells (HBMECs), end feet of astrocytes, and pericytes. HBMECs are glued together by tight-junction proteins (e.g., occulin and claudins) and scaffolding proteins (e.g., ZO-1 and Zo2) [115]. Using dynamic contrast-enhanced MRIs of the brains of humans, an age-dependent breakdown of the BBB in the hippocampus and injury to BBB-associated pericytes that correlated with mild cognitive impairment have been reported [116]. Decreased BBB integrity was observed in rats fed with a diet rich in saturated fat and cholesterol [117]. Increased BBB permeability is observed along with hippocampal-dependent cognitive dysfunction in rats fed with a high energy diet [118]. BBB damage and microglial activation in mice are caused by diet-induced obesity [119]. Aging further exacerbates the effects on oxidative stress, inflammation, and genes involved in the generation of Aβ. Treatment of cultured microglia with sera derived from aged high fat diet-induced obese mice leads to increased microglial activation and oxidative stress [119]. Another study demonstrated the reversal of high fat-induced BBB damage by anti-inflammatory and lipid-lowering agents [120]. The induction of oxidative stress and the decrease in sirtuin expression are observed in microvascular endothelial cells exposed to high glucose [121]. Antioxidants including vitamin C, free radical scavengers and mimetics of antioxidant enzymes have been shown to attenuate the deleterious effects of high glucose [122]. Exposure of HBMECs to AGEs results in increased MMP9 activation, leading to the degradation of TRKB, a BDNF receptor [123]. Decreased viability of endothelial cells is observed following the silencing of SIRT3, a mitochondrial sirtuin that increases the activities of metabolic enzymes by deacetylation [124]. Ouyang et al (2014) performed proteomic analysis in enriched microvessels from cerebral cortex of mice fed high-fat diet and observed the downregulation key proteins including chaperons, enzymes and transport-related proteins [125]. Thus, when BBB integrity is compromised, chronic low-grade inflammation and oxidative stress, observed in obesity and diabetes can lead to central inflammation.
10. Obesity and diabetes as risk factors for Alzheimer’s disease
AD is the most common cause of dementia, accounting for 60–80% of dementia cases. There are more than 35 million patients with AD worldwide. Recent studies suggest that the incidence of AD is likely to increase substantially as obesity and diabetes are risk factors for AD. A strong correlation between diabetes and AD was first reported in a large population-based Rotterdam study [126]. Later epidemiological studies also strongly suggested that T2D is a risk factor for AD (reviewed in [39, 40]). Diabetes has been found to decrease the threshold of the Aβ burden needed for the manifestation of dementia. An 11-year follow up study of a Taiwanese population found that diabetic patients are more susceptible to AD compared to non-diabetic patients [127]. MRI scans of 700 MCI and AD patients revealed that a higher body mass index is associated with reduced brain volume in the frontal, temporal, parietal, and occipital lobes of patients with AD [128]. A meta-analysis study reported that obesity and diabetes significantly and independently increased the risk for AD in elderly individuals [129]. However, the risk was less than that of persons with the APOE4 allele. Elderly diabetic women (over 65 years of age) were found to have a higher rate of developing AD than elderly diabetic men [130]. The Baltimore Longitudinal Study of Aging reported a higher midlife BMI associated with early onset AD and a greater burden of AD pathology [131]. The link between AD and diabetes has been further supported by studies using mouse models [132–134]. For example, diet-induced insulin resistance in Tg2576 mice leads to an increase in Aβ production [132]. An increase in Aβ, in Tg2576 AD mice fed a high-fat diet is associated with abnormal feeding behaviors and increased food intake [135]. Pathological features of AD, including tau phosphorylation and amyloid plaque deposition are exaggerated in the brains of APP transgenic mice following the induction of insulin-deficient diabetes [133]. The molecular link between AD and diabetes has been suggested to be the amylin receptor as it is a target for both amylin and Aβ in the brain [136]. Another important study reported that the crossing of APP transgenic mice with diabetic mice (ob/ob and NSY mice) leads to exacerbated cognitive dysfunction [134]. Interestingly these mice had an accelerated diabetic phenotype compared to the non-crossed diabetic mice.
11. Conclusions and Future Directions
Although the glucose-centered management of diabetes addresses the traditional complications of diabetes, long-term effects of diabetes-associated factors that are not downstream of hyperglycemia, on brain function cannot be ignored and need therapeutic attention. Pathways of oxidative stress and inflammation are commonly observed in obesity, diabetes, and AD. With the availability of a wide range of drugs for managing diabetes and its complications, the life expectancy of persons with diabetes is steadily increasing [137]. The association of obesity and diabetes with AD is likely to be more evident in the future than with the current older population because of survival bias. Health care systems worldwide will be facing growing populations of persons with AD and diabetes [138]. Aging diabetic patients are likely to present symptoms ranging from subtle executive dysfunction to overt dementia and memory loss. Cognitive dysfunction in the elderly diabetic patients makes it difficult for them to perform complex self-care tasks including glucose monitoring, adjusting the time and dose of insulin. Weight loss and aggressive glucose control are not ideal interventional strategies for the elderly diabetic patients [33]. They need to be initiated in the middle age rather than in the old age. Knowledge gained from studies examining the chronic neurodegenerative pathways associated with obesity and diabetes can lead to development of therapeutic agents that delay cognitive decline and improve the quality of life. Although glycemic control is the primary goal in the management of diabetes, current antidiabetic medications with known neuroprotective actions need to be given serious considerations.
Highlights.
Obesity and diabetes are among several modifiable risk factors of dementia
AGEs and their receptor RAGE provide critical links between diabetes and AD
Cross-talks between peripheral and central inflammation are observed in diabetes
Damage to the blood brain barrier causes infiltration of immune cells into the brain
Neuroinflammation provides a central mechanism for aging-associated ailments
Acknowledgments
Work presented in this article is supported by Merit Review grant (NEUD-004-07F) from the Veterans Administration (to S.P) and NIH grants (AG042178 and AG047812) and the Garrison Family Foundation (to P.H.R).
Abbreviations
- AD
Alzheimer’s disease
- BBB
Blood brain barrier
- RAGE
Receptor for Advanced Glycation End products
- AGE
Advanced glycation end products
- DHA
docosahexaenoic acid
- TLR4
Toll like receptor 4
- IL1β
interleukin 1 beta
- Aβ
Amyloid beta
- NLRP3
NACHT, LRR and PYD domains-containing protein 3
- SIRT1
Silence information regulator 1
- NAD+
Nicotinamide adenine dinucleotide
- PHA
Phytohemagglutinin
- PBMC
Peripheral blood mononuclear cells
- TNF∝
Tumor necrosis factor alpha
- MCI
Mild cognitive impairment
- TGFβ
Transforming growth factor beta
- HBMEC
Human brain microvascular endothelial cells
- TRKB
Tropomyosin receptor kinase B
- MMP9
Matrix metallopeptidase 9
- BDNF
Brain derived neurotrophic growth factor
- ApoE4
Apolipoprotein E4 genotype
- APP
Amyloid precursor protein
- BMI
Body mass index
- T2D
Type 2 diabetes mellitus
- MRI
magnetic resonance imaging
- NSY mice
Nagoya-Shibata-Yasuda mice
- HMGB1
High morbidity group protein 1
- NPCs
Neuroprogenitor cells
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 4.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62:1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 5.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, Johansson B. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes (Lond) 2009;33:893–898. doi: 10.1038/ijo.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hassing LB, Dahl AK, Pedersen NL, Johansson B. Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dementia and geriatric cognitive disorders. 2010;29:543–552. doi: 10.1159/000314874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 9.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O'Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L. Midlife overweight and obesity increase late-life dementia risk: a population-based twin study. Neurology. 2011;76:1568–1574. doi: 10.1212/WNL.0b013e3182190d09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wotton CJ, Goldacre MJ. Age at obesity and association with subsequent dementia: record linkage study. Postgraduate medical journal. 2014;90:547–551. doi: 10.1136/postgradmedj-2014-132571. [DOI] [PubMed] [Google Scholar]
- 12.Nepal B, Brown LJ, Anstey KJ. Rising midlife obesity will worsen future prevalence of dementia. PLoS One. 2014;9:e99305. doi: 10.1371/journal.pone.0099305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJ, Pocock SJ. BMI and risk of dementia in two million people over two decades: a retrospective cohort study. The lancet. Diabetes & endocrinology. 2015;3:431–436. doi: 10.1016/S2213-8587(15)00033-9. [DOI] [PubMed] [Google Scholar]
- 14.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62:194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. Journal of lipid research. 1997;38:526–530. [PubMed] [Google Scholar]
- 17.Karmi A, Iozzo P, Viljanen A, Hirvonen J, Fielding BA, Virtanen K, Oikonen V, Kemppainen J, Viljanen T, Guiducci L, Haaparanta-Solin M, Nagren K, Solin O, Nuutila P. Increased brain fatty acid uptake in metabolic syndrome. Diabetes. 2010;59:2171–2177. doi: 10.2337/db09-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutrition reviews. 2010;68(Suppl 2):S102–S111. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta S, Knight AG, Keller JN, Bruce-Keller AJ. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J Neurochem. 2012;120:1060–1071. doi: 10.1111/j.1471-4159.2012.07660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takalo M, Haapasalo A, Martiskainen H, Kurkinen KM, Koivisto H, Miettinen P, Khandelwal VK, Kemppainen S, Kaminska D, Makinen P, Leinonen V, Pihlajamaki J, Soininen H, Laakso M, Tanila H, Hiltunen M. High-fat diet increases tau expression in the brain of T2DM and AD mice independently of peripheral metabolic status. The Journal of nutritional biochemistry. 2014;25:634–641. doi: 10.1016/j.jnutbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DM, Binder LI. Free fatty acids stimulate the polymerization of tau and amyloid beta peptides. In vitro evidence for a common effector of pathogenesis in Alzheimer's disease. Am J Pathol. 1997;150:2181–2195. [PMC free article] [PubMed] [Google Scholar]
- 23.Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009;32:221–226. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohara T, Doi Y, Ninomiya T, Hirakawa Y, Hata J, Iwaki T, Kanba S, Kiyohara Y. Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology. 2011;77:1126–1134. doi: 10.1212/WNL.0b013e31822f0435. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, Strachan MW, Reynolds RM, Frier BM, Deary IJ, Fowkes FG, Lee AJ, McKnight J, Halpin P, Swa K, Price JF. Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes. 2010;59:2883–2889. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369:540–548. doi: 10.1056/NEJMoa1215740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spauwen PJ, Kohler S, Verhey FR, Stehouwer CD, van Boxtel MP. Effects of type 2 diabetes on 12-year cognitive change: results from the Maastricht Aging Study. Diabetes Care. 2013;36:1554–1561. doi: 10.2337/dc12-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. The journal of nutrition, health & aging. 2006;10:293–295. [PubMed] [Google Scholar]
- 30.Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care. 2004;27:2335–2340. doi: 10.2337/diacare.27.10.2335. [DOI] [PubMed] [Google Scholar]
- 31.Ravona-Springer R, Moshier E, Schmeidler J, Godbold J, Akrivos J, Rapp M, Grossman HT, Wysocki M, Silverman JM, Haroutunian V, Beeri MS. Changes in glycemic control are associated with changes in cognition in non-diabetic elderly. J Alzheimers Dis. 2012;30:299–309. doi: 10.3233/JAD-2012-120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravona-Springer R, Heymann A, Schmeidler J, Moshier E, Godbold J, Sano M, Leroith D, Johnson S, Preiss R, Koifman K, Hoffman H, Silverman JM, Beeri MS. Trajectories in glycemic control over time are associated with cognitive performance in elderly subjects with type 2 diabetes. PLoS One. 2014;9:e97384. doi: 10.1371/journal.pone.0097384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, Huang ES, Korytkowski MT, Munshi MN, Odegard PS, Pratley RE, Swift CS. Diabetes in older adults: a consensus report. Journal of the American Geriatrics Society. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Gong B, Zhao W, Tang C, Varghese M, Nguyen T, Bi W, Bilski A, Begum S, Vempati P, Knable L, Ho L, Pasinetti GM. Epigenetic mechanisms linking diabetes and synaptic impairments. Diabetes. 2014;63:645–654. doi: 10.2337/db13-1063. [DOI] [PubMed] [Google Scholar]
- 37.Qiu WQ, Folstein MF. Insulin, insulin-degrading enzyme and amyloid-beta peptide in Alzheimer's disease: review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 38.Haque R, Nazir A. Insulin-degrading enzyme: a link between Alzheimer's and type 2 diabetes mellitus. CNS Neurol Disord Drug Targets. 2014;13:259–264. doi: 10.2174/18715273113126660139. [DOI] [PubMed] [Google Scholar]
- 39.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer's disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroner Z. The relationship between Alzheimer's disease and diabetes: Type 3 diabetes? Altern Med Rev. 2009;14:373–379. [PubMed] [Google Scholar]
- 41.Ramos-Rodriguez JJ, Molina-Gil S, Ortiz-Barajas O, Jimenez-Palomares M, Perdomo G, Cozar-Castellano I, Lechuga-Sancho AM, Garcia-Alloza M. Central proliferation and neurogenesis is impaired in type 2 diabetes and prediabetes animal models. PLoS One. 2014;9:e89229. doi: 10.1371/journal.pone.0089229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 43.Lang BT, Yan Y, Dempsey RJ, Vemuganti R. Impaired neurogenesis in adult type-2 diabetic rats. Brain Res. 2009;1258:25–33. doi: 10.1016/j.brainres.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beauquis J, Saravia F, Coulaud J, Roig P, Dardenne M, Homo-Delarche F, De Nicola A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol. 2008;210:359–367. doi: 10.1016/j.expneurol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 45.Guo J, Yu C, Li H, Liu F, Feng R, Wang H, Meng Y, Li Z, Ju G, Wang J. Impaired neural stem/progenitor cell proliferation in streptozotocin-induced and spontaneous diabetic mice. Neurosci Res. 2010;68:329–336. doi: 10.1016/j.neures.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nature cell biology. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin L, Bouchard R, Pugazhenthi S. Regulation of cAMP response element binding protein during neuroglial interactions. J Neurochem. 2015 doi: 10.1111/jnc.13497. [DOI] [PubMed] [Google Scholar]
- 48.Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- 49.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 50.Toth C, Martinez J, Zochodne DW. RAGE, diabetes, and the nervous system. Curr Mol Med. 2007;7:766–776. doi: 10.2174/156652407783220705. [DOI] [PubMed] [Google Scholar]
- 51.Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, Catalano MG, Smith MA, Perry G, Danni O, Boccuzzi G, Tabaton M. AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging. 2012;33:196, e113–e127. doi: 10.1016/j.neurobiolaging.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 52.Kojro E, Postina R. Regulated proteolysis of RAGE and AbetaPP as possible link between type 2 diabetes mellitus and Alzheimer's disease. J Alzheimers Dis. 2009;16:865–878. doi: 10.3233/JAD-2009-0998. [DOI] [PubMed] [Google Scholar]
- 53.Liu LP, Hong H, Liao JM, Wang TS, Wu J, Chen SS, Li YQ, Long Y, Xia YZ. Upregulation of RAGE at the blood-brain barrier in streptozotocin-induced diabetic mice. Synapse. 2009;63:636–642. doi: 10.1002/syn.20644. [DOI] [PubMed] [Google Scholar]
- 54.Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M, Woodward M, Striker GE, Vlassara H. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. 2014;111:4940–4945. doi: 10.1073/pnas.1316013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beeri MS, Moshier E, Schmeidler J, Godbold J, Uribarri J, Reddy S, Sano M, Grossman HT, Cai W, Vlassara H, Silverman JM. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mechanisms of ageing and development. 2011;132:583–587. doi: 10.1016/j.mad.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, Kim SU, Yamamoto T, Won MH, Song BJ, Park YM, Lee B. Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer's disease. PLoS One. 2012;7:e37917. doi: 10.1371/journal.pone.0037917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 58.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 59.Choi BR, Cho WH, Kim J, Lee HJ, Chung C, Jeon WK, Han JS. Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease. Exp Mol Med. 2014;46:e75. doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, Takeuchi M, Makita Z. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- 61.Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, Silverberg GD, Stopa EG. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Yu S, Hu JP, Wang CY, Wang Y, Liu HX, Liu YL. Streptozotocin-induced diabetes increases amyloid plaque deposition in AD transgenic mice through modulating AGEs/RAGE/NF-kappaB pathway. The International journal of neuroscience. 2014;124:601–608. doi: 10.3109/00207454.2013.866110. [DOI] [PubMed] [Google Scholar]
- 63.Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. FASEB J. 2010;24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Du Yan SS. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. The EMBO journal. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deane R, Singh I, Sagare AP, Bell RD, Ross NT, LaRue B, Love R, Perry S, Paquette N, Deane RJ, Thiyagarajan M, Zarcone T, Fritz G, Friedman AE, Miller BL, Zlokovic BV. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galasko D, Bell J, Mancuso JY, Kupiec JW, Sabbagh MN, van Dyck C, Thomas RG, Aisen PS. Clinical trial of an inhibitor of RAGE-Abeta interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walker D, Lue LF, Paul G, Patel A, Sabbagh MN. Receptor for advanced glycation endproduct modulators: a new therapeutic target in Alzheimer's disease. Expert Opin Investig Drugs. 2015;24:393–399. doi: 10.1517/13543784.2015.1001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selvin E, Halushka MK, Rawlings AM, Hoogeveen RC, Ballantyne CM, Coresh J, Astor BC. sRAGE and risk of diabetes, cardiovascular disease, and death. Diabetes. 2013;62:2116–2121. doi: 10.2337/db12-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang F, Jia J, Wang S, Qin W, Liu G. Decreased plasma levels of soluble low density lipoprotein receptor-related protein-1 (sLRP) and the soluble form of the receptor for advanced glycation end products (sRAGE) in the clinical diagnosis of Alzheimer's disease. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2013;20:357–361. doi: 10.1016/j.jocn.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 70.Cai W, Ramdas M, Zhu L, Chen X, Striker GE, Vlassara H. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. Proc Natl Acad Sci U S A. 2012;109:15888–15893. doi: 10.1073/pnas.1205847109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;60:10–37. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 72.West RK, Moshier E, Lubitz I, Schmeidler J, Godbold J, Cai W, Uribarri J, Vlassara H, Silverman JM, Beeri MS. Dietary advanced glycation end products are associated with decline in memory in young elderly. Mechanisms of ageing and development. 2014;140:10–12. doi: 10.1016/j.mad.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carvalho C, Cardoso S, Correia SC, Santos RX, Santos MS, Baldeiras I, Oliveira CR, Moreira PI. Metabolic alterations induced by sucrose intake and Alzheimer's disease promote similar brain mitochondrial abnormalities. Diabetes. 2012;61:1234–1242. doi: 10.2337/db11-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurung P, Lukens JR, Kanneganti TD. Mitochondria: diversity in the regulation of the NLRP3 inflammasome. Trends in molecular medicine. 2015;21:193–201. doi: 10.1016/j.molmed.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell research. 2011;21:558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, Bestwick M, Duguay BA, Raimundo N, MacDuff DA, Kaech SM, Smiley JR, Means RE, Iwasaki A, Shadel GS. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015 doi: 10.1038/nature14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Laudisi F, Spreafico R, Evrard M, Hughes TR, Mandriani B, Kandasamy M, Morgan BP, Sivasankar B, Mortellaro A. Cutting edge: the NLRP3 inflammasome links complement-mediated inflammation and IL-1beta release. J Immunol. 2013;191:1006–1010. doi: 10.4049/jimmunol.1300489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, Ghetti B, Koller BH, LeBlanc AC. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015 doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Motori E, Puyal J, Toni N, Ghanem A, Angeloni C, Malaguti M, Cantelli-Forti G, Berninger B, Conzelmann KK, Gotz M, Winklhofer KF, Hrelia S, Bergami M. Inflammation-induced alteration of astrocyte mitochondrial dynamics requires autophagy for mitochondrial network maintenance. Cell Metab. 2013;18:844–859. doi: 10.1016/j.cmet.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature immunology. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burm SM, Zuiderwijk-Sick EA, t Jong AE, van der Putten C, Veth J, Kondova I, Bajramovic JJ. Inflammasome-induced IL-1beta secretion in microglia is characterized by delayed kinetics and is only partially dependent on inflammatory caspases. J Neurosci. 2015;35:678–687. doi: 10.1523/JNEUROSCI.2510-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cai D. One step from prediabetes to diabetes: hypothalamic inflammation? Endocrinology. 2012;153:1010–1013. doi: 10.1210/en.2011-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubartelli A. DAMP-Mediated Activation of NLRP3-Inflammasome in Brain Sterile Inflammation: The Fine Line between Healing and Neurodegeneration. Frontiers in immunology. 2014;5:99. doi: 10.3389/fimmu.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jabaut J, Ather JL, Taracanova A, Poynter ME, Ckless K. Mitochondria-targeted drugs enhance Nlrp3 inflammasome-dependent IL-1beta secretion in association with alterations in cellular redox and energy status. Free Radic Biol Med. 2013;60:233–245. doi: 10.1016/j.freeradbiomed.2013.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sokolowski JD, Chabanon-Hicks CN, Han CZ, Heffron DS, Mandell JW. Fractalkine is a "find-me" signal released by neurons undergoing ethanol-induced apoptosis. Frontiers in cellular neuroscience. 2014;8:360. doi: 10.3389/fncel.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Min SW, Sohn PD, Cho SH, Swanson RA, Gan L. Sirtuins in neurodegenerative diseases: an update on potential mechanisms. Frontiers in aging neuroscience. 2013;5:53. doi: 10.3389/fnagi.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng F, Wijaya L, Tang BL. SIRT1 in the brain-connections with aging-associated disorders and lifespan. Frontiers in cellular neuroscience. 2015;9:64. doi: 10.3389/fncel.2015.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008;58:10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 93.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wright AL, Zinn R, Hohensinn B, Konen LM, Beynon SB, Tan RP, Clark IA, Abdipranoto A, Vissel B. Neuroinflammation and neuronal loss precede Abeta plaque deposition in the hAPP-J20 mouse model of Alzheimer's disease. PLoS One. 2013;8:e59586. doi: 10.1371/journal.pone.0059586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanzel CE, Pichet-Binette A, Pimentel LS, Iulita MF, Allard S, Ducatenzeiler A, Do Carmo S, Cuello AC. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer's disease. Neurobiol Aging. 2014;35:2249–2262. doi: 10.1016/j.neurobiolaging.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 97.Di Filippo M, Chiasserini D, Gardoni F, Viviani B, Tozzi A, Giampa C, Costa C, Tantucci M, Zianni E, Boraso M, Siliquini S, de Iure A, Ghiglieri V, Colcelli E, Baker D, Sarchielli P, Fusco FR, Di Luca M, Calabresi P. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol Dis. 2013;52:229–236. doi: 10.1016/j.nbd.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutrition reviews. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 99.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1beta, [corrected] TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8:e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krstic D, Madhusudan A, Doehner J, Vogel P, Notter T, Imhof C, Manalastas A, Hilfiker M, Pfister S, Schwerdel C, Riether C, Meyer U, Knuesel I. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J Neuroinflammation. 2012;9:151. doi: 10.1186/1742-2094-9-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swardfager W, Lanctot K, Rothenburg L, Wong A, Cappell J, Herrmann N. A meta-analysis of cytokines in Alzheimer's disease. Biological psychiatry. 2010;68:930–941. doi: 10.1016/j.biopsych.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 103.Zhang R, Miller RG, Madison C, Jin X, Honrada R, Harris W, Katz J, Forshew DA, McGrath MS. Systemic immune system alterations in early stages of Alzheimer's disease. Journal of neuroimmunology. 2013;256:38–42. doi: 10.1016/j.jneuroim.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bermejo P, Martin-Aragon S, Benedi J, Susin C, Felici E, Gil P, Ribera JM, Villar AM. Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunology letters. 2008;117:198–202. doi: 10.1016/j.imlet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 105.van Himbergen TM, Beiser AS, Ai M, Seshadri S, Otokozawa S, Au R, Thongtang N, Wolf PA, Schaefer EJ. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and alzheimer disease: results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease: a meta-analysis. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013;68:433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tan ZS, Beiser AS, Vasan RS, Roubenoff R, Dinarello CA, Harris TB, Benjamin EJ, Au R, Kiel DP, Wolf PA, Seshadri S. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 108.Magaki S, Mueller C, Dickson C, Kirsch W. Increased production of inflammatory cytokines in mild cognitive impairment. Exp Gerontol. 2007;42:233–240. doi: 10.1016/j.exger.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peng L, Xu L, Ouyang W. Role of peripheral inflammatory markers in postoperative cognitive dysfunction (POCD): a meta-analysis. PLoS One. 2013;8:e79624. doi: 10.1371/journal.pone.0079624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Banks WA, Kastin AJ. Blood to brain transport of interleukin links the immune and central nervous systems. Life sciences. 1991;48:PL117–PL121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- 111.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 112.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. Journal of neuroimmunology. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 113.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Thomson CA, McColl A, Cavanagh J, Graham GJ. Peripheral inflammation is associated with remote global gene expression changes in the brain. J Neuroinflammation. 2014;11:73. doi: 10.1186/1742-2094-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haseloff RF, Dithmer S, Winkler L, Wolburg H, Blasig IE. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Seminars in cell & developmental biology. 2015;38:16–25. doi: 10.1016/j.semcdb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Freeman LR, Granholm AC. Vascular changes in rat hippocampus following a high saturated fat and cholesterol diet. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:643–653. doi: 10.1038/jcbfm.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davidson TL, Monnot A, Neal AU, Martin AA, Horton JJ, Zheng W. The effects of a high-energy diet on hippocampal-dependent discrimination performance and blood-brain barrier integrity differ for diet-induced obese and diet-resistant rats. Physiology & behavior. 2012;107:26–33. doi: 10.1016/j.physbeh.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer's disease. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014;69:1212–1226. doi: 10.1093/gerona/glt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pallebage-Gamarallage M, Lam V, Takechi R, Galloway S, Clark K, Mamo J. Restoration of dietary-fat induced blood-brain barrier dysfunction by anti-inflammatory lipid-modulating agents. Lipids in health and disease. 2012;11:117. doi: 10.1186/1476-511X-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mortuza R, Chen S, Feng B, Sen S, Chakrabarti S. High glucose induced alteration of SIRTs in endothelial cells causes rapid aging in a p300 and FOXO regulated pathway. PLoS One. 2013;8:e54514. doi: 10.1371/journal.pone.0054514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes Metab. 2009;11:480–490. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 123.Navaratna D, Fan X, Leung W, Lok J, Guo S, Xing C, Wang X, Lo EH. Cerebrovascular degradation of TRKB by MMP9 in the diabetic brain. J Clin Invest. 2013;123:3373–3377. doi: 10.1172/JCI65767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu G, Cao M, Xu Y, Li Y. SIRT3 protects endothelial cells from high glucose-induced cytotoxicity. International journal of clinical and experimental pathology. 2015;8:353–360. [PMC free article] [PubMed] [Google Scholar]
- 125.Ouyang S, Hsuchou H, Kastin AJ, Wang Y, Yu C, Pan W. Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2014;34:43–51. doi: 10.1038/jcbfm.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ott A, Stolk R, Harskamp Fv, Pols H, Hoffman A, Breteler M. Diabetes mellitus and the risk of dementia. The Rotterdam study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 127.Huang CC, Chung CM, Leu HB, Lin LY, Chiu CC, Hsu CY, Chiang CH, Huang PH, Chen TJ, Lin SJ, Chen JW, Chan WL. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS One. 2014;9:e87095. doi: 10.1371/journal.pone.0087095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, Jack CR, Jr, Weiner MW, Toga AW, Thompson PM. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol Aging. 2010;31:1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biological psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 130.Wang KC, Woung LC, Tsai MT, Liu CC, Su YH, Li CY. Risk of Alzheimer's disease in relation to diabetes: a population-based cohort study. Neuroepidemiology. 2012;38:237–244. doi: 10.1159/000337428. [DOI] [PubMed] [Google Scholar]
- 131.Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O'Brien RJ, Breitner JC, Ferruci L, Resnick SM, Thambisetty M. Midlife adiposity predicts earlier onset of Alzheimer's dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Molecular psychiatry. 2015 doi: 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. Faseb J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 133.Jolivalt CG, Hurford R, Lee CA, Dumaop W, Rockenstein E, Masliah E. Type 1 diabetes exaggerates features of Alzheimer's disease in APP transgenic mice. Exp Neurol. 2010;223:422–431. doi: 10.1016/j.expneurol.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kohjima M, Sun Y, Chan L. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer's disease mouse model. Endocrinology. 2010;151:1532–1540. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fu W, Patel A, Jhamandas JH. Amylin receptor: a common pathophysiological target in Alzheimer's disease and diabetes mellitus. Frontiers in aging neuroscience. 2013;5:42. doi: 10.3389/fnagi.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ioacara S, Guja C, Ionescu-Tirgoviste C, Fica S, Sabau S, Radu S, Micu A, Tiu C. Improvements in life expectancy in adult type 2 diabetes patients in the last six decades. Diabetes Res Clin Pract. 2011;92:400–404. doi: 10.1016/j.diabres.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 138.Han W, Li C. Linking type 2 diabetes and Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:6557–6558. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]