Abstract
Recently researchers proposed the term ‘Type-3-Diabetes’ for Alzheimer’s disease (AD) because of the shared molecular and cellular features among Type-1-Diabetes, Type-2-Diabetes and insulin resistance associated with memory deficits and cognitive decline in elderly individuals. Recent clinical and basic studies on patients with diabetes and AD revealed previously unreported cellular and pathological among diabetes, insulin resistance and AD. These studies are also strengthened by various basic biological studies that decipher the effects of insulin in the pathology of AD through cellular and molecular mechanisms. For instance, insulin is involved in the activation of glycogen synthase kinase 3β, which in turn causes phosphorylation of tau, which involved in the formation of neurofibrillary tangles. Interestingly, insulin also plays a crucial role in the formation amyloid plaques. In this review, we discussed significant shared mechanisms between AD and diabetes and we also provided therapeutic avenues for diabetes and AD.
Keywords: Alzheimer’s disease, Obesity, BMI, diabetes, Type-3-Diabetes, MCI amyloid beta, Tau, GSK3β
Graphical Abstract
1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by the progressive decline of memory, cognitive functions, and changes in behavior and personality. AD is the 6th leading cause of death in the United States and the 5th leading cause of death for those aged 65 and older. Currently, 5.4 million Americans suffer from AD, including an estimated 200,000 under the age of 65 and these numbers are expected to increase up to 16 million by 2015. Nearly two-thirds of those with AD are women (3.3 million). AD-related dementia has had a huge economic impact on medical resources, with the total estimated healthcare cost at about $818 billion in 2015, which is estimated to increase to 2 trillion by 2015 [1, 2, 3].
Histopathological examination of AD postmortem brains revealed that the presence of extracellular neuritic plaques, intracellular neurofibrillary tangles and neuronal loss. AD is also associated with the loss of synapses, oxidative stress & mitochondrial structural and functional abnormalities, inflammatory responses, changes in cholinergic neurotransmission, hormonal changes and cell cycle abnormalities [3, 4, 5, 6, 7].
AD is multifactorial, with both genetic and environmental factors implicated in its pathogenesis. A small proportion of AD cases show an autosomal dominant transmission of the disease, and currently mutations in the genes encoding APP, presenilin 1 and Presenilin 2 have been characterized in early-onset familial AD cases. The best described risk factors for AD are age and a positive family history of dementia, since more than one third of AD patients have one or more affected first degree relatives. Other risk factors that may be associated with the development of AD include severe head trauma, low levels of education, female gender, previous depression, and vascular factors [3,4].
The increase incidence in AD would be due to one of the emerging complication of Type 2 Diabetes mellitus (T2DM). In the United States alone there are more than 23 million T2DM patients are present. Currently, 366 million people have diabetes mellitus world-wide, and this number is expected to reach 552 million by 2030 (IDF, Diabetes atlas) [8]. T2DM is characterized by high blood sugar (hyperglycaemia), insulin resistance, and relative lack of insulin. This arises due to a reduced sensitivity of muscle, liver and fat cells to insulin (also called insulin resistance). In general, immediately after the meal there is increase in production of insulin by pancreas. The targeted organ for the insulin is adipose tissue, skeletal muscle, liver, and fat and induces the uptake of glucose from the blood and promotes glycogenesis by inhibiting glucose production. Another hallmark of diabetes is the formation of human islet amyloid polypeptide (hIAPP, amylin) that leads to pancreatic β-cells dysfunction. The resulting metabolic disturbance leads to chronic hyperglycemia, which is the immediate cause of many of the symptoms of diabetes such as retinopathy, peripheral neuropathy and nephropathy [2, 9].
Substantial epidemiological evidence suggests T2DM are strongly associated with cognitive impairment [10–14] due to failure in the action of glucose absorption in the neurons for energy production. The association between T2DM and AD is complex both are interlinked with insulin resistance, insulin growth factor (IGF) signalling, inflammatory response, oxidative stress, glycogen synthase kinase 3β (GSK3β) signalling mechanism, amyloid beta (Aβ) formation from amyloid precursor protein (APP), neurofibrillary tangle formation, Acetylcholine esterase activity regulation. Because of shared mechanisms among Type-1-Diabetes (T1DM), T2DM and AD; researchers termed “Type-3-Diabetes”. The purpose of the review article is to discuss the shared cellular and molecular connections between diabetes and AD for terming Type-3-Diabetes.
2. Impaired insulin and IGF actions in the brain
The insulin receptor (IR) is expressed both in neurons and glia of the brain and especially it is seen with highest in the hippocampus, hypothalamus, cerebral cortex and olfactory bulb [15, 16]. In the brain, insulin and IGF signalling mechanisms are important in establishing synaptic plasticity for cognitive function. Once insulin binds with IR there is the activation of various several tyrosine residues by auto phosphorylation (Fig. 1). These phospho-tyrosine residues are important for insulin receptor substrate (IRS) 1 and 2 for initiating several signalling cascades such as phosphatidylinositol 3-kinase (PI3K), GSK3β signalling, mitochondrial regulation for energy production and wnt signalling cascades. PI3K is associated with almost all of the metabolic actions of insulin [17, 18, 19]. PI3K converts phosphatidylinositol 4,5 bisphosphate (PIP2) to phosphatidylinositol 3,4,5 trisphosphate (PIP3). Then, PIP3 recruits protein kinase B (PKB, also known as Akt) to the plasma membrane, where it is phosphorylated and activated by specific protein kinases [20]. PKB has many important cellular targets including GSK3β Phosphorylation. This pathway connects IR at the cell surface with enzymes of glycogen metabolism within the cell. Several potent and selective inhibitors of GSK3 have been developed that mimic the action of insulin on glycogen synthesis [21], and these are being evaluated for the treatment of insulin resistance and T2DM.
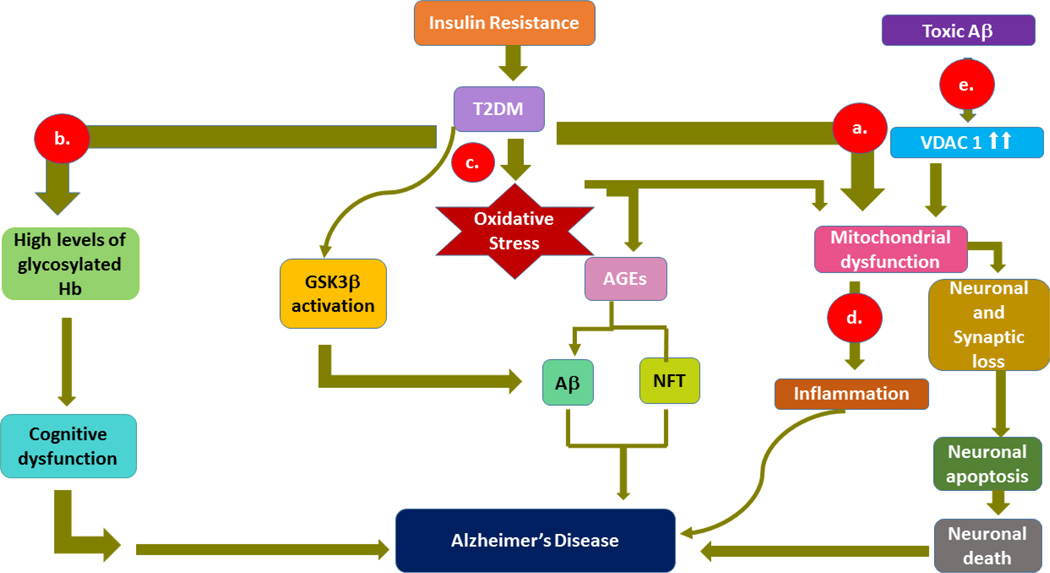
Figure 1.
Schematic representation of T2DM/insulin resistance in Alzheimer’s disease through a) mitochondrial dysfunction, which in turn causes synaptic damage, and neuronal death, b) glycosylated hemoglobin in impaired cognitive function by failure in the transport of glucose for neurons, c) oxidative stress-induced amyloid beta and phosphorylated tau formations through advanced glycation end products , d) inflammation by mitochondrial dysfunction and toxicities of amyloid beta and glycation end products, e) activation of voltage-dependent anion channel by amyloid beta-induction in neuronal loss.
Insulin regulates synaptic plasticity by internalization of neurotransmitter receptors. For example, insulin induces long term depression (LTD) by internalization of AMPA receptors, [22–28] and also promotes GABA receptor-mediated synaptic transmission by the recruitment of GABA receptors to postsynaptic membranes [29, 30]. Insulin also controls the internalization of β-adrenergic receptors [31] and GluR2 (of AMPA receptor) [25] and induces translation of dendritic synapse scaffolding protein PSD-95 [32]. These observations suggest that insulin not only involved for the glucose metabolism for the neuronal survival but also involved in the regulation of synaptic transmission neurotransmission for the establishment if synaptic plasticity. Other studies also explored neuronal functions of insulin such as neurite outgrowth [33] and enhancement of axonal regeneration in rat sensory neurons [34]. Till date, researchers have shown different types of cognitive defects in T2DM population but there are no studies on the role of insulin on spine density, synapse number and size. It is therefore of great interest to investigate whether T2DM, a disease of reduced insulin action, is associated with abnormal neuronal function. The increasing evidence that, support the hypothesis that neuronal as well as peripheral insulin sensitivity is defective in T2DM.
There are many studies that shown neurodegeneration and cognitive decline in insulin-resistant patients who does not show hyperglycaemia (pre-diabetes) [35, 36], concluding that hyperglycemia as important as loss of insulin action. In order to establish the molecular connection between these two conditions, it is important to first establish whether neuronal insulin resistance or neurotoxicity of hyperinsulinemias is responsible for the increased risk of AD type dementia. Intriguingly, studies have shown that in age advances glycemic control T2DM patients given the fact about insulin receptor (IR) presence in the cerebral cortex and hippocampus not limiting to the skeletal muscle, liver and fat [37].
3. Oxidative Stress, mitochondrial dysfunction, advanced glycation end products (AGE) in T2DM and AD
Oxidative reaction is a fundamental process that occurs in aerobic metabolism of every single cell in mammalian species. These reactions are considered “double edged swords” as they are essential for life but can be detrimental if unchecked or uncontrolled as already evidenced in various diseases processes including T2DM, Huntington’s disease (HD) and AD [38]. Oxidative stress (OS) occurs when there is an imbalance in reactive oxygen species (ROS) and reactive nitrogen species (RNS) production and inflammatory responses to counteract these free radicals [39]. Both AD and T2DM are prototypical examples of OS induced disease processes and hence AD is rightly proposed as type 3 diabetes by Suzanne et al. [40]. Free radicals are constantly produced in the cells as physiological byproducts of metabolism. To maintain homeostasis, antioxidants are produced by the local activation of enzymes, resulting in maintenance of cell integrity and prevention of damage and apoptosis. Free radicals, depending on their oxidative power can be divided into those with a lower reactivity, such as those produced in aerobic metabolism and those with longer reactivity as seen in AD and T2DM. The lower reactive free radicals generally induce minor cell damage and can be repaired relatively efficiently [41]. OS causes cell injury and death by apoptosis by activating various enzymatic cascades at different cell components which include mitochondria, cytoplasm, and cell membranes. Lipid rich membranes in the human brain are particularly vulnerable to oxidative stress. Other modes of cell injury can also be explained by structural alterations in proteins such as amyloid beta and tau. These mechanisms will be discussed briefly in the following sections (Figure 3).
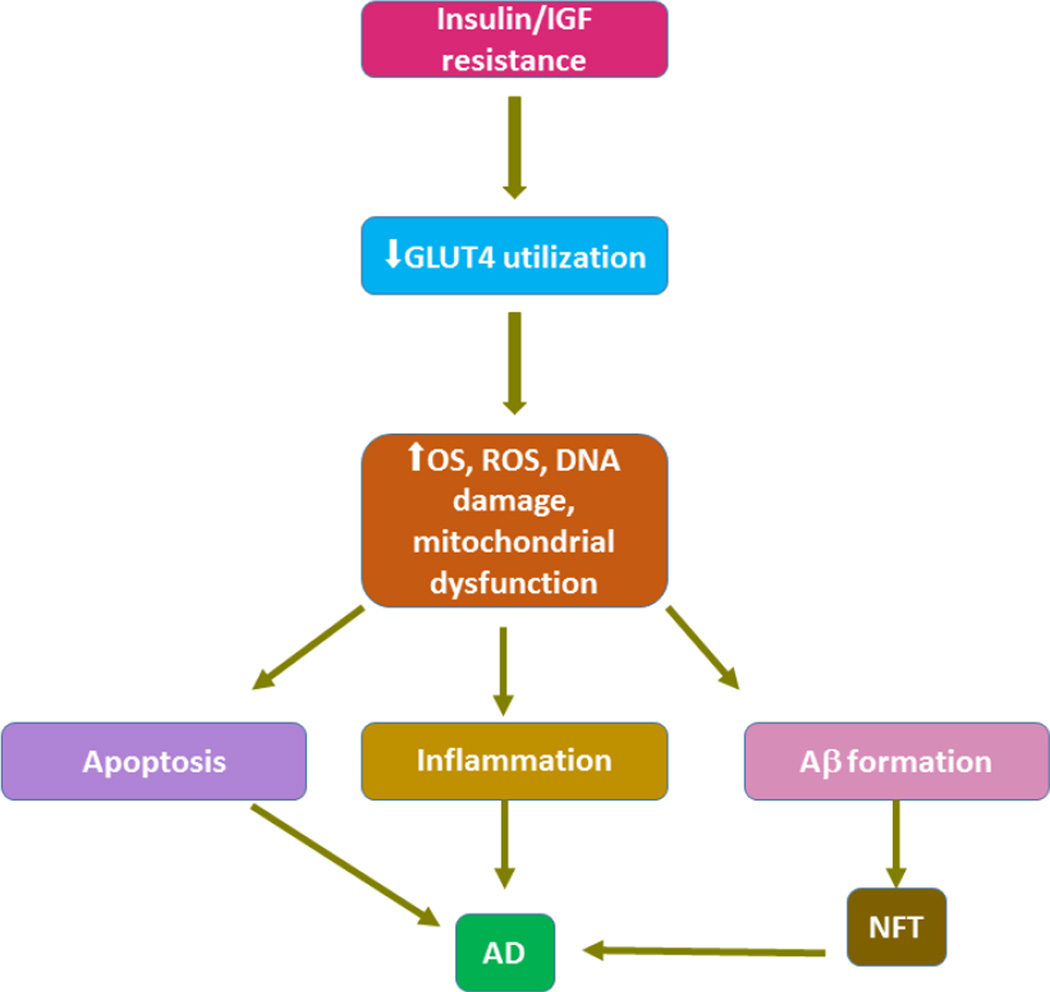
Figure 3.
Insulin resistance decreases glucose metabolism and plays a pivotal role in mitochondrial damage, DNA damage and ROS formation. This triplet action is involved in the formation of plaques.
3.1 Mitochondria and its dysfunction
Cell mitochondria, which are considered powerhouses of cells, are the key structures in the production of ROS and RNS. The mitochondrial membrane is very permeable to these products, which can enter the cell cytoplasm. However, most of these products are produced as a result of metabolism and can easily be converted into water and/or oxygen. This can occur in the mitochondria themselves or after entering into the cytoplasm in the presence of dismutase enzymes, thereby preventing cell damage. Despite this efficient system, oxidative imbalance can occur particularly when the mitochondria are dysfunctional and less efficient in the production of ATP, which results in increased production of ROS as observed in AD and T2DM. The production of ROS by mitochondria can be due to several enzymatic reactions [41]. These enzymes convert molecular oxygen of aerobic respiration to superoxide ion or hydrogen peroxide. The enzymes may be present on the outer or inner membranes of the mitochondria or the mitochondrial matrix itself. It has also been suggested that amyloid beta may play a direct role in the disruption of mitochondrial function (Figure 3). A recent study reported that mitochondrial localized amyloid beta induces increased production of free radicals and causes mitochondrial dysfunction and neuronal damage in the brains of AD mice [42].
3.2 Mechanism of OS secondary to amyloid and tau protein
Amyloid beta is formed due to proteolytic processing of APP. This is very similar h1APP, which results in islet cell dysfunction leading to T2DM, analogous to AD [9]. Studies indicate that amyloid beta may change the protective mechanisms that cells have against mitochondrial oxidative damage. Uncoupling proteins (UCPs) in the mitochondrial inner membrane have been shown to decrease free radical production [43]. This mechanism seems to be ineffective in AD brains, and amyloid beta accumulation may contribute to changes in the cell that lead to oxidative stress. Hyperphosphorylated tau protein causes neurofibrillary tangles, which are one of the hallmarks of AD pathology [41]. The mechanism by which these tau proteins and h1APP lead to oxidative stress is not well understood, but some investigators believe these are secondary processes. Su et al. reported that these proteins trigger cellular pathways such as MAPK and AKT, leading to OS and cell structure damage [44]. Many of these proteins have been studied and associated with tau phosphorylation (Figure 2).
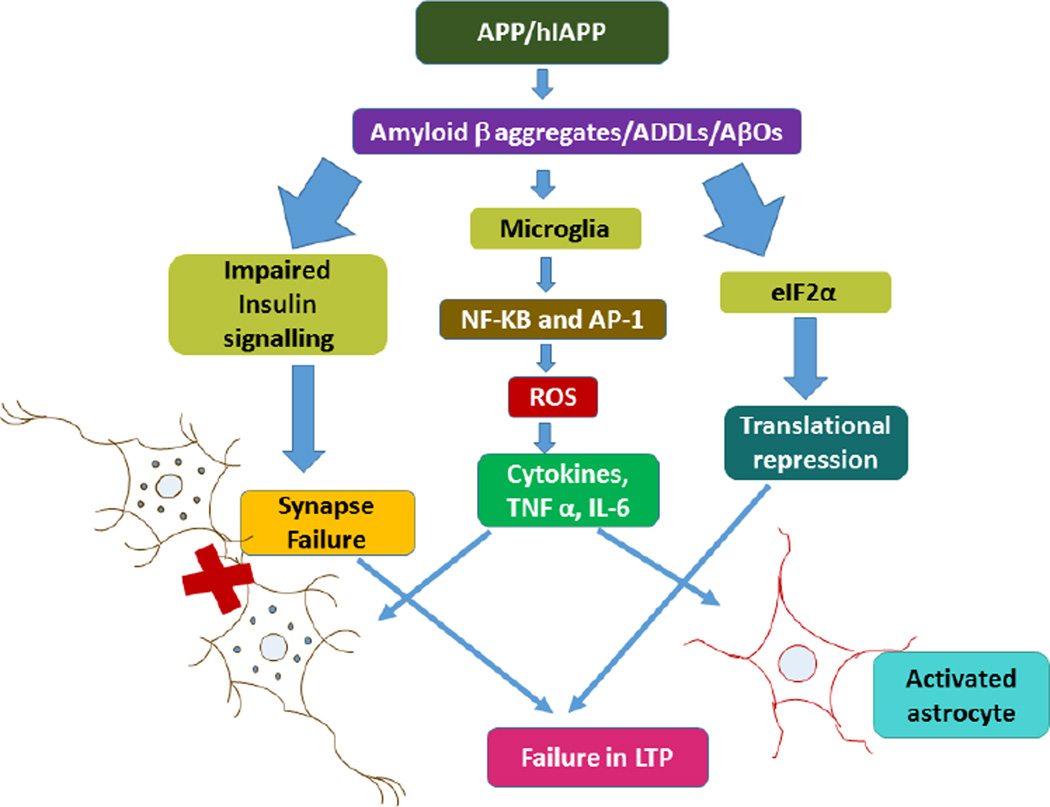
Figure 2.
Insulin resistance in T2DM causes mitochondrial dysfunction, which in turn triggers inflammation response through either APP/hIAPP catabolism. Amyloid beta oligomers activate microglia in the production of cytokines. An orchestrated action triggered by stress kinases promotes both the inhibition of brain insulin signaling and elevated eIF2a–P. While both events act to promote insulin resistance and metabolic deregulation in diabetes, they are likely to contribute to synapse loss and impaired long-term potentiation.
3.3 Hyperglycemia and oxidative stress
Hyperglycemia, either due to decreased insulin production by islet cells or due to impaired insulin receptors, can cause accumulation of advanced glycation end (AGE) products leading to ROS generation and cell damage. It has been shown that AGE products produce superoxide and H2O2 resulting in lipid peroxidation and cell damage in brains [40]. The link between oxidative stress and hyperglycemia is that the increase in free radicals that occurs in T2DM may be caused by varying levels of antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) [44]. This resulting imbalance in pro-oxidants and antioxidants which causes oxidative stress is observed in diseases such as AD and T2DM
3.4 Lipid peroxidation
The human brain is highly susceptible to OS due to the rich abundance of peroxidizable polyunsaturated fatty acids and relative paucity of antioxidants and enzymes. T2DM pathology induces changes in the lipid profile, which causes the cells to be more likely to undergo lipid peroxidation [45]. Similar processes have been observed in the pathology of AD. Lipid peroxidation is a key biomarker of oxidative stress as polyunsaturated fatty acids with multiple bonds in cells are very likely to associate with free radicals [45]. Therefore, lipid peroxidation is associated with increased levels of ROS and RNS in any disease process which advances with oxidative stress, including AD and T2DM.
3.5 Advanced glycation end (AGE) products
AGEs are peptide/protein molecules formed as a result of the Maillard reaction [46]. These molecules accumulate with aging and also found in both the types of diabetes. The formation of these molecules in diabetes due to the hyper glycemia which is generally accepted that many diabetic complications are potentiated or initiated by the accumulation of specific forms of AGE and their interaction with receptors for AGE [47]. These AGEs promote amyloid oligomer aggregation and thus involve the formation AD neurotoxicity [48], in addition glycation of tau may enhance the formation of paired helical filaments [49]. Sato et al ., has shown the addition of AGEs to primary cortical neurons reduces cell viability, confirming that these molecules are neurotoxic [47].
4 Cellular and molecular mechanisms of insulin in AD
The role of insulin in the brain has been discussed very little in comparison with muscle, adipose tissue and liver. Recent studies have shown important functions of insulin in brain such as metabolism of glucose (and transportation by GLUT4), regulating GSK3β signalling in maintaining neuronal plasticity, neurotrophic and neuroendocrine functions [50]. The key molecule for the neuroprotective function of IGF/insulin signaling is PKB (Akt) may be mediated by direct phosphorylation of known regulators of apoptosis, such as the pro-apoptotic mitochondrial protein Bad [51, 52] and the transcription factor FOXO [53, 54, 55], as well as the pro-survival transcription factors CREB [56] and NF-kB [57, 58]. FOXO controls the transcription of the pro-apoptotic Bcl2-family member BIM-1 [59], NF-kB controls transcription of the pro-survival Bcl2-family members Bcl-XL [60], A1 [61], and c-IAP2 [62], and CREB controls the expression of Bcl2 [63] and BDNF [64]. Both IR and insulin are present in the brain, and insulin is actively transported across the blood–brain barrier and might also be produced locally in the brain [65]. IRs regulate neurotransmitter release and receptor recruitment at synapse and thus responsible for synaptic/neuronal plasticity [66–69]. IRs is abundant in cerebral cortex and hippocampus and thus responsible for learning and memory processing which in turn responsible for cognitive functions [70–73]. Intracerebroventricular (i.c.v.) streptozotocin (STZ) studies has shown cognitive impairment in rats when IRs were disrupted [74] and in contrast, i.c.v. injection of insulin improves memory function in rats [75]. Based on these studies it concluded the role of insulin on the cellular and molecular events that underlie in AD pathology. In diabetes, insulin regulates the metabolism of amyloid beta and tau, in the formation AD pathology through three signalling cascades such as phospholipase C, PI3K and MAP kinases.
Tau is a neuronal cytoskeletal protein and responsible for micro tubulin polymerization and stabilization. GSK-3β is responsible for binding the tau protein to microtubules. This process is regulated by protein kinases through phosphorylation. GSK-3β (Figs. 1, 3, 5) activity is downregulated by either insulin or insulin growth factor 1(IGF-1) because it is downstream event of the insulin-signaling pathway. Both IGF-1 and IGF-1 its receptors are homologous and trigger similar intracellular signaling events [65, 76]. Hong et al. (1997) demonstrated a decrease in tau phosphorylation by insulin and IGF-1 and promotes binding of tau to microtubules by inhibition of GSK-3β through phosphoinositide 3-kinase pathway in human neuronal cultures [77]. Normally Akt signalling involves to phosphorylate the GSK-3β and inactivates glycogen synthase. Insulin resistance leads to dephosphorylation and activation of GSK-3β [9]. Other than tau phosphorylation activity, insulin may also regulate the metabolism of APP and balances Aβ anabolism and catabolism. Qiu et al. [78] and Vekrellis et al. [79] have proposed that insulin influences insulin-degrading enzyme (IDE) in the clearance of Aβ in the brains of AD patients. IDE is the major metalloprotease involved in the degradation of extracellular Aβ along with insulin itself and other peptides [78, 79]. Interestingly, insulin was shown to increase extracellular levels Aβ1–40 and Aβ1–42 along with soluble APPα in primary cultures of rat cortical neurons and in mouse neuroblastoma cells that overexpress wild-type APP [80, 81]. Insulin alters the extracellular concentration of Aβ by inhibiting the extracellular degradation of Aβ by IDE, and by insulin stimulating Aβ secretion which significantly reduces the intracellular concentrations of Aβ1–40 and Aβ1–42 [80].
Figure 5.
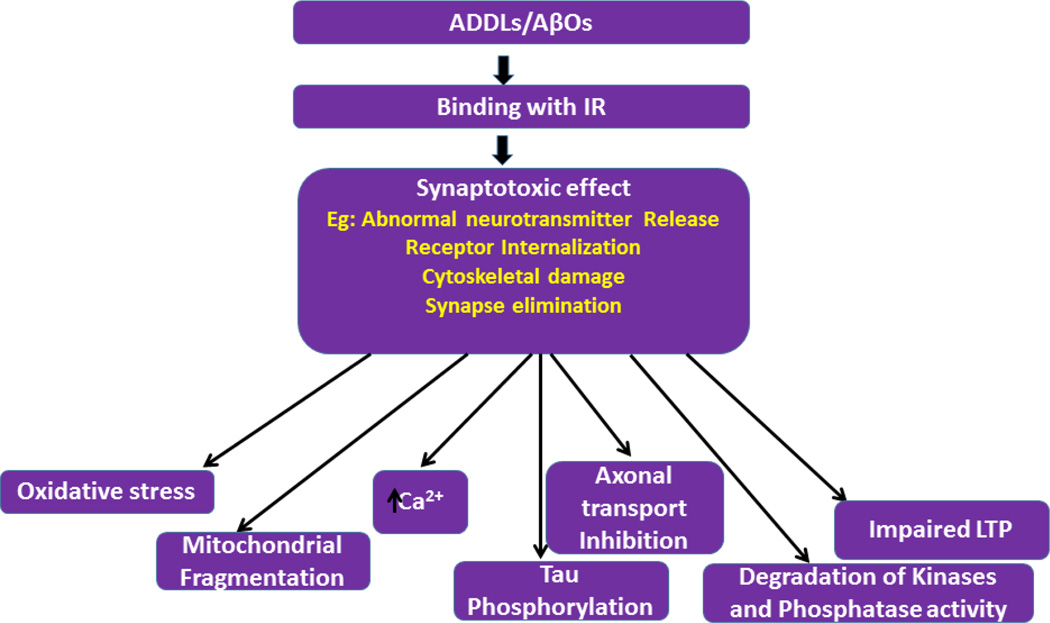
Role of amyloid beta oligomers on toxic effects of synapses, including synaptic degeneration, abnormal neurotransmitter release and cytoskeletal damage. Synaptotoxic effects cause defective axonal transport, mitochondrial fragmentation, degradation of kinases activity, oxidative stress, impaired LTP, increase in intracellular calcium levels.
Overall, these results indicate that insulin could play an important role in regulating tau protein, and Aβ and APP metabolism in neurons. Thus, dysfunction of insulin signaling might be involved in the pathological events that lead to the development plaques in AD brains.
5. Inflammation and AD
Insulin resistance in T2DM causes mitochondrial dysfunction, which in turn triggers inflammation response [82, 83]. In these conditions, insulin resistance increases the levels of cytokines such as IL-6, IL-1β and IL-18, tumor necrosis factor-alpha (TNF-α), alpha-1-antichymotrypsin and C-reactive protein [84–86]. Likewise, the same inflammatory mechanism triggers in AD as well [87–89]. It has also been reported that T2DM increased the neurodegeneration of the diabetic AD mouse model by promoting Aβ aggregation and cerebrovascular inflammation by up-regulating the receptors for AGEs [90], on the other hand there is elevated immunoreactivity to IL-6 was found in senile plaques and cerebrospinal fluid in patients with AD [87]. AGEs is expressed in neuronal cells, microglia astrocytes and in brain endothelial cells, and levels are increased in both AD and T2DM. AGEs and Aβ together induce the expression of the pro-inflammatory cytokines IL-6 and TNF-α. The Aβ-mediated activation of glial cells due to inflammation results in nerve cell death by promoting in the formation of NFTs and the progression to AD (Figure 2). Interestingly, it has been found that inflammation leads to elevation in the AGE, Tau and Aβ levels. To strengthen the role of diabetic inflammation in AD, recent reports have shown that incidence of AD is decreased in patients who consumed nonsteroidal anti-inflammatory drugs for pain or antidiabetic drugs peroxisome proliferator-activated receptor-G (PPARG) agonists [38, 39p1, 92]. AGE also causes the stimulation of TNF-α which in turn accelerates β-secretase (BACE) expression further causing APP processing for the formation of Aβ in astrocytes of diabetic models of AD [93, 94].
Recently, Lourenco et al. [95] described inflammation and cellular stress, are known to activate stress-sensitive kinases, some of which target eukaryotic initiation factor 2 alpha (eIF2a) [kinases namely PKR, PERK and GCN2] [95]. This is a mechanism initially conceived to avoid further cellular stress and to provide cells with response options to restore homeostasis. Nevertheless, prolonged brain metabolic stress and eIF2a kinase activity may lead to persistently increased eIF2a–P and exacerbated neuronal damage, in a parallel to what has been described for peripheral cells in diabetes [96]. Increased eIF2a–P levels lead to upregulated activating transcription factor 4 expression, recently described as a propagator of neurotoxic signals in AD [97], as well as to Aβ generation [98] and translational attenuation [99] (Figure 2). All these factors may contribute to neurological outcomes observed in AD. Furthermore, enhanced Aβ oligomerization, in conjunction with genetic and environmental factors (lifestyle, metabolic disease and/or cumulative infections), might instigate a feed-forward cycle that contributes to disease progression.
Aβ oligomers (AβOs) or ADDLs trigger diabetes-related toxic mechanisms in AD brains. AβOs accumulation in AD brains leads to removal of neuronal insulin receptor from cell surface [100–102] Aβ further increase microglial release of TNF-a in the brain, which in turn activates neuronal TNF-α receptors and instigates cytosolic stress-sensitive kinases (e.g. JNK, PKR, IKK) [103–105]. An orchestrated action triggered by stress kinases promotes both the inhibition of brain insulin signaling and elevated eIF2a–P [103, 106]. While both events act to promote insulin resistance and metabolic deregulation in diabetes, they are likely to contribute to synapse loss and impaired long-term potentiation in AD, resulting in memory impairment and behavioral outcomes (Figure 2).
All these findings suggest that T2DM insulin resistance generates oxidative stress which in turn causes mitochondrial dysfunction and activation inflammatory response. In one direction, it is responsible for the formation Aβ pathology and in another direction through abnormal expression of dynamin related protein causes the formation of NFTs in brain.
6 Insulin and IGF link to acetyl choline (ACh) in AD
Acetylcholine is a neurotransmitter associated with neuronal signal transmission and synaptic plasticity. Reduced levels of ACh are associated with the progression of AD, and a study done by Rivera et al. (2005) demonstrated the relationship between lower ChAT expression and IGF expression [107]. Using Real-time RT-PCR analysis, these authors showed that with increasing clinical AD Braak stage, the mRNA associated with IGF-I, IGF-II, and their receptors as well as tau protein regulated by IGF-I and Hu D neuronal protein are reduced. On the contrary, with increased Braak stage, progressively increased levels of amyloid beta, glial fibrilary acidic protein (GFAP) and microglial transcripts were observed. In a subsequent study, a year later, de la Monte et al. further supported the above findings using intracerebral Streptozotocin (ic-STZ) treated rat models [108]. In this study, STZ treated rats produced brain specific insulin depletion and resistance leading to progressive neurodegeneration simulating AD. Hence these authors propose “Type 3 DM” for AD and suggest possible early intervention and treatment using PPAR agonists (rosiglitazone and piloglitazone).
Therefore, insulin resistance and IGF1/II deficiency may impair in the establishment of synaptic/neuronal plasticity by altering neuronal structure and effect the production of acetylcholine thus establishes a cellular link between T2DM and AD by impairing the cognitive function.
7. Role of ADDLS/AβOs in AD pathogenesis through IR
Klein and colleagues demonstrated the existence of soluble and diffusible Aβ oligomers (AβOs) and can able trigger neurotoxic signaling [109]. These AβOs also called Aβ-derived diffusible ligands (ADDLs), the terminology originally proposed by Klein [109]. Various studies found are found at increased levels ADDLs / AβOs in the brains and cerebrospinal fluid AD patients [110–115]. However these oligomers also target excitatory synapses [113], promote toxic signaling and eventually cause failure in establishment of synaptic plasticity [116–118]. These events events likely to underlie rapid memory decline in AD (Figure 5) [102].
The AβOs when they bind with IR it causes synapse toxic effects such as abnormal neurotransmitter release, receptor internalization and removal synapse across the neurons. These cascades ultimately cause oxidative stress, mitochondrial fragmentation, increased cytosolic ca2+ levels which effects PI3K–MAPK-GSK3 signalling mechanisms, Inhibition of axonal transport and mitochondria, hyper phosphorylation tau. Furthermore, AβOs plays a role in the impairment of long term potentiation (LTP), to induce cognitive deficits in mice [102, 143, 144].
8. Is T2DM and late-onset dementia a Type 3 Diabetes: based on available evidences?
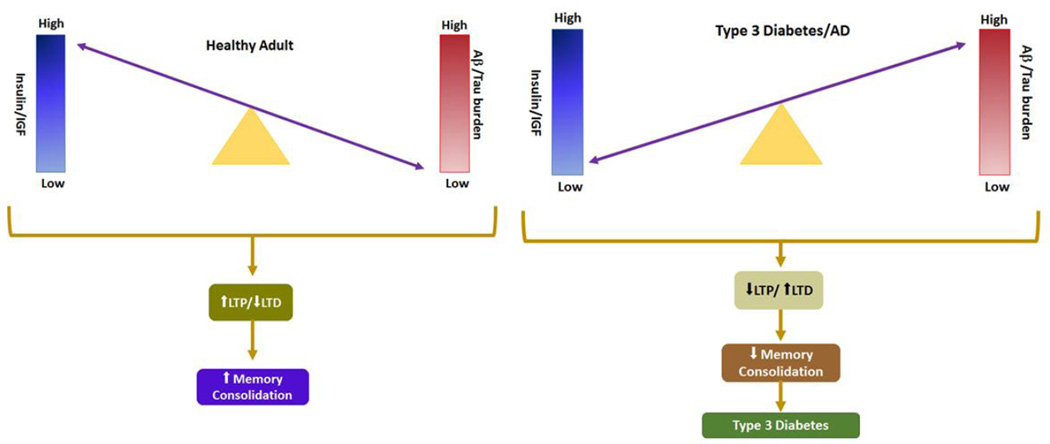
Recent concept suggests that AD represents a metabolic disease and the studies has shown that deficits in utilization of glucose by the brain in the early course of disease [119–122] and eventually leads to the cognitive dysfunction [123, 124]. Hoyer et al. has shown deficits in cerebral glucose utilization worsening in with progression of cognitive impairment [125]. In addition to the deficits in glucose metabolism, human postmortem studies shown insulin and IGF resistance and impairments in signal transduction [107, 126]. Insulin resistance in brain is manifested by reduced levels of insulin and IGF receptors [107, 126, 127], while insulin and IGF deficiencies are associated with altered expression of insulin and IGF polypeptides in brain and cerebrospinal fluid [124, 128, 107, 126]. These findings support the role of insulin/IGF signaling in the pathogenesis of AD [126]. Moreover, AD could be regarded as a brain disorder that has composite features of T1DM (insulin deficiency) and Type2DM (insulin resistance). To consolidate this concept, de la Monte proposed that AD be referred to as, “Type-3-Diabetes” (107, 126, 129]. As insulin stimulates cerebral glucose uptake and metabolism [130], cognition and memory [131–135], but failure in insulin signalling causes impairments in glucose metabolism and leads to the cerebral energy balance in turn causes ROS production, DNA damage, and mitochondrial dysfunction, all these cascades leads to pro-apoptosis, proinflammatory, and pro-AβPP-Aβ cascades [136, 130, 142]. Correspondingly, experimental suppression of brain insulin/receptor expression causes cognitive impairment [137–141]. So in T2DM because of insulin resistance the above stringent actions may takes place and this would be one of the reason researchers termed this metabolic syndrome as “Type-3-Diabetes”.
9. Clinical and preclinical evidence for obesity/diabetes contributing to type3 diabetes
Till date the role of obesity in cognitive impairment is not well understood. Some studies have shown direction association, others indirect association, inverse association or U shaped association of obesity in relation either with low or high BMI as increased risk factor for AD (20, 21, 22). Recently, several studies on T2DM, obesity, IR, and hyperinsulinemia have shown links with cognitive impairment and AD [146–148]. A recent meta-analysis on obesity (BMI> 30 kg/m2) has reported as obesity an increased risk factor for AD [149]; in another study it has shown that cardiovascular disease is also risk factor for memory loss [150]. Whitmer, 2007 has shown ‘mid-life obesity’ strong and independent association with an increased risk of dementia and AD [151]. In this study author has shown that both obesity and overweight, as measured by body mass index and skinfold thickness, in middle-age are strongly associated with an increased risk of all cause dementia, AD & Vascular dementia (VaD), independent of the development of diabetes and cardiovascular-related morbidities. There is also value in assessing regional body shape distributions of adiposity, particular the role of abdominal obesity. Mechanistic pathways such as adipocyte secreted proteins and hormones, and inflammatory cytokines could explain the association between obesity and increased risk of dementia [151].
Whereas “Midlife and late-life obesity and the risk of dementia: cardiovascular health study” has shown an increased risk of dementia was found for obese (BMI >30) vs normal-weight (BMI 20–25) persons, adjusted for demographics (hazard ratio [HR], 1.39; 95% confidence interval [CI], 1.03–1.87) and for cardiovascular risk factors (1.36; 0.94–1.95). The risk estimates were reversed in assessments of late-life BMI. Underweight persons (BMI <20) had an increased risk of dementia (1.62; 1.02–2.64), whereas being overweight (BMI >25–30) was not associated (0.92; 0.72–1.18) and being obese reduced the risk of dementia (0.63; 0.44–0.91) compared with those with normal BMI [152].
Luchsinger et al. (2007) results are little contradiction with above studies results and shown decreased risk of dementia with increasing BMI, but the subjects are ≥76 years of age, but a U-shaped association in subjects <76 years of age [153]. These results suggested that changes in body size and composition with age make BMI a poor measure of obesity in older subjects and also weight loss could be the preclinical condition of AD [151, 154, 157].
Till now the literature says the pathology of AD may develop in the advanced stage of AD so the current risk factors such as obesity and IR in mid-life may be far more important than they are in later life to treat mild cognitive loss. In the later life, it is also possible that insulin sensitivity may mediate the effects of obesity on dementia, VaD and AD risk. Interestingly, numerous studies shown increase in insulin concentrations in AD patients compared to controls [146, 147, 155, 156, 157].
All these clinical and preclinical studies have shown obesity, insulin resistance/hyperinsulinemia are would be the risk factors for AD, but whether adiposity and peripheral insulin sensitivity mediate incidence of AD remains unknown. However Baker et al., (2011) has shown little evidence on the role of peripheral insulin sensitivity on cognition [158]. In detail, greater IR was associated with an AD-like pattern of reduced cerebral glucose metabolic rate (CMRglu) in frontal, parietotemporal, and cingulate regions in adults with PD/T2D. The relationship between CMRglu and homeostasis model assessment insulin resistance (HOMA-IR) was independent of age, 2-hour OGTT glucose concentration, or apolipoprotein E ε4 allele carriage. During the memory encoding task, healthy adults showed activation in right anterior and inferior prefrontal cortices, right inferior temporal cortex, and medial and posterior cingulate regions. Adults with PD/T2D showed a qualitatively different pattern during the memory encoding task, characterized by more diffuse and extensive activation, and recalled fewer items on the delayed memory test [158]. All these changes suggest that IR may be a marker of AD risk that is associated with reduced CMRglu and subtle cognitive impairments at the earliest stage of disease, even before the onset of mild cognitive impairment [158]. In another study is has shown Higher levels of HOMA-IR along with hyperinsulinemia have also been linked to an increased burden of amyloid plaques over 10 years later in autopsy samples [159].
Overall, obesity/diabetes and type3 diabetes are directly and indirectly associated with AD. Further research is still needed to better understand the precise molecular links among obesity/diabetes, type3 diabetes and AD.
10. Effects of diabetes and obesity on the brain independent of type3 diabetes
Central obesity and diabetes are the key components of metabolic syndrome (MS) [160]. Several studies have shown the deleterious effects of MS on the brain both structurally and functionally, leading to neurodegeneration and dementia [161].
The co-occurrence of metabolic risk factors for T2DM and cardiovascular disease (CVD) which includes central obesity, hyperglycemia, dyslipidemia, and hypertension suggest the existence of a “metabolic syndrome” [160]. Other names for MS include syndrome X, the insulin resistance syndrome, and the obesity dyslipidemia syndrome. Genetic predisposition, lack of exercise, and body fat distribution all affect the likelihood that a given obese subject will become overtly diabetic. The mechanism by which obesity induces insulin resistance is poorly understood. Several studies have focused on the role of inflammation as a mediator linking obesity to both the pathogenesis and vascular changes in several organs including the brain [162]. The incidence of T2DM has been correlated with increased levels of markers of inflammation or so called “metaflammation” including c-reactive protein (C-RP), IL-6, plasminogen activator inhibitor 1 (PAI-1), TNF [3]. Evidence from various studies strongly favor that morbid obesity/MS cause a low grade inflammation inducing tissue and vascular damage [160].
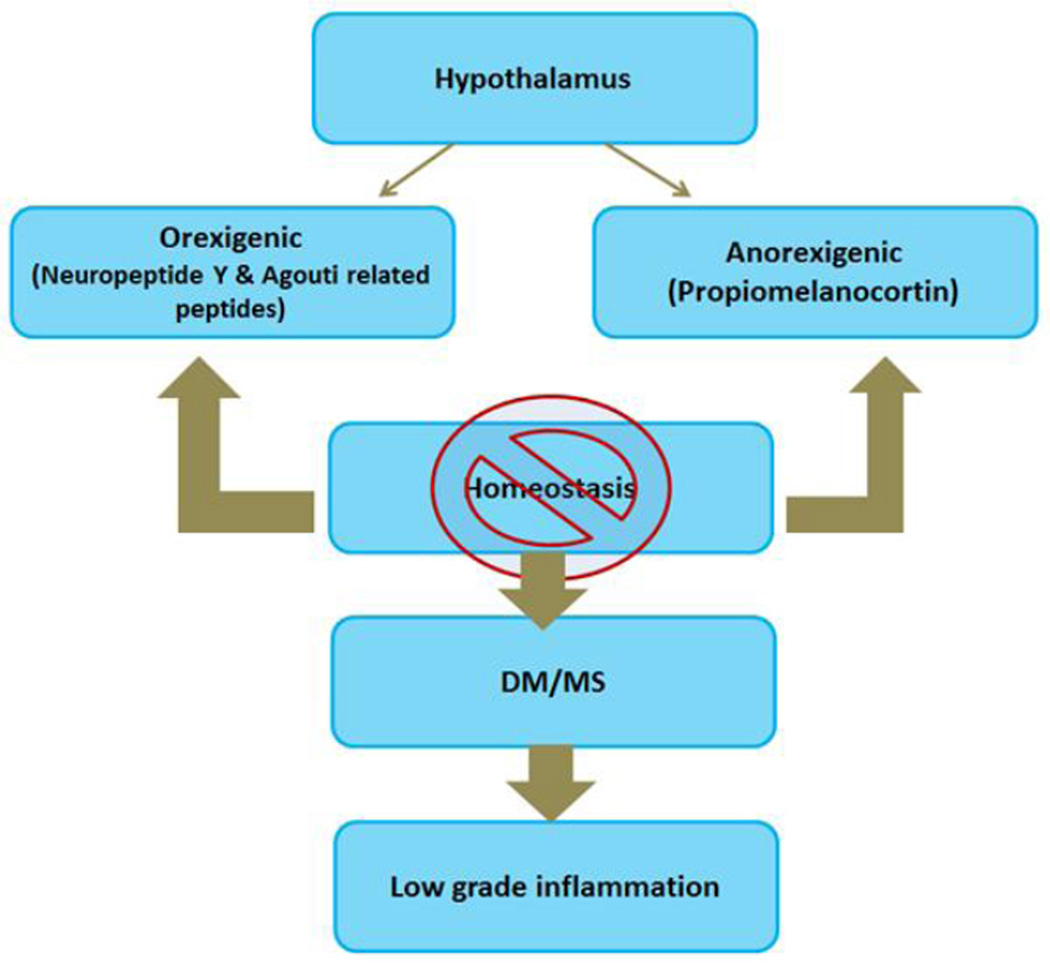
Recent studies have demonstrated the hypothalamus in the brain plays a crucial role in energy homeostasis by regulating two opposing neuronal axes, namely orexigenic axis and anorexigenic axis [4] (Figure 6). When the balance in this homeostasis mechanism is disturbed, it results in disturbances in appetite with changes in insulin secretion pattern, leading to T2DM. In addition, insulin resistance in the brain plays a significant role as described in section ‘2, 8, 9’ in this review article including promotion of amyloid beta protein and tau protein.
Figure 6.
Illustration of hypothalamus in the brain plays a crucial role in energy homeostasis by regulating two opposing neuronal axes, namely orexigenic axis and anorexigenic axis
As described elsewhere in this review, this met inflammation releases cytokines inducing cellular damage by way of free radicals or oxidative stress and autophagic effect. In addition, insulin resistance in the brain parenchyma causes microglial and astrocytic cellular abnormalities with resultant accumulation of amyloid beta protein and tau protein characteristic of AD.
Based on these it can be concluded that disturbances in hypothalamic function (a brain component) causes MS which in turn induces cellular damage in the rest of the brain causing AD. However, the cause and effect relationship is poorly understood and needs further research.
11. Type3 diabetes and astrocytes
Impairment of mitochondrial function is another common link between diabetes, obesity and AD. In the insulin resistance condition in T2DM, oxidative stress triggers mitochondrial injury, finally leads to the not only the activation of inflammatory markers but also the activation macrophages such as microglia in the brain. So in the T2DM patient’s brain microglia plays crucial role for the inflammation. The precise mechanism of microglia in this diabetic inflammation has not been fully investigated eventhough the microglial activation pathway in the hypothalamus has been widely discussed in T2DM. However, oxidative stress signals induce microglia based inflammation through chemokines and the high mobility group protein in advanced stage of T2DM [2]. On the other hand silent information regulator (SIRT) genes (sirtuins) plays crucial role in the inflammation of the brain in obesity and T2DM via macrophages [2]. Nicotinamide riboside (NAD+) is an activator of SIRT1 and increases neuronal mitochondrial biogenesis such as PGC1α, NRF1, NRF2 and TFAM and anti-inflammatory action in glial cells [2, 164–166]. However further research is needed to understand the therapeutic interventions of nicotinamide riboside in the brain.
Insulin resistance in T2DM not only increases the levels of cytokines, chemokines but also astrocytes. Earlier all the researchers were thinking astrocytes have nutritive role in addition with structural support and a physical scaffold for neurons. Recent studies have shown that astrocytes play a crucial role in glutamatergic neurotransmission and in synaptic transmission by “tripartite” synapse mechanism [astrocytes themselves excites and they communicate with neurons by sensing neurotransmitter release and in turn releases their own signaling molecules (glia-transmitters), and intimately associated with synapses physically]. In addition, astrocytes also associate with the cerebrovascular capillaries through “endfeet” processes. These astrocyte ‘endfeet’ ensheath intraparenchymal blood vessels in the brain and maintenance ionic and osmotic homeostasis and gliovascular signalling [167–169]. Thus astrocytes are in close contact with microglia as well, and there is a strong evidence for bidirectional signaling between the two cell types. Like microglia, astrocytes also activated by various stimuli such as stress, and astrocytic activation are increasingly appreciated event in AD and HD. Astrocytic involvement in the inflammation of brain is characterized by increased cytokine production and the release of signaling molecules. These events either directly or through microglial activation may affect the neuronal function. NF-kB activated astrocyte pathway releases complement protein C3, which can bind neuronal C3aR and induce neuron damage [170]. Another astrocytic signaling molecule CD40 ligand binds to the microglial cell surface receptor which in turn increases the production and release of TNFα. Insulin resistance in diabetes may cause changes such as the inflammatory response of both astrocytes and microglia may be seen in peak during MCI, ultimately causes cortical tissue destruction in AD [171].
12. Cerebral metabolic changes in type3 diabetes
Rich intake of carbohydrates and unsaturated fatty acids (omega 6), low antioxidant intake, lack of physical activity causes oxidative stress in the brain, ultimately leading to severe cognitive decline in T2DM. Recent research on T2DM strongly suggesting a connection between impaired glucose metabolism, insulin signaling with AD. In T2DM dysregulation between glucose metabolism and insulin signaling causing additional risk factor for developing AD. Clinically, AD patients have decreased cognitive function and lapses in memory that decline progressively and ultimately affect performance of tasks involved in everyday living. Physiological hall marks of AD such as insoluble extracellular plaques, intracellular NFTs, loss of hippocampal neurons, decrease in acetylcholine production, decrease in glucose consumption in cortex and hippocampus (associated with memory and learning) can be measured by biopsy, positron emission tomography (PET) scan, or autopsy [172–175]. All of these changes in the brain were resulting from long-term dysregulation of insulin signaling and glucose metabolism. In AD patients there is significant decrease in the rate of glucose metabolism especially in the regions where the memory processing and learning takes place [177, 173, 175, 178]. Interestingly, the PET scans of people who are at high risk for developing AD have shown decrease in the rate of glucose metabolism before the appearance of AD symptoms and can be detected before 2–3 decades [173]. These declines in the rate of glucose metabolism are associated with normal aging, but in people who are at risk for AD, they begin at a younger age and decline more aggressively.
In the CNS, Apo-E is mainly produced by astrocytes, and transports cholesterol to neurons via Apo-E receptors. Cholesterol synthesis is upregulated and absorption downregulated in insulin resistance and in T2DM. ApoE isoform ApoEε4 allele has shown the risk factor AD. The presence of this allele is associated with increased risk of both early-onset AD and Late onset AD. Localization studies demonstrate that Apo-E is deposited in the extracellular senile plaques AD patients. Aβ deposition in the form of senile plaques is more abundant in ApoEε4 carriers when compared with ApoEε4 non carriers. Whereas ApoEε3 expression and microglial phagocytosis, reduce soluble Aβ levels, and improve cognition. The formation of NFTs in the brain are regulated by glycogen synthase kinase 3β (GSK-3β) by hyper phosphorylation tau but insulin inhibits this hyperphosphorylation of tau action. In T2DM/insulin resistance hyper phosphorylation of tau is not inhibited but an interesting feature ties hyper phosphorylated tau back to ApoEε4. Of the three isoforms of ApoE, ε4 is unique in its inability to bind tau. The E3 isoform has been proven to bind to tau (with the same suspected for E2), thus preventing or minimizing its phosphorylation [4, 179, 180].
Increased production of Aβ inside the cell indicates that reduced extracellular clearance and causes Aβ to accumulate as senile plaques. Aβ is cleared primarily by insulin degrading enzyme (IDE). The affinity of IDE for insulin is so high, however, that the presence of even small amounts of insulin completely inhibits the degradation of Aβ [181]. Insulin acts as a competitive inhibitor for IDE and allows Aβ to accumulate. When age advances, the production of IDE declines with age, so there is an increasing amount of substrate combined with lower enzyme activity. Just as insulin can be seen as a competitive inhibitor of IDE for degradation of Aβ, Aβ can be viewed as a competitive inhibitor of insulin for its receptor. This has been proven in human cells in vitro—Aβ reduces the binding of insulin to its receptor in a dose-dependent manner [182].
It had long been believed that glucose uptake in the brain was entirely independent of insulin with GLUT1 and GLUT3 glucose transporters and are non-insulin-sensitive. However, it is now recognized that GLUT4 glucose transporter is insulin receptors and insulin-sensitive glucose transporter which is present at the blood brain barrier (BBB) and in some types of brain cells. Interestingly these transporters are rich in the regions where memory and learning processing takes place at high rate [183, 184]. Entry of insulin and glucose into the brain happens by saturable mechanism i.e when increased peripheral insulin levels no longer elevate levels in the CNS. In addition, GLUT1 transporters at the BBB are saturated by normal physiological concentrations of glucose [185]. Ultimately, increasing glucose uptake by the brain/CNS would require an increase regulation of GLUT4 or insulin receptors. But in AD when GLUT4 or insulin receptors have been compromised, it could cause dynamics to a functional hypoglycemia in the brain and thus decreases the rate of brain glucose metabolism. On the other hand, if there is deficiency in insulin this could causes increase in glycation (AGE) what can be seen in AD brains even though glucose enters in to the brain interstitial fluid. In one clinical study it has shown, patients with advanced AD has higher plasma insulin levels and lower CSF insulin levels when compared with healthy controls [184]. This indicating that, entered glucose in to the brain could not able to metabolize (insulin resistant) and eventually leads to the formation of AGEs and thus affects key enzymes in cognitive function. Intriguingly, in most biological mechanisms, acute administration of insulin improves performance on tests of memory and cognition, but chronically elevated insulin levels have the opposite effect [147, 185–187]. This is due to the pathology of T2DM, in which normal, acute injections of insulin help regulate glucose uptake, but chronically elevated levels lead to insulin resistance, hyperglycemia, and complimented with inflammation and vascular damage. Chronically elevated insulin levels in the periphery, it seems, depress insulin sensitivity at the BBB and therefore increases glucose utilization in the brain. Usually in the absence of an alternative fuel source, brain cells starve and neurons will show decrease in their metabolic activity for other physiological functions. Metabolic fuel is inside the body, but the brain cells are not able to able to derive energy from it. The parallels to T2DM are striking, making the term “type 3 diabetes”. However, further research is needed to better understand cerebral metabolic changes in type3 diabetes in relation to the progression of AD.
13. Conclusion and future directions
Earlier T2DM and AD were earlier considered as two independent metabolic disorders. But the recent literature on clincal and basic research has shown that there are common pathophysiological changes and signaling pathways such as PI3K–GSK3β signaling, neuronal stress signalling and inflammatory pathways which associates a relation between the two pathologies and termed as T3D diabetes. The current notion in AD is that abnormally activated neuronal stress signaling pathways have functional consequences in pathological conditions that affect the brain raises the possibility that targeting these mechanisms through anti-diabetic agents and/or small molecule inhibitors may constitute an approach to treat defective brain insulin signaling, cognitive impairment and neurodegeneration. Finally, understanding how disease modifiable risks factors such as aging, ApoE abnormalities, defective insulin signaling, and metabolism are critical in the development of therapeutic interventions. Several studies have reported diet and exercise in slowing the progression of AD. Exciting new therapies, including immunotherapy and deep brain stimulation (DBS), are in the horizon. The recent discovery of an immune system associated gene coding for the immune signal IL-1 receptor accessory protein (IL-1 RAP) provides another target in the treatment of AD. Abnormalities in this gene were reportedly associated with lower levels of microglial activity, faster cognitive decline, and progression to AD. In the future, targeting the IL-1 RAP pathway may be a viable approach to clearing amyloid from the brain and slowing the progression of AD. Further research is needed to better assess the impact of such exogenous mediators of neurodegeneration, and the spectrum of agents that can produce similar abnormalities leading to AD-type neurodegeneration.
Although, significant research has been done till date in understanding the cellular and molecular pathogenesis of diabetes and AD, still several questions remain unanswered on the shared mechanisms of AD and diabetes termed ‘Type-3-Diabetes’. How upstream components (IR and IRS) of brain insulin/IGF signalling successfully involved in AD pathogenesis. Further, investigation of how insulin acts on long term potentiation and long-term depression across synaptic contacts is required in order to understand how stimulators of insulin signaling act to promote neuroprotection in AD brains. The role of ApoEε4 allele on the cerebral insulin signalling in the pathogenesis of AD is not studied. Current research evidence is largely based on a few rodent models and on observational clinical studies. Genetically modified mouse models by ‘CRISPR-gene editing’ on the brain insulin signalling along with meta-analysis studies on insulin resistance and neurodegeneration may provide important clues in the pathogenesis of AD.
Figure 4.
Brief Illustration of insulin signaling pathway in a) healthy brain and b) AD brain. (Figure concept adapted from “Trends Neurosci. 2016 Jun 17. pii: S0166-2236(16)30037-6. “)
Highlights.
-
➢
Type3 diabetes
-
➢
Molecular and cellular mechanisms between Type2diabetes and Alzheimer’s disease
-
➢
Role of insulin signaling on amyloid beta oligomers
-
➢
Inflammatory response in Alzheimer’s disease
Acknowledgments
Work presented in this article is supported by NIH grants AG042178, AG047812 and the Garrison Family Foundation.
Abbreviations
- AD
Alzheimer’s disease
- BBB
blood brain barrier
- RAGE
Receptor for Advanced Glycation End products
- AGE
advanced glycation end products
- IL1β
interleukin 1 beta
- Aβ
amyloid beta
- TNFα
tumor necrosis factor alpha
- TGFβ
transforming growth factor beta
- ApoE4
apolipoprotein E4 genotype
- APP
amyloid precursor protein
- Aβ
β-amyloid
- T2DM
type 2 diabetes mellitus
- NF-kB
nuclear factor kappa-light-chain-enhancer of activated B
- GSK3β
Glucose synthase kinase 3 beta
- NMDAR
N-methyl-D-aspartate receptor
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- Bax
Bcl2 associated X protein
- Bak
Bcl2 associated K protein
- Bcl2
B-cell leukemia/lymphoma2
- Drp1
Dynamin-related protein1
- ADDLs
Amyloid-beta-derived diffusible ligands
- AβOs
β-amyloid oligomers
- hIAPP
Human islet amyloid polypeptide
- IR
insulin receptor
- IRS
insulin receptor substrate
- PI3K
phosphatidylinositol 3-kinase
- PIP2
phosphatidylinositol 4,5 bisphosphate
- PIP3
phosphatidylinositol 3,4,5 trisphosphate
- PKB
protein kinase B
- LTD
long term depression
- GABA
Gamma Amino Butyric Acid
- PSD-95
Post Synaptic Density95
- HD
Huntington’s disease
- ROS
Reactive oxygen species
- RNS
Reactive nitrogen species
- MAPK
Mitogen Activated Protein Kinase
- SOD
superoxide dismutase
- GSH-Px
Glutathione peroxidase
- CAT
Catalase
- Bad
Bcl2 associated death promoter
- FOXO
Forkhead box protein
- ATF4
activating transcription factor 4
- eIF2a
eukaryotic translation initiation factor 2a
- Icv
intra cerebro ventricular
- IGF-1
insulin-like growth factor 1
- JNK
c-Jun N-terminal kinase
- LTP
long term potentiation
- PERK
PKR-like endoplasmic reticulum kinase
- PKR
double-stranded RNA-dependent protein kinase
- CREB
cAMP-response element-binding protein
- PS1
presenilins 1
- PS2
presenilins 2
- BIM
Bcl like protein
- BDNF
Brain-derived neurotrophic factor
- IDE
Insulin degrading enzyme
- STZ
Streptozotocin
- PPAR
peroxisome proliferator-activated receptors
- VDAC1
Voltage dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer’s Association Report. 2015. [Google Scholar]
- 2.Pugazhenthi S, Qin L, Reddy PH. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbadis.2016.04.017. http://dx.doi.org/10.1016/j.bbadis.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandimalla R, Reddy PH. Multiple faces of dynamin-related protein 1 and its role in Alzheimer’s disease pathogenesis. Biochim Biophys Acta. 2016;1862:814–828. doi: 10.1016/j.bbadis.2015.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kandimalla RJ, Prabhakar S, Binukumar BK, Wani WY, Gupta N, Sharma DR, Sunkaria A, Grover VK, Bhardwaj N, Jain K, Gill KD. Apo-Eepsilon4 allele in conjunction with Abeta42 and tau in CSF: biomarker for Alzheimer’s disease. Curr Alzheimer Res. 2011;8:187–196. doi: 10.2174/156720511795256071. [DOI] [PubMed] [Google Scholar]
- 5.Kandimalla RJ, Anand R, Veeramanikandan R, Wani WY, Prabhakar S, Grover VK, Bharadwaj N, Jain K, Gill KD. CSF ubiquitin as a specific biomarker in Alzheimer’s disease. Curr Alzheimer Res. 2014;11:340–348. doi: 10.2174/1567205011666140331161027. [DOI] [PubMed] [Google Scholar]
- 6.Binukumar BK, Gupta N, Sunkaria A, Kandimalla R, Wani WY, Sharma DR, Bal A, Gill KD. Protective efficacy of coenzyme Q10 against DDVP-induced cognitive impairments and neurodegeneration in rats. Neurotox Res. 2012;21:345–357. doi: 10.1007/s12640-011-9289-0. [DOI] [PubMed] [Google Scholar]
- 7.Reddy PH, Tripathi R, Troung Q, Tirumala K, Reddy TP, Anekonda V, Shirendeb UP, Calkins MJ, Reddy AP, Mao P, Manczak M. Abnormal mitochondrial dynamics and synaptic degeneration as early events in Alzheimer’s disease: implications to mitochondria-targeted antioxidant therapeutics. Biochim Biophys Acta. 2012;1822:639–649. doi: 10.1016/j.bbadis.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verdile G, Fuller SJ, Martins RN. The role of type 2 diabetes in neurodegeneration. Neurobiol Dis. 2015;84:22–38. doi: 10.1016/j.nbd.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad W. Overlapped metabolic and therapeutic links between Alzheimer and diabetes. Mol Neurobiol. 2013;47:399–424. doi: 10.1007/s12035-012-8352-z. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Song W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience. 2013;250:140–150. doi: 10.1016/j.neuroscience.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 12.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care. 2005;28:726–735. doi: 10.2337/diacare.28.3.726. [DOI] [PubMed] [Google Scholar]
- 13.Stewart R, Liolitsa D, Type 2 diabetes mellitus. cognitive impairment and dementia. Diabet Med. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- 14.Strachan MW, Deary IJ, Ewing FM, Frier BM. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care. 1997;20:438–445. doi: 10.2337/diacare.20.3.438. [DOI] [PubMed] [Google Scholar]
- 15.Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31:1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Cole AR, Astell A, Green C, Sutherland C. Molecular connexions between dementia and diabetes. Neurosci Biobehav Rev. 2007;31:1046–1063. doi: 10.1016/j.neubiorev.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Fisher TL, White MF. Signaling pathways: the benefits of good communication. Curr Biol. 2004;14:R1005–R1007. doi: 10.1016/j.cub.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 18.Vanhaesebroeck B, Leevers SJ, Ahmadi K, Timms J, Katso R, Driscoll PC, Woscholski R, Parker PJ, Waterfield MD. Synthesis and function of 3-phosphorylated inositol lipids. Annu Rev Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333(Pt 3):471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lizcano JM, Alessi DR. The insulin signalling pathway. Curr Biol. 2002;12:R236–R238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- 21.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 22.Gross M, Sheinin A, Nesher E, Tikhonov T, Baranes D, Pinhasov A, Michaelevski I. Early onset of cognitive impairment is associated with altered synaptic plasticity and enhanced hippocampal GluA1 expression in a mouse model of depression. Neurobiol Aging. 2015;36:1938–1952. doi: 10.1016/j.neurobiolaging.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 23.Chen TJ, Wang DC, Hung HS, Ho HF. Insulin can induce the expression of a memory-related synaptic protein through facilitating AMPA receptor endocytosis in rat cortical neurons. Cell Mol Life Sci. 2014;71:4069–4080. doi: 10.1007/s00018-014-1620-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 25.Huang CC, Lee CC, Hsu KS. An investigation into signal transduction mechanisms involved in insulin-induced long-term depression in the CA1 region of the hippocampus. J Neurochem. 2004;89:217–231. doi: 10.1111/j.1471-4159.2003.02307.x. [DOI] [PubMed] [Google Scholar]
- 26.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 27.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 29.Korol SV, Jin Z, Babateen O, Birnir B. GLP-1 and exendin-4 transiently enhance GABAA receptor-mediated synaptic and tonic currents in rat hippocampal CA3 pyramidal neurons. Diabetes. 2015;64:79–89. doi: 10.2337/db14-0668. [DOI] [PubMed] [Google Scholar]
- 30.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 31.Karoor V, Wang L, Wang HY, Malbon CC. Insulin stimulates sequestration of beta-adrenergic receptors and enhanced association of beta-adrenergic receptors with Grb2 via tyrosine 350. J Biol Chem. 1998;273:33035–33041. doi: 10.1074/jbc.273.49.33035. [DOI] [PubMed] [Google Scholar]
- 32.Lee CC, Huang CC, Wu MY, Hsu KS. Insulin stimulates postsynaptic density-95 protein translation via the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway. J Biol Chem. 2005;280:18543–18550. doi: 10.1074/jbc.M414112200. [DOI] [PubMed] [Google Scholar]
- 33.Recio-Pinto E, Lang FF, Ishii DN. Insulin and insulin-like growth factor II permit nerve growth factor binding and the neurite formation response in cultured human neuroblastoma cells. Proc Natl Acad Sci U S A. 1984;81:2562–2566. doi: 10.1073/pnas.81.8.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernyhough P, Willars GB, Lindsay RM, Tomlinson DR. Insulin and insulin-like growth factor I enhance regeneration in cultured adult rat sensory neurones. Brain Res. 1993;607:117–124. doi: 10.1016/0006-8993(93)91496-f. [DOI] [PubMed] [Google Scholar]
- 35.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 36.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes impaired fasting glucose and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 37.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 38.Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 39.Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016;4:519–522. doi: 10.3892/br.2016.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–1247. doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Hum Mol Genet. 2006;15:1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- 43.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F, Ricquier D. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl 1):S130–S135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 44.Lipinski B. Pathophysiology of oxidative stress in diabetes mellitus. J Diabetes Complications. 2001;15:203–210. doi: 10.1016/s1056-8727(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Matute P, Zulet MA, Martinez JA. Reactive species and diabetes: counteracting oxidative stress to improve health. Curr Opin Pharmacol. 2009;9:771–779. doi: 10.1016/j.coph.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 46.Bucala R, Cerami A. Advanced glycosylation: chemistry biology and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34. doi: 10.1016/s1054-3589(08)60961-8. [DOI] [PubMed] [Google Scholar]
- 47.Sato T, Shimogaito N, Wu X, Kikuchi S, Yamagishi S, Takeuchi M. Toxic advanced glycation end products (TAGE) theory in Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2006;21:197–208. doi: 10.1177/1533317506289277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woltjer RL, Maezawa I, Ou JJ, Montine KS, Montine TJ. Advanced glycation endproduct precursor alters intracellular amyloid-beta/A beta PP carboxy-terminal fragment aggregation and cytotoxicity. J Alzheimers Dis. 2003;5:467–476. doi: 10.3233/jad-2003-5607. [DOI] [PubMed] [Google Scholar]
- 49.Kuhla B, Haase C, Flach K, Luth HJ, Arendt T, Munch G. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem. 2007;282:6984–6991. doi: 10.1074/jbc.M609521200. [DOI] [PubMed] [Google Scholar]
- 50.Gasparini L, Netzer WJ, Greengard P. H. Xu, Does insulin dysfunction play a role in Alzheimer’s disease? Trends Pharmacol Sci. 2002;23:288–293. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 51.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 52.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 53.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 54.Kops GJ, de Ruiter ND, De Vries-Smits AM, Powell DR, Bos JL, Burgering BM. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature. 1999;398:630–634. doi: 10.1038/19328. [DOI] [PubMed] [Google Scholar]
- 55.Biggs WH, 3rd, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci U S A. 1999;96:7421–7426. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 57.Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K–Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. doi: 10.1016/s0959-4388(00)00211-7. [DOI] [PubMed] [Google Scholar]
- 58.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 59.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zong WX, Edelstein LC, Chen C, Bash J, Gelinas C. The prosurvival Bcl-2 homolog Bfl-1/A1 is a direct transcriptional target of NF-kappaB that blocks TNFalpha-induced apoptosis. Genes Dev. 1999;13:382–387. doi: 10.1101/gad.13.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 64.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 65.Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: review and clinical implications. Neurosci Biobehav Rev. 2000;24:855–872. doi: 10.1016/s0149-7634(00)00040-3. [DOI] [PubMed] [Google Scholar]
- 66.Abbott MA, Wells DG, Fallon JR. The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci. 1999;19:7300–7308. doi: 10.1523/JNEUROSCI.19-17-07300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christie JM, Wenthold RJ, Monaghan DT. Insulin causes a transient tyrosine phosphorylation of NR2A and NR2B NMDA receptor subunits in rat hippocampus. J Neurochem. 1999;72:1523–1528. doi: 10.1046/j.1471-4159.1999.721523.x. [DOI] [PubMed] [Google Scholar]
- 68.Jonas EA, Knox RJ, Smith TC, Wayne NL, Connor JA, Kaczmarek LK. Regulation by insulin of a unique neuronal Ca2+ pool and of neuropeptide secretion. Nature. 1997;385:343–346. doi: 10.1038/385343a0. [DOI] [PubMed] [Google Scholar]
- 69.Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- 70.Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 71.Wickelgren I. Tracking insulin to the mind. Science. 1998;280:517–519. doi: 10.1126/science.280.5363.517. [DOI] [PubMed] [Google Scholar]
- 72.Zhao W, Chen H, Xu H, Moore E, Meiri N, Quon MJ, Alkon DL. Brain insulin receptors and spatial memory. Correlated changes in gene expression, tyrosine phosphorylation and signaling molecules in the hippocampus of water maze trained rats. J Biol Chem. 1999;274:34893–34902. doi: 10.1074/jbc.274.49.34893. [DOI] [PubMed] [Google Scholar]
- 73.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol. 2001;177:125–134. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 74.Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- 75.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68:509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 76.Siddle K, Urso B, Niesler CA, Cope DL, Molina L, Surinya KH, Soos MA. Specificity in ligand binding and intracellular signalling by insulin and insulin-like growth factor receptors. Biochem Soc Trans. 2001;29:513–525. doi: 10.1042/bst0290513. [DOI] [PubMed] [Google Scholar]
- 77.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 78.Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J Biol Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- 79.Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solano DC, Sironi M, Bonfini C, Solerte SB, Govoni S, Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 2000;14:1015–1022. doi: 10.1096/fasebj.14.7.1015. [DOI] [PubMed] [Google Scholar]
- 82.Dar TA, Sheikh IA, Ganie SA, Ali R, Singh LR, Gan SH, Kamal MA, Zargar MA. Molecular linkages between diabetes and Alzheimer’s disease: current scenario and future prospects. CNS Neurol Disord Drug Targets. 2014;13:290–298. doi: 10.2174/18715273113126660135. [DOI] [PubMed] [Google Scholar]
- 83.Watson GS, Craft S. The role of insulin resistance in the pathogenesis of Alzheimer’s disease: implications for treatment. CNS Drugs. 2003;17:27–45. doi: 10.2165/00023210-200317010-00003. [DOI] [PubMed] [Google Scholar]
- 84.Hak AE, Pols HA, Stehouwer CD, Meijer J, Kiliaan AJ, Hofman A, Breteler MM, Witteman JC. Markers of inflammation and cellular adhesion molecules in relation to insulin resistance in nondiabetic elderly: the Rotterdam study. J Clin Endocrinol Metab. 2001;86:4398–4405. doi: 10.1210/jcem.86.9.7873. [DOI] [PubMed] [Google Scholar]
- 85.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 86.Hotamisligil GS. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S53–S55. doi: 10.1038/sj.ijo.0802502. [DOI] [PubMed] [Google Scholar]
- 87.Akter K, Lanza EA, Martin SA, Myronyuk N, Rua M, Raffa RB. Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? Br J Clin Pharmacol. 2011;71:365–376. doi: 10.1111/j.1365-2125.2010.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52:168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 89.Engelhart MJ, Geerlings MI, Meijer J, Kiliaan A, Ruitenberg A, van Swieten JC, Stijnen T, Hofman A, Witteman JC, Breteler MM. Inflammatory proteins in plasma and the risk of dementia: the rotterdam study. Arch Neurol. 2004;61:668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 90.Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anthony JC, Breitner JC, Zandi PP, Meyer MR, Jurasova I, Norton MC, Stone SV. Reduced prevalence of AD in users of NSAIDs and H2 receptor antagonists: the Cache County study. Neurology. 2000;54:2066–2071. doi: 10.1212/wnl.54.11.2066. [DOI] [PubMed] [Google Scholar]
- 92.Broe GA, Grayson DA, Creasey HM, Waite LM, Casey BJ, Bennett HP, Brooks WS, Halliday GM. Anti-inflammatory drugs protect against Alzheimer disease at low doses. Arch Neurol. 2000;57:1586–1591. doi: 10.1001/archneur.57.11.1586. [DOI] [PubMed] [Google Scholar]
- 93.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Advanced glycation endproducts and their receptor RAGE in Alzheimer’s disease. Neurobiol Aging. 2011;32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 95.Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38. doi: 10.1146/annurev-neuro-071013-014100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baleriola J, Walker CA, Jean YY, Crary JF, Troy CM, Nagy PL, Hengst U. Axonally synthesized ATF4 transmits a neurodegenerative signal across brain regions. Cell. 2014;158:1159–1172. doi: 10.1016/j.cell.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Connor T, Sadleir KR, Maus E, Velliquette RA, Zhao J, Cole SL, Eimer WA, Hitt B, Bembinster LA, Lammich S, Lichtenthaler SF, Hebert SS, De Strooper B, Haass C, Bennett DA, Vassar R. Phosphorylation of the translation initiation factor eIF2alpha increases BACE1 levels and promotes amyloidogenesis. Neuron. 2008;60:988–1009. doi: 10.1016/j.neuron.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, Cavener DR, Klann E. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16:1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 101.De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J, Acton PJ, Shughrue PJ, Chen-Dodson E, Kinney GG, Klein WL. Alzheimer’s disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;29:1334–1347. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lourenco MV, Ferreira ST, De Felice FG. Neuronal stress signaling and eIF2alpha phosphorylation as molecular links between Alzheimer’s disease and diabetes. Prog Neurobiol. 2015;129:37–57. doi: 10.1016/j.pneurobio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Bomfim TR, Forny-Germano L, Sathler LB, Brito-Moreira J, Houzel JC, Decker H, Silverman MA, Kazi H, Melo HM, McClean PL, Holscher C, Arnold SE, Talbot K, Klein WL, Munoz DP, Ferreira ST, De Felice FG. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease-associated Abeta oligomers. J Clin Invest. 2012;122:1339–1353. doi: 10.1172/JCI57256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ledo JH, Azevedo EP, Clarke JR, Ribeiro FC, Figueiredo CP, Foguel D, De Felice FG, Ferreira ST. Amyloid-beta oligomers link depressive-like behavior and cognitive deficits in mice. Mol Psychiatry. 2013;18:1053–1054. doi: 10.1038/mp.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, Sathler LB, Brito-Moreira J, Amaral OB, Silva CA, Freitas-Correa L, Espirito-Santo S, Campello-Costa P, Houzel JC, Klein WL, Holscher C, Carvalheira JB, Silva AM, Velloso LA, Munoz DP, Ferreira ST, De Felice FG. TNF-alpha mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s beta-amyloid oligomers in mice and monkeys. Cell Metab. 2013;18:831–843. doi: 10.1016/j.cmet.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 106.Lourenco MV, Ledo JH. Targeting Alzheimer’s pathology through PPARgamma signaling: modulation of microglial function. J Neurosci. 2013;33:5083–5084. doi: 10.1523/JNEUROSCI.0172-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- 108.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 109.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Abeta1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Georganopoulou DG, Chang L, Nam JM, Thaxton CS, Mufson EJ, Klein WL, Mirkin CA. Nanoparticle-based detection in cerebral spinal fluid of a soluble pathogenic biomarker for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2005;102:2273–2276. doi: 10.1073/pnas.0409336102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, Krafft GA, Klein WL. Alzheimer’s disease-affected brain: presence of oligomeric A beta ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci U S A. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kokubo H, Kayed R, Glabe CG, Yamaguchi H. Soluble Abeta oligomers ultrastructurally localize to cell processes and might be related to synaptic dysfunction in Alzheimer’s disease brain. Brain Res. 2005;1031:222–228. doi: 10.1016/j.brainres.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 113.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, Lambert MP, Velasco PT, Bigio EH, Finch CE, Krafft GA, Klein WL. Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Viola KL, Sbarboro J, Sureka R, De M, Bicca MA, Wang J, Vasavada S, Satpathy S, Wu S, Joshi H, Velasco PT, MacRenaris K, Waters EA, Lu C, Phan J, Lacor P, Prasad P, Dravid VP, Klein WL. Towards non-invasive diagnostic imaging of early-stage Alzheimer’s disease. Nat Nanotechnol. 2015;10:91–98. doi: 10.1038/nnano.2014.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xia W, Yang T, Shankar G, Smith IM, Shen Y, Walsh DM, Selkoe DJ. A specific enzyme-linked immunosorbent assay for measuring beta-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch Neurol. 2009;66:190–199. doi: 10.1001/archneurol.2008.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL. Abeta oligomer-induced aberrations in synapse composition shape and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li S, Jin M, Koeglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Abeta oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B–containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Caselli RJ, Chen K, Lee W, Alexander GE, Reiman EM. Correlating cerebral hypometabolism with future memory decline in subsequent converters to amnestic pre-mild cognitive impairment. Arch Neurol. 2008;65:1231–1236. doi: 10.1001/archneurol.2008.1. [DOI] [PubMed] [Google Scholar]
- 120.Langbaum JB, Chen K, Caselli RJ, Lee W, Reschke C, Bandy D, Alexander GE, Burns CM, Kaszniak AW, Reeder SA, Corneveaux JJ, Allen AN, Pruzin J, Huentelman MJ, Fleisher AS, Reiman EM. Hypometabolism in Alzheimer-affected brain regions in cognitively healthy Latino individuals carrying the apolipoprotein E epsilon4 allele. Arch Neurol. 2010;67:462–468. doi: 10.1001/archneurol.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci. 2008;1147:180–195. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mosconi L, Mistur R, Switalski R, Tsui WH, Glodzik L, Li Y, Pirraglia E, De Santi S, Reisberg B, Wisniewski T, de Leon MJ. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–822. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Iwangoff P, Armbruster R, Enz A, Meier-Ruge W. Glycolytic enzymes from human autoptic brain cortex: normal aged and demented cases. Mech Ageing Dev. 1980;14:203–209. doi: 10.1016/0047-6374(80)90120-7. [DOI] [PubMed] [Google Scholar]
- 124.Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv Exp Med Biol. 2004;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- 125.Hoyer S, Nitsch R, Oesterreich K. Predominant abnormality in cerebral glucose utilization in late-onset dementia of the Alzheimer type: a cross-sectional comparison against advanced late-onset and incipient early-onset cases. J Neural Transm Park Dis Dement Sect. 1991;3:1–14. doi: 10.1007/BF02251132. [DOI] [PubMed] [Google Scholar]
- 126.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SMJR. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 127.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance IRS-1 dysregulation and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 129.de la Monte SM. Type 3 diabetes is sporadic Alzheimers disease: mini-review. Eur Neuropsychopharmacol. 2014;24:1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de la Monte SM, Wands JR. Review of insulin insulin-like growth factor expression, signaling and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 131.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 132.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 133.Krikorian R, Eliassen JC, Boespflug EL, Nash TA, Shidler MD. Improved cognitive-cerebral function in older adults with chromium supplementation. Nutr Neurosci. 2010;13:116–122. doi: 10.1179/147683010X12611460764084. [DOI] [PubMed] [Google Scholar]
- 134.Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 135.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 136.de la Monte SM, Longato L, Tong M, Wands JR. Insulin resistance and neurodegeneration: roles of obesity type 2 diabetes mellitus and non-alcoholic steatohepatitis. Curr Opin Investig Drugs. 2009;10:1049–1060. [PMC free article] [PubMed] [Google Scholar]
- 137.de la Monte SM. Contributions of brain insulin resistance and deficiency in amyloid-related neuro degeneration in Alzheimer’s disease. Drugs. 2011;72:1–18. doi: 10.2165/11597760-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- 139.Hoyer S, Lee SK, Loffler T, Schliebs R. Inhibition of the neuronal insulin receptor. An in vivo model for sporadic Alzheimer disease? Ann N Y Acad Sci. 2000;920:256–258. doi: 10.1111/j.1749-6632.2000.tb06932.x. [DOI] [PubMed] [Google Scholar]
- 140.Labak M, Foniok T, Kirk D, Rushforth D, Tomanek B, Jasinski A, Grieb P. Metabolic changes in rat brain following intracerebroventricular injections of streptozotocin: a model of sporadic Alzheimer’s disease. Acta Neurochir Suppl. 2010;106:177–181. doi: 10.1007/978-3-211-98811-4_32. [DOI] [PubMed] [Google Scholar]
- 141.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 142.Reddy PH. Inhibitors of mitochondrial fission as a therapeutic strategy for diseases with oxidative stress and mitochondrial dysfunction. J Alzheimers Dis. 2014;40:245–256. doi: 10.3233/JAD-132060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manczak M, Reddy PH. Abnormal interaction of oligomeric amyloid-beta with phosphorylated tau: implications to synaptic dysfunction and neuronal damage. J Alzheimers Dis. 2013;36:285–295. doi: 10.3233/JAD-130275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Calkins MJ, Manczak M, Mao P, Shirendeb U, Reddy PH. Impaired mitochondrial biogenesis defective axonal transport of mitochondria abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2011;20:4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel Mechanistic Pathway, Biomarker, and Treatment Target in the Pathology of Alzheimer’s Disease? Trends Neurosci. 2016 Jun 7; doi: 10.1016/j.tins.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kuusisto J, Koivisto K, Mykkanen L, Helkala EL, Vanhanen M, Hanninen T, Kervinen K, Kesaniemi YA, Riekkinen PJ, Laakso M. Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 148.Schrijvers EM, Witteman JC, Sijbrands EJ, Hofman A, Koudstaal PJ, Breteler MM. Insulin metabolism and the risk of Alzheimer disease: the Rotterdam Study. Neurology. 2010;75:1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer’s disease risk with obesity diabetes and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 150.Dorner TE, Rieder A. Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol. 2012;155:56–65. doi: 10.1016/j.ijcard.2011.01.076. [DOI] [PubMed] [Google Scholar]
- 151.Whitmer RA. The epidemiology of adiposity and dementia. Curr Alzheimer Res. 2007;4:117–122. doi: 10.2174/156720507780362065. [DOI] [PubMed] [Google Scholar]
- 152.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64:392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60:117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 155.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 156.Razay G, Wilcock GK. Hyperinsulinaemia and Alzheimer’s disease. Age Ageing. 1994;23:396–399. doi: 10.1093/ageing/23.5.396. [DOI] [PubMed] [Google Scholar]
- 157.Hildreth KL, Van Pelt RE, Schwartz RS. Obesity insulin resistance and Alzheimer’s disease. Obesity (Silver Spring) 2012;20:1549–1557. doi: 10.1038/oby.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010;75:764–770. doi: 10.1212/WNL.0b013e3181eee25f. [DOI] [PubMed] [Google Scholar]
- 160.Singh RB, Gupta S, Dherange P, De Meester F, Wilczynska A, Alam SE, Pella D, Wilson DW. Metabolic syndrome: a brain disease. Can J Physiol Pharmacol. 2012;90:1171–1183. doi: 10.1139/y2012-122. [DOI] [PubMed] [Google Scholar]
- 161.Ojo O, Brooke J. Evaluating the Association between Diabetes Cognitive Decline and Dementia. Int J Environ Res Public Health. 2015;12:8281–8294. doi: 10.3390/ijerph120708281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2:356–363. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bourdel-Marchasson I, Lapre E, Laksir H, Puget E. Insulin resistance diabetes and cognitive function: consequences for preventative strategies. Diabetes Metab. 2010;36:173–181. doi: 10.1016/j.diabet.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 164.Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008;58:10–14. doi: 10.1016/j.neuron.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects againstmicroglia-dependent amyloid-beta toxicity through inhibiting NFkappaB signaling. J. Biol. Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 166.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol. Cell. Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Van Eldik LJ, Carrillo MC, Cole PE, Feuerbach D, Greenberg BD, Hendrix JA, Kennedy M, Kozauer N, Margolin RA, Molinuevo JL, Mueller R R, Ransohoff RM, Wilcock DM, Bain L L, Bales K. The roles of inflammation and immune mechanisms in Alzheimer’s disease Alzheimer’s and Dementia: Translational Research and Clinical Interventions. 2016;2:99–109. doi: 10.1016/j.trci.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Harada K, Kamiya T, Tsuboi T. Gliotransmitter Release from Astrocytes: Functional Developmental and Pathological Implications in the Brain. Front Neurosci. 2016;9:499. doi: 10.3389/fnins.2015.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H. NFkappaB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron. 2015;85:101–115. doi: 10.1016/j.neuron.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Henderson ST. High carbohydrate diets and Alzheimer’s disease. Med Hypotheses. 2004;62:689–700. doi: 10.1016/j.mehy.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 173.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 175.Fukuyama H, Ogawa M, Yamauchi H, Yamaguchi S, Kimura J, Yonekura Y, Konishi J. Altered cerebral energy metabolism in Alzheimer’s disease: a PET study. J Nucl Med. 1994;35:1–6. [PubMed] [Google Scholar]
- 176.Correia SC, Santos RX, Carvalho C, Cardoso S, Candeias E, Santos MS, Oliveira CR, Moreira PI. Insulin signaling glucose metabolism and mitochondria: major players in Alzheimer’s disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 177.Mamelak M, Alzheimer’ s disease. oxidative stress and gammahydroxybutyrate. Neurobiol Aging. 2007;28:1340–1360. doi: 10.1016/j.neurobiolaging.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 178.Mosconi L, De Santi S, Li J, Tsui WH, Li Y, Boppana M, Laska E, Rusinek H, de Leon MJ. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–692. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer’s disease. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 180.Cook DG, Leverenz JB, McMillan PJ, Kulstad JJ, Ericksen S, Roth RA, Schellenberg GD, Jin LW, Kovacina KS, Craft S. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. Am J Pathol. 2003;162:313–319. doi: 10.1016/s0002-9440(10)63822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R. Alzheimer’s beta-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci. 2002;22:RC221. doi: 10.1523/JNEUROSCI.22-10-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 184.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 185.Convit A. Links between cognitive impairment in insulin resistance: an explanatory model. Neurobiol Aging. 2005;26(Suppl 1):31–35. doi: 10.1016/j.neurobiolaging.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 186.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol. 2004;3:169–178. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 187.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]