Abstract
Background
Abnormal responses to tactile stimuli are a common feature of Autism Spectrum Disorder (ASD). Several lines of evidence suggest that GABAergic function, which has a crucial role in tactile processing, is altered in ASD. In this study, we determine whether in vivo GABA levels are altered in children with ASD, and whether alterations in GABA levels are associated with abnormal tactile function in these children.
Methods
GABA-edited MRS was acquired in 37 children with Autism and 35 Typically Developing Children from voxels over primary sensorimotor and occipital cortices. Children performed tactile tasks previously shown to be altered in ASD, linked to inhibitory mechanisms. Detection threshold was measured with- and without the presence of a slowly increasing sub-threshold stimulus. Amplitude discrimination was measured with- and without the presence of an adapting stimulus, and frequency discrimination was measured.
Results
Sensorimotor GABA levels were significantly reduced in children with autism compared to healthy controls. Occipital GABA levels were normal. Sensorimotor GABA levels correlated with dynamic detection threshold as well as with the effect of sub-threshold stimulation. Sensorimotor GABA levels also correlated with amplitude discrimination after adaptation (an effect absent in autism) and frequency discrimination in controls, but not in children with autism.
Conclusions
GABA levels correlate with behavioral measures of inhibition. Children with autism have reduced GABA, associated with abnormalities in tactile performance. We show here that altered in vivo GABA levels might predict abnormal tactile information processing in ASD and that the GABA system may be a future target for therapies.
Keywords: Autism, Somatosensory, GABA, Magnetic Resonance Spectroscopy, Tactile, MRS, Touch
Introduction
Autism Spectrum Disorder (ASD) is characterized by impairments in social interaction, disordered communication, and repetitive behaviors. Despite decades of study, the neurophysiological basis of ASD remains poorly understood. Multiple lines of evidence suggest that γ-aminobutyric acid (GABA), the main inhibitory neurotransmitter in the brain, plays a role in the pathophysiology of ASD, including evidence from mouse models (Fukuda et al., 2005, Chao et al., 2010), expression of GABA receptor genes (Abrahams and Geschwind, 2008, DeLorey, 2005), altered GABA levels (Gaetz et al., 2014, Rojas et al., 2008), and altered cortical structure (Fatemi et al., 2009, Oblak et al., 2010, Casanova et al., 2006).
Difficulties in sensory (including tactile) processing are a long recognized feature of ASD (Rogers and Ozonoff, 2005). Impairments in the response to sensory stimulation were reported in Kanner’s original account of the disorder, are described as phenotypically characteristic of ASD, and have been added to DSM-5 criteria for ASD. However, the underlying cortical mechanisms remain unclear. We recently reported, using tightly controlled tactile psychophysics, that children with ASD have impairments in specific tactile tasks, as compared to Typically Developing Children (TDC).
Detection threshold in TDC increases after being exposed to dynamically increasing sub-threshold stimulation, compared to a static condition. This effect is absent in children with ASD, and children with ASD also show higher static detection threshold than TDC. Sub-threshold stimulation predominantly activates GABAergic feedforward mechanisms, suppressing cortical activity (Blankenburg et al., 2003, Connors et al., 1988, Zhang and Sun, 2011) and therefore reducing the detectability of subsequent stimuli. Given that this effect is absent in children with ASD (Puts et al., 2013, Puts et al., 2014a), it is suggestive of abnormal feed-forward inhibitory mechanisms. Mechanisms to suppress weak stimulation, such as ‘sensory gating’, have been implicated in filtering of sensory information, so that only relevant information is processed further (Blankenburg et al., 2003, Favorov and Kursun, 2011, Francisco et al., 2013).
In our previous study, we also showed higher-amplitude discrimination thresholds in ASD compared to TDC, and we showed that adaptation (habituation to prior sensory stimulation) is absent in ASD (Tannan et al., 2008, Puts et al., 2014a). As GABAergic lateral inhibitory connectivity in the cortex plays a role in separating tactile stimulus signals, as does the decrease in cortical firing rate that occurs during tactile habituation (Tommerdahl et al., 2010, Whitsel et al., 1989), these behavioral results are suggestive of altered inhibitory function in ASD.
Given the importance of GABA in the regulation and control of neuronal activity and in tactile processing, it is important to link specific aspects of tactile function that may be altered in ASD, to altered GABAergic function. This understanding is key in understanding the pathophysiology underlying tactile abnormalities in ASD.
It is possible to directly measure the concentration of GABA in vivo in the human brain using edited Magnetic Resonance Spectroscopy (MRS)(Puts and Edden, 2012). One study in children with ASD showed that motor cortex GABA levels were decreased in ASD, but that occipital GABA levels were normal (Gaetz et al., 2014), but little is known about the functional relevance of these changes (Ford and Crewther, 2016). In healthy controls, MRS of GABA has been used to show functional regionally specific correlations between brain GABA levels and behavior, including tactile frequency discrimination (Puts et al., 2011), brain activity (Donahue et al., 2010, Muthukumaraswamy et al., 2009), as well as learning (Floyer-Lea et al., 2006). Abnormal GABA levels have been shown in several neurodevelopmental, psychiatric and neurological disorders (for a review see (Puts and Edden, 2012)).
To date, no studies have attempted to investigate whether brain GABA levels in ASD are associated with tactile behavioral abnormalities found in this population. The aim of this study was to investigate the relationship between GABA levels and tactile performance in ASD, as measured using a battery of vibrotactile tests linked to GABAergic function. First we hypothesize that children with ASD will have lower GABA levels in the sensorimotor cortex compared to TDC. Second, as it is suggested that sub-threshold stimulation drives GABAergic feed-forward inhibition, we hypothesize that participants with higher levels of GABA show a stronger increase in dynamic detection threshold compared to the static condition and that children with ASD show an absence of this effect (Puts et al., 2014a), which correlates with lower GABA levels. Third, as tactile adaptation leads to a decrease in firing rate through GABAergic signaling, we expect that participants with higher GABA levels show higher amplitude discrimination thresholds (more GABA would lead to a stronger decrease in firing rate which would be reflected by a stronger effect of adaptation) after adaptation compared to amplitude discrimination without adaptation, and that children with ASD show no adaptation. Lastly, animal studies have shown that GABA plays an important role in encoding the periodicity of neuronal firing that encode stimulus frequency (McLaughlin and Juliano, 2005); in previous work we showed that tactile frequency discrimination correlates negatively with GABA levels in both healthy adults (Puts et al., 2011) and healthy children (Puts et al., 2015). In the present study we will explore this link in children with ASD.
Methods and materials
Population
In total, this study was performed on 37 children with ASD (10.69 ± 1.4 years, 6 female) and 35 typically developing children (TDC; average age 10.09 ± 1.25 years; 8 female) with all children between 8-12 years old. Of these, 16 children with ASD and 16 children with ASD children were included in previous behavioral studies (Puts et al., 2014a); GABA MRS data from 16 TDC were also presented in (Puts et al., 2015). Informed consent was obtained from a parent of each child (who also assented to testing), with the approval of Kennedy Krieger Institute and Johns Hopkins School of Medicine Institutional Review Boards.
Participants in the ASD cohort met the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria for ASD and this was confirmed with the Autism Diagnostic Observation Schedule-Generic (ADOS-G)(Lord et al., 2000) and Autism Diagnostic Interview-Revised (ADI-R) (Lord et al., 1994). Children with identifiable causes of autism (e.g., Fragile X syndrome) and neurological disorders including epilepsy were excluded. Standard intellectual functioning was assessed using the Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV) (Wechsler, 2003). Children with full-scale IQ scores below 80 were excluded from participation in all studies unless there was a 12 point or greater index discrepancy, in which case either the Verbal Comprehension Index or Perceptual Reasoning Index (PRI) was required to be > 80 and the lower of the two was required to be > 65. To avoid effects on cognitive and behavioral measures, stimulant medications were discontinued the day prior to and the day of testing. Participants were, however, allowed to continue treatment with other psychotropic medications that would normally require a longer washout period, for both ethical and practical reasons. All children in the TDC cohort were free of criteria for psychiatric disorders as assessed using the Diagnostic Interview for Children and Adolescents-Fourth Edition (DICA-IV). None of the children in the TDC cohort were prescribed psychoactive medications. Handedness was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971).
MRI Acquisition
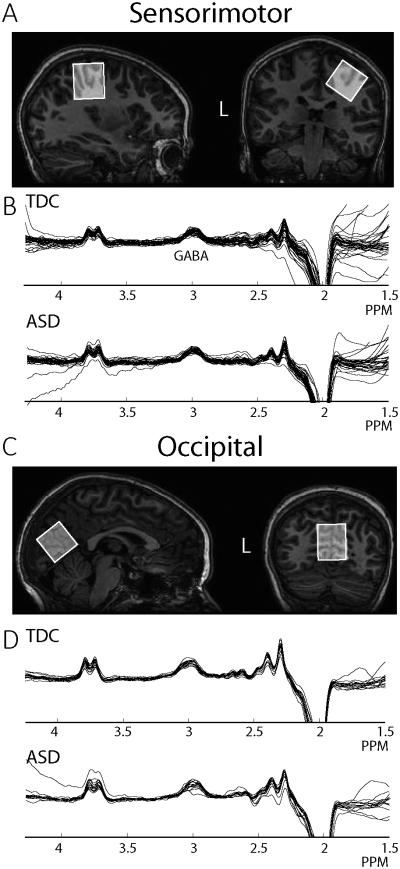
All GABA-edited MRS data were acquired using a Philips 3T Achieva MRI scanner (Best, the Netherlands; 32-channel head coil for receive and body coil for transmit). For 16 children with ASD and 16 TDC, MEGA-PRESS (Mescher et al., 1998) data were acquired from (3 cm)3 voxels placed on the right sensorimotor cortex (SM1; as tactile stimulation was performed on the left hand, Figure 1A) and midline occipital cortex (OCC; Figure 1C) using the following parameters for each voxel acquisition: 40 blocks of 8-step phase cycles in a ~10 min acquisition. A separate water scan was acquired for quantification. In a further 21 children with ASD and 19 TDC, MEGA-PRESS data were also acquired in a (3cm)3 voxel over sensorimotor cortex using a sequence where editing on and off scans were interleaved at the level of each 16-step phase cycle, acquiring for a total of 320 scans, with an unsuppressed water reference acquired as part of the sequence rather than separate. While there were no differences in data quality between the two acquisitions, interleaving at the level of each 16 step phase cycle allows for better post-hoc frequency correction and therefore reduce the potential effect of subject motion and scanner drift. The following parameters were common between the two acquisitions: TE 68 ms, 14 ms editing pulses at 7.46 ppm (edit-OFF) and 1.9 ppm (edit-ON) ordered OFF-first, TR 2s, 2048 datapoints, 2 kHz spectral width, VAPOR water suppression (Tkac et al., 1999). The SM1 voxel was centered on the central sulcus, posterior to the hand-knob (Yousry et al., 1997) in the axial plane (Figure 1A) and rotated to align with the cortical surface (previously described (Puts et al., 2011)). The OCC voxel (only acquired in 16 TDC and 16 ASD) was centered on the midline and aligned with the cerebellar tentorium (Fig 1C). Prior to voxel placement, a 1 mm3 isotropic T1-weighted image (MP-RAGE) was acquired for voxel localization and segmentation (TR = 7.99 ms, TE = 3.76 ms, Flip angle = 8°) for each participant. To motivate participant compliance during scanning, children were allowed to watch a movie and ‘points’ were given for lying still that could be exchanged for prizes.
Figure 1.
A. Sensorimotor (SM1) MRS voxel location. The voxel was placed on the hand area of the right primary motor cortex and rotated to align with the edge of the brain. B. Good quality spectra from all participants retained (6 ASD and 3 TDC excluded). C. Occipital voxel location. The voxel was placed on the midline and the bottom edge aligned with the cerebellar tentorium. D. Good quality spectra were acquired in 16 children with ASD and 16 TDC.
Tactile psychophysics
All children performed a battery of tactile tasks outside the scanner. The procedures have been described in detail elsewhere (Puts et al., 2013). This battery has been shown to be suitable for children and was acquired in 30 - 40 minutes, with a break half-way. A CM4 four-digit tactile stimulator (Cortical Metrics, North Carolina) was used for vibrotactile stimulation (Holden et al., 2012). Stimuli were delivered to the glabrous skin of left digits 2 (LD2) and 3 (LD3) and all stimuli were presented within the flutter range (25-50 Hz) using sinusoidal stimuli. Visual feedback, task responses, and data collection was performed on an Acer Onebook Netbook computer, running Cortical Metrics software (Holden et al., 2012). All tasks used stepwise tracking for threshold determination and stimulus order was pseudo-randomized. Children performed the following tasks:
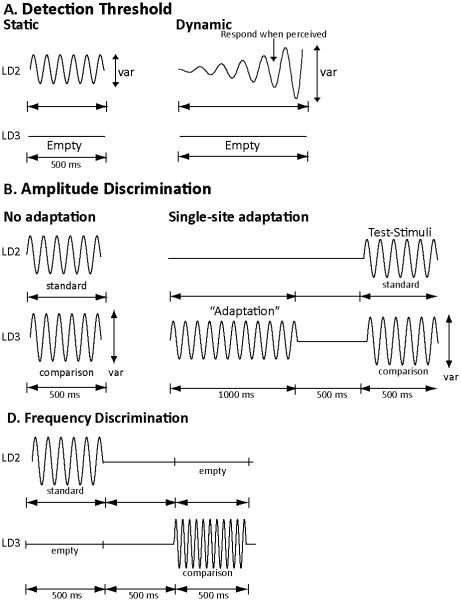
(1) Static and dynamic detection threshold (Figure 2A). In the static task, children were asked to detect a weak stimulus and indicate on which finger they felt the stimulus (starting amplitude 25 µm, 25 Hz, 500 ms; Inter Trial Interval = 5 s; 24 trials). In the dynamic task, stimulus amplitude started at zero after a variable delay (0-2500 ms) and increased with 2 microns per second (ITI 10 s; 7 trials). Participants were asked to indicate on which finger the stimulus was perceived.
Figure 2.
Psychophysical Tasks. A. Static and dynamic detection threshold. Participants were asked to detect a weak stimulus that was either Static, or Dynamic (increasing in amplitude) and determine stimulus location. B. Amplitude discrimination with- and without single site adaptation. In each trial, participants were asked to judge which of two simultaneously applied test stimuli was most intense, trials were preceded by 1s adapting stimulus in the adaptation condition. C. Sequential frequency discrimination. Participants were asked to judge which of two sequentially applied stimuli had the highest frequency.
(2) Amplitude discrimination without adaptation (un-adapted) and with single-site adaptation (Figure 2B). In the un-adapted condition, participants were asked to judge which of two simultaneously applied stimuli was more intense (both 25 Hz; 500 ms; Standard stimulus amplitude: 100 µm; initial comparison stimulus amplitude: 200 µm; ITI 5 s; 20 trials). In the adapted task, one of the two test stimuli was preceded by a 1 second long ‘adapting’ stimulus (Puts et al., 2013, Tannan et al., 2008) (also 25 Hz; amplitude 100 µm) which participants were told to ignore. The effect of adaption was calculated as the percentage difference between the threshold in the single-site adaptation task and the no-adaptation task (adapted - unadapted)/unadapted *100).
(3) Sequential frequency discrimination (Figure 2C). Participants were asked to indicate which of two stimuli (with the same amplitude but varying frequency; both 500 ms; 200 µm; standard stimulus frequency 30 Hz; comparison stimulus starting frequency 40 Hz) had higher frequency.
MRS Analysis
Using Gannet 2.0 (Edden et al., 2014), GABA data were frequency- and phase-corrected using spectral registration (Near et al., 2015) and filtered with a 3 Hz exponential line broadening and zero-filled to 32768 points. GABA concentration was estimated using a five-parameter Gaussian model, fitting between 2.79 and 3.55 ppm. GABA levels were calculated relative to the unsuppressed water signal from the same voxel. For each subject, GABA levels were tissue-corrected, assuming the concentration of GABA in grey matter (GM) is twice the concentration of GABA in while matter (WM) or alpha = 0.5, as per Harris et al., 2015b, then, also as per Harris et al 2015b, group normalization for mean tissue fractions was performed across all data. GM, WM and CSF fractions were calculated using the SPM “new segment” routine implemented within Gannet 2.0. Given the limited selectivity of the 14 ms editing pulses, all reported GABA values refer to GABA + co-edited macro-molecules (Harris et al., 2015b, Mullins et al., 2014). Movement during the scans, which may lead to underestimation of the GABA concentration (Harris et al., 2014a), was assessed from the standard deviation of the offset of the water signal across the scan (Harris et al., 2014a) and GABA signal fit errors (Evans et al., 2013), as calculated by Gannet 2.0, common metrics of data quality. Scans where no clear GABA signal could be identified, or where Gannet fitting failed to converge on a model, were excluded.
Statistical Methods
All statistical analyses were performed using SPSS (IBM SPSS Version 17.0). Behavioral tasks were analyzed using univariate statistical models including diagnosis and task-type (static/dynamic or adapted/unadapted) as fixed factors and outcome (threshold) as the dependent variables, in order to confirm previous behavioral findings in a larger cohort (Puts et al., 2014a). To confirm further previous findings of group differences in patterns of behavior (Puts et al. 2014a), Student’s t-tests were used. Student’s t-test was also used to test for group differences in tissue-corrected GABA levels and scan quality differences. A univariate model with ‘sequence’ as coviariate was used to test whether methodological differences impacted results. Pearson correlations were used to examine whether GABA concentration was correlated with behavioral performance. Robustness of these correlation was confirmed using Jackknife permutations. Differences between correlations in TDC and ASD were tested using Fisher r-to-z transformations.
Six children with ASD and 3 TDC had either poor GABA MRS data or poor T1-weighted images, and were therefore excluded from the analysis. Not all children were able to perform all vibrotactile tasks; 5 children with ASD and 1 TDC were excluded for the detection threshold tasks, 4 children with ASD and 2 TDC were excluded from the amplitude discrimination tasks, and 6 children with ASD were excluded for the frequency discrimination tasks.
Results
Sample characteristics and demographics
There were no group differences in age or sex (Table 1). While there was a group difference in full-scale IQ (FSIQ TD: 115.91 ± 10.62; FSIQ ASD: 103.21 ± 15.41, df = 67, t = −3.997, p < 0.001), there were no significant group differences on the PRI, which is thought to be a more valid measure of intellectual functioning in children with ASD (TDC: 111.94 ± 11.05; ASD: 107.82 ± 12.83, df = 65, t = −1.397, p = 0.168). Average ADOS score for the ASD cohort was 13.15 ± 3.57. 18 children with ASD had comorbidity for Attention-Deficit/Hyperactive Disorder (ADHD) combined-type, 1 for ADHD inattentive-type, 3 for Obsessive Compulsive Disorder (OCD), and 1 for Oppositional Defiance Disorder (ODD). Participants in the ASD group on prescribed psychotropic medications at the time of assessment, were as follows: 15 children were on stimulant medications (methylphenidate, dexmethylphenidate, dextroamphetamine, lisdexamfetamine, quillivant, and were removed from these medications on the day of and before testing). 1 child was on antidepressants (fluoxetine), 1 child on other medication (atomoxetine). None of the children with ASD were on anticonvulsants or benzodiazepines. None of the TDC were on prescribed psychotropic medications. 2 ASD and 5 TDC were left-handed. 6 children with ASD and 3 TDC had either poor GABA MRS data or poor T1-weighted images, and were therefore excluded from the analysis. Not all children were able to perform all vibrotactile tasks; 5 children with ASD and 1 TDC were excluded for the detection threshold tasks, 4 children with ASD and 2 TDC were excluded from the amplitude discrimination tasks, and 6 children with ASD were excluded for the frequency discrimination tasks.
Table 1.
Demographic, behavioral and imaging comparisons
| TDC | ASD | p-value – between groups | |||
|---|---|---|---|---|---|
| Participants | 37 (6F) | 35 (8F) | |||
| Age (years) | 10.09 ± 1.25 | 10.69 ± 1.4 | > 0.2 | ||
| Full IQ score | 103.21 ± 15.41 | 115.91 ± 10.62 | < 0.001* | ||
| Perceptual reasoning score | 111.94 ± 11.05 | 107.82 ± 12.83 | > 0.15 | ||
| ADOS score | na | 13.15 ± 3.57 | |||
| SM1 GABA conc. (tissue corrected) | 2.40 ± 0.25 | 2.20 ± 0.44 | = 0.016* | ||
| Average Grey Matter fraction (SM1) | 0.41 | 0.41 | >0.5 | ||
| Average White Matter fraction (SM1) | 0.51 | 0.5 | >0.3 | ||
| Average CSF fraction (SM1) | 0.075 | 0.086 | >0.2 | ||
| Static Detection threshold | 6.81 ± 2.66 | =0.001* within group |
9.01 ± 4.96 | = 0.12 within group |
* =0.03 |
| Dynamic Detection threshold | 9.72 ± 4.71 | 10.38 ± 3.98 | = 0.55 | ||
| Amplitude discrimination | 46.1 ± 29.61 | = 0.02* within group |
63.07 ± 37.01 | = 0.43 within group |
= 0.05* |
| Amplitude discrimination – adaptation | 67.88 ± 31.78 | 68.03 ± 46.10 | > 0.9 | ||
| Frequency Discrimination | 7.52 ± 3.48 | 8.4 ± 3.99 | >0.36 | ||
MR Spectroscopy
SM1 GABA levels were significantly lower in children with ASD compared to TDC (t = −2.472, p = 0.016, Table 1) but not occipital GABA (t = −0.032, p > 0.98). The difference in SM1 GABA values remained significant when covarying for sequence used (F = 4.690, p = 0.034). Water offset frequency standard deviation (which reflects motion, ASD: 1.92 ± 1.71 Hz; TDC: 2.48 ± 2.04 Hz, t = 1.177, p= 0.24) and fit error of the GABA peak (ASD: 7.23 ± 2.4 ; TDC: 7.07 ± 1.97, t =0.706, p = 0.45) did not differ significantly between groups indicating no significant differences in data quality between ASD and TDC.GABA did not correlate with age in either cohort or in both combined (R values between −0.068 and 0.04, p values > 0.7) or with full scale IQ (R values between –0.22 and −0.14, p values > 0.2). Given the prevalence of children with comorbid ADHD, we tested whether GABA levels differed in sub-cohorts with- and without comorbid ADHD, but found no differences in GABA concentration (1-way ANOVA; p > 0.5). There were no significant differences in SM1 grey matter, white matter, or CSF volumes between the two cohorts (Table 1), consistent with previous findings (Haar et al., 2014). There were no differences in fit error (p = 0.54) and water drift (p = 0.49) between the two acquisitions (40 blocks of 8 versus 2 NSA of 160 scans). GABA levels did not correlate between sensorimotor and occipital regions as previously shown in healthy adults (Puts et al. 2011).
Behavioral psychophysics
There was a significant effect of task (df = 1, F = 9.2, p = 0.003), and a one sided effect of diagnosis (df = 1, F = 3.61, p = 0.06) on detection threshold and no significant interaction between task and diagnosis (df = 1, F = 9.66, p = 0.33; Table 1). Post-hoc testing to investigate differences in the pattern of performance show that static detection threshold was significantly higher in ASD compared to TDC (df = 63, t = 2.220, p = 0.03), but dynamic detection threshold was not significantly different between cohorts (df = 61, t = 0.59, p = 0.55). Dynamic detection threshold was significantly higher than static detection in TDC (df = 31, t = −3.808, p = 0.001), but not in ASD (df = 29, t = −1.62, p = 0.12).
There was a significant effect of task (df = 1, F = 5.856, p = 0.017) but not of diagnosis df = 1, F = 0.798, p = 0.373) or task x diagnosis interaction (F = 2.753, p = 0.1) on amplitude discrimination threshold. Post-hoc analysis to test for differences in patterns of performance show that amplitude discrimination threshold was worse in ASD compared to TDC without adaptation at p = 0.05 (df = 61, t = 1.99, p = 0.05), but not when a single-site adapting stimulus was present (df = 60, t = 0.004, p > 0.9). Amplitude discrimination with adaptation was significantly worse than without adaptation in TDC (df = 28, t = −2.306, p = 0.02) but not in ASD (df = 28, t = −0.8, p = 0.43), reflecting the absence of adaptation in ASD. There were no significant differences in frequency discrimination performance (df = 62, t = 0.907, p = 0.36), although 8 children with ASD were not able to perform this talk successfully. Analysis did not show differences in behavioral performance related to ADHD co-morbidity.
Correlative analysis
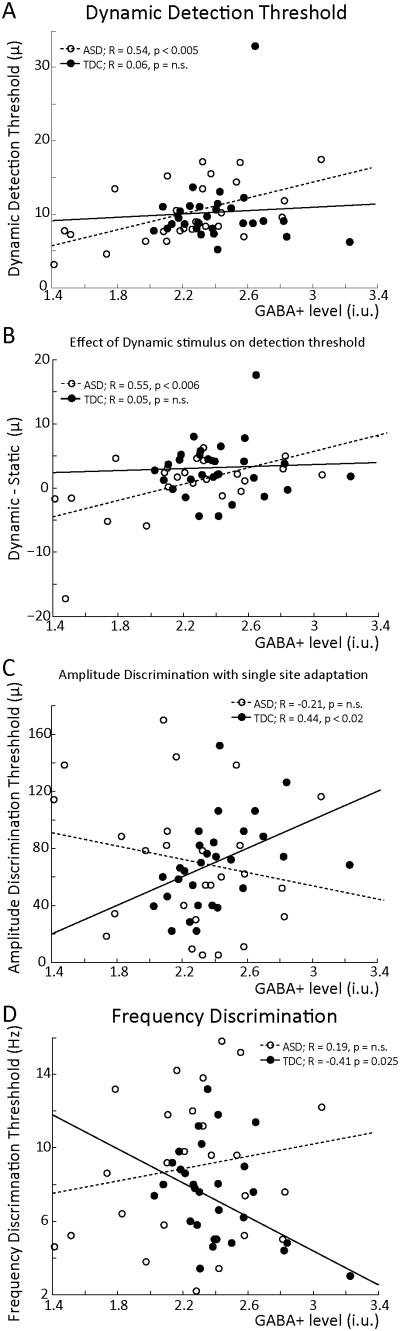
As shown in Figure 3A, GABA levels across both cohorts correlate positively with dynamic detection threshold, with higher GABA levels correlating with a higher detection threshold (R = 0.29, p = 0.029) . This correlation is significant for the ASD cohort alone (R = 0.54, p < 0.005, jackknife 0.54 ± 0.0148, R-values between 0.46 and 0.60, all significant at p = 0.05), but not for TDC alone (R = 0.06, p = 0.74, jackknife 0.052 ± 0.011, R-values between −0.23 and 0.19, none significant at p = 0.05). This correlation is significantly different between TDC and ASD (z = 2.02, p = 0.04). Furthermore, the individual difference between the static and dynamic detection threshold (Figure 3B) is significantly correlated with GABA levels for both groups (R = 0.39, p < 0.005) as well as for the ASD group alone (R = 0.55, p < 0.006, jackknife 0.54 ± 0.0058, R-values between 0.43 and 0.61, all significant at p = 0.05), but not for TDC alone (R = 0.06, p = 0.74, jackknife 0.051 ± 0.0202, R-values between −0.084 and 0.11, none significant at p = 0.05) . This correlation is significantly different between TDC and ASD (z = 2.01, p = 0.04). As shown in Figure 3C, amplitude discrimination performance after single-site adaptation correlates with GABA levels in TDC (R = 0.44, p = 0.019, jackknife 0.44 ± 0.0059, R-values between 0.36 and 0.55, p-values between 0.07 and 0.003) but not in ASD (R = −0.21, p = 0.3, jackknife −0.21 ± 0.0051, R-values between −0.33 and −0.11, none significant at p = 0.05) or when grouped. These correlations were significantly different between TDC and ASD (z = 2.39, p = 0.017). GABA did not correlate with the difference between adaptation and amplitude discrimination without adaptation. Finally, Figure 3D shows a strong negative correlation between GABA levels and tactile frequency discrimination in TDC, a replication (Puts et al., 2011, Puts et al., 2015) (R = −0.41, p =0.025, jackknife 0.41 ± 0.0049, R-values between −0.32 and −0.49, p-values between 0.08 and 0.003); this correlation is absent in ASD (R = 0.19, p = n.s, jackknife 0.20 ± 0.0076, R-values between 0.11 and 0.27, none significant at p = 0.05), and these correlations were significantly different (z = 2.19, p = 0.03). GABA values did not correlate with ADOS score or IQ (p > 0.5). Occipital GABA levels did not correlate with any of the tactile tasks (p > 0.2 for all comparisons).
Figure 3.
Correlative analysis. A. GABA concentration is correlated with dynamic detection threshold in cohorts combined as well as in ASD separately. B. GABA concentration is positively correlated with the difference between a static and dynamic detection threshold in cohorts combined and ASD separately, with higher GABA levels leading to a stronger effect. C. GABA concentration correlates with single-site adaptation amplitude discrimination performance in TDC but not in ASD. D. GABA concentration correlates with frequency discrimination performance in TDC but not in ASD.
Discussion
In this study, we aimed to investigate whether children with ASD have reduced GABA levels compared to typically developing children and whether GABA levels are associated with sensory abnormalities in ASD. Our results show reduced GABA levels in children with ASD in sensorimotor cortex, but not in occipital cortex, replicating a previous study (Gaetz et al., 2014). Our results also show GABA levels are associated with impaired tactile performance in these children, specifically in tasks we previously showed group differences in performance (Puts et al., 2014a) and hypothesized were driven by GABAergic inhibitory function. In this study we link abnormal tactile function to abnormal brain GABA levels.
Several studies have suggested altered GABA function in ASD, although the pathophysiological origin of these alterations remains unclear. Our study confirms that reductions in bulk GABA concentration are present in ASD, and that they are not shown globally throughout the brain.
Detection threshold
The correlations presented in this paper show that GABA levels in ASD are associated with the effect of sub-threshold stimulation on detection threshold, where children with lower GABA levels show an absence (or even reversal) of the effect of sub-threshold stimulation. It has been suggested that sub-threshold stimulation predominantly affects feed-forward inhibitory mechanisms (Favorov and Kursun, 2011, Tommerdahl et al., 2010, Blankenburg et al., 2003, Connors et al., 1988, Zhang and Sun, 2011), and that detection threshold is raised by sub-threshold stimulation due to the activation of inhibitory drive. It is therefore consistent that participants with higher GABA levels show increased dynamic detection threshold and an increased effect of sub-threshold stimulation, and therefore that children with ASD, having lower GABA levels, show a reduced effect. Interestingly, this correlation (between GABA levels and the dynamic-static detection threshold increment) is significant for both groups combined and ASD alone, but not for TDC alone, which may stem from reduced variance (and thus power to reveal correlation) in controls. Interestingly, while GABA plays a role in static detection, as GABA may decrease the amount of neuronal noise in the system (Tavassoli et al., 2016), no correlation is seen in this study. It is also possible that this process occurs via e.g. thalamic mechanisms rather than cortical.
Adaptation and Amplitude discrimination
Children with ASD typically do not show an effect of adaptation on amplitude discrimination. While GABA levels did not correlate with the effect of adaptation per se, they did correlate with amplitude discrimination performance after adaptation in TDC, but not ASD. Strictly, this did not follow our predictions that it is the “effect of adaptation” that is predicted by GABA levels. However, studies have suggested that single site adaptation makes separation of subsequent signals more difficult through inhibition of firing rates (representing stimulus intensity)(Tannan et al., 2008, Simons et al., 2005), but also that amplitude discrimination in itself is driven by lateral inhibition (Whitsel et al., 2003, Tommerdahl et al., 2010). Therefore, performance in the amplitude discrimination with adaptation tasks likely involves both lateral inhibitory connectivity (to separate signals) and GABA-driven reductions in firing rate due to adaptation, and may therefore be reflected by bulk GABA more clearly than those separate mechanisms. Given this possibility, it is therefore expected that participants with higher GABA levels show worse performance, as is shown in TDC. It must be noted that while the jackknife correlation is significant on a one-sided alpha level of 0.1, it is not at 0.05 and specific individuals may drive the correlations, although behaviorally there was no reason for exclusion. Behavioral performance of children with ASD in the adaptation condition itself is not different from TDC, but they do not show an effect of adaptation compared to the unadapted condition.
Frequency discrimination
Finally, we show that GABA levels correlate with frequency discrimination performance in TDC but not ASD. GABAergic inhibition plays an important role in encoding tactile frequencies (McLaughlin and Juliano, 2005), and participants with higher GABA levels may be able to better encode tactile frequencies, therefore improving their ability to distinguish them. This TDC result is consistent with our previous work showing this correlation in healthy adults (Puts et al., 2011), and while the jackknife correlation is not significant at all comparisons at 0.05, the directionality does follow our previous work, and behaviorally there was no reason for exclusion of specific individuals. TDC and ASD do not show significant differences in frequency discrimination performance, so the different correlative outcomes may reflect differences in mechanisms underlying frequency discrimination that warrant further investigation.
One common feature of these results is the effects of sub-threshold stimulation, adaptation, and frequency discrimination; rely on either temporal changes in neuronal signals or changes in synchrony. Modulatory effects of prior stimulation (whether sub- or supra-threshold) are behaviorally absent in ASD. The absence of sub-threshold activity is reflected in reduced GABA levels. Correlations between GABA levels and differences in (supra-threshold) adaptation, and differences in frequency discrimination, are absent in ASD while they do exist in TDC. This may suggest that children with ASD respond abnormally to changes in sensation which may be driven by impaired GABA-driven adjustments in cortical processing. These autism-associated impairments in GABA-mediated tactile functions might reflect impaired synchronization or modulation of incoming neuronal signals, or the inhibitory capacity of cortex to filter, or habituate to, to sensory information. (However, it should be emphasized that these mechanisms do not solely depend on GABAergic inhibition.) Performance in TDC may be linked more closely to specific inhibitory mechanisms, than it is in ASD, as discussed in more detail below.
The behavioral data presented here follow a similar pattern as shown in our previous study that used larger cohorts (and includes several of the current cohort that underwent MRI scans). In this smaller cohort, static detection threshold is also higher in ASD, and children with ASD do not show a significant increase in detection threshold whereas TDC do. Amplitude discrimination without adaptation is only significantly different between groups at p =.05 and does not pass correction for multiple comparisons. However, the trend is similar to the statistically significant data presented in previous work (Puts et al., 2014a). Differences in study populations might help to explain the discrepancies. Inclusion in the current study required understanding instructions and being able to lie still in the scanner. It is therefore possible that the current study sample of children with ASD were less affected as compared with those in the previous study.
Interestingly, while occipital GABA levels appear normal, studies have shown differences in visual processing in ASD (Freyberg et al., 2015, Dickinson et al., 2014, Robertson et al., 2016), so it remains unclear how normal GABA levels associate with abnormal visual processing. Robertson et al. (Robertson et al., 2016) did, however, show that occipital GABA levels correlate with visual binocular rivalry in healthy participants, but not in ASD, showing an absence of association between GABA and visual processing in ASD. This study did not find reduced GABA levels in occipital cortex (consistent with our results), yet, still showed abnormal inhibitory visual signaling in ASD. GABA reductions (as detected by MRS) are not global throughout the brain, and are not universally linearly linked to altered sensory processing. It is important to emphasize that the behavioral tasks used in this study were specifically chosen to tap into inhibitory mechanisms, based on prior human and animal work (Tommerdahl et al., 2010, McLaughlin and Juliano, 2005, Zhang and Sun, 2011). In our own findings, we do see that reduced sensorimotor GABA levels predicting altered tactile function in one task (the effect of sub-threshold stimulation), but that, similar to Robertson et al., (Robertson et al., 2016) correlative brain-behavior correlations that are seen in TDC may be absent in ASD. It is important to note that behavioral performance in ASD is not always reduced (Dickinson and Milne, 2014, Puts et al., 2014b) and that all behaviors arise from the complex interaction of multiple neurotransmitter systems across broad networks.
There is currently an increased interest in investigating the link between GABA and sensory function in ASD, although the literature is still developing. Auditory GABA has been shown to be reduced in ASD (Gaetz et al., 2014), corresponding to reduction in the oscillatory response to auditory stimulation (Rojas et al., 2013, Rojas et al., 2008). MRS of GABA is an emerging methodology and thus far, few studies link GABA levels to (within-domain) sensory impairments in ASD; cross-domain associations remain unexplored. GABA levels do not generally correlate between functionally distinct regions, as shown here and previously (Boy et al., 2011, Harris et al., 2015a). In our data at least, none of the tactile tasks associated with occipital GABA levels, which may not be expected; occipital GABA levels were only acquired in a subset of participants.
While the behavioral tasks used in this study are all thought to reflect aspects of inhibitory function, there does not exist a unilateral or linear association between brain GABA levels and behavioral performance in TDC and ASD. As described in a previous study (Puts et al., 2015), it is unlikely that our measurements of GABA concentration using MRS reflect simple up- and down-regulation of all GABA function. Detection threshold correlates with GABA along the same axis in both cohorts, but dissociations exist in the adaptation and frequency discrimination outcomes. MRS of GABA suffers from limited spatial resolution (all GABA within a large region is measured), so while our tactile tasks, and their basis in the animal literature, might allow for a discussion of specific GABAergic mechanisms (e.g. feed-forward inhibition), a direct link between MRS-GABA and synaptic GABA levels cannot be drawn. The GABA levels measured by MRS likely reflect inhibitory tone or “the ability of the system to inhibit”. It is more likely that TDC and ASD differ in specific genetic or receptor functions, leading to changes in GABAergic processes.
The current measurements of GABA are limited by the fact that edited MRS of GABA has a low signal-to-noise (Mullins et al., 2014) so that the regional specificity of our measurements is limited to a voxel that includes both S1 and M1. In addition, the GABA signal is contaminated by co-edited macromolecular signal (MM)(Mullins et al., 2014) to the order of 50% (Harris et al., 2015c). While there are techniques that remove MM signal from GABA spectra, these techniques are more susceptible to frequency drift and motion (Harris et al., 2015c) than the GABA+ method, limiting their applicability to pediatric cohorts. In the current study, a typical MEGA-PRESS acquisition was used to retain sufficient SNR (Mullins et al., 2014). Two slightly different MRS acquisitions were used during the study, albeit with the same pulses and editing parameters. In the current study, no differences between acquisitions were found and while we believe these are interchangeable under circumstances without excessive motion, such as in the case of children with normal IQ and relatively mild ADOS scores as in the current study, the second acquisition with better interleaving and an internal water reference allows for better post-hoc corrections and will perform better in more difficult, e.g. pediatric, studies, for instance in children with more severe autism and/or lower IQ.
A common limitation in studying pediatric neurodevelopmental cohorts are effects of comorbid diagnosis and medication. The most common comorbidity in this cohort is ADHD and it has previously been show that children with ADHD also show reduced GABA levels (Edden et al., 2012, Bollmann et al., 2015). In the current study, there was no clear difference in GABA levels between children with ASD with and without comorbid ADHD. Other measures may provide better discriminatory power to assess differences between these groups. All children were refrained from stimulant medication prior to the experiments, but were allowed to take other medications. While this study is underpowered to determine potential medication effects, it is possible that medications affect GABA levels, and possibly behavioral performance, to some degree and larger studies are therefore needed to examine the impact of medication status.
In summary, we showed that children with ASD have significantly reduced SM1 GABA levels compared to TDC. Further, we show that GABA levels correlated with dynamic detection threshold as well as with the effect of sub-threshold stimulation. GABA levels also correlated with amplitude discrimination after adaptation (an effect absent in autism) and frequency discrimination in TDC, but not in children with autism. These data suggest that cortical inhibition at least in the somatosensory cortex, and especially inhibition related to the synchronization and processing of incoming sensory information, is altered in ASD, and is reflected by a bulk reduction in brain GABA levels. This may reflect behavioral abnormalities in filtering of tactile information and impaired habituation of repetitive stimulation. The altered in vivo GABA levels might therefore explain abnormal tactile information processing in autism. The GABA system may be a future target for therapies that can alleviate these symptoms.
GABA and touch are abnormal in ASD.
Sensory (e.g. touch) abnormalities are common in children with Autism Spectrum Disorder (ASD). In previous work we have shown that children with ASD have altered sensitivity to touch. This altered sensitivity is specific to tasks that involve filtering and habituation to touch.
GABA is the main inhibitory neurotransmitter in the human brain, and plays a key role in encoding touch. Previous studies suggest that the GABA system is altered in ASD. Using Magnetic Resonance Spectroscopy (MRS), it is now possible to measure GABA levels in the human brain. Recent MRS work suggests that GABA levels are reduced in ASD.
In this study we investigated whether brain GABA levels are altered in ASD and whether changes in brain GABA levels can predict differences in tactile sensitivity. Our results suggest that GABA levels are reduced in sensorimotor areas, but not in occipital visual areas. Lower GABA levels are associated with less "filtering" of touch information, and are not associated with habituation in ASD, whereas they are in typically developing children.
Further research is necessary to elucidate the specific GABAergic mechanisms altered in ASD. Our results suggest that reduced brain GABA levels could underlie altered tactile function in ASD, and that altered GABA function in ASD disrupts the link between GABA and behavior. Understanding the link between brain GABA and tactile behavior is an important step in understanding brain behavior links in ASD, potentially leading to future therapies to reduce the severity of sensory symptoms.
Acknowledgments
Grant information
NAJP was funded by an Autism Speaks Translational Post-doctoral Fellowship. This work was further supported by NIH P41 EB015909, R21 MH098228, R01 MH078160, and R01 EB016089. We thank Kristie Sweeney, Terri Brawner, Kathleen Kahl, and Ivana Kusevic, for their research support.
Footnotes
Financial Disclosures
M. Tommerdahl discloses that he is cofounder of Cortical Metrics that built and provided the stimulator used in these experiments and that he receives royalties through this.
Nicolaas A.J. Puts reported no biomedical financial interests or potential conflicts of interest. Ericka L. Wodka reported no biomedical financial interests or potential conflicts of interest. Ashley D. Harris reported no biomedical financial interests or potential conflicts of interest. Deana Crocetti reported no biomedical financial interests or potential conflicts of interest. Stewart H. Mostofsky reported no biomedical financial interests or potential conflicts of interest. Richard A.E. Edden reported no biomedical financial interests or potential conflicts of interest.
References
- ABRAHAMS BS, GESCHWIND DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9:341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANKENBURG F, TASKIN B, RUBEN J, MOOSMANN M, RITTER P, CURIO G, VILLRINGER A. Imperceptible stimuli and sensory processing impediment. Science. 2003;299:1864. doi: 10.1126/science.1080806. [DOI] [PubMed] [Google Scholar]
- BOLLMANN S, GHISLENI C, POIL SS, MARTIN E, BALL J, EICH-HOCHLI D, EDDEN RA, KLAVER P, MICHELS L, BRANDEIS D, O'GORMAN RL. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry. 2015;5:e589. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOY F, EVANS CJ, EDDEN RA, LAWRENCE AD, SINGH KD, HUSAIN M, SUMNER P. Dorsolateral prefrontal gamma-aminobutyric acid in men predicts individual differences in rash impulsivity. Biol Psychiatry. 2011;70:866–72. doi: 10.1016/j.biopsych.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASANOVA MF, VAN KOOTEN IA, SWITALA AE, VAN ENGELAND H, HEINSEN H, STEINBUSCH HW, HOF PR, TRIPPE J, STONE J, SCHMITZ C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- CHAO HT, CHEN H, SAMACO RC, XUE M, CHAHROUR M, YOO J, NEUL JL, GONG S, LU HC, HEINTZ N, EKKER M, RUBENSTEIN JL, NOEBELS JL, ROSENMUND C, ZOGHBI HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNORS BW, MALENKA RC, SILVA LR. Two inhibitory postsynaptic potentials, and GABAA and GABAB receptor-mediated responses in neocortex of rat and cat. J Physiol. 1988;406:443–68. doi: 10.1113/jphysiol.1988.sp017390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELOREY TM. GABRB3 gene deficient mice: a potential model of autism spectrum disorder. Int Rev Neurobiol. 2005;71:359–82. doi: 10.1016/s0074-7742(05)71015-1. [DOI] [PubMed] [Google Scholar]
- DICKINSON A, JONES M, MILNE E. Oblique orientation discrimination thresholds are superior in those with a high level of autistic traits. J Autism Dev Disord. 2014;44:2844–50. doi: 10.1007/s10803-014-2147-1. [DOI] [PubMed] [Google Scholar]
- DICKINSON A, MILNE E. Enhanced and impaired sensory discrimination in autism. J Neurophysiol. 2014;112:1599. doi: 10.1152/jn.00288.2014. [DOI] [PubMed] [Google Scholar]
- DONAHUE MJ, NEAR J, BLICHER JU, JEZZARD P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–8. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- EDDEN RA, CROCETTI D, ZHU H, GILBERT DL, MOSTOFSKY SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69:750–3. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDEN RA, PUTS NA, HARRIS AD, BARKER PB, EVANS CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS CJ, PUTS NA, ROBSON SE, BOY F, MCGONIGLE DJ, SUMNER P, SINGH KD, EDDEN RA. Subtraction artifacts and frequency (mis-)alignment in J-difference GABA editing. J Magn Reson Imaging. 2013;38:970–5. doi: 10.1002/jmri.23923. [DOI] [PubMed] [Google Scholar]
- FATEMI SH, FOLSOM TD, REUTIMAN TJ, THURAS PD. Expression of GABA(B) receptors is altered in brains of subjects with autism. Cerebellum. 2009;8:64–9. doi: 10.1007/s12311-008-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVOROV OV, KURSUN O. Neocortical layer 4 as a pluripotent function linearizer. J Neurophysiol. 2011;105:1342–60. doi: 10.1152/jn.00708.2010. [DOI] [PubMed] [Google Scholar]
- FLOYER-LEA A, WYLEZINSKA M, KINCSES T, MATTHEWS PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–44. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- FORD TC, CREWTHER DP. A Comprehensive Review of the (1)H-MRS Metabolite Spectrum in Autism Spectrum Disorder. Front Mol Neurosci. 2016;9:14. doi: 10.3389/fnmol.2016.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCISCO EM, FAVOROV O, TOMMERDAHL M. The Role of Cortical Modularity in Tactile Information Processing: An Approach to Measuring Information Processing Deficits in Autism. In: Fitzgerald Michael., editor. Recent Advances in Autism Spectrum Disorders. InTech; 2013. ISBN 978-953-51-1022-4, 194 pages. Chapters published March 06, 2013 under CC BY 3.0 license. DOI: 10.5772/50854. [Google Scholar]
- FREYBERG J, ROBERTSON CE, BARON-COHEN S. Reduced perceptual exclusivity during object and grating rivalry in autism. J Vis. 2015;15:11. doi: 10.1167/15.13.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUDA T, ITOH M, ICHIKAWA T, WASHIYAMA K, GOTO Y. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J Neuropathol Exp Neurol. 2005;64:537–44. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- GAETZ W, BLOY L, WANG DJ, PORT RG, BLASKEY L, LEVY SE, ROBERTS TP. GABA estimation in the brains of children on the autism spectrum: measurement precision and regional cortical variation. Neuroimage. 2014;86:1–9. doi: 10.1016/j.neuroimage.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAAR S, BERMAN S, BEHRMANN M, DINSTEIN I. Anatomical Abnormalities in Autism? Cereb Cortex. 2014;26:1440–52. doi: 10.1093/cercor/bhu242. [DOI] [PubMed] [Google Scholar]
- HARRIS AD, GLAUBITZ B, NEAR J, JOHN EVANS C, PUTS NA, SCHMIDT-WILCKE T, TEGENTHOFF M, BARKER PB, EDDEN RA. Impact of frequency drift on gamma-aminobutyric acid-edited MR spectroscopy. Magn Reson Med. 2014a;72:941–8. doi: 10.1002/mrm.25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS AD, PUTS NA, ANDERSON BA, YANTIS S, PEKAR JJ, BARKER PB, EDDEN RA. Multi-regional investigation of the relationship between functional MRI blood oxygenation level dependent (BOLD) activation and GABA concentration. PLoS One. 2015a;10:e0117531. doi: 10.1371/journal.pone.0117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Barker PB, Edden RA. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn. Reson. Med. 2015c;74:1523–9. doi: 10.1002/mrm.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS AD, PUTS NA, EDDEN RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015b;42:1431–40. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN JK, NGUYEN RH, FRANCISCO EM, ZHANG Z, DENNIS RG, TOMMERDAHL M. A novel device for the study of somatosensory information processing. J Neurosci Methods. 2012;204:215–20. doi: 10.1016/j.jneumeth.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORD C, RISI S, LAMBRECHT L, COOK EH, JR., LEVENTHAL BL, DILAVORE PC, PICKLES A, RUTTER M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–23. [PubMed] [Google Scholar]
- LORD C, RUTTER M, LE COUTEUR A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- MCLAUGHLIN DF, JULIANO SL. Disruption of layer 4 development alters laminar processing in ferret somatosensory cortex. Cereb Cortex. 2005;15:1791–803. doi: 10.1093/cercor/bhi056. [DOI] [PubMed] [Google Scholar]
- MESCHER M, MERKLE H, KIRSCH J, GARWOOD M, GRUETTER R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- MULLINS PG, MCGONIGLE DJ, O'GORMAN RL, PUTS NA, VIDYASAGAR R, EVANS CJ, CARDIFF SYMPOSIUM ON, M. R. S. O. G. EDDEN RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUTHUKUMARASWAMY SD, EDDEN RA, JONES DK, SWETTENHAM JB, SINGH KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–61. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEAR J, EDDEN R, EVANS CJ, PAQUIN R, HARRIS A, JEZZARD P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73:44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OBLAK AL, GIBBS TT, BLATT GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2010;1380:218–28. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLDFIELD RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- PUTS NA, EDDEN RA. In vivo magnetic resonance spectroscopy of GABA: a methodological review. Prog Nucl Magn Reson Spectrosc. 2012;60:29–41. doi: 10.1016/j.pnmrs.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTS NA, EDDEN RA, EVANS CJ, MCGLONE F, MCGONIGLE DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31:16556–60. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTS NA, EDDEN RA, WODKA EL, MOSTOFSKY SH, TOMMERDAHL M. A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J Neurosci Methods. 2013;218:39–47. doi: 10.1016/j.jneumeth.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTS NA, HARRIS AD, CROCETTI D, NETTLES C, SINGER HS, TOMMERDAHL M, EDDEN RA, MOSTOFSKY SH. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol. 2015;114:808–17. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTS NA, WODKA EL, TOMMERDAHL M, MOSTOFSKY SH, EDDEN RA. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014a;111:1803–11. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUTS NA, WODKA EL, TOMMERDAHL M, MOSTOFSKY SH, EDDEN RA. Reply to Dickinson and Milne. J Neurophysiol. 2014b;112:1600–1. doi: 10.1152/jn.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Curr. Biol. 2016;26:80–5. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- ROGERS SJ, OZONOFF S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. J Child Psychol Psychiatry. 2005;46:1255–68. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- ROJAS DC, MAHARAJH K, TEALE P, ROGERS SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROJAS DC, SINGEL D, STEINMETZ S, HEPBURN S, BROWN MS. Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMONS SB, TANNAN V, CHIU J, FAVOROV OV, WHITSEL BL, TOMMERDAHL M. Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci. 2005;6:43. doi: 10.1186/1471-2202-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANNAN V, HOLDEN JK, ZHANG Z, BARANEK GT, TOMMERDAHL MA. Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res. 2008;1:223–30. doi: 10.1002/aur.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAVASSOLI T, BELLESHEIM K, TOMMERDAHL M, HOLDEN JM, KOLEVZON A, BUXBAUM JD. Altered tactile processing in children with autism spectrum disorder. Autism Res. 2016;9:616–20. doi: 10.1002/aur.1563. [DOI] [PubMed] [Google Scholar]
- TKAC I, STARCUK Z, CHOI IY, GRUETTER R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- TOMMERDAHL M, FAVOROV OV, WHITSEL BL. Dynamic representations of the somatosensory cortex. Neuroscience and Biobehavioral Reviews. 2010;34:160–70. doi: 10.1016/j.neubiorev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- WECHSLER D. Wechsler Intelligence Scale for Children. 4th The Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- WHITSEL BL, FAVOROV O, TOMMERDAHL M, DIAMOND M, JULIANO SJ, KELLY D. Sensory Processing in the Mammalian Brain: Neural substrates and experimental strategies. Oxford University Press; New York: 1989. Dynamic processes govern the somatosensory cortical response to natural stimulation; pp. 79–107. 1989. [Google Scholar]
- WHITSEL BL, KELLY EF, QUIBRERA M, TOMMERDAHL M, LI Y, FAVOROV OV, XU M, METZ CB. Time-dependence of SI RA neuron response to cutaneous flutter stimulation. Somatosens Mot Res. 2003;20:45–69. doi: 10.1080/0899022031000083834. [DOI] [PubMed] [Google Scholar]
- YOUSRY TA, SCHMID UD, ALKADHI H, SCHMIDT D, PERAUD A, BUETTNER A, WINKLER P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–57. doi: 10.1093/brain/120.1.141. Pt 1. [DOI] [PubMed] [Google Scholar]
- ZHANG Z, SUN QQ. The balance between excitation and inhibition and functional sensory processing in the somatosensory cortex. Int Rev Neurobiol. 2011;97:305–33. doi: 10.1016/B978-0-12-385198-7.00012-6. [DOI] [PubMed] [Google Scholar]