Abstract
Human disease commonly manifests as a result of complex genetic and environmental interactions. In the case of neurodegenerative diseases, such as Parkinson's disease (PD), understanding how environmental exposures collude with genetic polymorphisms in the central nervous system (CNS) to cause dysfunction is critical in order to develop better treatment strategies, therapies, and a more cohesive paradigm for future research. The intersection of genetics and the environment in disease etiology is particularly relevant in the context of their shared pathophysiological mechanisms. This review offers an integrated view of disease-toxicant interactions in PD. Particular attention is dedicated to how mutations in the genes SNCA, parkin, leucine-rich repeat kinase 2 (LRRK2) and DJ-1, as well as dysfunction of the ubiquitin proteasome system, may contribute to PD and how exposure to heavy metals, pesticides and illicit drugs may further the consequences of these mutations to exacerbate PD and PD-like disorders. Although the toxic effects induced by exposure to these environmental factors may not be the primary causes of PD, their mechanisms of action are critical for our current understanding of the neuropathologies driving PD. Elucidating how environment and genetics collude to cause pathogenesis of PD will facilitate the development of more effective treatments for the disease. Additionally, we discuss the neuroprotection exerted by estrogen and other compounds that may prevent PD and provide an overview of current treatment strategies and therapies.
Keywords: Disease-toxicant interaction, gene-environment interaction, Parkinson's disease (PD), neurodegeneration, heavy metals, illicit drugs, pesticides, estrogen
1. Parkinson's disease overview
Parkinson's disease (PD) is the second most frequently diagnosed neurodegenerative disease, afflicting more than 4 million globally [1]. Its symptoms include motoric abnormalities due to dopamine deficits in the striatum resulting in resting tremor, stiffness and slow movements, in addition to non-dopaminergic related symptoms of dementia and depression, anxiety and autonomic dysfunction [2, 3]. Approximately 85–90% of PD cases are sporadic, i.e. only about 10–15% of patients report a positive family history or familial form of the disease, suggesting that exposure to specific environmental agents may play a strong role in PD pathogenesis [4–7]. This conclusion is supported by studies that show a low concordance rate in monozygotic and dizygotic twins [8–10]. Other studies have shown that early-onset PD cases have a higher concordance rate in monozygotic compared to dizygotic twins, indicating that genetic factors may also be at play [11]. Five to fifteen percent of PD patients have monogentically-inherited forms of the disease that are associated with one of the six identified genes. Of these genes, mutations in SNCA (PARK1–4) and LRRK2 (PARK8) are responsible for autosomal-dominant PD forms, while Parkin (PARK2), DJ-1 (PARK7), ATP13A2 (PARK9), PTEN-induced putative kinase 1 (PINK1), and leucine-rich repeat kinase 2, LRRK2 (PARK8) account for autosomal recessive mode of inheritance of PD [3, 12, 13]. These mutations exacerbate neurodegenerative mechanisms, such as protein aggregation, oxidative stress and impaired protein degradation that contribute to PD pathogenesis. Exposures to toxins such as heavy metals, MPTP and pesticides also result in the aforementioned pathophysiological mechanisms, causing PD-like symptoms. Although exposure to these compounds may not be the primary causes of PD, their mechanisms of action are critical for our current understanding of the neuropathologies driving PD. Thus, many cases of PD may not be the result of disparate mutations or exposures to environmental factors, but rather a product of how genetics and environment interact with one another to cause damage in the nigrostriatal pathway. This hypothesis for PD pathogenesis is referred to as disease-toxicant interactions within this article. Exploring interactions between genes of interest in PD and the environment are imperative in order to develop more effective therapies for the disease.
This review discusses the contribution of genes and the intersection between genes and toxicants, such as heavy metals, illicit drugs and pesticides that may be necessary for our understanding of the cellular mechanisms that underlie the neuropathology of PD. Additionally, we discuss the neuroprotective effects of estrogen and other environmental neuroprotective agents, such as tobacco and caffeine in PD neuropathology (Figure 1 and Table 1).
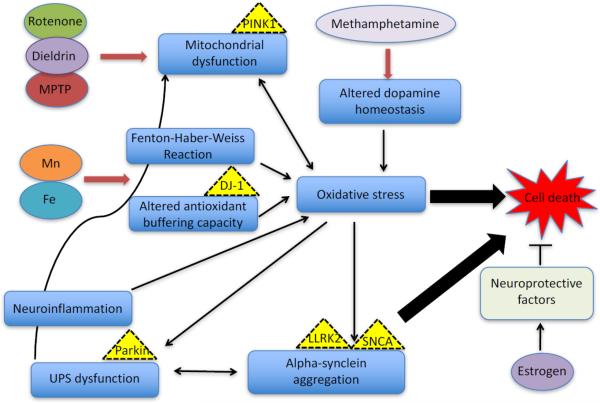
Figure 1.
Proposed mechanisms of action for some of the major toxicants associated with PD pathogenesis. Several cellular pathways have been implicated in the neuropathology of PD, including oxidative stress, mitochondrial dysfunction, protein aggregation, UPS dysfunction and neuroinflammation. Exposure to toxicants result in similar neurotoxicity as the aforementioned. Heavy metals, such as Mn (II) and Fe (III) induce oxidative stress by causing increase in the production of reactive oxygen species (ROS) via the Fenton-Haber Weiss reaction and alter the antioxidant system (levels and activity of important enzymes, including glutathione, superoxide dismutase, catalase, arginase, etc.) in the cell. By provoking this imbalance between free radicals and antioxidants, these toxicants exacerbate oxidative stress processes in the cell to cause cell death. Neuroinflammation exacerbates oxidative stress mechanisms that can lead to protein aggregation via alteration in the ubiquitin proteasome system (UPS) function. The impaired protein degradation machinery can lead to the accumulation of protein aggregates capable of disrupting cellular processes and causing cell death. The toxicants rotenone, dieldrin and MPTP inhibit complexes in the electron transport chain of the mitochondria, leading not only to impaired metabolic processes but also to the increased production of ROS and oxidative stress. Methamphetamine use can cause increased dopamine levels at the synapse, leading to dopamine auto-oxidation, increased production of free radicals and subsequently oxidative stress. Estrogen is hypothesized to protect against PD via enhanced secretion of nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF) or by preventing apoptosis via estrogen receptor mediated pathways
Table 1.
Some of the toxicants implicated in PD pathogenesis, their targets in the brain, mechanisms of action and the resulting symptoms upon over exposure. ETC: electron transport chain
| Toxicant | Type | Target | Mechanisms of toxicity | Genes that may interact with toxicant | Symptoms |
|---|---|---|---|---|---|
| Manganese | Heavy metal | Nigrostriatal system (chronic exposure), Globus pallidus (acute exposure) | Oxidative stress, mitochondrial dysfunction, UPS dysfunction, protein aggregation | DJ-1, LLRK2, SNCA | Tremors, bradykinesia, hallucinations, rigidity |
| Iron | Heavy metal | Substantia nigra pars compacta | Oxidative stress | DJ-1, LLRK2, SNCA | Vomiting, seizures, liver failure |
| MPTP | Illicit drug | Substantia nigra; blocks complex I of the ETC | Mitochondrial Dysfunction, excitotoxicity, oxidative stress, neuroinflammation | PINK1, Parkin, LLRK2, SNCA | Difficulty moving, rigidity, resting tremor |
| Methamphetamine | Illicit drug | Nigrostriatal and mesolimbic dopaminergic pathways | Altered dopamine homeostasis, oxidative stress, UPS dysfunction | Parkin, LLRK2, SNCA | Psychosis, memory loss, mood disturbances |
| Rotenone | Rotenoid pesticide, insecticide | Nigrostriatal dopaminergic neurons, blocks complex I of the ETC | Mitochondrial dysfunction, oxidative stress, neuroinflammation | PINK1, Parkin, LLRK2, SNCA | Headache, tremor, unconsciousness, vomiting |
| Dieldrin | Organochloride pesticide | Complex III of the ETC | Mitochondrial dysfunction, UPS dysfunction, oxidative stress | Pink1, LLRK2, SNCA | Headache, vomiting, seizures |
2. Genetic factors
2.1. Alpha-synuclein (SNCA)
Degeneration of nigrostriatal dopaminergic neurons is a hallmark of PD, with clinical symptoms of the disease manifesting when 50–60% of these neurons are lost [1]. SNCA is a gene that encodes for alpha-synuclein. Intracellular accumulation of Lewy bodies, inclusions composed primarily of alpha-synuclein, within the substantia nigra and other brain regions are a defining characteristic of PD [14, 15]. Alpha-synuclein is a 140 amino acid protein expressed in both the central and peripheral nervous system and though its function in the brain is not well characterized, it is present in neuronal presynaptic terminals and associated with neurotransmitter release, and suggested to mediate neurotoxicity via its oligomerization [16–20]. Collectively, alpha-synuclein plays a physiological role in regulating dopamine biosynthesis and in its aggregated form, is deleterious. In its native state, alpha-synuclein is highly soluble and cytosolic, and under physiological conditions assumes a helically folded tetramer formation [21]. Some literature indicates that wild-type alpha-synuclein may serve as an anti-apoptotic molecule after exposure to some neurotoxins, serving to protect dopaminergic neurons in the substantia nigra pars compacta [22]. Specifically, Jensen et al. reported that wild-type alpha-synuclein, but not those with A53T or A30P mutant forms, protected non-differentiated dopaminergic neurons from the neurotoxic effects of the complex I inhibitors, MPP+ and rotenone [23]. This appears to indicate that alpha-synuclein is a neuroprotective protein, and may even help to ameliorate neurodegenerative mechanisms in the brain. However, other studies have suggested otherwise. For example, it has been revealed that alpha-synuclein self-assembles into proto-fibrils and fibrils and aggregates to form insoluble amyloid fibrils composing Lewy bodies and neurites in the brain that may by cytotoxic [1, 3]. These aggregates are thought to contribute to PD symptomology by disrupting cellular homeostasis and causing neuron death through their toxic effects on neighboring cells and synapses [24]. Additionally, impaired clearance of misfolded alpha-synuclein may result in proteolytic stress as well as increased aggregate formation [25].
The Lewy pathology in PD has been suggested to spread throughout the brain as the disease progresses. Evidence from neurons grafted into the brains of PD patients has further supported the occurrence of Lewy pathology in PD and suggested the propagation of pathology from the host tissues to the grafts, similar to the one observed in prion diseases [26, 27]. For example, Lee et al. [28] reported that a small percentage but significant proportion of newly synthesized alpha-synuclein and its aggregates are rapidly secreted from neuronal cells via unconventional, endoplasmic reticulum/Golgi-independent exocytosis. Previously, alpha-synuclein was considered an intracellular protein, present mostly in the cytosol with some partitioning to vesicle fractions that mediate its toxic effects intracellularly [29, 30], thus meriting the assumption that alpha-synuclein harbors only cell-autonomous functions. This assumption was challenged by findings from studies reporting the presence of nanomolar concentrations of alpha-synuclein in human CSF and blood plasma samples in both PD and neurotypical subjects [31, 32]. Moreover, there was a two-fold increase in the levels of alpha-synuclein present in the blood of familial PD patients with alpha-synuclein gene locus triplication [33]. Another study demonstrated that a portion of alpha-synuclein present in the lumen of vesicles can be secreted through an unconventional exocytic pathway in a constitutive manner. In addition, vesicular alpha-synuclein was found to be highly prone to aggregation and the aggregated forms were also secreted from the cells [28]. Alpha-synuclein can be directly transmitted from neuronal cells overexpressing alpha-synuclein to transplanted embryonic stem cells both in tissue culture and in transgenic animals [34]. Cell produced alpha-synuclein oligomers can be secreted and taken up by neighboring cells where they cause toxicity [35]. A first hint of how intracellular alpha-synuclein may be secreted into the extracellular space via externalized vesicles that have hallmarks of exosomes was uncovered through studies that examined the role of secreted alpha-synuclein in neuronal homeostasis in cultured SH-SY5Y dopaminergic cells [36, 37]. A recent study used a novel protein fragment complementation assay to demonstrate that alpha-synuclein oligomers are present on both the outside and inside of exosomes. Importantly, the mode of secretion of alpha-synuclein oligomers was shown to be strongly influenced by autophagic activity [35]. Collectively, these and other studies suggest that the pathogenicity of alpha-synuclein oligomers are not restricted to the donor cells but can propagate into the extracellular space and alter the physiology of neighboring cells.
Wild-type alpha-synuclein inhibits tyrosine hydroxylase (TH) activity [38]. Nevertheless, aggregated forms are unable to inhibit TH activity with higher TH phosphorylation present [39]. This suggests a physiological role of wild-type alpha-synuclein in regulating dopamine biosynthesis. Mitochondrial dysfunction and oxidative stress are reported to contribute to the misfolding of alpha-synuclein protein, activating a deleterious cycle of protein accumulation and cellular damage, leading to dopaminergic neurodegeneration [40].
The presence of aggregated or pathological forms of alpha-synuclein in both idiopathic and familial PD cases suggests a mechanistic link between genetically inherited and environmentally derived forms of the disease. As of yet, three missense mutations in the gene coding for alpha-synuclein (SNCA) have been associated with familial PD. These mutations include Ala30Pro, Ala53Thr, and Glu46Lys, all of which alter the structure of the protein, and increase the probability of fibril formation and oligomerization in vitro as well as neurodegeneration in vivo [41, 42]. PD patients with point mutations in SNCA display alpha-synuclein aggregation and Lewy body formation that results in severe PD symptoms [43, 44]. Duplications or triplications of wild-type SNCA may lead to similar PD pathology [45, 46]. This indicates that excess amounts of wild-type alpha-synuclein may contribute to neurodegeneration and that environmental toxins that directly or indirectly contribute to alpha-synuclein accumulation may render individuals more vulnerable to the disease. Apart from duplication of SNCA or relatively rare mutations in the gene, variability in promoter regions of SNCA have been identified as susceptibility factors to PD and two different loci in the gene have been established as risk factors for PD pathogenesis [47].
Enteric nervous system (ENS) dysfunction is characteristic of early PD progression, accounting for symptoms including gastrointestinal distress and difficulty swallowing [48]. Exposure to various environmental factors modulates alpha-synuclein transport within the neuron. For example, intragastric rotenone administration in mice promotes the release of alpha-synuclein by enteric neurons and the released enteric alpha-synuclein is up-taken by presynaptic sympathetic neurites and retrogradely transported to the soma, where it accumulates in the enteric nervous system (ENS) and CNS following the same pattern of progression as hypothesized by Braak [49]. These findings suggest that pesticides can activate pathophysiological mechanisms in PD through transneuronal and retrograde axonal transport of alpha-synuclein. Exposure to pesticides may alter the homeostasis of alpha-synuclein and contribute to its aggregation. This prospect is particularly exciting in light of emerging evidence that genetic variability in SNCA may render populations more susceptible to PD [50]. However, as of yet there is insufficient evidence to definitively say whether certain polymorphisms of alpha-synuclein are more vulnerable to such insults than others. Genome-wide association studies have sought to find links between single nucleotide polymorphisms (SNPs) of SNCA, vulnerability to certain environmental exposures and risk for PD. Such studies have failed to establish significant interactions between genetic susceptibility loci and exposures to pesticides, coffee and alcohol consumption [51]. Thus, in order to evaluate the possibility that different polymorphisms of SNCA leave individuals more vulnerable to alpha-synuclein aggregation subsequent to environmental exposures, further studies are needed from both epidemiological and molecular perspectives.
2.2. LRRK2
Aggregation of alpha-synuclein is observed in aged mice with a germ-line deletion of the gene, Leucine-rich repeat kinase 2 (LRRK2) [52]. This indicates that wild-type LRRK2 may play an essential role in alpha-synuclein homeostasis, likely through regulation of protein degradation [1]. LRRK2 is a protein composed of 2,327 amino acids and is primarily cytoplasmic; however, it is also found on the outer membrane of the mitochondrial [53]. Wild-type LRRK2 is neuroprotective, serving to counter cell death induced by oxidative stress [54]. Mutations in LRRK2 are the most commonly identified cases of inherited PD and have an average frequency of 4% in these populations, with frequencies up to 25% in specific ethnic populations [55, 56]. Genetic variation in the LRRK2 gene has been found in 1% of sporadic PD cases [57], suggesting that interactions between LRRK2 polymorphisms and environmental exposures may contribute to PD progression in some populations.
In combination with the incomplete penetrance that characterizes most point mutations in LRRK2, it is likely that individuals heterozygous for mutations in this gene may be at a greater risk for developing PD in combination with exposure to certain toxins or other genetic factors. Animal models including Drosophila melanogaster and Caenorhabditis elegans (C. elegans) over-expressing mutant LRRK2 show increased susceptibility to the toxic effects of pesticides, such as rotenone and paraquat, and experienced increased dopaminergic neurodegeneration [58, 59]. Future studies are necessary in order to assess whether LRRK2 directly or indirectly interacts with environmental factors in order to modulate pathways associated with the protein aggregation and neurodegeneration characteristic of PD. This is a particuarly intriging avenue of study given that mutations in LRRK2 typically have incomplete penetrance and thus, toxin exposure may modulate PD risk in these populations.
2.3. Parkin and DJ-1
While mutations in SNCA and LRRK2 are dominantly inherited, a number of loss-of-function mutations have also been associated with PD, including Parkin, PINK1, and PARK7, commonly referred to as DJ-1. These types of mutations are particularly interesting in the context of the hypothesis that disease-toxicant interactions drive many cases of idiopathic PD, as they may constitute genetic risk factors leaving individuals more vulnerable to disease pathogenesis.
Parkin is an E2-dependent E3 protein-ubiquitin ligase that serves to link polyubiquitin chains to misfolded proteins via the ubiquitin proteasome system (UPS) [60]. Parkin is selectively recruited to altered mitochondria with reduced membrane potential in mammalian cells where it mediates autophagy [61]. Thus, the UPS facilitates intracellular protein degradation with the help of parkin. Prior to degradation, dysfunctional proteins or organelles are tagged with ubiquitin through the actions of ubiquitin activating (E1), conjugating (E2) and ligating enzymes (E3, or parkin) before they are broken down into their amino acid components by proteolytic enzymes in the proteosome [62]. Parkin's essential role in UPS function suggests that mechanistic failures in this protein degradation pathway may lead to accumulation of alpha-synuclein in the PD patient, promoting Lewy Body formation and cellular dysfunction. Parkin knock-out mice show decreased proteins associated with protection from mitochondrial dysfunction and oxidative stress, while Drosophila melanogaster with mutant parkin exhibit swollen and disordered mitochondria, disrupted spermatogenesis and loss of dopaminergic neurons [63–66]. Loss-of -function mutations in parkin are associated with nigrostriatal degeneration via the aforementioned pathophysiological mechanisms. It is possible that mutations in parkin may render individuals more susceptible to the deleterious effects of certain neurotoxins. For example, an individual heterozygous for the parkin mutation may be more vulnerable to toxins that inhibit the UPS or increase alpha-synuclein aggregation due to lower production of functional parkin within the CNS. This might lead to accumulation of cytotoxic proteins and contribute to PD pathogenesis.
Similar neurodegenerative mechanisms are exacerbated by mutant forms of DJ-1, a protein that regulates antioxidant gene expression and known to protect mitochondria against oxidative stress [56]. Furthermore, the interactions of Parkin, DJ-1 and PINK1 with mitochondria suggest the possibility of shared genetic pathways and gene-gene interactions in both familial and sporadic cases of PD [1]. Chronic treatment of rats with rotenone is sufficient to induce oxidative modification of DJ-1 to a more acidic form and redistribute the protein to the mitochondria, resulting in alpha-synuclein accumulation and dysfunction of the UPS [60]. UPS dysfunction may damage other essential cellular mechanisms including axonal transport and energy homeostasis, contributing to neurodegeneration of nigrostriatal dopaminergic neurons. Oxidative stress and mitochondrial dysfunction induced by environmental factors may be sufficient to influence genetic factors, contributing to PD pathogenesis. Exposure to heavy metals or other compounds that exacerbate oxidative stress pathways may impact those with mutations in DJ-1 to a greater extent than in wild-type DJ-1 due to decreased antioxidant capacity. Further studies are necessary to discern whether these deficits modulate PD pathogenesis.
3. Environmental modulators in PD
3.1. Heavy metal exposure
Overexposure to specific heavy metals has been implicated to exacerbate or cause neurodegeneration. Epidemiological studies have demonstrated a positive association between heavy metal exposed populations and incidence of PD [67]. For example, exposure to manganese (Mn) and iron (Fe) are known to cause a parkinsonism-like disorder, a condition that features some of the PD symptoms such as tremor, bradykinesia and rigidity, resulting from dopaminergic cell death [68, 69].
Mn is an essential metal for normal development and growth [70, 71]. However, chronic exposure via inhalation of Mn particulates in occupational and industrial settings such as mining and welding results in its accumulation in selected brain regions causing CNS dysfunctions and an extrapyramidal motor disorder similar to PD, referred to as Mn-induced parkinsonism or manganism [69, 72–75]. Thus, chronic occupational exposure to Mn represents a risk factor for PD [76]. PD and Mn-induced parkinsonism share some similar cellular mechanisms including energy deficits, altered mitochondria function, protein aggregation, UPS dysfunction, and neurotoxic effects on dopamine neurons [69, 77]. Patients receiving total parenteral nutrition [78, 79] as well as those with chronic liver failure who are unable to efficiently excrete Mn in bile [80] are more likely to exhibit symptoms of Mn-induced parkinsonism. Despite the similarities in some of the shared pathophysiological mechanisms between PD and Mn-induced Parkinsonism, there are distinguishable features between the two disorders. Acute exposure to Mn is characterized by early symptoms including hallucinations and psychoses while the later gives rise to motor deficits including progressive dystonia [81]. Notably, dopamine neuron loss occurs despite the absence of the Lewy bodies, which are characteristic of PD. However, the main target of neurotoxicity in these cases appears to be the globus pallidus rather than the nigrostriatal system, which is affected in idiopathic and familiar PD cases [82]. While PD results in altered dopaminergic neuron morphology, Mn-induced parkinsonism alters dopaminergic neurotransmission without causing such morphological changes [83]. Mn-induced parkinsonism cause decrease levels of monoaminergic neurotransmitters, including norepinephrine and dopamine, something not characteristic of PD [84]. Individuals with Mn-induced parkinsonism do not generally respond to levodopa (a drug used to treat early stages of PD) treatment, exhibit more frequent dystonia, and less resting tremor compared to PD patients [82].
Chronic exposure to Mn may also be sufficient to exacerbate or influence PD pathology. Specifically, Mn is capable of causing increased fibril formation of alpha-synuclein, leading to Lewy body formation that is characteristic of idiopathic PD and other synucleinopathies [77] that may result in neuronal cell death. Gitler and colleagues have reported that PARK9 encodes a metal cation transporter and interacts with alpha-synuclein in yeast to cause neuroprotection against Mn-induced toxicity [85] thus, illustrating gene-toxicant (PARK9 and Mn) and gene-gene (alpha-synuclein and PARK9) interactions in PD etiology. Over-expression of alpha-synuclein may be capable of activating transcription factors, kinases, and apoptotic signaling cascades associated with toxicity.
A link between Mn overexposure and PD has been established [86], suggesting that Mn may exert neurotoxic effects on the nigrostriatal system. Overexposure to Mn causes oxidative stress and mitochondrial dysfunction, which also characterizes PD [87, 88]. It is worth noting that intracellular Mn is taken up by the Ca2+ uniporter, facilitating its storage in mitochondria, while Mn activates heme oxygenase-1 that triggers oxidative damage in the mitochondria [89–91]. Mn exposure may serve to up-regulate parkin, an ER-stress factor that in turn up regulates PINK1, which has been associated with mitochondrial dysfunction in PD patients [77]. Over-expression of alpha-synuclein is sufficient to increase vulnerability to Mn neurotoxicity in a mesencephalic cell line, while Mn induces the over-expression of alpha-synuclein in PC12 cells, suggesting a cyclical effect between the genetic and environmental factors [92, 93].
Recent studies suggest that alpha-synuclein may protect against Mn toxicity in the context of certain mutations linked to PD pathogenesis. For example, wild-type alpha-synuclein exhibit protective effects against Mn-induced neurotoxicity during the early stages of exposure in a N27 dopaminergic neuronal model of PD [94]. The invertebrate Caenorhabditis elegans (C. elegans) model system has revealed the neurotoxic or neuroprotective role of alpha-synuclein upon acute Mn exposure in the background of mutated pdr1, pink1 or djr1.1. Specifically, the pdr1 and djr1.1 mutants exhibit increased Mn accumulation and oxidative stress that was rescued by alpha-synuclein. The loss-of-function mutations in these genes may exacerbate Mn neurotoxicity through impairing clearance of damaged mitochondria, leading to increased free radical production. Additionally, while dopaminergic neurodegeneration was unchanged with Mn exposure, it returned to wild-type levels for pdr1, but not djr1.1 mutants expressing α-synuclein [95]. This study indicates a neuroprotective role for wild-type human alpha-synuclein in mitigating Mn-induced cytotoxicity in the background of PD-associated genes, and further supports the role of extracellular dopamine in potentiating Mn neurotoxicity in vivo. Mn interacts with alpha-synuclein to promote aggregation of the protein [96]. Alpha-synuclein may help protect against Mn toxicity through its ability to segregate this metal, thus altering Mn homeostasis in dopaminergic neurons in the worm.
Other heavy metals have also been associated with PD. For example, analyses of postmortem PD brain tissues have reported cases of increased iron deposition in the substantia nigra pars compacta compared to neurotypical control tissues [97, 98]. Additional studies have found decreased levels of ferritin in this region of the brain [99]. Ferritin is a protein that binds to iron and renders it non-reactive, which may suggest that the decreased binding ability and increased iron accumulation may cause neurotoxicity in PD patients. Iron can mediate the Fenton-Haber-Weiss reaction to produce hydroxyl radicals that cause lipid, protein, and DNA oxidation, potentially resulting in dopamine neuron cell death [100]. Future studies are necessary to further understand the influence of Mn, Fe, and other heavy metal-mediated toxicity in other neurotransmitter systems, cellular and circuitry mechanisms that underlie dopamine neuron survival and function. Epidemiological studies have indicated that countries in the United States with heavy metal emissions, including copper (Cu) and Mn have a greater incidence of PD [101], further highlighting the necessity for future research to evaluate the potential role of other metals in PD pathogenesis.
3.2. Illicit drugs
3.2.1. MPTP
Evidence for the influence of neurotoxins in illicit drug use as causal agents in PD began with the discovery of the neurotoxin 1-methyl-4-pheyl-1,2,3,6-tetrahydropyrine (MPTP), which was sufficient to reproduce PD-like symptoms that can be attenuated with levodopa treatment [102, 103]. Moreover, a postmortem study of three patient brains who had been afflicted with MPTP-induced parkinsonism revealed an inordinate reduction of pigmented dopaminergic neurons within the substantia nigra, a characteristic of idiopathic PD, indicating the possibility of a shared pathology between MPTP exposure and PD [104].
MPTP is lipophilic and readily crosses the blood-brain-barrier where it is metabolized in glial cells into its unstable and toxic metabolite, 1-methyl-4-phenylpyridinium (MPP+). MPP+ is released into the extracellular space where it is taken up by dopamine transporter (DAT) on dopaminergic neurons and inhibits complex I of the mitochondrial electron transport chain, blocking electron flow and thus impairing ATP production and generating reactive oxygen species (ROS) [100]. Thus, it is possible that genetic variability in genes controlling the antioxidant buffering capacity may increase vulnerability to MPTP toxicity due to decreased ability to neutralize free radicals. Upon MPP+ entry into dopaminergic neurons, it is sequestered into synaptic vesicles by the vesicular monoamine transporter (VMAT2), effectively decreasing the amount of MPP+ that accumulates in the mitochondria [105]. The ratio between DAT and VMAT2 may present a means to explain why dopaminergic neurons are especially vulnerable to MPTP-induced toxicity. Transgenic mice bereft of DAT showed complete resistance to MPTP toxicity, implying that the accumulation of MPP+ in dopaminergic neurons facilitated by DAT is an essential step in MPTP toxicity and nigrostriatal neurodegeneration [106]. Therefore, higher levels of DAT may predispose neurons to MPTP-induced toxicity. Conversely, VMAT2 heterozygote knockout mice experience twice as much nigral dopaminergic neuron loss subsequent to MPTP exposure than wild-type mice [107]. This suggests that higher levels of DAT may serve to exacerbate MPTP toxicity and VMAT2 exerts a neuroprotective effect. It is also possible that chemical interactions may alter the ratio between DAT and VMAT2, thus affecting dopaminergic neurons' vulnerability to MPTP and similar toxicants. A study by Vaccari and Saba found that heptachlor, an organochlorine insecticide increase DAT levels in the mouse striatum [108], while Miller and colleagues show that treatment with heptachlor inhibits VMAT2 in vitro [109]. A synergistic epiphenomenon may be possible wherein a toxic insult may increase DAT or reduce VMAT2 rendering the neuron more vulnerable to a second insult. Future studies are necessary to elucidate how exposure to heavy metals and pesticides may modulate MPP+-induced toxicity.
Additional mechanisms including oxidative stress have been implicated in causing nigrostriatal damage and MPTP-induced energy deficits. The substrata nigra is the most vulnerable region of the brain in PD due to its high metabolic rate combined with its high content of oxidizable species, including dopamine. In addition to disrupting electron flow at complex one, MPP+ is thought to increase superoxide production. Oxidative stress may arise as a result of MPP+ disrupting dopamine homeostasis and triggering the release of dopamine from vesicular storage, causing the formation of oxidizing metabolites including dopamine quinones and hydrogen peroxide [110]. In conjunction with generating destructive oxidizing agents, MPTP may employ mechanisms, such as excitotoxicity, the formation of nitric oxide, and apoptosis to cause nigrostriatal degeneration. Nitric oxide is a free radical that gives rise to peroxynitrite when it interacts with superoxide anion. Peroxynitrite causes damage to cellular macromolecules and eventually death in neurons. Peroxynitrate can lead to mitochondria dysfunction, as can nitric oxide, thus exacerbating the toxic effects of MPTP [100]. Though the source of the nitric oxide in MPTP neurotoxicity is unclear, mice lacking the inducible nitric oxide synthase (iNOS) gene show greater resistance to the effects of MPTP than wild-type littermates, implying that iNOS may play a crucial role in generating nitric oxide in this process [111].
iNOS is typically generated by microglia, implicating the inflammatory cascade in MPTP-induced toxicity. This is particularly intriguing given the evidence of the role neuroinflammation plays in idiopathic PD where chronic exposure to environmental triggers may activate microglia causing them to overproduce a neurotoxic inflammatory mediator. There is an especially high density of microglia in the midbrain that may make the substantia nigra particularly vulnerable [112]. Epidemiological studies show support for a potential role of neuroinflammation in causing cell death in dopaminergic neurons. For example, the incidence of idiopathic PD in individuals who use non-steroidal anti-inflammatory (NSAID) drugs chronically was 46% lower than age-matched controls [113]. Presumably, this may be due to NSAIDs' ability to scavenge free oxygen radicals and inhibit cyclo-oxygenase activity triggered, reducing neuroinflammation.
As MPTP is not readily available to the general population, it is not a major factor in inducing non-familial PD pathology. However, understanding the mechanisms by which MPTP acts to cause PD symptoms may lead to a better understanding of the mechanisms that cause PD neuropathology. Additionally, there exist many heterocyclic molecules that structurally resemble MPTP and identified as potential parkinsonogenic agents. Among them are tetrahydroisoquinoline (TIQ) and ß-carboline (ß-C) that are alkaloids found in a number of foods [114], cause nigrostriatal damage in rat brains [115]. Future research is needed to determine whether TIQs and ß-Cs are capable of exerting the same neurotic effects as MPTP. It may be possible that chronic exposure to TIQs and ß-Cs may damage the nigrostriatal system and result in PD neuropathology.
There is a relationship between alpha-synuclein and exposure to MPTP. For example, squirrel monkeys experience up-regulation of alpha-synuclein in response to injection with MPTP [116]. RT-PCR analysis of genes in the dopaminergic system of murine cells revealed MPTP-induced changes in the expression levels of genes implicated in PD, including the rate limiting enzyme tyrosine hydroxylase (TH), DAT, VMAT, and alpha-synuclein [117]. Thus, MPTP induced Parkinsonism presents a good model to further explore disease-toxicant interactions, and genes and proteins altered in PD pathogenesis.
3.2.2. Methamphetamine
Other drugs of abuse are implicated in PD pathogenesis. A 2015 epidemiological study examined medical records between 1996 and 2011 within the state of Utah and reported that abusers of methamphetamine and its metabolite, amphetamine have a near three-fold chance of being diagnosed with PD in comparison to nonusers. The same study also found that female methamphetamine users were five times more likely to develop PD in comparison to their male counterparts [118].
Methamphetamine is a notoriously addictive synthetic stimulant that readily crosses the blood-brain barrier (BBB). Within the brain, it triggers signaling cascades leading to the release of inordinate amounts of dopamine and other monoamines such as serotonin and inhibits dopamine uptake into the presynaptic terminal via DATs [119]. Both these mechanisms lead to increased dopamine at the synapse, stimulating the nigrostriatal and mesolimbic reward dopaminergic pathways, which results in feelings of euphoria and makes users prone to addiction [120].
Methamphetamine is a potent neurotoxin that cause neuronal loss of dopaminergic neurons, leading to decreased overall dopamine concentrations and tyrosine hydroxylase (TH) levels subsequent to abuse of the drug [121]. The dopamine depletion may be due to the activation of oxidative stress pathways by methamphetamine. Specifically, methamphetamine-induced dopamine accumulation at the synapse results in dopamine auto-oxidation, causing increased production of free radicals and reactive quinones [122]. These reactive species can cause lipid, protein and DNA peroxidation, leading to increased cellular damage and dopaminergic cell death.
Excessive production of free radicals may alter UPS subunit and protein expression leading to its dysfunction and subsequently the accumulation of misfolded proteins, potentially aiding alpha-synuclein aggregation and Lewy body formation. Fornai and colleagues showed that amphetamines could cause the formation of neuronal inclusions within the nigrostriatal system of mice, likely by negatively affecting essential proteins in the UPS. These inclusions share common components of Lewy bodies such as alpha-synuclein and ubiquitin [123].
The neurodegenerative effects of methamphetamine on dopaminergic neurons parallels that of PD pathogenesis, suggesting that abusing this drug may contribute to PD-like phenotypes. This link is especially troubling given that as many as half a million people in the United States may use methamphetamine every week [124]. Studies have demonstrated that UPS dysfunction can contribute to methamphetamine toxicity in dopaminergic neurons and that methamphetamine can cause oxidative damage of parkin in vivo. [125]. Conversely, over-expression of Parkin protects rat dopaminergic neurons from the toxic effects of acute methamphetamine, in vivo [126]. Future studies are necessary in order to examine whether genetic variability in Parkin and other UPS proteins renders the individual more susceptible to dopaminergic cell damage resulting from methamphetamine use.
3.3. Pesticides
3.3.1. Rotenone
Rotenone is a natural botanical pesticide used in organic farming and to control fish populations [127]. Due to its hydrophobic structure, rotenone can easily cross the blood-brain-barrier without the assistance of a transporter where it accumulates in the mitochondria and exerts its toxic effects by inhibiting mitochondrial complex I [128]. Inhibition of the mitochondria leads to insufficient ATP synthesis, increased production and accumulation of ROS in the cell, and subsequent cell death. Based on the studies that have reported 15–30 % reduction in mitochondrial complex I activity in nonfamilial PD patients, it has been hypothesized that rotenone exposure may be an environmental factor that leads to PD-related pathogenesis [2]. Additional studies have found that rotenone infusion generates many of the motor symptoms of non-familial PD, including rigidity, hypokinesia, resting tremor, and as a result dopaminergic neurodegeneration [129]. Similar to MPP+, rotenone interacts with complex I and reduces electron flow at upstream sites thus causing electrons to remain at complex I for an inordinate amount of time. This fosters the creation of superoxide radicals, which is released within the mitochondria. Notably, rotenone is selectively toxic to nigrostriatal dopamine neurons and causes the formation of Lewy-body-like inclusions containing alpha-synuclein [129]. The selective vulnerability of rotenone-induced inhibition of mitochondrial complex I in dopaminergic neurons remain unclear and may be a result of dopamine metabolism, which results in the generation of hydrogen peroxide that induces oxidative damage in dopaminergic neurons [130].
In addition to ROS generation, rotenone also triggers glial cell activation and a neuroinflammatory response [130]. Knock-out studies in mice have indicated that rotenone releases superoxide from microglia. Inhibition of NADPH oxidase in these microglia dramatically reduces rotenone's neurotoxic effects, indicating that the microglial NADPH oxidase generated ROS are the primary contributor to rotenone-induced neurotoxicity [131]. Rotenone may modify alpha-synuclein structure and function, making it more likely to aggregate and contribute to oxidative damage [130]. This prompts the question of whether genetic polymorphisms leading to impaired function of the UPS may leave individuals more likely to develop PD after rotenone exposure.
While a number of factors render rotenone a flawed model of PD (including its toxic effects on peripheral organs) [129], epidemiological studies have indicated that the incidence of PD-related disorders is more common in individuals exposed to rotenone than control groups with similar demographic backgrounds [127]. Chronic treatment with rotenone is sufficient to cause oxidative modification of DJ-1 (a protein associated with oxidative stress), alpha-synuclein accumulation, and proteasome inhibition [60]. The genetic forms of PD target similar cellular pathways to cause PD. Recessive loss-of-function mutations in DJ-1 are associated with early-onset Parkinsonism, while mutations in SNCA cause alpha-synuclein modification and accumulation. Likewise, loss-of-function mutations in Parkin eliminate its ability to regulate protein degradation via the UPS processes. The fact that rotenone and genetic forms of PD share some similar pathophysiological mechanisms, including oxidative stress, mitochondrial complex I inhibition, proteasomal dysfunction, protein aggregation, and many others suggests an important link between genetic and idiopathic cases of PD. In the future, epidemiological studies should be conducted to identify whether these shared mechanisms may leave certain genetic populations more vulnerable to rotenone neurotoxicity and PD.
3.3.2. Dieldrin
In addition to mitochondria complex I deficiency revealed by MPP+ and rotenone, deficits in other parts of the mitochondrial respiratory chain may also play a role in PD pathogenesis. Dieldrin and Maneb inhibit complex III of the respiratory chain and implicated in epidemiological and in vivo studies to cause PD-related disorders [132, 133].
Dieldrin is an organochlorine insecticide that was used between 1950 and the mid-1970s in the United States before it was restricted and finally banned based on its potential carcinogenic capacity and bioaccumulation [134]. However, due to the compounds long half-life and lipophilicity, significant levels of the pesticide remain in the ecosystem after use. Post-mortem studies have revealed that PD patients are more likely to have perceivable amounts of dieldrin in their brains in comparison to those afflicted with other illnesses. For example, other studies show elevated amounts of the dieldrin in the caudate nucleus of PD patients when compared to healthy age-matched controls [135, 136].
Dieldrin readily crosses the BBB due to its lipophilicity where it exerts neurotoxicity via similar mechanisms that are prominent in PD: production of ROS, ubiquitin-proteasome-system dysfunction, up-regulation of alpha-synuclein aggregation, reduction and release of intracellular dopamine, and mitochondrial dysfunction [128]. Dopaminergic neurons are more vulnerable to the neurotoxic effects of dieldrin than other neuronal populations [137]. Kanthasamy and colleagues utilized flow cytometry, biochemical and immunochemical techniques to demonstrate that acute exposure of N27 and PC12 dopaminergic cell models of PD and rat brain slices to dieldrin results in a significant increase in ROS production, DNA fragmentation, and cytochrome c release into the cytosol, which activates a caspase-dependent apoptotic pathway via proteolytic cleavage of protein kinase C delta (PKC.1 into 41 kDa catalytic and 38 kDa regulatory subunits [138, 139]. Single high doses of dieldrin administered to mice failed to induce any noticeable changes in striatal dopamine levels; however, chronic doses reduced dopamine levels in several animal models, while also altering DAT and VMAT levels [140–142]. For example, chronic low doses of dieldrin administered to mice for 30 days altered dopamine-handling by significantly decreasing DAT expression and the dopamine metabolites, DOPAC (3,4-Dihydroxyphenylacetic acid) and HVA (homovanillic acid), and increasing cysteinyl-catechol and alpha-synuclein levels in the striatum. In addition, exposure to dieldrin significantly reduced total glutathione and increased protein carbonyls levels in the striatum indicating oxidative stress. Despite these impairments in dopamine metabolism and induction of oxidative stress pathways, there were no dopamine neuron loss in the substantia nigra pars compacta. These effects indicate that low doses of dieldrin enhances the susceptibility of dopamine neurons in the substantia nigra by activating oxidative stress pathways that may eventually lead to PD neuropathology [142]. Exposure to dieldrin further cause impairment in UPS function and increase apoptosis [143]. Thus, dieldrin exposure may further exacerbate the aggregation of alpha synuclein in the context of genetic variation leading to either increase alpha synuclein production, or its impaired degredation. These results should be translated in epidemiological studies assessing whether individuals with genetic polymorphisms in SNCA exhibit UPS dysfunction and how prior exposure to dieldrin coupled with SNCA polymorphism may contribute to PD pathogenesis.
4. Estrogen and other neuroprotective compounds
Epidemiological studies revealed the incidence of PD to be 1.5–2 times greater in men than women with women who do develop PD having a later age of onset by an average of 2.2 years, and greater motor skills than men afflicted with the disease [144]. Thus, women appear to be more protected against PD pathogenesis than men. This protection may be a result of higher estrogen levels. In support of this hypothesis, dopaminergic neurons in the substantial nigra of animal models of PD experience greater vulnerability to lesions at low levels of estrogen than those treated with higher levels of estrogen [145]. Women who have undergone ovariectomy or hysterectomy have a greater chance of developing PD [145]. However, this seemingly clear neuroprotective function of estrogen is complicated by studies that found that estrogen supplementation in postmenopausal women after PD symptom onset did not alter the course or severity of the disease [145]. Therefore, estrogen may have neuroprotective potential in preventing or mitigating PD prior to symptoms onset.
The exact mechanism by which estrogen exerts its neuroprotective effects is unclear. It is possible that estrogen acts to prevent apoptosis via estrogen receptor mediated pathways [145] and that the absence of estrogen in MPTP-induced neurotoxic dopaminergic cells may induce apoptosis [146]. Alternatively, estrogen may protect dopaminergic neurons through more indirect means, such as its capacity to up-regulate the secretion of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). Estradiol and estrogen-like phytoestrogens rescue PC12 neural cells from MPP+-induced cell death [147, 148]. These findings bolster epidemiological studies suggesting that estrogen may protect against PD likely by protecting dopaminergic neurons, thus slowing the progression of PD [149].
Environmental neuroprotective agents, such as tobacco and caffeine have been identified and linked with PD. Epidemiological studies show that smokers have decreased PD incidence independent of selective mortality due to the health complications of tobacco exposure [150, 151]. This protective effect could be due to the nicotine found in tobacco. This mechanism is supported by several studies, one of which found that repeated nicotine exposure was sufficient to impede nigrostriatal cell loss as a result of a hemitransection of the medial forebrain of rats [152]. However, the ability of nicotine to improve the motor symptoms of PD is less disputed, with some clinical studies finding positive results [153], and others no significant correlation [154, 155]. Additional studies report an association between high caffeine consumption with lower PD incidence [100]. Caffeine is an adenosine receptor antagonist, and may be able to encourage dopamine release through repressing adenosine inhibition [156]. Alternatively, caffeine may exert an anti-apoptotic function via activation of the phosphatidyl-linositol-3-kinase/Akt pathway as reported in the human dopaminergic neuroblastoma SH-SY5Y cell model of PD [157]. It still remains somewhat controversial whether caffeine is truly able to exert neuroprotective effects in PD neuropathology. Most notably, it is unclear whether caffeine is neuroprotective in women, with some studies finding a significantly reduced risk of PD in women who consume coffee [158], while others finding no correlation between the two, presumably due to modifications caused by estrogen [159], and/or other genetic and environmental factors that were not controlled for in the studies. Future studies should assess the possibility that genetic polymorphisms may alter the neuroprotective effects of estrogen, thus leaving some women more vulnerable to environmental factors associated with PD pathogenesis.
4.1. Treatment strategies and therapies used in PD
Though the mechanisms underlying PD and Parkinsonism have been better characterized in recent years, no cure has yet been found. Rather, many current treatments focus on allaying symptoms of dopaminergic neuron loss by replenishing dopamine within the brain. One such treatment, levodopa is one of the most effective drugs for treating PD due to its ability to ameliorate the low levels of dopamine characteristic of the disease. Administered orally in conjunction with carboxylate-inhibitors such as carbidopa to prevent its conversion to dopamine in the periphery, levodopa crosses the blood brain barrier and is converted to dopamine by L-amino acid decarboxylase or dopa decarboxylase in the presence of the cofactor pyridoxal phosphate (vitamin B6) [160]. Increased dopamine in the PD brain through this treatment helps to improve symptoms such as tremors, rigidity, and hypokinesia. However, long-term use of levodopa results in disabling motor and sensory fluctuations, neuropsychiatric and circadian complications, and dyskinesias negating its beneficial effects. [161, 162]. The surgical method deep brain stimulation (DBS) is used to alleviate some of the motor complications such as tremor, stiffness, bradykinesia, and dystonia that cannot be controlled my medications in in PD individuals [163].
5. Summary
The etiology of PD has long been controversial. Genome-wide association studies have revealed gene polymorphisms related to the incidence of the disease, dispelling the previous belief that genetics did not play a role in its pathogenesis. While these discoveries are significant, majority of PD cases are sporadic, indicating environmental factors are essential to consider in order to uncover the triggers, understand the disease and find a cure for PD. Epidemiological studies have linked exposures to pesticides and heavy metals to higher incidence of PD, yet the exact role these factors play in disease progression remain elusive in most cases. An emerging perspective towards PD etiology seeks to integrate interest in these environmental agents and genetic predispositions or susceptibility factors (disease-toxicant interactions), in order to understand how the two may interact or up-regulate PD-associated pathways such as impaired protein degradation and aggregation in neurons, mitochondrial dysfunction, and subsequent oxidative stress resulting in dopaminergic neuron loss. In combining a whole genome approach in order to understand genetic susceptibility to PD and epidemiological and toxicology studies, a better understanding of PD pathogenesis can be reached and new, more effective therapies developed and utilized in conjunction with current treatments, such as levodopa and deep brain stimulation.
Acknowledgements
GFK was supported in part by Oberlin College Office of Foundation, Government, and Corporate Grants. RAM was supported in part by Robert Rich Student Research Grant at Oberlin College. MA was supported in part by NIEHS grants R01ES07331, R01ES10563 and R01ES020852.
References
- 1.Gao H-M, Hong J-S. Gene-environment interactions: key to unraveling the mystery of Parkinson's disease. Prog Neurobiol. 2011;94:1–19. doi: 10.1016/j.pneurobio.2011.03.005. doi: 10.1016/j.pneurobio.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schapira AH, Cooper JM, Dexter D, et al. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez-Alvarado M, Kelly JW, Dobson CM. Protein Misfolding Diseases. 2010 doi: 10.1002/9780470572702. [Google Scholar]
- 4.Sveinbjörnsdottir S, Hicks AA, Jonsson T, et al. Familial aggregation of Parkinson's disease in Iceland. N Engl J Med. 2000;343:1765–1770. doi: 10.1056/NEJM200012143432404. doi: 10.1056/NEJM200012143432404. [DOI] [PubMed] [Google Scholar]
- 5.Rocca WA, McDonnell SK, Strain KJ, et al. Familial aggregation of Parkinson's disease: The Mayo Clinic family study. Ann Neurol. 2004;56:495–502. doi: 10.1002/ana.20228. doi: 10.1002/ana.20228. [DOI] [PubMed] [Google Scholar]
- 6.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat Rev Genet. 2006;7:306–318. doi: 10.1038/nrg1831. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 7.Thomas B, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16:R183–94. doi: 10.1093/hmg/ddm159. Spec No. 2. doi: 10. 1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 8.Duvoisin RC, Eldridge R, Williams A, et al. Twin study of Parkinson disease. Neurology. 1981;31:77–80. doi: 10.1212/wnl.31.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Tanner CM. Is the cause of Parkinson's disease environmental or hereditary? Evidence from twin studies. Adv Neurol. 2003;91:133–142. [PubMed] [Google Scholar]
- 10.Wirdefeldt K, Gatz M, Schalling M, Pedersen NL. No evidence for heritability of Parkinson disease in Swedish twins. Neurology. 2004;63:305–311. doi: 10.1212/01.wnl.0000129841.30587.9d. [DOI] [PubMed] [Google Scholar]
- 11.Tanner CM, Ottman R, Goldman SM, et al. Parkinson disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- 12.Lesage S, Brice A. Parkinson's disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18:R48–59. doi: 10.1093/hmg/ddp012. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 13.Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cookson MR. alpha-Synuclein and neuronal cell death. Mol Neurodegener. 2009;4:9. doi: 10.1186/1750-1326-4-9. doi: 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baba M, Nakajo S, Tu PH, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 16.Darios F, Ruipérez V, López I, et al. Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010;11:528–533. doi: 10.1038/embor.2010.66. doi: 10.1038/embor.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J Biol Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- 18.Sharon R, Bar-Joseph I, Frosch MP, et al. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson's disease. Neuron. 2003;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 19.Assayag K, Yakunin E, Loeb V, et al. Polyunsaturated fatty acids induce alpha-synuclein-related pathogenic changes in neuronal cells. Am J Pathol. 2007;171:2000–2011. doi: 10.2353/ajpath.2007.070373. doi: 10.2353/ajpath.2007.070373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009399. doi: 10.1101/cshperspect.a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartels T, Choi JG, Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature. 2011;477:107–110. doi: 10.1038/nature10324. doi: 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.da Costa CA, Ancolio K, Checler F. Wild-type but not Parkinson's disease-related ala-53 --> Thr mutant alpha -synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 23.Jensen PJ, Alter BJ, O'Malley KL. Alpha-synuclein protects naive but not dbcAMP-treated dopaminergic cell types from 1-methyl-4-phenylpyridinium toxicity. J Neurochem. 2003;86:196–209. doi: 10.1046/j.1471-4159.2003.01835.x. [DOI] [PubMed] [Google Scholar]
- 24.Rockenstein E, Nuber S, Overk CR, et al. Accumulation of oligomer-prone α-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain. 2014;137:1496–1513. doi: 10.1093/brain/awu057. doi: 10.1093/brain/awu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNaught KSP, Olanow CW. Proteolytic stress: a unifying concept for the etiopathogenesis of Parkinson's disease. Ann Neurol. 2003;53(Suppl 3):S73–84. doi: 10.1002/ana.10512. discussion S84. doi: 10.1002/ana.10512. [DOI] [PubMed] [Google Scholar]
- 26.Li J-Y, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 27.Kordower JH, Chu Y, Hauser RA, et al. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 28.Lee H-J, Patel S, Lee S-J. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–6024. doi: 10.1523/JNEUROSCI.0692-05.2005. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H-J, Choi C, Lee S-J. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- 30.George JM, Jin H, Woods WS, Clayton DF. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 31.Borghi R, Marchese R, Negro A, et al. Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett. 2000;287:65–67. doi: 10.1016/s0304-3940(00)01153-8. doi: 10.1016/S0304-3940(00)01153-8. [DOI] [PubMed] [Google Scholar]
- 32.El-Agnaf OMA, Salem SA, Paleologou KE, et al. Alpha-synuclein implicated in Parkinson's disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 33.Miller DW, Hague SM, Clarimon J, et al. Alpha-synuclein in blood and brain from familial Parkinson disease with SNCA locus triplication. Neurology. 2004;62:1835–1838. doi: 10.1212/01.wnl.0000127517.33208.f4. [DOI] [PubMed] [Google Scholar]
- 34.Desplats P, Lee H-J, Bae E-J, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danzer KM, Kranich LR, Ruf WP, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alvarez-Erviti L, Seow Y, Schapira AH, et al. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42:360–367. doi: 10.1016/j.nbd.2011.01.029. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmanouilidou E, Melachroinou K, Roumeliotis T, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30:6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez RG, Waymire JC, Lin E, et al. A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci. 2002;22:3090–3099. doi: 10.1523/JNEUROSCI.22-08-03090.2002. doi: 20026307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alerte TNM, Akinfolarin AA, Friedrich EE, et al. Alpha-synuclein aggregation alters tyrosine hydroxylase phosphorylation and immunoreactivity: lessons from viral transduction of knockout mice. Neurosci Lett. 2008;435:24–29. doi: 10.1016/j.neulet.2008.02.014. doi: 10.1016/j.neulet.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson's disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 41.Conway KA, Lee SJ, Rochet JC, et al. Acceleration of oligomerization, not fibrillization, is a shared property of both alpha-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci U S A. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karpinar DP, Balija MBG, Kügler S, et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson's disease models. EMBO J. 2009;28:3256–3268. doi: 10.1038/emboj.2009.257. doi: 10.1038/emboj.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duda JE, Lee VM, Trojanowski JQ. Neuropathology of synuclein aggregates. J Neurosci Res. 2000;61:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 44.Seidel K, Schöls L, Nuber S, et al. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol. 2010;67:684–689. doi: 10.1002/ana.21966. doi: 10.1002/ana.21966. [DOI] [PubMed] [Google Scholar]
- 45.Chartier-Harlin M-C, Kachergus J, Roumier C, et al. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. The Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 46.Nishioka K, Hayashi S, Farrer MJ, et al. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 47.Maraganore DM, de Andrade M, Elbaz A, et al. Collaborative analysis of alpha-synuclein gene promoter variability and Parkinson disease. JAMA. 2006;296:661–670. doi: 10.1001/jama.296.6.661. doi: 10.1001/jama.296.6.661. [DOI] [PubMed] [Google Scholar]
- 48.Cersosimo MG, Benarroch EE. Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord. 2008;23:1065–1075. doi: 10.1002/mds.22051. doi: 10.1002/mds.22051. [DOI] [PubMed] [Google Scholar]
- 49.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 50.Farrer M, Maraganore DM, Lockhart P, et al. alpha-Synuclein gene haplotypes are associated with Parkinson's disease. Hum Mol Genet. 2001;10:1847–1851. doi: 10.1093/hmg/10.17.1847. [DOI] [PubMed] [Google Scholar]
- 51.Chung SJ, Armasu SM, Anderson KJ, et al. Genetic susceptibility loci, environmental exposures, and Parkinson's disease: a case-control study of gene-environment interactions. Parkinsonism Relat Disord. 2013;19:595–599. doi: 10.1016/j.parkreldis.2013.02.008. doi: 10.1016/j.parkreldis.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tong Y, Yamaguchi H, Giaime E, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith WW, Pei Z, Jiang H, et al. Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration. Proc Natl Acad Sci U S A. 2005;102:18676–18681. doi: 10.1073/pnas.0508052102. doi: 10.1073/pnas.0508052102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liou AKF, Leak RK, Li L, Zigmond MJ. Wild-type LRRK2 but not its mutant attenuates stress-induced cell death via ERK pathway. Neurobiol Dis. 2008;32:116–124. doi: 10.1016/j.nbd.2008.06.016. doi: 10.1016/j.nbd.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross CA, Smith WW. Gene-environment interactions in Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S309–15. doi: 10.1016/S1353-8020(08)70022-1. doi: 10.1016/S1353-8020(08)70022-1. [DOI] [PubMed] [Google Scholar]
- 57.Paisán-Ruiz C, Washecka N, Nath P, et al. Parkinson's disease and low frequency alleles found together throughout LRRK2. Ann Hum Genet. 2009;73:391–403. doi: 10.1111/j.1469-1809.2009.00524.x. doi: 10.1111/j.1469-1809.2009.00524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha S, Guillily MD, Ferree A, et al. LRRK2 modulates vulnerability to mitochondrial dysfunction in Caenorhabditis elegans. J Neurosci. 2009;29:9210–9218. doi: 10.1523/JNEUROSCI.2281-09.2009. doi: 10.1523/JNEUROSCI.2281-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng C-H, Mok SZS, Koh C, et al. Parkin protects against LRRK2 G2019S mutant-induced dopaminergic neurodegeneration in Drosophila. J Neurosci. 2009;29:11257–11262. doi: 10.1523/JNEUROSCI.2375-09.2009. doi: 10.1523/JNEUROSCI.2375-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Betarbet R, Canet-Aviles RM, Sherer TB, et al. Intersecting pathways to neurodegeneration in Parkinson's disease: effects of the pesticide rotenone on DJ-1, alpha-synuclein, and the ubiquitin-proteasome system. Neurobiol Dis. 2006;22:404–420. doi: 10.1016/j.nbd.2005.12.003. doi: 10.1016/j.nbd.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Narendra D, Tanaka A, Suen D-F, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim K-L, Tan JMM. Role of the ubiquitin proteasome system in Parkinson's disease. BMC Biochem. 2007;8(Suppl 1):S13. doi: 10.1186/1471-2091-8-S1-S13. doi: 10.1186/1471-2091-8-S1-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greene JC, Whitworth AJ, Kuo I, et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci U S A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitworth AJ, Theodore DA, Greene JC, et al. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc Natl Acad Sci U S A. 2005;102:8024–8029. doi: 10.1073/pnas.0501078102. doi: 10.1073/pnas.0501078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palacino JJ, Sagi D, Goldberg MS, et al. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- 66.Poole AC, Thomas RE, Andrews LA, et al. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc Natl Acad Sci U S A. 2008;105:1638–1643. doi: 10.1073/pnas.0709336105. doi: 10.1073/pnas.0709336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seidler A, Hellenbrand W, Robra BP, et al. Possible environmental, occupational, and other etiologic factors for Parkinson's disease: a case-control study in Germany. Neurology. 1996;46:1275–1284. doi: 10.1212/wnl.46.5.1275. [DOI] [PubMed] [Google Scholar]
- 68.Aschner M, Erikson KM, Herrero Hernández E, et al. Manganese and its role in Parkinson's disease: from transport to neuropathology. Neuromolecular Med. 2009;11:252–266. doi: 10.1007/s12017-009-8083-0. doi: 10.1007/s12017-009-8083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwakye GF, Paoliello MMB, Mukhopadhyay S, et al. Manganese-Induced Parkinsonism and Parkinson's Disease: Shared and Distinguishable Features. Int J Environ Res Public Health. 2015;12:7519–7540. doi: 10.3390/ijerph120707519. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeda A. Manganese action in brain function. Brain Res Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 71.Aschner M, Erikson KM, Dorman DC. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- 72.Perl DP, Olanow CW. The neuropathology of manganese-induced Parkinsonism. J Neuropathol Exp Neurol. 2007;66:675–682. doi: 10.1097/nen.0b013e31812503cf. doi: 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- 73.Erikson KM, Syversen T, Aschner JL, Aschner M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ Toxicol Pharmacol. 2005;19:415–421. doi: 10.1016/j.etap.2004.12.053. doi: 10.1016/j.etap.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 74.Dobson AW, Erikson KM, Aschner M. Manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:115–128. doi: 10.1196/annals.1306.009. [DOI] [PubMed] [Google Scholar]
- 75.Keen CL, Ensunsa JL, Clegg MS. Manganese metabolism in animals and humans including the toxicity of manganese. Met Ions Biol Syst. 2000;37:89–121. [PubMed] [Google Scholar]
- 76.Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational metal exposures and the risk of Parkinson's disease. Neuroepidemiology. 1999;18:303–308. doi: 10.1159/000026225. doi: 26225. [DOI] [PubMed] [Google Scholar]
- 77.Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M. Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol. 2011;25:191–203. doi: 10.1016/j.jtemb.2011.08.144. doi: 10.1016/j.jtemb.2011.08.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fitzgerald K, Mikalunas V, Rubin H, et al. Hypermanganesemia in patients receiving total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1999;23:333–336. doi: 10.1177/0148607199023006333. [DOI] [PubMed] [Google Scholar]
- 79.Bertinet DB, Tinivella M, Balzola FA, et al. Brain manganese deposition and blood levels in patients undergoing home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2000;24:223–227. doi: 10.1177/0148607100024004223. [DOI] [PubMed] [Google Scholar]
- 80.Hauser RA, Zesiewicz TA, Rosemurgy AS, et al. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–875. doi: 10.1002/ana.410360611. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- 81.Verhoeven WM, Egger JI, Kuijpers HJ. Manganese and acute paranoid psychosis: a case report. J Med Case Reports. 2011;5:146. doi: 10.1186/1752-1947-5-146. doi: 10.1186/1752-1947-5-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pal PK, Samii A, Calne DB. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- 83.Kim Y, Kim JM, Kim JW, et al. Dopamine transporter density is decreased in parkinsonian patients with a history of manganese exposure: what does it mean? Mov Disord. 2002;17:568–575. doi: 10.1002/mds.10089. doi: 10.1002/mds.10089. [DOI] [PubMed] [Google Scholar]
- 84.Struve MF, McManus BE, Wong BA, Dorman DC. Basal ganglia neurotransmitter concentrations in rhesus monkeys following subchronic manganese sulfate inhalation. Am J Ind Med. 2007;50:772–778. doi: 10.1002/ajim.20489. doi: 10.1002/ajim.20489. [DOI] [PubMed] [Google Scholar]
- 85.Gitler AD, Chesi A, Geddie ML, et al. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cowan DM, Zheng W, Zou Y, et al. Manganese exposure among smelting workers: relationship between blood manganese-iron ratio and early onset neurobehavioral alterations. Neurotoxicology. 2009;30:1214–1222. doi: 10.1016/j.neuro.2009.02.005. doi: 10.1016/j.neuro.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malecki EA. Manganese toxicity is associated with mitochondrial dysfunction and DNA fragmentation in rat primary striatal neurons. Brain Res Bull. 2001;55:225–228. doi: 10.1016/s0361-9230(01)00456-7. [DOI] [PubMed] [Google Scholar]
- 88.Xu B, Wu S-W, Lu C-W, et al. Oxidative stress involvement in manganese-induced alpha-synuclein oligomerization in organotypic brain slice cultures. Toxicology. 2013;305:71–78. doi: 10.1016/j.tox.2013.01.006. doi: 10.1016/j.tox.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 89.Hsu LJ, Sagara Y, Arroyo A, et al. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157:401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migheli R, Godani C, Sciola L, et al. Enhancing effect of manganese on L-DOPA-induced apoptosis in PC12 cells: role of oxidative stress. J Neurochem. 1999;73:1155–1163. doi: 10.1046/j.1471-4159.1999.0731155.x. [DOI] [PubMed] [Google Scholar]
- 91.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium transport in mitochondria: implications for manganese toxicity. Neurotoxicology. 1999;20:445–453. [PubMed] [Google Scholar]
- 92.Prabhakaran K, Chapman GD, Gunasekar PG. α-Synuclein overexpression enhances manganese-induced neurotoxicity through the NF-κB-mediated pathway. Toxicol Mech Methods. 2011;21:435–443. doi: 10.3109/15376516.2011.560210. doi: 10.3109/15376516.2011.560210. [DOI] [PubMed] [Google Scholar]
- 93.Cai T, Yao T, Zheng G, et al. Manganese induces the overexpression of α-synuclein in PC12 cells via ERK activation. Brain Res. 2010;1359:201–207. doi: 10.1016/j.brainres.2010.08.055. doi: 10.1016/j.brainres.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 94.Harischandra DS, Jin H, Anantharam V, et al. α-Synuclein protects against manganese neurotoxic insult during the early stages of exposure in a dopaminergic cell model of Parkinson's disease. Toxicol Sci. 2015;143:454–468. doi: 10.1093/toxsci/kfu247. doi: 10.1093/toxsci/kfu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bornhorst J, Chakraborty S, Meyer S, et al. The effects of pdr1, djr1.1 and pink1 loss in manganese-induced toxicity and the role of α-synuclein in C. elegans. Metallomics. 2014;6:476–490. doi: 10.1039/c3mt00325f. doi: 10.1039/c3mt00325f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human alpha-synuclein. A possible molecular NK between Parkinson's disease and heavy metal exposure. J Biol Chem. 2001;276:44284–44296. doi: 10.1074/jbc.M105343200. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 97.Earle KM. Studies on Parkinson's disease including x-ray fluorescent spectroscopy of formalin fixed brain tissue. J Neuropathol Exp Neurol. 1968;27:1–14. doi: 10.1097/00005072-196801000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Sofic E, Riederer P, Heinsen H, et al. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205. doi: 10.1007/BF01244786. [DOI] [PubMed] [Google Scholar]
- 99.Dexter DT, Carayon A, Javoy-Agid F, et al. Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114(Pt 4):1953–1975. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- 100.Di Monte DA, Lavasani M, Manning-Bog AB. Environmental factors in Parkinson's disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 101.Willis AW, Evanoff BA, Lian M, et al. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol. 2010;172:1357–1363. doi: 10.1093/aje/kwq303. doi: 10.1093/aje/kwq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 103.Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- 104.Langston JW, Forno LS, Tetrud J, et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 105.Daniels AJ, Reinhard JF. Energy-driven uptake of the neurotoxin 1-methyl-4-phenylpyridinium into chromaffin granules via the catecholamine transporter. J Biol Chem. 1988;263:5034–5036. [PubMed] [Google Scholar]
- 106.Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- 107.Takahashi N, Miner LL, Sora I, et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion, and enhanced MPTP toxicity. Proc Natl Acad Sci U S A. 1997;94:9938–9943. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vaccari A, Saba P. The tyramine-labelled vesicular transporter for dopamine: a putative target of pesticides and neurotoxins. Eur J Pharmacol. 1995;292:309–314. doi: 10.1016/0926-6917(95)90037-3. [DOI] [PubMed] [Google Scholar]
- 109.Miller GW, Kirby ML, Levey AI, Bloomquist JR. Heptachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999;20:631–637. [PubMed] [Google Scholar]
- 110.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- 111.Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 112.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 113.Chen H, Zhang SM, Hernán MA, et al. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 114.Makino Y, Ohta S, Tachikawa O, Hirobe M. Presence of tetrahydroisoquinoline and 1-methyl-tetrahydro-isoquinoline in foods: compounds related to Parkinson's disease. Life Sci. 1988;43:373–378. doi: 10.1016/0024-3205(88)90115-4. [DOI] [PubMed] [Google Scholar]
- 115.Neafsey EJ, Albores R, Gearhart D, et al. Methyl-beta-carbolinium analogs of MPP+ cause nigrostriatal toxicity after substantia nigra injections in rats. Brain Res. 1995;675:279–288. doi: 10.1016/0006-8993(95)00082-2. [DOI] [PubMed] [Google Scholar]
- 116.Purisai MG, McCormack AL, Langston WJ, et al. Alpha-synuclein expression in the substantia nigra of MPTP-lesioned non-human primates. Neurobiol Dis. 2005;20:898–906. doi: 10.1016/j.nbd.2005.05.028. doi: 10.1016/j.nbd.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 117.Xu Z, Cawthon D, McCastlain KA, et al. Selective alterations of gene expression in mice induced by MPTP. Synapse. 2005;55:45–51. doi: 10.1002/syn.20089. doi: 10.1002/syn.20089. [DOI] [PubMed] [Google Scholar]
- 118.Curtin K, Fleckenstein AE, Robison RJ, et al. Methamphetamine/amphetamine abuse and risk of Parkinson's disease in Utah: a population-based assessment. Drug Alcohol Depend. 2015;146:30–38. doi: 10.1016/j.drugalcdep.2014.10.027. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Granado N, Ares-Santos S, Moratalla R. Methamphetamine and Parkinson's disease. Parkinsons Dis. 2013;2013:308052. doi: 10.1155/2013/308052. doi: 10.1155/2013/308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kohno M, Morales AM, Ghahremani DG, et al. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ares-Santos S, Granado N, Espadas I, et al. Methamphetamine causes degeneration of dopamine cell bodies and terminals of the nigrostriatal pathway evidenced by silver staining. Neuropsychopharmacology. 2014;39:1066–1080. doi: 10.1038/npp.2013.307. doi: 10.1038/npp.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cubells JF, Rayport S, Rajendran G, Sulzer D. Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci. 1994;14:2260–2271. doi: 10.1523/JNEUROSCI.14-04-02260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fornai F, Lenzi P, Gesi M, et al. Similarities between methamphetamine toxicity and proteasome inhibition. Ann N Y Acad Sci. 2004;1025:162–170. doi: 10.1196/annals.1316.021. doi: 10.1196/annals.1316.021. [DOI] [PubMed] [Google Scholar]
- 124.Roehr B. Half a million Americans use methamphetamine every week. BMJ. 2005;331:476. doi: 10.1136/bmj.331.7515.476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Moszczynska A, Yamamoto BK. Methamphetamine oxidatively damages parkin and decreases the activity of 26S proteasome in vivo. J Neurochem. 2011;116:1005–1017. doi: 10.1111/j.1471-4159.2010.07147.x. doi: 10.1111/j.1471-4159.2010.07147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu B, Traini R, Killinger B, et al. Overexpression of parkin in the rat nigrostriatal dopamine system protects against methamphetamine neurotoxicity. Exp Neurol. 2013;247:359–372. doi: 10.1016/j.expneurol.2013.01.001. doi: 10.1016/j.expneurol.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hatcher JM, Pennell KD, Miller GW. Parkinson's disease and pesticides: a toxicological perspective. Trends Pharmacol Sci. 2008;29:322–329. doi: 10.1016/j.tips.2008.03.007. doi: 10.1016/j.tips.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 130.Sherer TB, Betarbet R, Testa CM, et al. Mechanism of toxicity in rotenone models of Parkinson's disease. J Neurosci. 2003;23:10756–10764. doi: 10.1523/JNEUROSCI.23-34-10756.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao H-M, Hong J-S, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bergen WG. The in vitro effect of dieldrin on respiration of rat liver mitochondria. Proc Soc Exp Biol Med. 1971;136:732–735. doi: 10.3181/00379727-136-35352. [DOI] [PubMed] [Google Scholar]
- 133.Zhang J, Fitsanakis VA, Gu G, et al. Manganese ethylene-bis-dithiocarbamate and selective dopaminergic neurodegeneration in rat: a link through mitochondrial dysfunction. J Neurochem. 2003;84:336–346. doi: 10.1046/j.1471-4159.2003.01525.x. [DOI] [PubMed] [Google Scholar]
- 134.Drechsel DA, Patel M. Role of reactive oxygen species in the neurotoxicity of environmental agents implicated in Parkinson's disease. Free Radic Biol Med. 2008;44:1873–1886. doi: 10.1016/j.freeradbiomed.2008.02.008. doi: 10.1016/j.freeradbiomed.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corrigan FM, Wienburg CL, Shore RF, et al. Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health Part A. 2000;59:229–234. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- 136.Fleming L, Mann JB, Bean J, et al. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–103. doi: 10.1002/ana.410360119. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- 137.Sanchez-Ramos J, Facca A, Basit A, Song S. Toxicity of dieldrin for dopaminergic neurons in mesencephalic cultures. Exp Neurol. 1998;150:263–271. doi: 10.1006/exnr.1997.6770. doi: 10.1006/exnr.1997.6770. [DOI] [PubMed] [Google Scholar]
- 138.Kanthasamy AG, Kitazawa M, Yang Y, et al. Environmental neurotoxin dieldrin induces apoptosis via caspase-3-dependent proteolytic activation of protein kinase C delta (PKCdelta): Implications for neurodegeneration in Parkinson's disease. Mol Brain. 2008;1:12. doi: 10.1186/1756-6606-1-12. doi: 10.1186/1756-6606-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kitazawa M, Anantharam V, Kanthasamy AG. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free Radic Biol Med. 2001;31:1473–1485. doi: 10.1016/s0891-5849(01)00726-2. [DOI] [PubMed] [Google Scholar]
- 140.Heinz GH, Hill EF, Contrera JF. Dopamine and norepinephrine depletion in ring doves fed DDE, dieldrin, and Aroclor 1254. Toxicol Appl Pharmacol. 1980;53:75–82. doi: 10.1016/0041-008x(80)90383-x. [DOI] [PubMed] [Google Scholar]
- 141.Richardson JR, Caudle WM, Wang M, et al. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease. FASEB J. 2006;20:1695–1697. doi: 10.1096/fj.06-5864fje. doi: 10.1096/fj.06-5864fje. [DOI] [PubMed] [Google Scholar]
- 142.Hatcher JM, Richardson JR, Guillot TS, et al. Dieldrin exposure induces oxidative damage in the mouse nigrostriatal dopamine system. Exp Neurol. 2007;204:619–630. doi: 10.1016/j.expneurol.2006.12.020. doi: 10.1016/j.expneurol.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sun F, Anantharam V, Latchoumycandane C, et al. Dieldrin induces ubiquitin-proteasome dysfunction in alpha-synuclein overexpressing dopaminergic neuronal cells and enhances susceptibility to apoptotic cell death. J Pharmacol Exp Ther. 2005;315:69–79. doi: 10.1124/jpet.105.084632. doi: 10.1124/jpet.105.084632. [DOI] [PubMed] [Google Scholar]
- 144.Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatr. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Spyridopoulos I, Principe N, Krasinski KL, et al. Restoration of E2F expression rescues vascular endothelial cells from tumor necrosis factor-alpha-induced apoptosis. Circulation. 1998;98:2883–2890. doi: 10.1161/01.cir.98.25.2883. [DOI] [PubMed] [Google Scholar]
- 146.Dluzen DE, McDermott JL, Liu B. Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/B1 mice. Neurotoxicol Teratol. 1996;18:603–606. doi: 10.1016/0892-0362(96)00086-4. [DOI] [PubMed] [Google Scholar]
- 147.Miranda R, Sohrabji F, Singh M, Toran-Allerand D. Nerve growth factor (NGF) regulation of estrogen receptors in explant cultures of the developing forebrain. J Neurobiol. 1996;31:77–87. doi: 10.1002/(SICI)1097-4695(199609)31:1<77::AID-NEU7>3.0.CO;2-C. doi: 10.1002/(SICI)1097-4695(199609)31:1<77::AID-NEU7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 148.Son JH, Chun HS, Joh TH, et al. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J Neurosci. 1999;19:10–20. doi: 10.1523/JNEUROSCI.19-01-00010.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gatto NM, Deapen D, Stoyanoff S, et al. Lifetime exposure to estrogens and Parkinson's disease in California teachers. Parkinsonism Relat Disord. 2014;20:1149–1156. doi: 10.1016/j.parkreldis.2014.08.003. doi: 10.1016/j.parkreldis.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Gorell JM, Rybicki BA, Johnson CC, Peterson EL. Smoking and Parkinson's disease: a dose-response relationship. Neurology. 1999;52:115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- 151.Baumann RJ, Jameson HD, McKean HE, et al. Cigarette smoking and Parkinson disease: 1. Comparison of cases with matched neighbors. Neurology. 1980;30:839–843. doi: 10.1212/wnl.30.8.839. [DOI] [PubMed] [Google Scholar]
- 152.Janson AM, Møller A. Chronic nicotine treatment counteracts nigral cell loss induced by a partial mesodiencephalic hemitransection: an analysis of the total number and mean volume of neurons and glia in substantia nigra of the male rat. Neuroscience. 1993;57:931–941. doi: 10.1016/0306-4522(93)90039-i. [DOI] [PubMed] [Google Scholar]
- 153.Mitsuoka T, Kaseda Y, Yamashita H, et al. Effects of nicotine chewing gum on UPDRS score and P300 in early-onset parkinsonism. Hiroshima J Med Sci. 2002;51:33–39. [PubMed] [Google Scholar]
- 154.Clemens P, Baron JA, Coffey D, Reeves A. The short-term effect of nicotine chewing gum in patients with Parkinson's disease. Psychopharmacology (Berl) 1995;117:253–256. doi: 10.1007/BF02245195. [DOI] [PubMed] [Google Scholar]
- 155.Ebersbach G, Stöck M, Müller J, et al. Worsening of motor performance in patients with Parkinson's disease following transdermal nicotine administration. Mov Disord. 1999;14:1011–1013. doi: 10.1002/1531-8257(199911)14:6<1011::aid-mds1016>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 156.Watanabe H, Uramoto H. Caffeine mimics dopamine receptor agonists without stimulation of dopamine receptors. Neuropharmacology. 1986;25:577–581. doi: 10.1016/0028-3908(86)90208-x. [DOI] [PubMed] [Google Scholar]
- 157.Nakaso K, Ito S, Nakashima K. Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci Lett. 2008;432:146–150. doi: 10.1016/j.neulet.2007.12.034. doi: 10.1016/j.neulet.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 158.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol. 2001;50:56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 159.Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology. 2000;55:1350–1358. doi: 10.1212/wnl.55.9.1350. [DOI] [PubMed] [Google Scholar]
- 160.Perry TL, Yong VW, Ito M, et al. Nigrostriatal dopaminergic neurons remain undamaged in rats given high doses of L-DOPA and carbidopa chronically. J Neurochem. 1984;43:990–993. doi: 10.1111/j.1471-4159.1984.tb12834.x. [DOI] [PubMed] [Google Scholar]
- 161.Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- 162.Thanvi B, Lo N, Robinson T. Levodopa-induced dyskinesia in Parkinson's disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J. 2007;83:384–388. doi: 10.1136/pgmj.2006.054759. doi: 10.1136/pgmj.2006.054759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moldovan A-S, Groiss SJ, Elben S, et al. The treatment of Parkinson's disease with deep brain stimulation: current issues. Neural regeneration research. 2015;10:1018–1022. doi: 10.4103/1673-5374.160094. doi: 10.4103/1673-5374.160094. [DOI] [PMC free article] [PubMed] [Google Scholar]