Abstract
Background
The selection of the most appropriate treatment combinations requires the balancing of benefits and harms of these treatment options as well as the patients’ preferences for the resulting outcomes.
Objective
This research aimed at estimating and comparing the utility weights between elderly women with early stage hormone receptor positive (HR+) breast cancer receiving a combination of radiotherapy and hormonal therapy after breast conserving surgery (BCS) and those receiving a combination of BCS and hormonal therapy.
Methods
The Surveillance, Epidemiology, and End Results (SEER) linked with Medicare Health Outcomes Survey (MHOS) was used as the data source. Health utility weights were derived from the VR-12 health-related quality of life instrument using a mapping algorithm. Descriptive statistics of the sample were provided. Two sample t-tests were performed to determine potential differences in mean health utility weights between the two groups after propensity score matching.
Results
The average age at diagnosis was 72 vs. 76 years for the treated and the untreated groups, respectively. The results showed an inverse relationship between the receipt of radiotherapy and age. Patients who received radiotherapy had, on average, a higher health utility weight (0.70; SD = 0.123) compared with those who did not receive radiotherapy (0.676; SD = 0.130). Only treated patients who had more than two comorbid conditions had significantly higher health utility weights compared with patients who were not treated.
Conclusions
The mean health utility weights estimated for the radiotherapy and no radiotherapy groups can be used to inform a comparative cost-effectiveness analysis of the treatment options. However, the results of this study may not be generalizable to those who are outside a managed care plan because MHOS data is collected on managed care beneficiaries.
Keywords: Breast cancer, comparative effectiveness research, health utility weight, radiotherapy
Introduction
Breast cancer is the most commonly diagnosed cancer among women and it is projected that one in eight women will develop breast cancer in her lifetime1. Fifty percent of breast cancers are diagnosed at aged 61 and approximately 41% of breast cancers occur in women aged 652.
Treatment options for early stage breast cancer often include some combination of surgery, radiation, chemotherapy, and hormonal therapy and/or targeted therapy3. That being said, the selection of optimal treatment depends on balancing the benefits and harms of these treatment options as well as the preferences patients might have for the resulting outcomes. Compared with no radiation therapy after breast conserving surgery (BCS) for early stage breast cancer, radiation therapy has been associated with improved survival and has reduced the risk of recurrence. However, there is no clear evidence as to whether elderly patients with early stage hormone receptor positive (HR+) should or not receive radiation therapy after breast conserving surgery (BCS). On one hand, the results from randomized controlled trials suggest that the addition of radiation therapy after BCS does not improve the survival benefit for patients with less aggressive early stage breast cancer compared with those who were treated with hormonal therapy alone after BCS4–8. On the other hand, observational studies that reported the addition of radiation therapy after BCS had shown that the risk of death is reduced compared with no radiation and suggested the consideration of radiation therapy9,10.
The lack of clear evidence regarding the survival benefits of radiotherapy after BCS in elderly patients suffering from early stage HR + breast cancer poses challenges for optimal shared decision-making. Complete evidence regarding real-life survival benefits, quality of life, and costs associated with the addition or omission of radiotherapy to BCS and hormonal therapy are key to facilitate such a decision-making problem.
The focus of this study is on estimating and comparing the mean health utility weights for elderly patients presenting with early stage HR + breast cancer who were treated with a combination of radiotherapy and hormonal therapy after BCS versus those receiving hormonal therapy alone after BCS.
Methods
Dataset
The Surveillance, Epidemiology, and End Results (SEER) – Medicare Health Outcomes Survey (MHOS) was used. The data provides individual patient demographics, clinical, and cause of death information from nationwide registries and patient reported outcomes of elderly patients who are enrolled in Medicare advantage organizations (managed care health plans). The collection of patient reported outcomes data, by Centers for Medicare & Medicaid Services (CMS), started since 1998. The purpose of the survey is a Medicare Advantage quality improvement initiative to provide health status information to consumers, beneficiaries and health plans11. Since then, multiple cohorts have been recruited (14 cohorts), covering data for the years 1998 to 2013. The cohorts include patients with and without cancer. The SEER-MHOS dataset provides HRQOL information measured via Short Form-36 (SF-36) until 2005 and Veteran Rand-12 (VR-12) starting 200612. A baseline survey is administered every year to a new cohort. Each cohort is resurveyed two years after baseline to provide two waves of data. Ambs and colleagues provided details about the SEER-MHOS dataset12.
Patient population
The patient population was composed of elderly women diagnosed with HR + early stage breast cancer. Those who received radiotherapy served as the treatment group while those did not receive radiotherapy were treated as the control group. Since MHOS changed the data collection instrument in 2006, the patient population was selected from six cohorts starting cohort 9 to 14 (2006 and 2008, 2007 and 2009, 2008 and 2010, 2009 and 2011, 2010 and 2012, 2011 and 2013). The following inclusion and exclusion criteria were applied to obtain the study samples.
Inclusion criteria
Patient diagnosed with breast cancer as a primary cancer.
Patient diagnosed with early stage hormone receptor positive breast cancer. Stage of breast cancer was defined based on the American Joint Committee on Cancer (AJCC) staging criteria (early stage [stage 1 and 2] when T1–T3/N0, or M0).
Patient lived in SEER area at the time of survey.
Patient had undergone breast conserving surgery.
Elderly patient (age 65 and older at the time of diagnosis).
The first survey after the most recent breast cancer diagnoses for those who completed multiple surveys.
Exclusion criteria
Patient was male.
Patient surveyed before diagnosed.
Patient diagnosed with other cancer.
Cases with missing or unknown treatment status (surgery and/or radiation surgery sequence).
Patient diagnosed with stage III and IV breast cancer and unknown stage status.
Patient diagnosed with autopsy and death certificate.
To minimize recall bias, patients diagnosed before the year 2004 were excluded in the analysis.
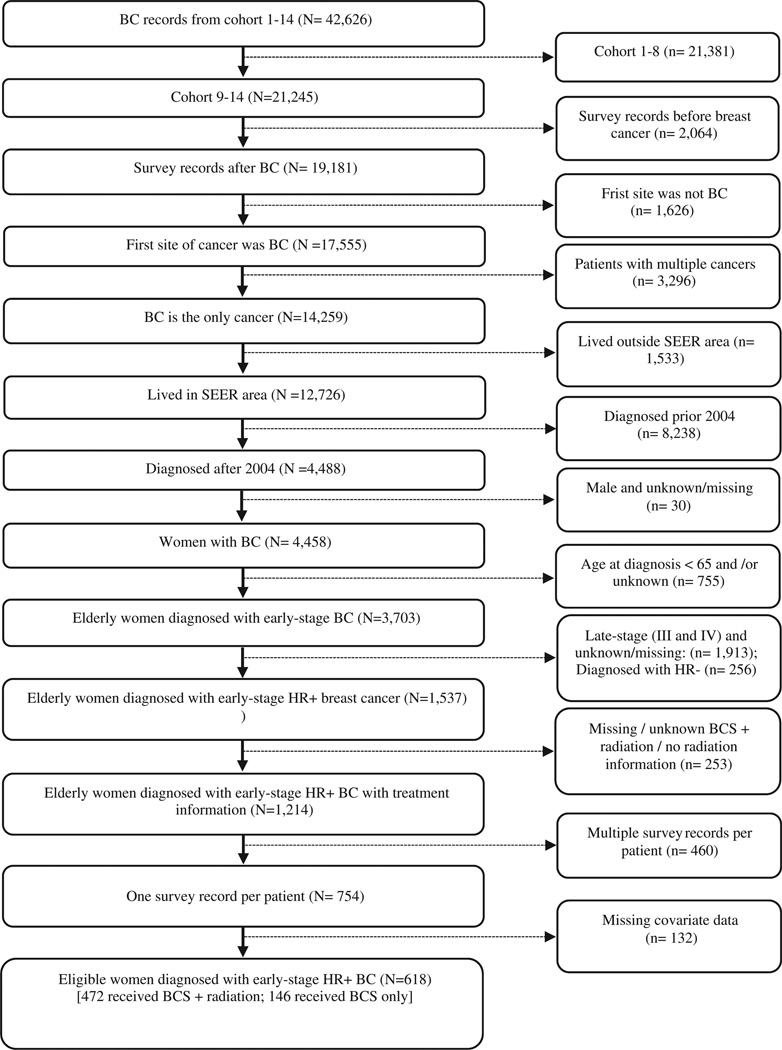
Duplicate cases were excluded by retaining the first case recorded. Between the years 2004 to 2011, 754 elderly women were identified who were diagnosed with early stage hormone receptor breast cancer, received BCS alone or BCS plus radiation treatment and met the other pre-specified inclusion criteria. Among 754 patients, 562 received BCS plus radiation (BCS plus radiation plus hormonal therapy) and 192 of them received BCS alone (BCS + hormonal therapy). The data extraction process is summarized in Figure 1.
Figure 1.
Summary of data extraction. Abbreviations. BC, breast cancer; BCS, breast conserving surgery.
Variable identification and measurement
We controlled for socio-demographic (age, race/ethnicity, and marital status) factors, comorbidities, tumor grade, and tumor size. Age represented age at the time of diagnosis. Race (white, black, and others) was defined as the patient reported at the time of diagnosis. Marital status represented the marital status, which was a dichotomous variable indicating the marital status (married or not) of the women at the time of diagnosis. Patients’ education levels were identified from MHOS. Education is coded as follows: high school graduate or less, some college education, a 4 year college education, and more than 4 year education. Any chronic conditions that were reported by the patient were considered as comorbidities. The following comorbidities were reported by the patients: hypertension/high blood pressure, angina pectoris/coronary artery disease, congestive heart failure, myocardial infarction, other heart conditions, stroke, emphysema, asthma, or chronic obstructive pulmonary disease (COPD), Crohn’s ulcerative colitis or inflammatory bowel diseases (IBD), arthritis of hip/knee, arthritis of hand/wrist, sciatica, and diabetes. These comorbidities were collapsed into a categorical variable with three levels: no comorbidity, 1–2 comorbidities, and >2 comorbidities. A proxy measure (yes/no) was yes if the survey was filled out by the third party. The tumor grade was identified from the SEER dataset. Tumor grade was a categorical variable coded as well differentiated, moderately differentiated, and combined poorly differentiated and undifferentiated. The definition and measurement of the variables used in this study are presented in Table 1.
Table 1.
Variable definition and measurement used in the analysis.
| Variable name | Definition and measurement |
|---|---|
| VR-6D index | A single utility index generated from VR-12 |
| Radiation therapy | 1 if the patient received radiation therapy after BCS, otherwise 0 |
| Race | 0 if the patient was white |
| 1 if the patient was black | |
| 2 if the patient was not white nor black (others) | |
| Marital status | 0 if the patient was unmarried (single, divorced, and widowed) |
| 1 if the patient was married | |
| Age at diagnosis | 0 if the patient age was 65–69 years |
| 1 if the patient age was 70–74 years | |
| 2 if the patient age was 75–79 years | |
| 3 if the patient age was 80 years or older | |
| Tumor grade | 0 if the tumor was well differentiated |
| 1 if the tumor was moderately differentiated | |
| 2 if the tumor was poorly differentiated or undifferentiated |
|
| Education | 0 if the patient was high-school graduate or less (≤high school) |
| 1 if the patient had some college degree or 2 years degree |
|
| 2 if the patient was college graduate or higher | |
| Comorbidity | 0 if the patient had no comorbidity |
| 1 if the patient had 1–2 comorbidities | |
| 2 if the patient had >2 comorbidities | |
| Proxy | If the survey was completed by a third party, i.e. not self-reported |
| 0 if the survey was completed by the patient herself | |
| 1 if the survey was completed by a third party | |
| Time from cancer diagnosis to survey |
0 if time from cancer diagnosis was ≥0 months but <7 months |
| 1 if time from cancer diagnosis was ≥7 months but <12 months |
|
| 2 if time from cancer diagnosis was ≥13 months but <19 months |
|
| Time from diagnosis to treatment |
0 if time from diagnosis to treatment <2 months |
| 1 if time from diagnosis to treatment is ≥2 months | |
| Area of residence | 0 if the women reside in non-metro area at the time diagnosis |
| 1 if the women reside in metro area at the time of diagnosis |
|
| Year of diagnosis | The year the patient diagnosis breast cancer |
Treatments
This study examined and compared the health utility of patients treated with radiation therapy (radiation plus hormonal) and no radiation therapy (hormonal therapy only) after breast conserving surgery. However, SEER data does not provide information regarding hormonal therapy. Since hormonal therapy is a recommended treatment for patients diagnosed with hormone receptor positive breast cancer, we assumed that hormonal therapy was administered if the patient was diagnosed with hormone receptor positive breast cancer.
Surgical treatment is categorized as mastectomy or breast conserving surgery (BCS). BCS is defined as partial mastectomy with nipple resection, lumpectomy or excisional biopsy, re-excision of the biopsy site for gross or microscopic residual disease and segmental mastectomy (including wedge resection, quadrantectomy, and tylectomy. Mastectomy is defined as a subcutaneous mastectomy, total (simple) mastectomy, bilateral mastectomy, modified radical mastectomy, and extended radical mastectomy.
The receipt of radiation therapy after BCS was a dichotomous variable: yes if the patient received radiation after surgery and no if the patient did not receive radiation therapy after surgery. This variable was constructed using the radiation surgery sequence and surgery information. Seven categories of radiation surgery sequence are available in the SEER dataset: no radiation and/or cancer directed surgery, radiation prior to surgery, radiation after surgery, radiation before and after surgery, intraoperative radiation, intraoperative radiation with and other radiation before/after surgery, and sequence unknown, but both were given. A patient was considered as having received radiation therapy after BCS if any of the following criteria were met: the woman has to receive BCS, radiation after surgery, radiation before and after surgery, and intraoperative radiation with and other radiation before/after surgery. If the patients received BCS but “no radiation and/or cancer directed surgery, radiation prior to surgery, and intraoperative radiation” then treatment was defined as BCS only. We included the cases where the treatment sequences were known.
Outcome measures
The outcome of interest in this study was health utility. We used a novel multi-stage mapping algorithm to convert VR-12 into VR-6D13 for the radiation therapy and no radiation therapy groups. The multi-stage mapping approach involves the use of different health-related quality of life (HRQOL) questionnaires (SF-36, VR-36 and VR-12) to derive preference-based measures (SF-6D and VR-6D).
SF-36, VR-36 and VR-12 questionnaires are composed of eight health dimensions, and these dimensions are physical functioning, role limitations due to physical health problems, bodily pain, general health, vitality (fatigue), social functioning, role limitations due to emotional problems, and mental health14. For each of the eight dimensions, these questionnaires generate a score on a 0–100 scale, 0 representing the worst health and 100 representing perfect health14.
The SF-6D was derived from SF-36 with six dimensional wellbeing states to determine patients’ preferences for these states. The six dimensions are physical working, part restrictions, social working, agony, psychological well-being, and imperativeness13.
The VR-6D, a preference-based measure derived from the VR-12, has comparable distributional properties to the SF-6D13. The VR-6D represents health utility scores ranging between 0 and 1, 0 representing death and 1 representing optimal health13. The multi-stage mapping algorithm operates as follows13:
Step 1: The prediction of SF-6D scores from responses to VR-36 questionnaire using the coefficient of the regression of the Brazier’s SF-6D scores onto the SF-36 questionnaire.
Step 2: The predicted SF-6D scores from responses to VR-36 were compared to the Brazier’s SF-6D scores. Some adjustments were made for the SF-6D scores from VR-36 to have a maximum utility value of 1.
Step 3: The adjusted SF-6D scores from VR-36 were regressed on the non-native VR-12 questionnaire. Missing data and differences related to the structure of the non-native and native VR-12 as well as mode of administration of the questionnaire (phone or mail) were controlled for.
Step 4: The estimation of VR-6D scores from VR-12 was based on steps 2 and 3.
Statistical analysis
The statistical analysis was performed according to the following steps using SAS 9.4 and Stata 14.0 statistical software. First, we computed descriptive statistics for the treatment and control groups (Table 2). Second, we matched the radiation therapy and no radiation therapy groups using propensity score. The rationale for using the propensity score is to mimic randomized controlled trials by ensuring that observed confounders in the dataset (listed in Table 2) are equally distributed between the radiation therapy and no radiation therapy groups. The application of propensity score matching consisted of estimating the probability of receiving treatment using Equation 1. To this aim, we used the pscore Stata command with logit option.
| (1) |
Table 2.
Characteristics of the breast cancer original cohort by treatment type before propensity matching.
| Characteristics | Women with breast cancer (N = 618) |
Breast cancer cases by treatment type | ||
|---|---|---|---|---|
| No radiation (N = 146; 23.62%) |
Radiation (N = 472; 76.38%) |
p-value | ||
| Mean utility | 0.695 (0.125) | 0.673 | 0.701 | .0164 |
| Age in years, N (%) | <.0001 | |||
| Mean age at diagnosis, mean (SD) | 73.72 (6.21) | 76.64 (7.09) | 72.53 (5.41) | <.0001 |
| Between 65 and 69 | 193 (31.23) | 29 (19.86) | 164 (34.75) | |
| Between 70 and 74 | 192 (31.07) | 35 (23.97) | 157 (33.26) | |
| Between 75 and 79 | 120 (19.42) | 30(20.55) | 90 (19.07) | |
| 80 and above | 113 (18.28) | 52 (35.62) | 61 (12.92) | |
| Race, N (%) | .3974 | |||
| White | 497 (79.94) | 120 (82.19) | 374 (79.24) | |
| Black | 59 (9.55) | 15 (10.27) | 44 (9.32) | |
| Others | 65 (10.52) | 11 (7.53) | 54 (11.44) | |
| Marital status at baseline, N (%) | .0034 | |||
| Married | 311 (49.32) | 58(39.75) | 253 (53.60) | |
| Unmarried | 307 (49.68) | 88 (60.27) | 307 (53.60) | |
| Education, N (%) | .327 | |||
| High school graduates or less (≤high school) | 343 (55.50) | 87 (59.59) | 256 (54.24) | |
| Some college degree or 2 years degree | 171 (27.67) | 40 (27.40) | 131 (27.75) | |
| College graduate or higher | 104 (16.83) | 19(13.01) | 85 (18.38) | |
| Area of residence | .2778 | |||
| Metro area | 579 (93.69) | 134 (91.78) | 445 (94.28) | |
| Non-metro area | 39 (6.31) | 12 (8.22) | 27 (5.72) | |
| Comorbidity, N (%) | .4250 | |||
| Healthy (0 comorbidities) | 62 (10.03) | 12 (8.22) | 50 (10.59) | |
| 1–2 comorbidities | 253 (40.94) | 66 (45.21) | 187 (39.62) | |
| >2 comorbidities | 303 (49.03) | 68 (46.58) | 235 (49.79) | |
| Grade, N (%) | .8780 | |||
| Well differentiated | 243 (39.32) | 60 (41.10) | 183 (38.77) | |
| Moderately differentiated | 304 (49.19) | 70 (47.95) | 234 (49.58) | |
| Poorly differentiated and undifferentiated | 71 (11.49) | 16(10.96) | 55 (11.65) | |
| Proxy, N (%) | .0184 | |||
| Yes | 85 (13.75) | 18(12.33) | 30 (6.36) | |
| No | 533 (86.25) | 128 (87.67) | 442 (93.64) | |
| Survey time point, N (%) | ||||
| Baseline | 492 (79.61) | 112 (76.71) | 380 (80.51) | .3197 |
| Follow-up | 126 (20.39) | 34 (23.29) | 92 (19.49) | |
| Survey disposition, N (%) | .4222 | |||
| Mailed survey | 533 (56.25) | 23 (15.75) | 62 (13.14) | |
| Telephone survey | 85 (13.75) | 123 (84.25) | 410 (86.86) | |
| Time from cancer diagnosis to survey, mean (SD), months | 27.31 (20.57) | 25.32 (19.89) | 27.93 (20.76) | .1812 |
| Time from cancer diagnosis to survey, N (%) | .6227 | |||
| 0–6 months | 89 (14.40) | 23 (15.75) | 66 (13.98) | |
| 7–12 months | 78 (12.62) | 20 (13.70) | 58 (12.29) | |
| 13–18 months | 88 (14.24) | 24 (16.44) | 64 (13.56) | |
| 19+ months | 368 (58.74) | 79 (54.11) | 284 (60.17) | |
| Time from diagnosis to treatment, Mean (SD), months | 1.81 (1.00) | 1.79 (1.40) | 1.81 (0.84) | .7799 |
| Time from diagnosis to treatment, N (%) | .8396 | |||
| <2 months | 503 (81.39) | 118(80.82) | 385 (81.57) | |
| ≥2 months | 115 (18.61) | 28 (19.18) | 87 (18.43) | |
| Year of diagnosis | .2458 | |||
| 2004 | 76 (12.30) | 13 (8.90) | 63 (13.35) | |
| 2005 | 80 (12.94) | 16(10.96) | 64 (13.56) | |
| 2006 | 91 (14.72) | 29 (19.86) | 62 (13.14) | |
| 2007 | 93 (15.05) | 21 (14.38) | 72 (15.25) | |
| 2008 | 84 (13.59) | 23 (15.75) | 61 (12.92) | |
| 2009 | 77 (12.46) | 22 (15.06) | 55 (11.65) | |
| 2010 | 66 (10.68) | 12 (8.22) | 54 (11.44) | |
| 2011 | 51 (8.25) | 10 (6.85) | 41 (8.69) | |
SD: standard deviation; N: number of observations; %: percent and column percent.
Treated and control groups were matched based on the probability of receiving treatment. The corresponding Stata command psmatch2 was used with the following specifications: the three nearest neighbors with replacement, tied observations, and common support condition. The balancing property of the covariates between the radiation and no radiation groups was checked graphically and analytically. The graphical test involved plotting and comparing the distributions of the propensity score for radiation and no radiation therapy groups before and after matching. In case the balancing property is satisfied, we would expect the distribution of propensity scores in the treated and untreated groups to superimpose after matching. In addition, a post-estimation test (pstest) was conducted to check for the mean equality of the propensity scores for the treated and untreated groups before and after matching. This test was further used to quantify the reduction of bias after matching.
Third, we compared the average (mean) health utility of patients who received radiation therapy to that of those who did not receive it after the matching procedure, using a two sample t-test. Institutional Review Board (IRB) exemption was granted from Florida Agricultural and Mechanical University.
Results
Patient characteristics before propensity matching
Only complete cases and those who underwent BCS were included in this analysis, meaning no data imputation rule was used for missing values except for the days of diagnosis and treatment with known year and month. Since the SEER-MHOS data does not include the day of diagnosis and treatment, we imputed these dates as follows: the first of the month was considered as the day of diagnosis while the 28th day of the month was considered as the day of the initiation of treatment.
Among patients who met the inclusion criteria (754), 132 observations were excluded from further analyses due to unknown or missing covariates data (list of covariates in Table 2). As a result, 618 observations were retained for further analyses.
Most of the patients received BCS plus radiation 472 (76.38%). Overall, a large proportion of patients were white (79.94%), high school graduates or less (55.50%), diagnosed with moderately differentiated tumor grade (49.19%), and resided in a metro area (93.69%). The mean time from diagnosis to survey time was 27.31 (SD = 20.57) months and the mean waiting time between diagnoses to initial treatment was approximately 2 months. On average, patients who did not receive radiation were significantly older (76.64) than women who received radiation therapy after BCS (72.53). On one hand, the proportion of patients who did not receive radiation therapy after BCS increased from women diagnosed age between 65 and 69 (19.86%) to age 80 and above (35.62%). On the other hand, the likelihood of receiving radiation therapy decreases when women are diagnosed at older age. Detailed descriptive statistics are provided in Table 2.
Utility before propensity matching
The overall average health utility weight before the propensity score matching was 0.695 (0.125 SD). Compared with patients who did not receive radiotherapy (0.673), women who received radiotherapy after BCS had significantly higher health utility (0.701; p = .0164), on average.
Assessment of the propensity score matching
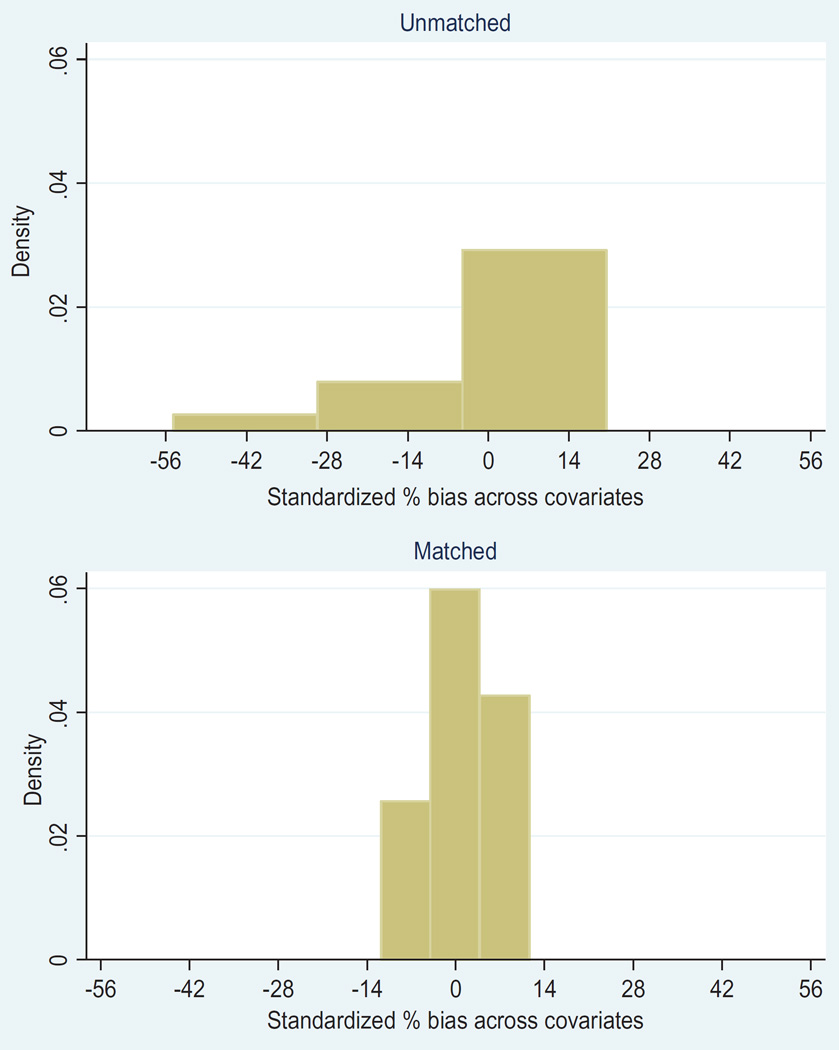
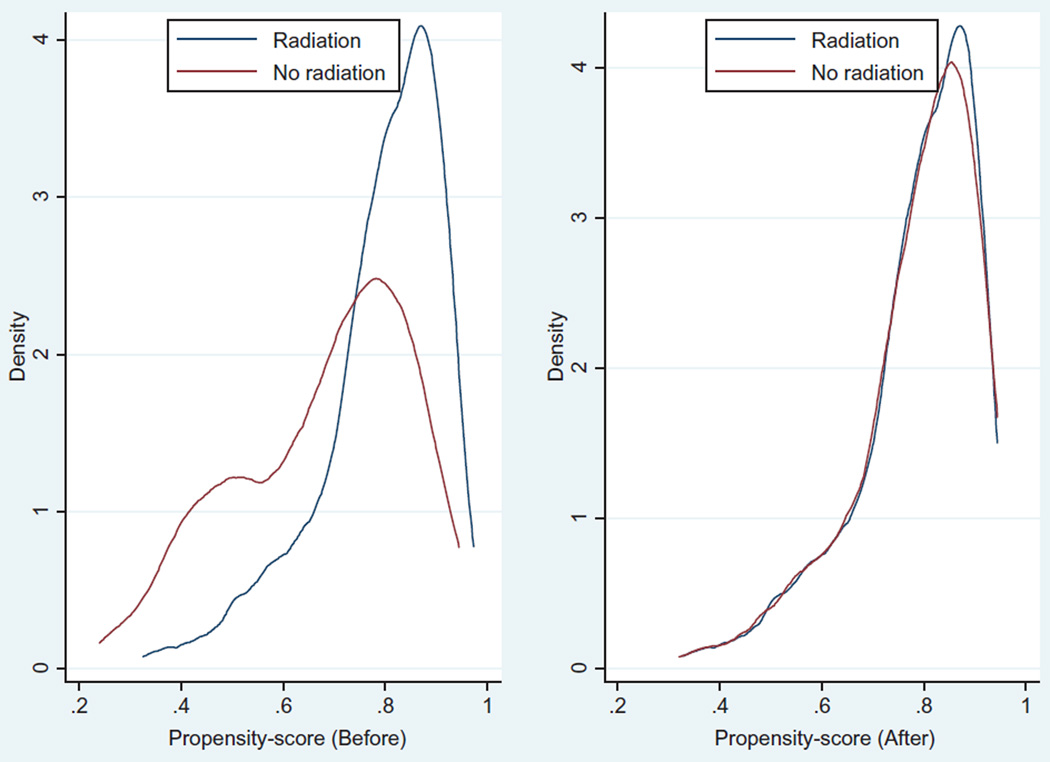
The balancing property of propensity score matching was satisfied. As shown in Figure 2, the standardized percentage biased across the covariates was lower in the matched data compared to the unmatched data. Furthermore, the distribution of the propensity score before matching and after matching indicates that the propensity score approach creates balance over the covariates for the radiation group and BCS only group. In addition, Figures 2 and 3 show that the balancing property of the matching is met. Figure 3 shows a non-parametric density estimate of the distribution of the estimated propensity before and after matching for patients who received radiation therapy and those who did not receive radiation therapy after BCS separately. The mean and median bias for the unmatched data was 77.4 whereas the mean and median bias for the matched data was 0.3. In general, the propensity score reduces the bias by 99.6%. This indicates that there is a greater likelihood that the covariates are balanced in both groups, after propensity score matching. Patients who did not receive radiation therapy tend to have lower propensity score before matching. After propensity score matching, both groups had approximately similar propensity score distributions. The mean propensity scores of the matched data for the radiation and no radiation groups are 0.78659 and 0.78616 respectively.
Figure 2.
Standardized percentage bias across covariates for unmatched and matched data.
Figure 3.
Propensity score density graph for radiation and no radiation therapy groups before and after matching.
Post-estimation
The propensity score matching generated 459 treated and 133 control patients. Table 3 reports the estimated average utility by radiation and no radiation groups after the propensity score matching method. The Stata command “Psmach2” generates a weight variable, which had missing values for some observations that were not matched. The matched data were obtained after deleting observations that had missing weights. The average utility was 0.696 (range 0.686 to 0.706). This finding suggests that there is a statistically significant difference between patients who were treated with radiation and those who were not treated with radiation therapy after BCS. Those who received radiation therapy had a higher average utility (0.701) compared to those who were not treated with radiation therapy (0.676) after BCS.
Table 3.
Comparison of health utility between treated and untreated groups after propensity score matching.
| Overall mean | Treatment groups | |||||
|---|---|---|---|---|---|---|
| Mean | No radiation (N = 133) | Radiation (N = 459) | p-value | |||
| N | Mean (SD) | N | Mean (SD) | |||
| Utility | 0.696 | 0.676 (0.130) | 0.701 (0.123) | .0423 | ||
| Age | ||||||
| 65–69 | 0.720 | 29 | 0.686 (0.132) | 155 | 0.727 (0.116) | .091 |
| 70–74 | 0.709 | 35 | 0.698 (0.135) | 154 | 0.711 (0.123) | .566 |
| 75–79 | 0.668 | 30 | 0.655 (0.123) | 89 | 0.673 (0.114) | .465 |
| 80+ | 0.657 | 39 | 0.669 (0.117) | 61 | 0.652 (0.131) | .570 |
| Race | ||||||
| White | 0.697 | 110 | 0.675 (0.128) | 372 | 0.704 (0.120) | .031 |
| Black | 0.688 | 12 | 0.679 (0.106) | 44 | 0.691 (0.143) | .786 |
| Others | 0.689 | 11 | 0.687 (0.142) | 43 | 0.690 (0.125) | .958 |
| Marital status | ||||||
| Married | 0.717 | 55 | 0.690 (0.119) | 244 | 0.722 (0.109) | .053 |
| Unmarried | 0.674 | 78 | 0.666 (0.132) | 251 | 0.677 (0.133) | .2813 |
| Comorbidity | ||||||
| Healthy (0 comorbidities) | 0.801 | 11 | 0.794 (0.105) | 44 | 0.803 (0.107) | .818 |
| 1–2 comorbidities | 0.744 | 59 | 0.729 (0.110) | 186 | 0.744 (0.105) | .327 |
| >2 comorbidities | 0.638 | 63 | 0.607 (0.105) | 229 | 0.647 (0.113) | .012 |
| Time from diagnosis to treatment | ||||||
| <2 months | 0.696 | 110 | 0.674 (0.124) | 375 | 0.702 (0.122) | .034 |
| ≥2 months | 0.693 | 23 | 0.686 (0.142) | 84 | 0.695 (0.127) | .765 |
| Time from diagnosis to survey | ||||||
| 0–6 months | 0.687 | 22 | 0.679 (0.128) | 64 | 0.691 (0.137) | .766 |
| 7–12 months | 0.693 | 19 | 0.667 (0.122) | 56 | 0.701 (0.111) | .253 |
| 13–18 months | 0.737 | 21 | 0.720 (0.129) | 63 | 0.742 (0.115) | .462 |
| 19+ months | 0.688 | 71 | 0.665 (0.137) | 276 | 0.694 (0.122) | .073 |
| Education | ||||||
| High school or less | 0.684 | 79 | 0.663 (0.126) | 250 | 0.691 (0.129) | .102 |
| Some college or 2 years degree | 0.698 | 37 | 0.695 (0.131) | 129 | 0.699 (0.115) | .864 |
| College graduate or higher | 0.730 | 17 | 0.695 (0.122) | 80 | 0.737 (0.107) | .1545 |
On the one hand, women who received radiotherapy after BCS and were aged between 65 and 79 had a higher health utility. On the other hand, women who were not treated with radiation and were 80 years old and above had relatively higher health utility weight. This difference was not statistically significant. White elderly women treated with radiation therapy had significantly better health utility than white elderly women who were not treated with radiation therapy after BCS (0.704 versus 0.675).
Only treated patients who had more than two comorbid conditions had significantly higher health utility compared with women who were not treated after BCS. Patients who were initially treated within 2 months of breast cancer diagnosis, and then received radiotherapy, had significantly higher health utility (0.702) than those who were not (0.674).
Among patients who received radiotherapy, the treatment was predicted to non-significantly increase utility by 1.41% on average.
Discussion
This study aimed to estimate and compare the health utility associated with the use of radiotherapy in combination with hormonal therapy after BCS in elderly patients diagnosed with early stage hormone receptor positive breast cancer. To meet the objective of this research, we used a population-based cancer registry data linked with patient reported outcomes data. Propensity score matching was employed to overcome the limitations of observational studies regarding imbalance between the treated and control groups over observed covariates. In addition, it was expected that the application of propensity score matching would improve the internal validity of this study.
The findings of this study suggest that elderly patients who received radiotherapy had, on average, a higher health utility weight compared with those who were not treated with radiotherapy after BCS, although the effect of radiotherapy on health utility on those who received radiation therapy was not statistically significant. The insignificance of average treatment effect among the treated could be attributed to several factors including the small sample size and response heterogeneity and unmeasured confounders. Previous studies have investigated the impact of the addition of radiotherapy on the quality of life of older breast cancer patients15–18. Overall, they reported similar quality of life between the treated and the control patients, although they varied in their designs, sample size, and participants’ age.
Even though there was no significant difference in health utility whether the patients were treated or not by age, we were able to observe that the health utility benefit of receiving radiotherapy waned as the patients were getting older. The inverse relationship of quality of life and the presence of comorbid conditions in cancer patients have been documented in previous studies19–22. Similar to these studies results, our study found that the health utility in both the treated and untreated groups declined as the number of comorbidities increases. Nevertheless, the decrease in health utility in those patients with comorbidities appeared to be worse in the untreated compared to the treated.
Patients who initiated their breast cancer treatment within 2 months of their diagnosis and had membership in the radiotherapy group have reported a higher health utility compared to the non-members. This may suggest that patients benefit more from receiving radiotherapy as compared to not receiving radiotherapy. The positive association between the health utility weight and early initiation of treatment could be explained by self-selection.
We observed that only white patients who were treated had significantly higher health utility than untreated patients. The relationship between race and treatment and the mechanisms through which they impact health utility are not clear and need further research.
This study has strengths and limitations. The main strength of this study relates to the minimization of selection bias by creating comparable treatment and control groups using propensity score matching. The adoption of propensity score matching allowed us to make causal inference. To our knowledge, this study is the first study that estimated and compared the health utility weights among elderly women with early stage HR + BC, receiving radiation therapy compared to those who did not receive radiation therapy.
Even though propensity score matching is a powerful tool to adjust for a large number of covariates, it can only adjust for observed covariates23. When unobserved or hidden biases exist, propensity score method does not account for them, resulting in biased estimates24. Second, the result of this study may not be generalizable to those who are out of managed care plan because MHOS data is collected on managed care beneficiaries. In addition, SEER does not collect data in areas where elderly patients reside (such as Florida). Therefore, the generalizability of this study to non-SEER areas may be limited. Third, cancer-specific HRQOL information may be important in order to make informed treatment decisions. However, MHOS data offers only the general HRQOL of the women. Fourth, SEER-MHOS data does not provide hormonal therapy information: we assumed that all women diagnosed with hormone receptor positive breast cancer received hormonal therapy. This assumption may overestimate the use of hormonal therapy.
Furthermore, misclassification of some variables including comorbidity may be an issue since the information collected was self-reported. The difference in the choice of treatment could be attributed to the patient’s physician and individual perceived benefit. Due to lack of data, we were not able to control for physicians’ influence on the selection of treatment and individual self-selection behavior.
This study illustrates the need to explicitly consider other factors such as comorbidities in making decisions to treat elderly patients diagnosed with early stage hormone receptor positive breast cancer with radiotherapy. The estimated mean health utility weights for the treated and untreated groups can be used in a cost-effectiveness analysis of the treatment options assessed in this study to inform reimbursement policy decision-making.
Acknowledgments
This study used the linked SEER–Medicare Health Outcomes Survey data. The interpretation and reporting of this data are the sole responsibility of the authors. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should it be inferred. The author acknowledges the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS) Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare Medicare Health Outcomes Survey data.
The authors thank Dr. Kazis and the research team for sharing the algorithm to convert VR12 into VR6-D. The authors are thankful to Mr. Abdrahmane Berthe, for his unwavering help in econometrics and STATA. My special thanks go to Florida State University Stats Consulting Team, particularly Melanie and Jane for their tremendous assistance in study design and statistical analyses.
Declaration of funding
This study is funded by the National Institute on Minority Health and Health Disparities of the National Institutes of Health. There was no commercial funding for this study.
V.D. and R.T. have disclosed that they are funded by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007582. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Minority Health and Health Disparities of the National Institutes of Health.
Footnotes
Transparency
Declaration of financial/other relationships
A.A.A., H.X., R.T., E.C., A.S., A.J.M., and V.D. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.American Cancer Society. American cancer society: cancer facts and figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 3.American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 4.Albert JM, Pan I, Shih YT, et al. Effectiveness of radiation for prevention of mastectomy in older breast cancer patients treated with conservative surgery. Cancer. 2012;118:4642–4651. doi: 10.1002/cncr.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher B, Bryant J, Dignam JJ, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20:4141–4149. doi: 10.1200/JCO.2002.11.101. [DOI] [PubMed] [Google Scholar]
- 6.Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31:2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winzer K, Sauer R, Sauerbrei W, et al. Radiation therapy after breast-conserving surgery: first results of a randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2004;40:998–1005. doi: 10.1016/j.ejca.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen R, Li L, Citron W, et al. Improved survival with adjuvant radiation in elderly women with early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:S33–S34. [Google Scholar]
- 10.Korah M, Sener S, Tripathy D. Implications of omitting radiation after breast conserving surgery in elderly women with low-risk invasive breast cancer. Int J Radiat Oncol Biol Phys. 2012;84:S34. [Google Scholar]
- 11.Brief description of the SEER-MHOS database. [accessed 27 Feb 2015];2015 Available at: http://healthcaredelivery.cancer.gov/seer-mhos/overview/
- 12.Ambs A, Warren JL, Bellizzi KM, et al. Overview of the SEER–Medicare health outcomes survey linked dataset. Health Care Financing Review. 2008;29:5–21. [PMC free article] [PubMed] [Google Scholar]
- 13.Selim AJ, Rogers W, Qian SX, et al. A preference-based measure of health: the VR-6D derived from the veterans RAND 12-item health survey. Qual Life Res. 2011;20:1337–1347. doi: 10.1007/s11136-011-9866-y. [DOI] [PubMed] [Google Scholar]
- 14.Selim AJ, Rogers W, Fleishman JA, et al. Updated US population standard for the veterans RAND 12-item health survey (VR-12) Qual Life Res. 2009;18:43–52. doi: 10.1007/s11136-008-9418-2. [DOI] [PubMed] [Google Scholar]
- 15.Hopwood P, Haviland J, Mills J, et al. The impact of age and clinical factors on quality of life in early breast cancer: an analysis of 2208 women recruited to the UK START trial (standardisation of breast radiotherapy trial) Breast. 2007;16:241–251. doi: 10.1016/j.breast.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Mbarek B, Galalae R, Michel J, et al. Impact of age on health-related quality of life (HR-QOL) in women with breast cancer treated by conserving surgery and postoperative 3D-radiotherapy. Radiotherapy and Oncology. Radiother Oncol. 2004;73:S520. [Google Scholar]
- 17.Prescott R, Kunkler I, Williams L, et al. A randomised controlled trial of postoperative radiotherapy following breast-conserving surgery in a minimum-risk older population. The PRIME trial. Health Technol Assess. 2007;11:1–170. doi: 10.3310/hta11310. [DOI] [PubMed] [Google Scholar]
- 18.Rayan G, Dawson LA, Bezjak A, et al. Prospective comparison of breast pain in patients participating in a randomized trial of breast-conserving surgery and tamoxifen with or without radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55:154–161. doi: 10.1016/s0360-3016(02)03826-9. [DOI] [PubMed] [Google Scholar]
- 19.Fu MR, Axelrod D, Guth AA, et al. Comorbidities and quality of life among breast cancer survivors: a prospective study. J Personalized Med. 2015;5:229–242. doi: 10.3390/jpm5030229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinkawa M, Fischedick K, Gagel B, et al. Impact of age and comorbidities on health-related quality of life for patients with prostate cancer: evaluation before a curative treatment. BMC Cancer. 2009;9:1. doi: 10.1186/1471-2407-9-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20:2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 22.Smith AW, Reeve BB, Bellizzi KM, et al. Cancer, comorbidities, and health-related quality of life of older adults. Health Care Financ Rev. 2008;29:41–56. [PMC free article] [PubMed] [Google Scholar]
- 23.Ertefaie A, Stephens DA. Comparing approaches to causal inference for longitudinal data: inverse probability weighting versus propensity scores. Int J Biostatistics. 2010;6:Article 14. doi: 10.2202/1557-4679.1198. [DOI] [PubMed] [Google Scholar]
- 24.Johnson ML, Crown W, Martin BC, et al. Good research practices for comparative effectiveness research: analytic methods to improve causal inference from nonrandomized studies of treatment effects using secondary data sources: the ISPOR good research practices for retrospective database analysis task force Report – Part III. Value Health. 2009;12:1062–1073. doi: 10.1111/j.1524-4733.2009.00602.x. [DOI] [PubMed] [Google Scholar]