Abstract
Objective
To investigate the effect of intravenous infusion of peripheral blood mononuclear cells (mPBMC) mobilized by granulocyte-colony stimulating factor (G-CSF) on upper extremity function in children with cerebral palsy (CP).
Methods
Fifty-seven children with CP were enrolled. Ten patients were excluded due to follow-up loss. In total, 47 patients (30 males and 17 females) were analyzed. All patients' parents provided signed consent before the start of the study. After administration of G-CSF for 5 days, mPBMC was collected and cryopreserved. Patients were randomized into two groups 1 month later. Twenty-two patients were administered mPBMC and 25 patients received normal saline as placebo. Six months later, the two groups were switched, and administered mPBMC and placebo, respectively. Quality of Upper Extremity Skills Test (QUEST) and the Manual Ability Classification System (MACS) were used to evaluate upper motor function.
Results
All subdomain and total scores of QUEST were significantly improved after mPBMC and placebo infusion, without significant differences between mPBMC and placebo groups. A month after G-CSF, all subdomain and total scores of QUEST were improved. The level of MACS remained unchanged in both mPBMC and placebo groups.
Conclusion
In this study, intravenously infused mPBMC showed no significant effect on upper extremity function in children with CP, as compared to placebo. The effect of mPBMC was likely masked by the effect of G-CSF, which was used in both groups and/or G-CSF itself might have other neurotrophic potentials in children with CP.
Keywords: Cerebral palsy, Peripheral blood stem cell transplantation, Granulocyte colony-stimulating factor, Upper extremity
INTRODUCTION
Cerebral palsy (CP) describes a group of permanent disorders of the development of movement and posture, causing limitation in activity attributed to non-progressive disturbances in the developing fetal or infant brain [1]. CP is the most common cause of physical disability in children. The overall reported prevalence of CP in children aged 3–10 years is 2–4 per 1,000, with gender variability [2]. Risk factors for CP are variable. A systemic review in 2013 reported 10 risk factors for CP including placental abnormalities, major and minor birth defects, low birth weight, meconium aspiration, emergency caesarean section, birth asphyxia, neonatal seizures, respiratory distress syndrome, hypoglycemia, and neonatal infections [3].
In current clinical practice, rehabilitation and supportive care such as physical therapy, occupational therapy, speech therapy, use of assistive devices, and medication are the basic treatments for typical CP. However, these treatments are not cures based on pathophysiology of CP, but instead improve children's motor skills in daily life activities. Recently, several studies conducted on stem cell treatment for CP showed effectiveness to some degree [4,5,6]. Deng et al. [7] reported that mesenchymal cells from peripheral blood integrate into traumatically injured cerebral tissue. Multipotent precursor cells exist in peripheral blood; moreover, peripheral blood mononuclear cells meet the criteria for mesenchymal stem cells [8]. These studies suggest that mononuclear cells from peripheral blood might help reduce the disability of children with CP. In this study, we comparatively evaluated the upper extremity function of children with CP who were intravenously injected mobilized peripheral blood mononuclear cells (mPBMC) or placebo.
MATERIALS AND METHODS
Enrollment of subjects
The participants were volunteers who were diagnosed with CP, and registered in cord blood clinic, Hanyang Medical Center, Seoul, Korea from 2011 to 2013. The diagnosis of CP was confirmed on evaluation by more than two professionals in the pediatric and rehabilitation department. The evaluation included delayed motor milestones, abnormal movements, persistent primitive reflexes, and abnormal postural reactions based on the definition of CP.
The inclusion criteria were as follows: (1) corrected age of 2 to 10 years, (2) clinically apparent functional disability in upper extremity (Manual Ability Classification System [MACS] grade II–V) related to CP, and (3) any radiologic evidence of white matter abnormalities of the brain and/or any grade of periventricular leukomalacia in brain MRI. The exclusion criteria were as follows: (1) children who had received any treatments including medications within a year that have known neuroprotective effects such as erythropoietin and granulocyte-colony stimulating factors (G-CSF), (2) children with genetic or congenital disorder such as chromosomal abnormalities, (3) children who had unstable vital sign, refractory seizure, bleeding tendency, and major organ dysfunction, and (4) children who were considered not suitable for this study by researchers.
The Institutional Review Board (IRB) of our institute reviewed and approved the detailed study protocol before it was initiated. A written informed consent was also obtained from the subject's parent.
G-CSF injection and mPBMC collection
At first, the participants received G-CSF (Leucostim; Dong-A Pharmaceutical Co., Seoul, Korea) injection subcutaneously at 10 µg/kg/day for 5 days to mobilize the mononuclear cells to the peripheral blood systems. On the fifth day, peripheral blood was collected from external jugular vein using a central venous catheter. mPBMC were collected using a blood cell separator (CS-3000; BAXTER, Deerfield, IL, USA). Separated mPBMC included >1×108/kg total nucleated cell (TNC) or 1×106/kg of CD34+ cells. The collected mPBMC were stored in the freezer until use (−196℃, LN2 TANK).
mPBMC infusion and evaluation
The participants were randomly assigned to two groups, i.e., T1 and T7 groups. The T1 group was injected with mPBMC at 1 month post-G-CSF infusion and T7 group was injected with mPBMC at 7 months post-G-CSF infusion. Randomization was performed by permuted block randomization. The researchers, physical assessor, and caregiver of the subjects were blinded to the random group assignment until the study's end. After storage in the freezer for 1 month, thawed mPBMC 1 unit (150 mL) was administered intravenously to subjects in the T1 group and the same amount of placebo (normal saline, 150 mL) was administered to the T7 group. Six months later, T7 group was injected with mPBMC and T1 group was injected with placebo with the same methods, respectively. The reagents administered to both groups were visually indistinguishable and the researchers and patients' caregiver were blinded to the treatment.
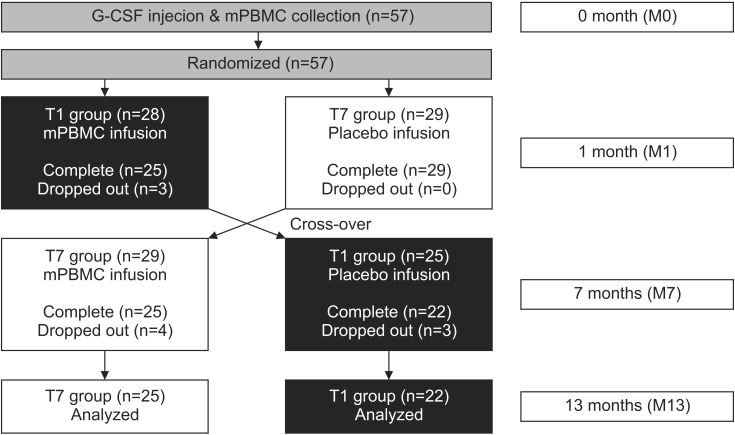
The subject's upper extremity function was evaluated by the MACS and Quality of Upper Extremity Skills Test (QUEST). All participants were evaluated for 1 or 2 days before G-CSF was injected (M0), when mPBMC or placebo was injected (the first injection, 1 month after G-CSF; M1), when cross injection was performed (6 months after the first injection; M7), and 6 months after the cross injection (M13) (Fig. 1). During the study period, ongoing personalized physiotherapy and occupational therapies were left unmodified, but other therapies that could affect upper extremity functions, such as botulinum toxin injection and neuro-protective medication were strictly regulated.
Fig. 1. A flowchart of this study. mPBMC, mobilized peripheral blood mononuclear cell; G-CSF, granulocyte-colony stimulating factor.
The QUEST is an evaluation tool to measure the upper extremity movement and function in children aged 18 months to 8 years [9]. It includes four domains: dissociated movement, grasp, protective extension, and weight-bearing. The QUEST has been commonly used as a standardized outcome measure of upper limb movement and activity limitation in studies to evaluate the efficacy of treatments for children with CP. It is also used widely in clinical practice, as it is one of the only assessments that can be used with both unilateral and bilateral distributions to provide an overview of upper limb movements in children with CP [10]. The MACS is a 5-level classification system for the manual ability of children with CP on the basis of self-initiated ability to manipulate objects in the home, school, and community. Children at level I can perform all manual tasks and can handle objects easily and successfully, whereas children at level V are completely dependent and demonstrate very limited ability in performing even simple actions [11].
Statistical analysis
IBM SPSS software ver. 21.0 (IBM, Armonk, NY, USA) was used for statistical analysis. The participant's continuous personal variables were compared using independent t-test, and categorical variables were compared using Pearson chi-square test. The repeated measure analysis of variance was used to analyze the differences in QUEST scores at baseline and progression over time. A post-hoc test was conducted by Bonferroni method. The Wilcoxon signed-rank test was used for intragroup analysis (M1 and M13) of each group, and the Mann-Whitney U-test was performed for intergroup analysis between the mPBMC phase of T1 group and control phase of T7 group. The p-value of <0.05 was considered significant.
RESULTS
Fifty-seven children participated in this study, but 47 patients were analyzed because 10 subjects were lost to follow-up. Parents of subjects who dropped out refused to participate in research due to personal reasons. Twenty-two children (T1 group) received mPBMC injections at 1 month after the G-CSF injections, and 25 children (T7 group) received mPBMC injections at 7 months after the G-CSF injections. The mean age of all subjects was 54.9±23.9 months (median 51, range 24–128), 62.2±27.5 months (median 55, range 24–128) for T1 group and 48.2±18.3 months (median 46, range 25–102) for T7 group. T1 group was significantly older than T7 group (p=0.044). There was no specific difference in gender, CP type, MACS, and QUEST scores between T1 and T7 groups (Table 1).
Table 1. Characteristics of subjects.
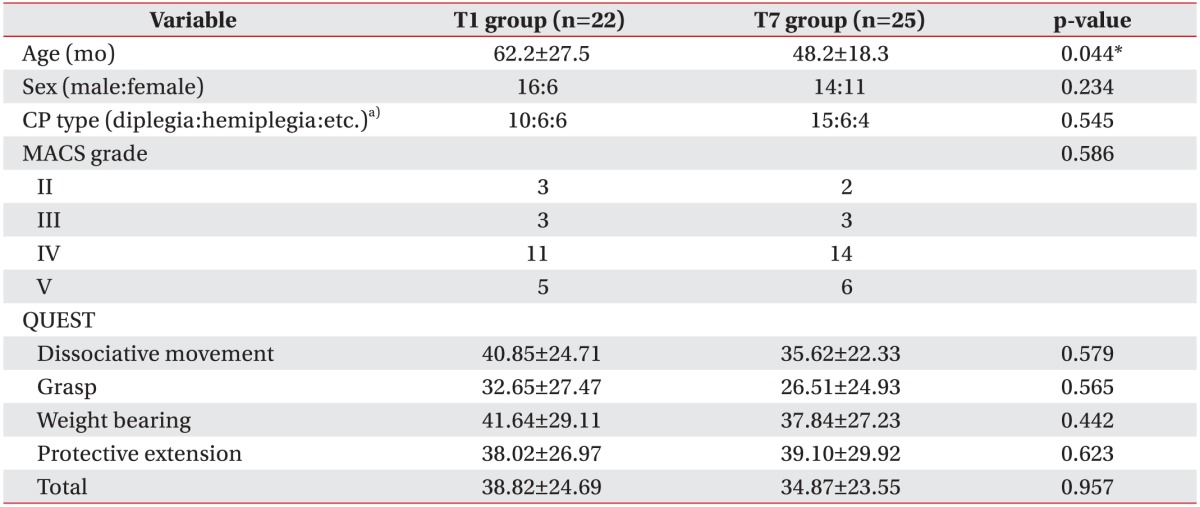
Values are presented as mean±standard deviation or number.
CP, cerebral palsy; MACS, manual ability classification system; QUEST, quality of upper extremity skills test.
a)The ‘etc.’ includes tetraplegia, ataxia, and athetoid type.
*p<0.05, statistically significant.
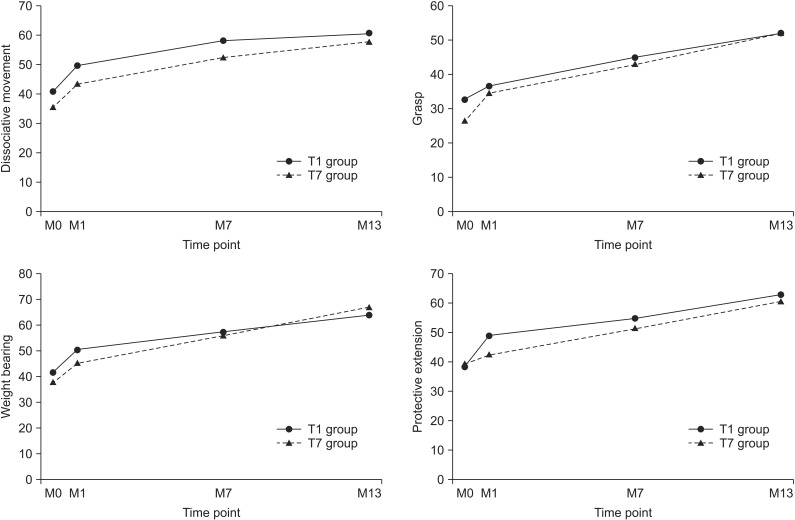
The total and subdomain scores of QUEST at 6- and 12-month evaluations were improved in both groups, as compared to baseline evaluation. The means scores of the subdomain of QUEST over time were shown Fig. 2. All scores showed improvement in both groups (p<0.001). However, there was no significant difference between the changes of M7–M1 and the changes of M13–M7 in both groups. The total and subdomain scores of QUEST were improved in both T1 and T7 groups at the 13-month evaluation, as compared to 1-month evaluation (Table 2). MACS level was unchanged in all patients.
Fig. 2. Changes of mean scores of subdomain of quality of upper extremity skills test in both groups during the study. M0, G-CSF injection time point; M1, mPBMC or placebo injection time point; M7, cross injection time point; M13, 6 months after cross injection; G-CSF, granulocyte-colony stimulating factor; mPBMC, mobilized peripheral blood mononuclear cells.
Table 2. QUEST score between 1 and 13 months within T1 and T7 groups.
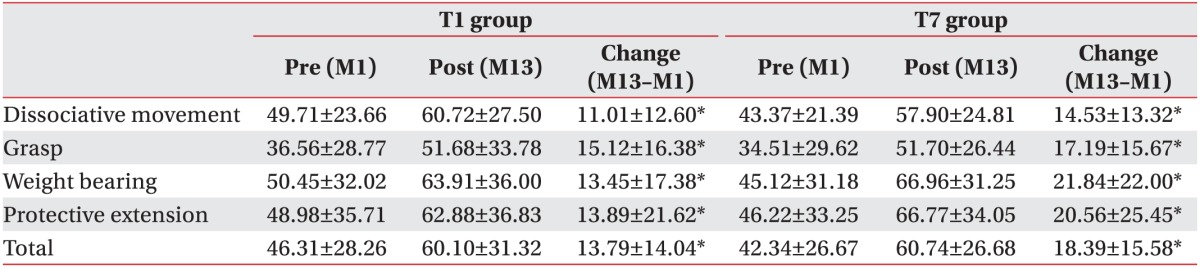
Values are presented as mean±standard deviation.
QUEST, Quality of Upper Extremity Skills Test.
*p<0.05, statistically significant.
To evaluate the effectiveness of mPBMC, the QUEST scores were compared before and after mPBMC (between 1 month and 7 months in T1 group), and before and after placebo (between 1 month and 7 months in T7 group). The QUEST scores were improved at 6 months post-mPBMC injection as well as post-placebo injection. QUEST scores showed no significant difference between mPBMC and placebo injection groups (Table 3). The scores of QUEST were improved at 1 month post-G-CSF injection, as compared to baseline scores (Table 4).
Table 3. QUEST score after mPBMC and placebo injection.
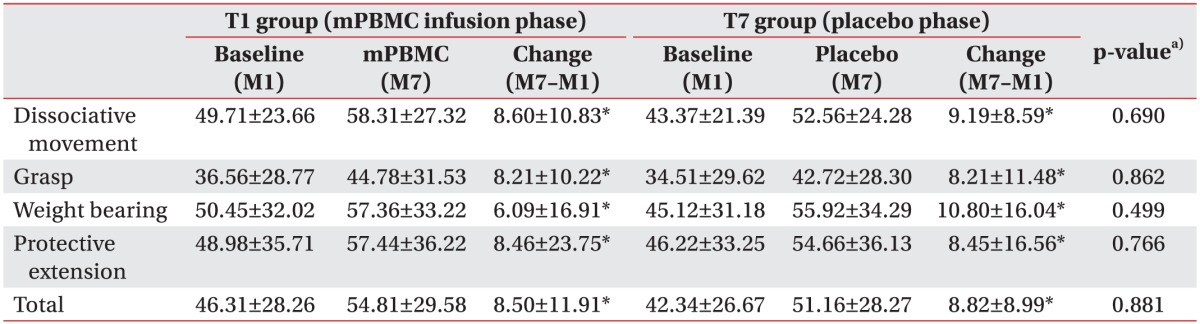
Values are presented as mean±standard deviation.
QUEST, Quality of Upper Extremity Skills Test; mPBMC, mobilized peripheral blood mononuclear cells.
a)p-value for comparison between changes of T1 group and changes of T7 group.
*p<0.05, statistically significant.
Table 4. QUEST score after G-CSF infusion (n=47).
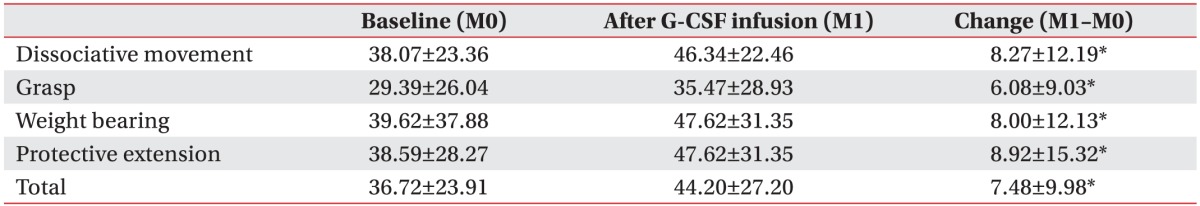
Values are presented as mean±standard deviation.
QUEST, Quality of Upper Extremity Skills Test; G-CSF, granulocyte-colony stimulating factor.
*p<0.05, statistically significant.
DISCUSSION
Stem cells potentially stimulate the repair process by homing to the injured sites of the brain and carrying out regeneration [12]. The mechanism of stem cell therapy remains uncertain, but the three possible mechanisms include structural support for damaged or surrounding tissues [13], re-myelination of damaged axon, and induction of regeneration via neurotrophic growth factors [14]. In addition, stem cells reduce TNF-α, IL-β, and IL-1α level, and increase IL-6 level to improve endogenous brain repair [15].
The stem cell therapy for CP patients mostly involves mesenchymal stem cells from bone marrow (BM) or cord blood (CB) [16,17]. Sharma et al. [18] reported therapeutic effects at 6 months after intrathecal injection of autologous bone marrow mononuclear cells in CP patients. The mesenchymal stem cell is an important source for stem cell therapy but mononuclear cells from peripheral blood are another potential source [19]. Effectiveness of peripheral blood-derived mesenchymal stem cells has been reported for ischemic stroke [20]. Based on this previous report, we collected and cryopreserved mPBMC after intravenous administration of G-CSF. A month later, mPBMC was infused in patients from the T1 group, and 6 months later, mPBMC was infused in patients from the T7 group. We performed this clinical trial based on reported safety and therapeutic potentials of G-CSF in neurodegenerative disease [21]. Safety of administering G-CSF and collecting mPBMC in CP children is already documented [22]. Moreover, mPBMC contains multipotent stem cells and secreted neurotrophic cytokines that could have an effect on neuro-regeneration [23].
In this study, the total and subdomain QUEST scores in both groups were improved regardless of timing of mPBMC and placebo injection; however, there were no differences in effect between mPBMC and placebo injection. In T7 group that received the placebo injection first, the scores of QUEST were also improved only after placebo was injected. This result suggested the possibility that the effect of mPBMC was masked by the effect of G-CSF and/or G-CSF itself might have other neurotrophic potentials in children with CP. Several studies suggest a neurotrophic effect of G-CSF in neurological diseases [24,25,26]. As shown in Table 4, QUEST scores at 1 month post-G-CSF injection were similar to QUEST scores at 6 months post-mPBMC injection. In this study, G-CSF was injected in both T1 and T7 groups to mobilize the stem cell from bone marrow to peripheral blood. This was unavoidable because it was an ethical crossover study in which all the participants were given the same mPMBC injection. Further studies are required to exclude the therapeutic potentials of G-CSF itself by using a control group of CP patients without G-CSF injection.
The study had a few limitations. First, it lacked strict control for other treatments, such as conventional rehabilitative therapies including physical and occupational therapy that could affect QUEST scores. Patients were allowed their ongoing physical and occupational therapy. We recommended no change in the dosage or types of ongoing therapy throughout pre- and post-study period. But, we strongly recommended exclusion of other therapies such as botulinum toxin injection, and neuroprotective medication during screening and throughout the study duration. Secondly, this study did not enroll a sufficient number of patients based on the severity and type of CP because of the research budget and invasiveness of study design. Improvement related to natural growth of children with CP was another inherent limitation. However, the recruited patients aged ≥2 years old and conducted group-wise comparisons in a randomized controlled trial with cross over design, which could somewhat reduce the effect of natural growth than study with younger children (<2 years old). Nevertheless, the hand function of children improves steadily after 2 years of age. Despite the study design, we were unable to exclude the improving effect of natural growth on QUEST scores. The last limitation was the difference in age between the older T1 group and T7 group. Age could have a role on the effect of G-CSF or mPBMC. However, there was no significant difference in changes of QUEST scores between T1 and T7 groups.
In this study, QUEST and MACS indicated no significant difference in the upper extremity function in the children with CP between mPBMC and placebo injection. The effect of mPBMC was possibly masked by the effect of G-CSF, which was used in both groups and/or G-CSF itself might have other neurotrophic potentials in children with CP. Further studies are needed to delineate the effect of mPBMC and G-CSF in patients with CP.
ACKNOWLEDGMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project (A101712), Ministry for Health & Welfare, Republic of Korea.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Colver A, Fairhurst C, Pharoah PO. Cerebral palsy. Lancet. 2014;383:1240–1249. doi: 10.1016/S0140-6736(13)61835-8. [DOI] [PubMed] [Google Scholar]
- 2.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N, Blair E. A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol. 2013;55:499–508. doi: 10.1111/dmcn.12017. [DOI] [PubMed] [Google Scholar]
- 4.Mancias-Guerra C, Marroquin-Escamilla AR, Gonzalez-Llano O, Villarreal-Martinez L, Jaime-Perez JC, Garcia-Rodriguez F, et al. Safety and tolerability of intrathecal delivery of autologous bone marrow nucleated cells in children with cerebral palsy: an open-label phase I trial. Cytotherapy. 2014;16:810–820. doi: 10.1016/j.jcyt.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Cheng H, Hua R, Yang J, Dai G, Zhang Z, et al. Effects of bone marrow mesenchymal stromal cells on gross motor function measure scores of children with cerebral palsy: a preliminary clinical study. Cytotherapy. 2013;15:1549–1562. doi: 10.1016/j.jcyt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Purandare C, Shitole DG, Belle V, Kedari A, Bora N, Joshi M. Therapeutic potential of autologous stem cell transplantation for cerebral palsy. Case Rep Transplant. 2012;2012:825289. doi: 10.1155/2012/825289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J, Zou ZM, Zhou TL, Su YP, Ai GP, Wang JP, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32:641–651. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 8.Huss R, Lange C, Weissinger EM, Kolb HJ, Thalmeier K. Evidence of peripheral blood-derived, plastic-adherent CD34(−/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000;18:252–260. doi: 10.1634/stemcells.18-4-252. [DOI] [PubMed] [Google Scholar]
- 9.Law M, Cadman D, Rosenbaum P, Walter S, Russell D, DeMatteo C. Neurodevelopmental therapy and upper-extremity inhibitive casting for children with cerebral palsy. Dev Med Child Neurol. 1991;33:379–387. doi: 10.1111/j.1469-8749.1991.tb14897.x. [DOI] [PubMed] [Google Scholar]
- 10.Thorley M, Lannin N, Cusick A, Novak I, Boyd R. Reliability of the quality of upper extremity skills test for children with cerebral palsy aged 2 to 12 years. Phys Occup Ther Pediatr. 2012;32:4–21. doi: 10.3109/01942638.2011.602389. [DOI] [PubMed] [Google Scholar]
- 11.Jeevanantham D, Dyszuk E, Bartlett D. The manual ability classification system: a scoping review. Pediatr Phys Ther. 2015;27:236–241. doi: 10.1097/PEP.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez P, Carrillo E, Velez C, Hita-Contreras F, Martinez-Amat A, Rodriguez-Serrano F, et al. Regulatory systems in bone marrow for hematopoietic stem/progenitor cells mobilization and homing. Biomed Res Int. 2013;2013:312656. doi: 10.1155/2013/312656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulkner SD, Ruff CA, Fehlings MG. The potential for stem cells in cerebral palsy: piecing together the puzzle. Semin Pediatr Neurol. 2013;20:146–153. doi: 10.1016/j.spen.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carroll JE, Mays RW. Update on stem cell therapy for cerebral palsy. Expert Opin Biol Ther. 2011;11:463–471. doi: 10.1517/14712598.2011.557060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathieu M, Bartunek J, El Oumeiri B, Touihri K, Hadad I, Thoma P, et al. Cell therapy with autologous bone marrow mononuclear stem cells is associated with superior cardiac recovery compared with use of nonmodified mesenchymal stem cells in a canine model of chronic myocardial infarction. J Thorac Cardiovasc Surg. 2009;138:646–653. doi: 10.1016/j.jtcvs.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Sharma A, Sane H, Gokulchandran N, Kulkarni P, Gandhi S, Sundaram J, et al. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: a new frontier. Stem Cells Int. 2015;2015:905874. doi: 10.1155/2015/905874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider A, Kuhn HG, Schabitz WR. A role for G-CSF (granulocyte-colony stimulating factor) in the central nervous system. Cell Cycle. 2005;4:1753–1757. doi: 10.4161/cc.4.12.2213. [DOI] [PubMed] [Google Scholar]
- 22.Moon JH, Kim MJ, Song SY, Lee YJ, Choi YY, Kim SH, et al. Safety and efficacy of G-CSF mobilization and collection of autologous peripheral blood stem cells in children with cerebral palsy. Transfus Apher Sci. 2013;49:516–521. doi: 10.1016/j.transci.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Koh H, Hwang K, Lim HY, Kim YJ, Lee YH. Mononuclear cells from the cord blood and granulocytecolony stimulating factor-mobilized peripheral blood: is there a potential for treatment of cerebral palsy? Neural Regen Res. 2015;10:2018–2024. doi: 10.4103/1673-5374.172321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.England TJ, Abaei M, Auer DP, Lowe J, Jones DR, Sare G, et al. Granulocyte-colony stimulating factor for mobilizing bone marrow stem cells in subacute stroke: the stem cell trial of recovery enhancement after stroke 2 randomized controlled trial. Stroke. 2012;43:405–411. doi: 10.1161/STROKEAHA.111.636449. [DOI] [PubMed] [Google Scholar]
- 25.Grassinger J, Khomenko A, Hart C, Baldaranov D, Johannesen SW, Mueller G, et al. Safety and feasibility of long term administration of recombinant human granulocyte-colony stimulating factor in patients with amyotrophic lateral sclerosis. Cytokine. 2014;67:21–28. doi: 10.1016/j.cyto.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Schabitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, et al. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]