Abstract
Purpose of Review
Distal radius fractures are one of the most common upper extremity fractures. Athletes with distal radius fractures are treated according to the same principles as non-athletes but present several unique considerations. At all levels of sport, injured athletes desire to return to play as rapidly as possible.
Recent Findings
Earlier operative fixation may allow an athlete to return to play more quickly. Volar locking plates are most commonly used for operative treatment of distal radius fractures due to their stability and low incidence of complications.
Summary
Although the majority of distal radius fractures in athletes are treated non-operatively, operative intervention is offered when required to restore and maintain acceptable skeletal alignment. Return to sport is individualized guided by fracture stability, athlete age, and wrist-specific demands for competition.
Keywords: Athlete, Distal radius, Fracture, Rehabilitation, Sport, Surgery
Introduction
Distal radius fractures are the most common upper extremity fracture in patients in the USA [1], accounting for 0.7–2.5% of emergency department visits [2, 3]. Worldwide, the incidence of distal radius fractures has increased over the past 40–50 years, almost doubling in certain populations [4, 5].
Distal radius fractures occur in a bimodal distribution with the highest frequency in youths under the age of 18 and a secondary peak in adults over 50 years old [1]. In the older adult, osteoporosis and poor postural stability are associated with these fractures after falls onto an outstretched hand [1, 6–8, 9•]. Distal radius fracture in young patients usually occurs in the setting of play or sports and accounts for 23% of all sports-related fractures in adolescents [10].
The athlete presenting with a distal radius fracture tends to be both younger and healthier than the average patient presenting with a distal radius fracture [11]. Athletes in particular have better bone quality when compared to age-matched controls [12–14], but they typically sustain fractures after higher impact falls than those in the more sedentary population. The incidence of distal radius fracture is heightened in sports that risk high energy falls onto the hand or direct impact to the hand or wrist [15, 16]. Perhaps because this group represents only 12.5% of adult distal radius fractures in adults [11], literature guiding their treatment is limited. However, the overall principles in management remain the same. First, the fracture must be stabilized and any secondary injuries evaluated. Next, the determination of operative versus non-operative treatment must be made. This is an important decision for athletes with stable fractures who desire to return to play but remains primarily based on the fracture severity and displacement, patient age, and the timing of the fracture relative to the sport season. Currently, the most common surgical procedure for distal radius fractures in adults is volar plating with locking screws, but the specific procedure should be tailored to the individual patient. The most common question for athletes, and perhaps the most difficult to answer, is predicting the timing of return to play. We will discuss all of these components as they pertain to the treatment of distal radius fracture in athletes.
Relevant anatomy
The distal radius has three articular facets, the scaphoid facet, lunate facet, and sigmoid notch articulating with the scaphoid, lunate, and distal ulna, respectively. Several measurements describe the normal distal radius: height (radius styloid 12-mm longer than the ulnar corner of the lunate facet), lateral tilt (11 degrees volar), inclination (22°), and length relative to the ulna (neutral variance) [17]. After fracture, change from these normative values or change compared to the opposite uninjured radius is assessed and often guides the decision to pursue operative treatment versus non-operative immobilization. Decreased radial height and inclination result in a hand that appears radially deviated with increased prominence of the ulnar head. Altered lateral tilt of the articular surface may produce visible deformity but is more important as a cause of adaptive midcarpal instability which is one cause of long-term wrist pain secondary to the malalignment between the lunate and capitate. When clinically relevant changes in lateral tilt or radius shortening occur, patients may also experience restricted forearm rotation secondary to altered distal radial-ulnar joint mechanics. Finally, loss of radius length producing an ulnar positive wrist increases the chance of symptomatic ulnar impaction syndrome.
The median nerve and flexor tendons for the fingers course volar to the distal radius. Distal radius fractures increase pressure within the carpal tunnel, a finding potentiated by immobilization in a position of wrist flexion [18]. Excessive pressure may produce symptomatic carpal tunnel syndrome that may necessitate emergent release or contribute to the development of complex regional pain syndrome after distal radius fracture.
Several anatomic features of the wrist are relevant to operative management of the distal radius. A volar approach to the radius through the flexor carpi ulnaris sheath requires awareness of the palmar cutaneous branch of the median nerve. Although most commonly located between the flexor carpi radialis and the palmaris longus, this causalgic nerve is located within the flexor carpi radius sheath in up to 6% of individuals (Fig. 1) [19]. The watershed line, a prominent transverse ridge on the volar aspect of the distal radius, is the most volar prominence off the distal radius. Volar plates placed on the distal radius are ideally positioned and tightly applied to the bone proximal to this ridge to prevent the flexor tendons from contacting the plate (Fig. 2a, b). Plate prominence otherwise risks tenosynovitis of the flexor tendons and potential tendon ruptures, most commonly affecting the flexor pollicis longus and index finger flexor digitorum profundus [20]. During volar plate fixation, direct visualization of the radiocarpal articular reduction is not possible secondary to the radioscaphocapitate and radiolunate ligaments, which originate from the volar articular margin of the distal radius. These must be noted and preserved during a volar approach to the distal radius to prevent an iatrogenic ulnar carpal translation.
Fig. 1.
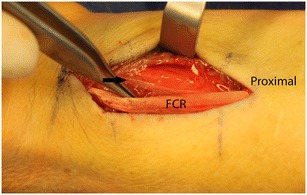
Intraoperative view of the volar wrist with the flexor carpi radialis (FCR) retracted revealing the palmar cutaneous branch of the median nerve (arrow) coursing within its sheath
Fig. 2.
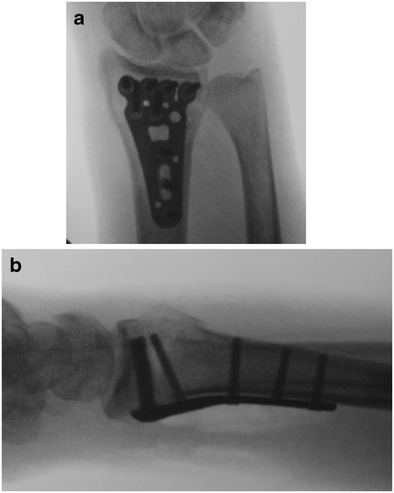
Anteroposterior (a) and lateral (b) of a well-reduced distal radius fracture with a volar plate. Subchondral screw placement optimizes support of the articular surface and plate placement so that the distal plate edge is proximal and less prominent than the volar watershed line of the radius
The utilization of a dorsal approach for distal radius fractures depends on surgeon preference, fracture configuration, or need to directly visualize the radiocarpal articular reduction. This approach requires the dissection and exposure of the extensor compartments. When elevating the subcutaneous tissues off this retinaculum, branches of the superficial radial nerve and dorsal cutaneous branch of the ulnar nerve are at risk. At the wrist, the extensor tendons run under the extensor retinaculum in six separate fibro-osseous sheaths. The EPL is the only extensor tendon that deviates substantially from a longitudinal course. As such, care must be taken to identify and protect the EPL during a dorsal approach to the distal radius, which is most frequently done by first opening the extensor retinaculum into the third extensor compartment (Fig. 3). The fourth extensor compartment contains the posterior interosseous nerve, which is commonly excised as a partial wrist denervation for pain relief during dorsal approaches. Careful elevation of the fourth extensor compartment overlying the DRUJ is essential to preservation of the dorsal radioulnar ligament during a dorsal exposure of the wrist. The EDM, located in the fifth extensor compartment, is a commonly used landmark for operative exposure of the DRUJ and TFCC. Deep to the extensor tendons, the dorsal intercarpal and radiocarpal ligaments stabilize the carpus. The primary intrinsic carpal ligaments of the proximal carpal row are the scapholunate and lunotriquetral, which are strongest dorsally and volarly, respectively. Care is taken during all dorsal exposures of the wrist to avoid iatrogenic injury to the scapholunate ligament which is located just ulnar to Lister’s tubercle of the distal radius.
Fig. 3.
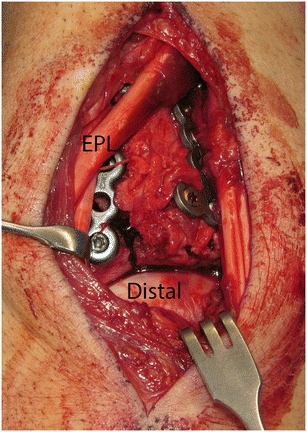
Intraoperative view after dorsal plates placed on the distal radius with direct visualization of the carpus and articular reduction. Extensor pollicis longus (EPL) crossing superficial to radial wrist extensor tendons
Epidemiology of distal radius fractures in athletes
Athletes represent a limited but sizable subset of the population with distal radius fractures. Over a 1-year period, 131 sports-related distal radius fractures were presented to the Royal Infirmary of Edinburgh, representing 12.5% of all distal radius fractures. The most commonly involved sports in this population were soccer, snowboarding, and rugby with 65, 16, and 8 associated fractures, respectively [11]. Lawson et al. retrospectively reviewed 225 sports-related distal radius fractures occurring over a 5-year period presenting to a single hospital in Edinburgh, Scotland. Of these fractures, 126 (56%) were minimally or non-displaced. Fifty percent of these fractures were soccer related (n = 112), with soccer being the most popular sport in the region [15]. Robertson et al. encountered 367 soccer-associated fractures in athletes of varying skill levels, 73 (20%) of which were distal radius fractures, in a 1-year period [21]. They found rugby-associated distal radius fractures to be less common, with only 11 encountered in a 2-year period [22]. Soccer players most commonly sustain distal radius fractures from falls, with the ball striking the hand a distant second, 79 and 21%, respectively. Higher velocity injuries from sports such as skiing and horseback riding tended to cause more complex fractures while soccer and rugby injuries were more likely to be simple, extra-articular fractures [15].
Differences in injury patterns can be seen within athletes in the same sport, where fractures seen in novice snowboarders were more likely to be extra-articular, while expert athletes suffered more intra-articular fractures [16]. The most common fracture types were simple extra-articular (A2) and simple intra-articular (C1), representing 36.8 and 27.0% of radius fractures in this population. Using the National Trauma Data Bank, Basgues et al. determined that distal radius fractures are more prevalent in snowboarders relative to skiers (10.5% of all presenting injuries vs 2.7%) [23]. Others have found a similar pattern, with distal radius fractures representing 2.2% of injuries in skiers and 14.5–17.4% in snowboarders [16, 24].
As expected, distal radius fractures can impart substantial time away from sporting activity when mobility and use of the hand and wrist are needed for participation. Among professional football players, wrist fractures are the seventh most commonly reported upper extremity injury, representing 17% of all wrist injuries. These players lost an average of 42 days playing time, or about 1/3 of the season [25].
Evaluation
After assessing for neurovascular injury, distal radius fractures require lateral and anterior-posterior radiographs. Fractures are examined for displacement according to the normative values for radius height, length, inclination, and lateral tilt. The articular surfaces of the radiocarpal joint and the distal radioulnar joint are assessed for step off and gapping. Loss of normal alignment with articular incongruity over 2 mm, radius shortening over 3 mm, or dorsal tilt over 10° generally requires reduction. Failure to obtain or maintain reduction better than these parameters is accepted as indicating the need for operative treatment.
Multiple fracture classifications exist for distal radius fractures. The AO/OTA classification offers a simple descriptive classification system which may imperfectly predict treatment but facilitates communication about the injury and is readily understood. A-type fractures are extra-articular injuries typically in the metaphysis of the radius. B-type fractures are partial articular injuries that are frequently associated with abnormal carpal translation in the direction of the displaced fragment. Partial articular fractures of the dorsal and volar rim of the lunate facet are notoriously unstable and are routinely stabilized operatively. C-type fractures are complete articular injuries where metaphyseal fracture completed separates each of the articular fragments from the diaphysis.
Distal radius fractures are frequently accompanied by soft tissue injuries. This holds true in the athlete presenting with a complex fracture. Hanker et al. reviewed the cases of 173 athletes presenting with distal radius fractures. The most common soft tissue injury was TFCC tears in 61% of patients, followed by carpal instability in 20% and distal radioulnar joint instability in 9% [26]. Although these associated injuries are often partial or low-grade injuries that do not require dedicated treatment, surgeons are obligated to remain vigilant when screening for these associated injuries on physical and radiographic examination.
In addition to assessing the injured wrist, athletes should be queried about the sport(s) they play, their position, and the timing of the injury relative to their season responsibilities. Late in a season, a displaced fracture requiring surgery may be treated on a delayed fashion by waiting 1–2 weeks if the athlete could otherwise effectively compete in a cast. This is possible for athletes who are not substantially limited by pain from the acute injury. Unlike scaphoid fractures where increased pre-operative activity may risk displacement that necessitates more complex surgery, distal radius fractures that are displaced enough to warrant surgery are unlikely to displace in a manner that would change the surgical approach. In that situation, an immediate surgery may be disadvantageous in necessitating missing the end of a season. Early in a season, delaying treatment is often not an option as allowing a fracture to progress to malunion increases the operative complexity and would likely impair the ultimate outcome. During the off-season, athletes with fractures that are presumed to be at risk for displacement despite immobilization may reasonably request immediate operative stabilization to avoid the chance of delayed surgery extending the time required for healing if that would encroach into the upcoming season.
Non-operative treatment
Non-operative treatment can be considered in the non-displaced, extra-articular fracture or the stable, reduced fracture. However, even if no instability criteria are met, there is still the chance of secondary displacement in a reduced fracture. Lafontaine’s criteria of stability predict secondary displacement after reduction if three or more of the following criteria are met: age >60 years, dorsal comminution, intra-articular fracture, associated ulnar fracture, and dorsal angulation >20°. The most highly predictive factor is age, which does not include the majority of athletic fractures, suggesting a higher chance of stable reduction in the athlete [27, 28••]. Displaced fractures require reduction, but in the patient who desires to undergo surgical fixation, there is no clear additional benefit to pre-operative reduction [29••]. We recommend clinical and radiographic evaluation 2–3 times over the first 3 weeks after fracture so that any displacement will be detected and can be corrected prior to bony union.
In the competitive athlete, the risks of displacement and prolonged time away from sport if surgery is required on a delayed fashion must be balanced with the inherent risks of surgery. If the patient presents with a non-displaced fracture, they can be treated with 4 to 6 weeks of a short arm cast followed by use of an orthosis as needed for comfort. If the fracture required closed reduction prior to casting, a short arm or sugar tong splint should be applied followed by plain films at 1 and 2 weeks to evaluate the fracture stability and assess for maintenance of reduction followed by transition to a short arm cast to complete 6 weeks of immobilization. Displacement during healing prompts surgical fixation.
Range of motion exercises for the fingers, elbow, and shoulder should be started immediately. These are performed without resistance and can be gentle active and passive motions. Forearm rotation is often possible as fractures begin to consolidate by 3 weeks and can be performed within the confines of a short arm cast. Casts are removed only when radiographs demonstrate fracture healing (disappearance of fracture line, callus formation). Wrist ROM commences once the cast is removed but is often associated with soreness following weeks of immobilization. Strengthening exercises should be delayed until there is radiographic evidence of union accompanied by a resolution of fracture tenderness. After cast removal, patients are transitioned to a removable brace or orthosis to be used for comfort over the first week. If a sport does not directly require use of the hand patients may return to sport in a brace, but otherwise, athletes are often reevaluated at 1–2 weeks after cast removal for potential return to play. Although not based on specific objective numbers, return to play is recommended after demonstration of a comfortable functional range of motion (fingers and wrist) and ability to use the affected hand without hesitation or guarding during daily activities. In the case of a non-displaced simple fracture, we may discontinue the cast at 4 weeks and start active range of motion (wrist and forearm rotation) but delay strengthening until 6 weeks.
Operative treatment
There are several options for surgery in patients with unstable fractures, or in those who value a more rapid rehabilitation and want anatomic restoration. Most studies show equivalent results at 1–2 years after adequate fracture reduction regardless of fixation choice, leaving the specific treatment dependent on fracture characteristics [30, 31].
Internal fixation will generally provide an earlier return to function in first 6–12 weeks compared to pin fixation or wrist spanning fixation. The most commonly used method is open reduction and internal fixation with volar plating. While some earlier studies showed no difference, more recent studies have demonstrated both improved functional outcomes and fewer complications with the use of volar plates relative to both external fixation and dorsal plating [34–37]. Along with the ease of the volar approach, the advent of pre-contoured volar locking plates has made the technical aspect of the procedure simpler as well. The volar aspect of the radius is frequently less comminuted, allowing for easier assessment of reduction.
Although volar would be our routine approach for most displaced fractures, an exception should be made for the displaced intra-articular fracture that requires reduction under direct visualization. In this situation, dorsal plating would be recommended in order to clearly visualize the articular surface.
Dorsal spanning plates have largely replaced the use of external fixation but should be reserved as a last resort in the athlete to avoid loss of motion at the wrist. However, it may be required in the setting of a highly comminuted articular surface to maintain reduction and restore radial length.
The open distal radius physis in the child or adolescent athlete requires alternative operative approaches to preserve future growth. Closed reduction with Kirchner wires is typically sufficient for young athletes with extra-articular fractures with open reduction performed only if required [38]. However, these patients should be monitored closely for subsequent displacement as this technique provides only relative fracture stability.
Surgical approach for volar plate fixation
The limb is exsanguinated and a tourniquet inflated after the arm is prepped and draped. A volar longitudinal incision through the skin and subcutaneous tissue is made over the distal aspect of the flexor carpi radialis (FCR) tendon. The FCR tendon sheath is then opened, with care to avoid injury of the palmar cutaneous branch of the median nerve (Fig. 1). The floor of the sheath is incised and blunt dissection can proceed radial to the flexor pollicis longus (FPL) muscle to allow identification of the pronator quadratus, which is then released distally and radially. If additional length and radial inclination are not readily restored, the brachioradialis tendon fibers are released from the radial styloid. The fracture is manually reduced and the volar plate positioned on the distal radius proximal to the watershed line (Fig. 2a, b). We typically stabilize the plate in position with a screw through the oblong hole proximal to the fracture and a K-wire through a wire hole in the distal plate, followed by fluoroscopic confirmation of adequate reduction and plate placement. A second and third screw are then placed into the radial diaphysis. For metaphyseal fractures, we utilize only unicortical distal screws to minimize the risk of either rupturing the extensor tendons or placing intra-articular hardware. Small dorsal fracture fragments are routinely ignored except when encountering of dorsal subluxation of the carpus resulting from partial articular fracture of the dorsal lunate facet. After fixation, clinical evaluation of the distal radius alignment, DRUJ stability, and forearm range of motion are performed. Fluoroscopic confirmation of appropriate alignment and plate placement is obtained prior to tourniquet deflation and skin closure. A short arm volar splint is then placed prior to leaving the operating room.
Risks of volar plate fixation
Although any surgical procedure carries inherent risk, the incidence of complications after volar plating for a distal radius fracture repair is low. There is an increased risk of median nerve dysfunction after volar approaches to the distal radius with reported incidence from 0 to 10.2% [39–44]. However, most of these symptoms are mild and self-limited resolving in the first few months after surgery.
Although the flexor tendons are not as tightly applied to the radius as the extensor tendons, even low profile volar plates may irritate the flexor tendons if the tendons glide repetitively over the distal edge of the plate. Rates of postoperative flexor tendon rupture are reported from 0 to 1.8% [20, 39, 41–44]. This risk can be reduced by ensuring the entirety of the plate remains proximal to the transverse ridge at the distal aspect of the radius, keeping the distal plate tightly applied to the bone, and restoring the volar tilt of the radius [20, 45, 46].
Extensor tendon ruptures, especially the EPL, have been reported as well. Although this can occur following non-operatively treated fractures, prominent screws placed from volar plates have been implicated in postoperative ruptures. Extensor tendon ruptures complicate up to 6.5% of distal radius fractures treated with a volar locking plate [39, 41–44].
There is also a risk of intra-articular hardware when treating distal radius fractures. Although no single method of prevention is perfect, preventative measures include checking for crepitus during motion in the operating room, using fluoroscopy to profile the articular surfaces of the radius or to provide live rotational imaging, supplementing open fixation with arthroscopic examination, and placing unicortical distal screws.
Role of arthroscopy
Distal radius fractures are frequently accompanied by soft tissue injuries, particularly in the high energy injuries associated with many athletic fractures [26, 47–49]. Despite the high frequency of these associated injuries, routine arthroscopic examination has not been accepted as routinely improving outcomes. We do not routinely incorporate arthroscopy in the treatment of distal radius fractures but believe there are several settings in which arthroscopy can be a useful adjunct. Arthroscopy offers a unique ability to provide assurance that screws do not penetrate the radiocarpal joint and is invaluable when assessing for suspected scapholunate ligament injury while treating a distal radius fracture with a volar approach. It can also assist in the reduction of small displaced articular fractures, such as in a die punch impaction, by providing better visualization of the articular surface. Furthermore, in the setting of distal radioulnar joint instability persisting after volar plate fixation, arthroscopy can facilitate TFCC repair in a minimally invasive fashion.
Postoperative rehabilitation
Following rigid internal fixation, patients are immobilized in a non-removable plaster splint for 10–14 days. When sutures are removed at 10–14 days, a removable orthosis is fashioned (Fig. 4). Active wrist motion and forearm rotation is initiated between 2 and 6 weeks depending on fracture severity and presumed construct rigidity. Although earlier range of motion exercises typically speeds early recover, it is not anticipated to change the ultimate outcome [50]. We routinely follow patients every 2 weeks until healing. At 6 weeks, or when healing is demonstrated radiographically, we then introduce passive range of motion while also discontinuing orthotic use and allowing gradual strengthening.
Fig. 4.
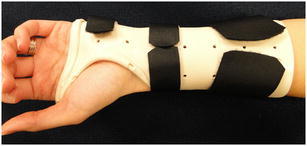
A typical resting orthosis that allows finger motion while supporting the healing distal radius. Copyright Rhonda Powell (Milliken Hand Therapy, St. Louis MO), reprinted with permission
Although the majority of motion and function is regained within 3 months of treatment, gradual improvement in motion, strength, endurance, and patient-reported function continue for at least a year after distal radius fracture [51, 52].
Finger motion
For both non-operatively and operatively treated distal radius fractures, achieving full finger motion is stressed as a primary goal beginning at the initial evaluation. Patients are recommended to work toward making a full fist without any resistance and to fully extend digits. A common misperception among patients is that squeezing a ball achieves this goal. We explain that squeezing a ball only adds resistance and actually blocks fingers from making a full fist. In our experience, prolonged finger stiffness after distal radius fracture is difficult to treat and imparts greater impairment than wrist stiffness. Finger motion is stressed at every office visit.
Outcomes
When the distal radius heals in reasonable alignment, regardless of the type of treatment delivered, most patients ultimately do well [30–33]. Non-union of the distal radius is rare following non-operative and operative treatment as these fractures routinely heal in 6–8 weeks [53, 54•]. Return of range of motion occurs primarily within the first 3 months, with pronation/supination reaching 92% of the uninjured wrist by this time. Flexion and extension are slower to return but on average regain 87 and 90% of the contralateral wrist’s motion by 1 year [52]. Return of strength progresses more slowly, but patients often experience a return of grip strength to approximately 81–94% of the opposite wrist [52, 54•]. Most importantly, patients tend to demonstrate excellent functional outcomes [52–55]. Rozental and Blazer reviewed 41 patients treated with volar locking plates at an average follow-up of 17 months [43]. These patients reported exceptional return to function, with an average Disabilities of the Arm, Shoulder, and Hand Questionnaire scores of 14/100 (lower scores indicate less disability). Higher energy trauma is associated with worse wrist range of motion, grip strength, and functional scores at 3 and 6 months, but this difference may diminish by 9 months [56•].
The athlete tends to recover more quickly, with most returning to play by 6–9 weeks. Robertson et al. found that at an average of 8.9 weeks, 77% of soccer players were able to return to the same level of play [21]. In the subset of professional athletes, this reached 100%. Lawson et al. found a similar rate of return to play, where 72.5% of athletes among varying disciplines and skill levels returned at an average follow-up of 27 months, with novices representing the majority of those choosing to not return [15].
Better short-term outcomes can be achieved with early surgical rather than delayed surgical fixation. At 12 weeks, patients surgically treated within the first few days of injury have shown significantly improved range of motion, strength, and functional outcome scores compared to patients treated one or more weeks after their injury [57, 58•]. These differences disappear by 1 year, suggesting that while earlier surgery may lead to earlier mobilization, long-term outcomes are the same whether or not surgery is delayed.
Radiocarpal arthritic changes are frequently noted on long-term follow-up after intra-articular distal radius fractures. Goldfarb et al. found radiocarpal arthrosis in 13 out of 16 patients 15 years after surgery [59]. However, these changes had no effect on the patients’ self-evaluation of function or their clinical exam. Knirk and Jupiter found that this delayed arthritis was best correlated with the accuracy of articular reduction, where 91% of patients with incongruous joint surfaces after surgery developed arthritis versus only 11% of those with congruent articular surfaces [60]. An increased articular gapping and postoperative displacement of articular fragments have also been associated with higher stages of arthritis at long-term follow-up [55, 61].
Return to sport
Perhaps the most pressing decision in the rehabilitation of a fracture in an athlete is the decision of when to return to play. Even non-contact sports can risk either collision with other participants or falling on the wrist. Although many athletes and those around them would prefer a rapid return to play, the athlete’s health and ultimate wrist outcome is paramount. Henn and Wolfe recommend at least 80% of the baseline range of motion and strength should be demonstrated in therapy, as well as radiographic healing prior to return [62]. In our practice, the athlete participating in a sport that does not require use of the wrist (e.g., field position in soccer, snowboarding, cross country running) can return to sport prior to fracture healing while in a cast. Understanding that a fall or collision could risk further injury, this decision is made with consideration of the level of competition and age of the athlete with a thorough discussion of the risks and benefits of participation. For athletes that require a functional wrist for their sport, we consider return to play only after healing of the fracture. This is expected as early as 4 weeks in the pediatric athlete and likely after 6 weeks in the adult. Increasing fracture severity and medical comorbidities (e.g., diabetes) may slow healing and are taken into account. Although return to play recommendations are individualized based on the anticipated sport demands, we have advocated for return to play after restoration of pain-free functional range of motion. In preparation of return to sport, we recommend a graduated progression of use to ensure the wrist will function comfortably for daily activities, followed by sport-specific drills, then practice, and finally in the competitive environment. We have not used a specific numeric threshold but see comfortable use of the wrist as paramount to allow for return to sport without inadvertent guarding of the wrist that may place the athlete at risk for other injuries.
The older athlete
Older adult athletes (>60 years) are being seen with increasing frequency in our practice. Although consistently reporting fewer medical comorbidities than others in their age group and appearing physiologically younger, these athletes’ chronologic age is relevant. Bone density is frequently compromised with distal radius fractures in older adults. Regardless of mechanism, suffering a distal radius fracture as an older adult is associated with both osteoporosis and subsequent risk of hip fracture in men and women. Advanced age predicts lost alignment after reduction and casting of distal radius fractures. In this age group, final radiographic appearance of the distal radius most often mirrors the appearance at the time of injury as opposed to post-reduction images. Although this would seemingly suggest that surgical intervention is preferred, the impact of distal radius malunion in the older adult is controversial. Macdermid found that the number needed to harm from malunion was greater in older adults compared to young adults [63]. Randomized trials have not demonstrated superior objective or patient-reported outcomes with operative treatment for displaced distal radius fractures in older adults, and a cross-sectional evaluation was unable to demonstrate any significant negative impact of distal radius malunion in older adults stratified by activity level [39, 64•]. Notably though, outside of randomized trials, investigators acknowledge that they have little to no experience treating particular fracture patterns (e.g., partial articular fractures associated with carpal subluxation) or fractures with more extreme displacement non-operatively. In our practice, we explain to the older athlete that operative treatment for mildly displaced fractures (even those somewhat exceeding those values for an acceptable reduction detailed earlier) may not change the ultimate functional outcome. The risks of surgery are therefore weighed against the advantages of earlier rehabilitation and more normal wrist appearance.
The child athlete
Children at all levels of sport competition present with distal radius fractures. Anatomically, these pediatric patients are unique in their ability to remodel bones with growth. Socially, children are more frequently involved in recreational-level sports and it is frequently the parents as opposed to the athlete who are most eager for a return to sport. Young children (<10 years) are rarely high-level athletes and although their sports are expected to be lower impact, we routinely recommend delaying return to play until fracture healing. Adolescent athletes (11–14 years) may be participants on traveling teams and select sports. However, they are still too young for most to receive any attention for collegiate sports and we recommend returning to play only after healing the fracture. Athletes in the middle or late years of high school may be playing in front of scouts competing for potentially valuable collegiate scholarships. If one of these athletes plays a sport that does not require use of the wrist, then we will discuss playing while immobilized as the benefit may exceed the risk. In these situations with high-level high school athletes, the discussion regarding the risks and benefits of return to play is held as if that athlete were an adult although the conversation engages both the athlete and their family to come to a consensus plan.
Conclusions
Distal radius fractures commonly affect athletes of all ages. Although treatment principles are similar for athletes and non-athletes, athletes are often rehabilitated aiming for minimizing time away from sport. The timing of return to play balances individualized risks and benefits for each athlete based on their age, sport requirements, fracture healing, and comfort.
Compliance with ethical standards
Conflict of interest
Casey Beleckas does not have any conflict of interest.
Ryan Calfee reports research support from Medartis outside of the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Hand and Wrist Sports Medicine
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Karl JW, Olson PR, Rosenwasser MP. The epidemiology of upper extremity fractures in the United States, 2009. J Orthop Trauma. 2015;29:242–4. doi: 10.1097/BOT.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 2.Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J. Hand. Surg. 2001;26:908–15. doi: 10.1053/jhsu.2001.26322. [DOI] [PubMed] [Google Scholar]
- 3.Larsen CF, Lauritsen J. Epidemiology of acute wrist trauma. Int J Epidemiol. 1993;22:911–6. doi: 10.1093/ije/22.5.911. [DOI] [PubMed] [Google Scholar]
- 4.Bengner U, Johnell O. Increasing incidence of forearm fractures. A comparison of epidemiologic patterns 25 years apart. Acta Orthop Scand. 1985;56:158–60. doi: 10.3109/17453678508994345. [DOI] [PubMed] [Google Scholar]
- 5.Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28:113–25. doi: 10.1016/j.hcl.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtis EM, van der Velde R, Moon RJ, et al. Epidemiology of fractures in the United Kingdom 1988–2012: variation with age, sex, geography, ethnicity and socioeconomic status. Bone. 2016;87:19–26. doi: 10.1016/j.bone.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ismail AA, Pye SR, Cockerill WC, et al. Incidence of limb fracture across Europe: results from the European Prospective Osteoporosis Study (EPOS) Osteoporos Int. 2014;13:565–71. doi: 10.1007/s001980200074. [DOI] [PubMed] [Google Scholar]
- 8.Sakai A, Oshige T, Zenke Y, Suzuki M, Yamanaka Y, Nakamura T. Association of bone mineral density with deformity of the distal radius in low-energy Colles’ fractures in Japanese women above 50 years of age. J. Hand. Surg. 2008;33:820–6. doi: 10.1016/j.jhsa.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 9.Louer CR, Boone SL, Guthrie AK, Motley JR, Calfee RP, Wall LB. Postural stability in older adults with a distal radial fracture. J. Bone Joint Surg. 2016;98:1176–82. doi: 10.2106/JBJS.15.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood AM, Robertson GA, Rennie L, Caesar BC, Court-Brown CM. The epidemiology of sports-related fractures in adolescents. Injury. 2010;41:834–8. doi: 10.1016/j.injury.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Court-Brown CM, Wood AM, Aitken S. The epidemiology of acute sports-related fractures in adults. Injury. 2008;39:1365–72. doi: 10.1016/j.injury.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Schipilow J, Macdonald H, Liphardt A, Kan M, Boyd S. Bone micro-architecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56(2):281–9. doi: 10.1016/j.bone.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Sherk VD, Bemben MG, Bemben DA. Comparisons of bone mineral density and bone quality in adult rock climbers, resistance-trained men, and untrained men. J. Strength Cond. Res. 2010;24:2468–74. doi: 10.1519/JSC.0b013e3181b60407. [DOI] [PubMed] [Google Scholar]
- 14.Tenforde AS, Fredericson M. Influence of sports participation on bone health in the young athlete: a review of the literature. PMR. 2011;3(9):861–7. doi: 10.1016/j.pmrj.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Lawson G, Hajducka C, Mcqueen M. Sports fractures of the distal radius—epidemiology and outcome. Injury. 1995;26:33–6. doi: 10.1016/0020-1383(95)90549-D. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K, Sumi H, Sumi Y, Shimizu K. Wrist fractures from snowboarding. Clin J Sport Med. 2004;14:64–71. doi: 10.1097/00042752-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez DL, Wolfe SW. Distal radius fractures. Green’s operative hand surgery 5th Ed. Editors green Hotchkiss, Pederson, Wolfe. Philadephia: Elsevier; 2005. pp. 645–710. [Google Scholar]
- 18.Gelberman RH, Szabo RM, Mortensen WW. Carpal tunnel pressures and wrist position in patients with Collesʼ fractures. J Trauma. 1984;24:747–9. doi: 10.1097/00005373-198408000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Jones C, Beredjiklian P, Matzon J, Kim N, Lutzky K. Incidence of an anomalous course of the palmar cutaneous branch of the median nerve during volar plate fixation of distal radius fractures. JHS. 2016;41(8):841–4. doi: 10.1016/j.jhsa.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Soong M, Earp BE, Bishop G, Leung A, Blazer P. Volar locking plate implant prominence and flexor tendon rupture. J Bone Joint Surg. 2011;93:328–35. doi: 10.2106/JBJS.J.00193. [DOI] [PubMed] [Google Scholar]
- 21.Robertson GA, Wood AM, Bakker-Dyos J, et al. The epidemiology, morbidity, and outcome of soccer-related fractures in a standard population. Am J Sports Med. 2012;40:1851–7. doi: 10.1177/0363546512448318. [DOI] [PubMed] [Google Scholar]
- 22.Robertson GA, Wood AM, Heil K, Aitken SA, Court-Brown CM. The epidemiology, morbidity and outcome of fractures in rugby union from a standard population. Injury. 2014;45:677–83. doi: 10.1016/j.injury.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Basques BA, Gardner EC, Samuel AM, Webb ML, Lukasiewicz AM, Bohl DD, Grauer JN. Injury patterns and risk factors for orthopaedic trauma from snowboarding and skiing: a national perspective. Knee Surgery, Sports Traumatology, Arthroscopy Knee Surg Sports Traumatol Arthrosc. 2016 10.1007/s00167-016-4137-7 [DOI] [PubMed]
- 24.Matsumoto K, Miyamoto K, Sumi H, Sumi Y, Shimizu K. Upper extremity injuries in snowboarding and skiing: a comparative study. Clin J Sport Med. 2002;12:354–9. doi: 10.1097/00042752-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Carlisle JC, Goldfarb CA, Mall N, et al. Upper extremity injuries in the National Football League: part II: elbow, forearm, and wrist injuries. Am J Sports Med. 2008;36:1945–52. doi: 10.1177/0363546508318198. [DOI] [PubMed] [Google Scholar]
- 26.Hanker GJ. Radius fractures in the athlete. Clin Sports Med. 2001;20:189–201. doi: 10.1016/S0278-5919(05)70255-6. [DOI] [PubMed] [Google Scholar]
- 27.Lafontaine M, Hardy D, Delince P. Stability assessment of distal radius fractures. Injury. 1989;20:208–10. doi: 10.1016/0020-1383(89)90113-7. [DOI] [PubMed] [Google Scholar]
- 28.Lamartina J, Jawa A, Stucken C, Merlin G, Tornetta P. Predicting alignment after closed reduction and casting of distal radius fractures. J. Hand. Surg. 2015;40:934–9. doi: 10.1016/j.jhsa.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 29.Teunis T, Mulder F, Nota SP, Milne LW, Dyer GS, Ring D. No difference in adverse events between surgically treated reduced and unreduced distal radius fractures. J Orthop Trauma. 2015;29:521–5. doi: 10.1097/BOT.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 30.Gartland JJ, Jr, Werley CW. Evaluation of healed Colles’ fractures. J Bone Joint Surg Am. 1951;33-A:895–907. doi: 10.2106/00004623-195133040-00009. [DOI] [PubMed] [Google Scholar]
- 31.Karnezis IA, Fragkiadakis EG. Association between objective clinical variables and patient-rated disability of the wrist. J Bone Joint Surg (Br) 2002;84:967–70. doi: 10.1302/0301-620X.84B7.12673. [DOI] [PubMed] [Google Scholar]
- 32.McQueen M, Caspers J. Colles fracture: does the anatomical result affect the final function? J Bone Joint Surg (Br) 1988;70:649–51. doi: 10.1302/0301-620X.70B4.3403617. [DOI] [PubMed] [Google Scholar]
- 33.Wilcke MK, Abbaszadegan H, Adolphson PY. Patient perceived outcome after displaced distal radius fractures. A comparison between radiological parameters, objective physical variables, and the DASH score. J Hand Ther. 2007;20:290–8. doi: 10.1197/j.jht.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Chappuis J, Boute P, Putz P. Dorsally displaced extra-articular distal radius fractures fixation: dorsal IM nailing versus volar plating. A randomized controlled trial. Orthop. Traumatol. Surg. Res. 2011;97:471–8. doi: 10.1016/j.otsr.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Grewal R, Perey B, Wilmink M, et al. A randomized prospective study on the treatment of intra-articular distal radius fractures: open reduction and internal fixation with dorsal plating versus mini open reduction, percutaneous fixation, and external fixation. J Hand Surg [Am] 2005;30:764–72. doi: 10.1016/j.jhsa.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 36.Ruch DS, Papadonikolakis A. Volar versus dorsal plating in the management of intra-articular distal radius fractures. J Hand Surg [Am] 2006;31:9–16. doi: 10.1016/j.jhsa.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 37.Wei DH, Poolman RW, Bhandari M, et al. External fixation versus internal fixation for unstable distal radius fractures: a systematic review and meta-analysis of comparative clinical trials. J Orthop Trauma. 2012;26:386–94. doi: 10.1097/BOT.0b013e318225f63c. [DOI] [PubMed] [Google Scholar]
- 38.Clancey GJ. Percutaneous kirschner-wire fixation of colles fractures. A prospective study of thirty cases. J Bone Joint Surg Am. 1984;66:1008–14. doi: 10.2106/00004623-198466070-00006. [DOI] [PubMed] [Google Scholar]
- 39.Arora R, Lutz M, Hennerbichler A, Krappinger D, Md DE, Gabl M. Complications following internal fixation of unstable distal radius fracture with a palmar locking-plate. J Orthop Trauma. 2007;21:316–22. doi: 10.1097/BOT.0b013e318059b993. [DOI] [PubMed] [Google Scholar]
- 40.Ho AW, Ho ST, Koo SC, Wong KH. Hand numbness and carpal tunnel syndrome after volar plating of distal radius fracture. Hand. 2011;6:34–8. doi: 10.1007/s11552-010-9283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hove LM, Nilsen P, Furnes O, Oulie HE, Solheim E, Mölster AO. Open reduction and internal fixation of displaced intraarticular fractures of the distal radius: 31 patients followed for 3–7 years. Acta Orthop Scand. 1997;68:59–63. doi: 10.3109/17453679709003977. [DOI] [PubMed] [Google Scholar]
- 42.Musgrave DS, Idler RS. Volar fixation of dorsally displaced distal radius fractures using the 2.4-mm locking compression plates. J. Hand. Surg. 2005;30:743–9. doi: 10.1016/j.jhsa.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 43.Rozental TD, Blazar PE. Functional outcome and complications after volar plating for dorsally displaced, unstable fractures of the distal radius. J. Hand. Surg. 2006;31:359–65. doi: 10.1016/j.jhsa.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Ward CM, Kuhl TL, Adams BD. Early complications of volar plating of distal radius fractures and their relationship to surgeon experience. Hand. 2010;6:185–9. doi: 10.1007/s11552-010-9313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitay A, Swanstrom M, Schreiber JJ, Carlson MG, Nguyen JT, Weiland AJ, et al. Volar plate position and flexor tendon rupture following distal radius fracture fixation. J. Hand. Surg. 2013;38:1091–6. doi: 10.1016/j.jhsa.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka Y, Aoki M, Izumi T, Fujimiya M, Yamashita T, Imai T. Effect of distal radius volar plate position on contact pressure between the flexor pollicis longus tendon and the distal plate edge. J Hand Surg [Am] 2011;36:1790–797. doi: 10.1016/j.jhsa.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Carlsen BT, Rizzo M, Moran SL. Soft-tissue injuries associated with distal radius fractures. Oper Tech Orthop. 2009;19:107–18. doi: 10.1053/j.oto.2009.05.003. [DOI] [Google Scholar]
- 48.Geissler WB, Freeland AE, Savoie FH, McIntyre LW, Whipple TL. Intracarpal soft-tissue lesions associated with an intra-articular fracture of the distal end of the radius. J Bone Joint Surg Am. 1996;78:357–65. doi: 10.2106/00004623-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Ogawa, T., Tanaka, T., Yanai, T., Kumagai, H., & Ochiai, N. Analysis of soft tissue injuries associated with distal radius fractures. BMC Sports Sci Med Rehabil Sports Medicine, Arthroscopy, Rehabilitation, Therapy & Technology. 2013:5:19. [DOI] [PMC free article] [PubMed]
- 50.Lozano-Calderón SA, Souer S, Mudgal C, Jupiter JB, Ring D. Wrist mobilization following volar plate fixation of fractures of the distal part of the radius. J Bone Joint Surg Am. 2008;90:1297–304. doi: 10.2106/JBJS.G.01368. [DOI] [PubMed] [Google Scholar]
- 51.Abramo A, Kopylov P, Tägil M. Evaluation of a treatment protocol in distal radius fractures. Acta Orthop. 2008;79:376–85. doi: 10.1080/17453670710015283. [DOI] [PubMed] [Google Scholar]
- 52.Dillingham C, Horodyski M, Struk AM, Wright T. Rate of improvement following volar plate open reduction and internal fixation of distal radius fractures. Advances in Orthopedics 2011:1–4 [DOI] [PMC free article] [PubMed]
- 53.Fok M, Klausmeyer M, Fernandez D, Orbay J, Bergada A. Volar plate fixation of intra-articular distal radius fractures: a retrospective study. J Wrist Surg. 2013;02:247–54. doi: 10.1055/s-0033-1350086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Macfarlane RJ, Miller D, Wilson L, Meyer C, Kerin C, Ford DJ, et al. Functional outcome and complications at 2.5 years following volar locking plate fixation of distal radius fractures. J. Hand Microsurg. 2015;7:18–24. doi: 10.1007/s12593-014-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catalano LW, III, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J., Jr Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg. 1997;79:1290–302. doi: 10.2106/00004623-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Roh YH, Lee BK, Noh JH, Oh JH, Gong HS, Baek GH. Factors delaying recovery after volar plate fixation of distal radius fractures. J. Hand. Surg. 2014;39:1465–70. doi: 10.1016/j.jhsa.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 57.Weil YA, Mosheiff R, Firman S, Liebergall M, Khoury A. Outcome of delayed primary internal fixation of distal radius fractures: a comparative study. Injury. 2014;45:960–4. doi: 10.1016/j.injury.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 58.Yamashita K, Zenke Y, Sakai A, Oshige T, Moritani S, Maehara T. Comparison of functional outcome between early and delayed internal fixation using volar locking plate for distal radius fractures. J UOEH. 2015;37:111–9. doi: 10.7888/juoeh.37.111. [DOI] [PubMed] [Google Scholar]
- 59.Goldfarb CA, Rudzki JR, Catalano LW, Hughes M, Borrelli J. Fifteen-year outcome of displaced intra-articular fractures of the distal radius. J. Hand. Surg. 2006;31:633–9. doi: 10.1016/j.jhsa.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 60.Knirk J, Jupiter J. Intra-articular fractures of the distal end of the radius in young adults. Orthop Trauma Dir. 2006;4:27–31. doi: 10.1055/s-2006-944282. [DOI] [PubMed] [Google Scholar]
- 61.Lutz M, Arora R, Krappinger D, Wambacher M, Rieger M, Pechlaner S. Arthritis predicting factors in distal intraarticular radius fractures. Arch Orthop Trauma Surg. 2010;131:1121–6. doi: 10.1007/s00402-010-1211-3. [DOI] [PubMed] [Google Scholar]
- 62.Henn CM, Wolfe SW. Distal radius fractures in athletes. Sports Med Arthrosc Rev. 2014;22:29–38. doi: 10.1097/JSA.0000000000000003. [DOI] [PubMed] [Google Scholar]
- 63.Grewal R, Macdermid JC. The risk of adverse outcomes in extra-articular distal radius fractures is increased with malalignment in patients of all ages but mitigated in older patients. J. Hand. Surg. 2007;32:962–70. doi: 10.1016/j.jhsa.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Nelson GN, Stepan JG, Osei DA, Calfee RP. The impact of patient activity level on wrist disability after distal radius malunion in older adults. J Orthop Trauma. 2015;29:195–200. doi: 10.1097/BOT.0000000000000235. [DOI] [PMC free article] [PubMed] [Google Scholar]


