Abstract
Purpose of review
Osteochondral lesions of the talus (OLT) are common injuries in athletes. The purpose of this study is to comprehensively review the clinical results and return to sport capacity in athletes following treatment for OLT.
Recent findings
Reparative procedures, such as bone marrow stimulation, and replacement procedures, such as autologous osteochondral transplantation, provide good clinical outcomes in short- and mid-term follow-up in the athlete. Recently, biological augmentation and scaffold-based therapies have been shown to improve clinical and radiological outcomes in OLT in both the general population and athletes.
Summary
Most studies are of a low level of evidence. Studies analyzing the return to sport capability in athletes are further lacking. High-level evidence and well-designed clinical trials are required to establish the most effective treatment protocol.
Keywords: Osteochondral lesions of talus, Bone marrow stimulations, Autologous osteochondral transplantation, Biologic
Introduction
Osteochondral lesions of the talus (OLT) are the most common ankle articular cartilage injury. OLT occur in up to 50% of acute ankle sprains and are frequently associated with sports-related injuries [1]. While ankle injuries are suffered by approximately 1 per 10,000 people in the general US population per day, the athletic injury rate is much higher at 5.2 injuries per 10,000 athlete exposures and as high as 9.4 injuries per 10,000 athlete exposures during competitive events [2, 3].
Recent clinical evidence has suggested that operative treatment of OLT provides good-to-excellent clinical outcomes in up to 85% of cases [4]. However, concerns regarding long-term outcomes and biologic deterioration of the repair still remain. In particular, despite the high frequency of OLT in the athletic population, little is reported regarding return to sport following surgical treatment of OLT in this population.
This comprehensive review provides an evidence-based overview of clinical results following the operative treatments for OLT in the athletic population.
Conservative treatment
Conservative treatment strategies are indicated as the primary treatment for symptomatic patients with OLT. The treatment traditionally consists of one or more regimens, which include rest, restriction of activities, immobilization, and non-steroidal anti-inflammatory drugs (NSAIDs). A systematic review by Zengerink et al. [4] reported that 45% of patients had successful outcomes when conservatively treated with a weightbearing as tolerated protocol. The authors also demonstrated that 53% of patients who underwent cast immobilization for a duration of 3 weeks to 4 months reported successful clinical outcomes. However, almost all studies are dated back more than 20 years and success was determined by symptomatic complaints, not on the physiological healing of the OLT nor on the ability of an athlete to return to sport. Recently, platelet-rich plasma (PRP) has been employed as a conservative treatment option for OLT, with reduced pain and improved function after intra-articular injection. In a prospective study, Mei-Dan et al. [5] compared the short-term clinical efficacy of hyaluronic acid injections with PRP injections for OLT in 32 patients and reported that the PRP group had significantly better clinical outcomes.
As most patients with a symptomatic OLT will have had some form of trauma, ligamentous stability should be addressed. Triple-phase rehabilitation is the most common and most effective physical therapy for most ankle sprains. In addition, closed-chain balance and proprioception activities, along with peroneal muscle strengthening, improve neuromuscular control of the ankle, preventing recurrence [6]. External ankle supports using tape or orthosis are also effective in the early stages of rehabilitation but have limited role in long-term treatment [7].
Although conservative treatment may relieve symptoms in the short term, the long-term outcome of these treatment strategies has not been established. In addition, little evidence is available in the conservative treatment for OLT in the athletic population. To draw definitive conclusions, further well-designed clinical trials of high methodological quality are required.
Operative treatment
A wide variety of procedures have been described for the operative treatment of OLT. Conventionally, operative treatment for OLT can be divided into two broad categories: reparative procedures, including bone marrow stimulation (BMS), and replacement procedures, including autologous osteochondral transplantation (AOT). Current indication to proceed with either BMS or AOT is primarily based on the size of the lesion. It is traditionally accepted that smaller lesions up to 150 mm2 in size or 15 mm in diameter are treated with BMS [8, 9] and larger lesions are treated with AOT [10]. In addition, there has been recent evidence recommending AOT for the treatment after failed BMS [11]. However, recently this traditional paradigm has been challenged. In a consensus paper from the society for cartilage repair of the ankle, the optimal indication sizes to treat with BMS were established as those lesions of 10 mm or less [12].
While BMS is a common technique, the process promotes a fibrous cartilage healing response that is biomechanically inferior to hyaline cartilage. This led to the establishment of autologous chondrocyte implantation (ACI) to regenerate damaged cartilage with hyaline-like tissue. More recently, various techniques relying on engineered scaffolds, such as matrix-induced autologous chondrocyte implantation (MACI), autologous matrix-induced chondrogenesis (AMIC), and bone marrow-derived cells transplantation (BMDCT), have been developed.
Reparative procedure
Bone marrow stimulation
BMS is a reparative technique that aims to promote the formation of regenerative fibrous cartilage repair tissue in the osteochondral defect. BMS is performed arthroscopically in the following steps: (1) debridement of unstable cartilage and necrotic bone, (2) debridement of the calcified cartilage layer, and (3) creating several holes in the subchondral bone plate (SBP) at 3- to 4-mm intervals using microfracture pick or small-diameter drill (Fig. 1). This procedure is low cost, technically undemanding, and minimally invasive.
Fig. 1.
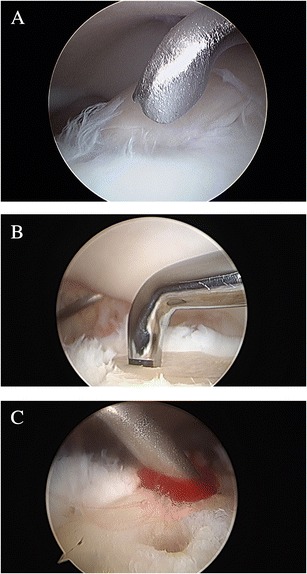
a Arthroscopic image showing unstable cartilage of an osteochondral lesion of the talus. b Bone marrow stimulation is performed using a microfracture pick. c Bleeding from the created holes is confirmed
Several clinical studies have shown that approximately 85% of patients that underwent BMS reported good-to-excellent clinical results at short- and mid-term outcomes [1, 4, 13]. Two studies have shown the long-term results of BMS. Van Bergen et al. [14] reported the long-term clinical outcomes of 50 patients with a mean follow-up of 141 months. The authors demonstrated that the median American Orthopaedic Foot & Ankle Society (AOFAS) ankle-hindfoot score was 88 out of 100 possible points. Polat et al. [15•] in a study of 82 patients at a mean 121-month follow-up reported that 42.6% of patients had no symptoms and 23.1% had pain after walking more than 2 h or after competitive sports activities.
In the athletic populations, Saxena et al. [16] demonstrated that out of 26 patients, the AOFAS score was 94 and return to high-demand sports activity occurred at a mean 15.1 weeks. The authors reported that anterolateral lesions had significantly faster return to sports activity and higher clinical outcomes compared with other lesion locations. Seijas et al. [17] reported excellent clinical outcomes of 16 soccer players with a mean follow-up of 3.6 years and resumption of sports activities at preinjury level in 93.8% of patients. Many investigators have supported good functional outcomes following BMS with short- and mid-term follow-up in athletes [14, 16, 17]. Nevertheless, there is a distinct lack of long-term data in athletic populations.
Despite successful outcomes following BMS for OLT, it is of increasing concern that deterioration of reparative fibrous cartilage tissue is inevitable over time [18–20]. Ferkel et al. [18] found deterioration of clinical scores in up to 35% of patients within 5 years following BMS. Lee et al. [19] reported that only 30% of patients had integration of the repair tissue with the surrounding native cartilage at second look arthroscopy 12 months after BMS. Van Bergen et al. [14] reported that 33% of patients showed progression of osteoarthritis by one grade on standard radiographs with a mean follow-up of 141 months.
Both mechanical and biologic factors are considered as causative for deterioration of reparative fibrous cartilage over time. Mechanically, regenerated fibrous cartilage is inferior to hyaline cartilage [21, 22]. Subchondral bone that has cross talk with articular cartilage [23, 24] may not restore after the subchondral bone plate is damaged by BMS in animal osteochondral models [25, 26]. In a human clinical study, Reilingh et al. [26] recently revealed that subchondral bone was not filled completely in 78.6% of OLT at 1 year after BMS. In both animal and clinical studies, the subchondral bone is not likely to adequately remodel, suggesting it may be important to reduce the damage to the subchondral bone in order to improve long-term outcomes. In accordance with this premise, recent studies have focused on the diameter and depth of microfracture and drilling to improve overall outcome with an emphasis on both the quality of the cartilage repair tissue and the restorative morphology of the subchondral bone [27–31].
Unfortunately, the methodological quality of clinical studies and reported outcomes may be insufficient to accurately assess the outcome of BMS. A systematic review by Hannon et al. found gross inconsistencies and an underreporting of data between these studies on BMS [32], making long-term meaningful analysis challenging.
Autologous chondrocyte implantation
ACI is categorized as a reparative procedure. Historically, ACI was first developed for knee cartilage injury and subsequently has been indicated for OLT patients who had larger lesions or failed prior BMS surgery. The aim of this operative technique is to regenerate damaged cartilage with hyaline-like tissue [33]. ACI is a cell-based, two-stage procedure. In the first procedure, healthy articular cartilage is harvested and the chondrocytes isolated by filtration are cultured for approximately 2 to 3 weeks [34]. In the second step, viable cultured chondrocytes are implanted into the defect site and covered by a periosteal flap harvested from the distal end of the tibia, facilitating a hyaline-like repair tissue. The disadvantages of ACI include the need for two surgical procedures, which increases the cost and the potential for morbidity while it decreases durability of the graft [2]. Overgrowth of repair tissue which most likely represented periosteal hypertrophy is also an inherent disadvantage [35].
In a recent systematic review, Niemeyer et al. [36] evaluated the effectiveness of ACI for OLT treatment and reported a clinical success rate of 89.9% in 213 patients included in 16 level IV studies. Battaglia et al. [37] evaluated 20 patients following ACI at a mean follow-up of 5 years and found that the mean AOFAS score improved from 59 preoperatively to 84 postoperatively. The authors found that, on MRI evaluation, all patients showed a T2 mapping value consistent with normal hyaline cartilage. Kwak et al. [38] reported that 21 of 29 patients who underwent ACI with a mean lesion size of 198 mm2 had good-to-excellent clinical outcomes at a mean follow-up of 70 months. Additionally, it was found that the mean Tegner activity score improved from 1.6 to 4.3. Giannini et al. [34] reported clinical and MRI outcomes at a 10-year follow-up following ACI for OLT. The authors evaluated 10 OLT patients with a mean lesion size of 3.1 cm2 treated with ACI at a mean follow-up of 119 months. It was reported that the AOFAS score improved from 37.9 preoperatively to 92.7 postoperatively with well-modeled restoration of the articular surface on MRI. In addition, among the eight patients who played sports, five resumed sports at preinjury level, two resumed sports at a lower level, and one patient gave up sports.
Matrix-induced autologous chondrocyte implantation
MACI is a second generation of ACI. Similar to ACI, MACI is a two-stage procedure, but the periosteal flap is replaced by biodegradable polymer scaffolds. The chondrocytes are embedded in a scaffold, which contains type I/III collagen, hyaluronan, and polyglycolic/polylactic acid [39, 40] and is placed over the cartilage defect, not requiring fixation with sutures. Traditional ACI procedure requires a tibial or fibular osteotomy and is associated with intrinsic limitations, increasing harvesting and suturing of the periosteum, delamination of the graft, and periosteal hypertrophy [41]. The advantage of MACI, however, is that the periosteal graft harvest is avoided, and the procedure can be performed arthroscopically allowing for a shorter surgery and less morbidity.
Evidence in the literature has demonstrated arthroscopic MACI as a safe alternative for the treatment of OLT with good overall clinical and radiologic results. Giza et al. [39] assessed the clinical outcome following MACI in 10 patients over 2 years. The mean AOFAS score improved by 13 points after 1 year and was maintained after 2 years. Aurich et al. [42] reported, in a case series of 19 ankles, significant improvement of the AOFAS score from 58.6 to 80.4 at a mean follow-up of 24 months. In the athletic population, 81% patients returned to sports after MACI, of which 56% returned to preinjury level. In an additional study, Magnan et al. [43] evaluated 30 patients with a mean lesion size of 2.36 cm2. The mean AOFAS score improved from 36.9 preoperatively to 83.9 postoperatively, and 50% patients returned to previous sports. Giannini et al. [44] reported, in what we believe to be the largest case series with the longest follow-up, a study involving 46 ankles with a mean follow-up of 87.2 months. The authors reported that the mean AOFAS score was 92 at final follow-up. Among 25 patients who practiced sports, 20 returned to preinjury sports level, 3 resumed the same sport at a lower level, 2 shifted to a non-contact sport, and 4 patients gave up sports. Four professional soccer players included in the study were all able to resume their previous activity. Currently, this procedure is not approved in the USA for generalized use.
Autologous matrix-induced chondrogenesis
AMIC is a one-step scaffold-based therapy that combines BMS with the use of a porcine collagen I/III matrix. Despite AMIC showing good functional outcomes in the literature, most studies are small case series and, to our knowledge, no study has described the long-term outcomes. Kubosch et al. [45] reported 17 patients following AMIC for medial OLT with a mean 39.5 months of follow-up and found that the mean postoperative AOFAS score was 82.6 and the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score was 52.7. MRI assessment demonstrated a good quality of regenerative tissue similar to the MRI ultrastructure of the surrounding cartilage. Wiewiorski et al. [46] investigated the outcomes of 23 patients at a mean of 23 months postoperatively and reported the improvement of mean AOFAS scores from 60.3 to 90.9. The mean MOCART score at follow-up was 62.6. In a recent case series by Valderrabano et al. [47], the mean AOFAS score improved from 60 to 89 in 26 patients who underwent AMIC. The authors reported that 35% of patients had complete filling of the defect and 84% of patients had normal or nearly normal signal intensity of the repair tissue compared with the adjacent native cartilage on MRI. The authors also assessed the athletic population and reported that 45% of patients participating in sports activity before surgery returned to previous activity level at follow-up.
Bone marrow-derived cell transplantation
Bone marrow-derived cell transplantation (BMDCT), a combination of concentrated bone marrow aspirate (CBMA) and scaffold material, is also a one-step procedure that has been proposed as an alternative procedure capable of repairing a defect with hyaline cartilage [48]. Several clinical studies have shown that clinical outcomes improved using this procedure [49–53, 54•]. Giannini et al. [53] evaluated patients who underwent BMDCT and demonstrated improved AOFAS scores from 63.7 to 82.2 in 49 patients at a mean follow-up of 48 months.
More recently, Vannini et al. [54•] reported a case series completely focused on the return to sports of athletes with OLT treated with a one-step BMDCT procedure. They demonstrated that in 140 athletes, the mean AOFAS score improved from 58.7 preoperatively to 90.9 at 48 months after surgery. At the final follow-up, 72.8% were able to resume sports at preinjury level. Buda et al. [52] compared clinical outcomes of two clusters of patients who underwent ACI or BMDCT for OLT and demonstrated that clinical results were similar in both groups at 48 months of follow-up. The rate of return to sports activity showed slightly better results for BMDCT compared to ACI; however, the difference was not statistically significant.
The timing of return to sports activity following BMDCT in the athletes may appear shorter than in ACI because of the advantage of a single surgical stage. Similarly, the period is longer when compared with BMS as would be expected [16, 54•, 55–56].
Replacement procedure
Autologous osteochondral transplantation
AOT is a cartilage replacement treatment performed by inserting a cylindrical osteochondral graft typically harvested from a non-weightbearing portion of the ipsilateral knee into the prepared site of the defect on the talus. The goal of this procedure is to reproduce the mechanical, structural, and biochemical properties of the native hyaline articular cartilage. This technique is typically carried out in the following steps: (1) tibial osteotomy to provide a visualization of lesion, (2) removal of the talar lesion trephine with controlled taps of the mallet, (3) BMS to the surrounding healthy bone followed by overdrill to make the recipient site slightly longer than the harvested graft, (4) harvesting of the osteochondral graft from a non-weightbearing portion of the ipsilateral femoral condyle, and (5) insertion of the graft into the created recipient site (Figs. 2 and 3).
Fig. 2.
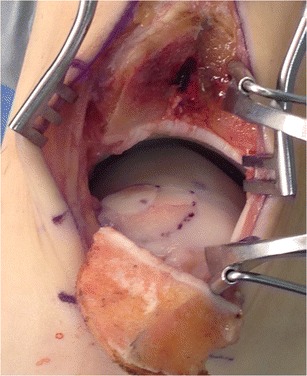
Intraoperative photograph of exposure of the medial talus by Chevron-type medial malleolar osteotomy. Double osteochondral autograft transplantation has been performed
Fig. 3.
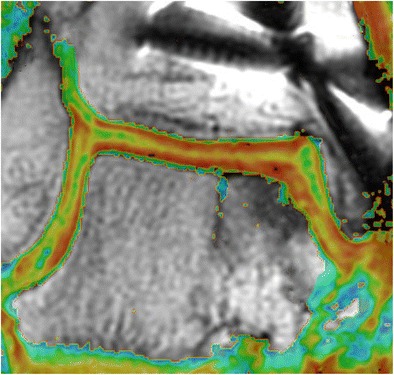
Coronal quantitative T2 mapping image showing normal stratification of the graft and adjacent native articular cartilage
Several studies have reported good clinical outcomes following AOT at both short- and mid-term follow-up [10, 57, 58]. A recent case series of 85 patients who underwent AOT found improved Foot and Ankle Outcome Score (FAOS) at mean 47.2 months of follow-up and MOCART scores postoperatively at mean 24.8 months of follow-up [59].
In the athletic population study, Fraser et al. [60•] reported improved AOFAS scores to 89.4 and found that 90% of professional athletes and 87% of recreational athletes had full return to preinjury activity levels at final follow-up of 24 months. Other studies showed that 63–85% of athletes who underwent AOT were able to return to preinjury levels of sports activity [59, 61]. However, in a study by Paul et al. [61], patients engaged in high-impact and contact sports required a partial modification of the sporting activities and a reduction of participation. With regards to long-term follow-up, to our knowledge, no study has been established.
Similar to BMS, there are some mechanical and biologic concerns following AOT. A biomechanical study by Fansa et al. [62] demonstrated increased contact pressure on the graft surface by almost sevenfold with a mere 1.0 mm of graft protrusion above the level of native cartilage. This study highlighted the need for accurate placement of osteochondral grafts. Additionally, the use of a tibial osteotomy carries the potential risk of mal/non-union at the site of osteotomy. Recently, Lamb et al. [63] addressed this concern and found that 94% patients were asymptomatic at the osteotomy site at a mean 34.5 months of follow-up and T2 mapping relaxation values were similar to that of the native cartilage at the osteotomy site.
With regards to biological concern, it is known that ankle fractures may cause activation of intra-articular inflammatory cytokines, which leads to progressive deterioration of OLT over time. Theoretically, this same principle may occur with malleolar osteotomy resulting in posttraumatic osteoarthritis [64–66]. Furthermore, poor integration of the graft surface with native tissue, cyst formation around the graft, and deterioration of graft cartilage over time have been reported as potential consequences following AOT procedure [63, 67, 68]. However, Savage-Elliott et al. [69] reported that that, although increasing age was related to increased cyst prevalence, the clinical impact of cyst formation was not found to be significant at a mean short-term follow-up of 15 months after AOT surgery. Gül et al. [70] also reported that the clinical outcomes following AOT with subchondral cyst formation were as successful as those without a subchondral cyst. Donor site morbidity is also a concern in some series [71]. More recent studies, however, demonstrated that OLT treated with AOT have a low incidence of donor site morbidity with good functional outcomes. Yoon et al. [11] found that 9% patients had early donor site morbidity with 100% resolution at 48 months of follow-up. Fraser et al. [72] reported a retrospective case series of 39 patients who underwent AOT and demonstrated that at mean 41.8 months of follow-up, donor site morbidity was present in only 5% of patients and Lysholm scores for the entire cohort were at 99.4 at final follow-up.
Osteochondral allograft transplantation
Osteochondral allograft transplantation is a similar replacement procedure in which a graft of articular cartilage and bone is taken from a cadaveric donor. Generally, this procedure is considered as a rescue procedure if previous surgeries fail, but can be performed as a first treatment in larger lesions that cannot be successfully treated with other procedures [73]. Some surgeons prefer this procedure over AOT because it avoids donor site morbidity. Although both fresh and frozen grafts can be used, the decline in the viability of chondrocytes within frozen graft tissue has led to an increase in the use of fresh allografts.
There are several papers about osteochondral allograft transplantation for the treatment of OLT, and most are small prospective and retrospective case series [74–80]. El-Rashidy et al. [79] performed one of the largest studies in patients who received small cylindrical allografts and reported good or excellent outcomes in 28 of 38 patients at mean 37.7 months of follow-up. However, graft failure defined as the need for arthrodesis or arthroplasty occurred in four patients (11%). Using large allograft segments, Raikin et al. [75] prospectively evaluated 15 patients with a mean follow-up of 44 months and demonstrated that the mean AOFAS score had improved from 38 preoperatively to 83 postoperatively. Radiographic findings revealed evidence of collapse or resorption of the graft in 10 ankles (67%) and narrowing of the joint space overlying the graft area in 9 ankles (60%). Haene et al. [80] reported that only 10 of 17 cases had good or excellent results at a mean follow-up of 4.1 years. The results of this procedure in the athletic population have not been reported.
Cartilage allograft
DeNovo NT (Zimmer Biomet, Inc., Warsaw, IN) is a prepacked, particulate juvenile cartilage allograft from donors aged younger than 13 years, more often below 2 years of age. The theory behind DeNovo NT is that juvenile chondrocytes have the potential with higher metabolic activity level to reproduce more hyaline cartilage than adult chondrocyte [81]. Several in vitro studies demonstrated that juvenile cartilage was superior to adult cartilage in terms of chondrogenesis [82–84]. However, no published animal studies have investigated the histological and structural behavior of particulate juvenile cartilage allograft implantation in osteochondral defects. DeNovo NT is indicated for large lesions or patients who have failed previous BMS procedure. Coetzee et al. [85] reported clinical outcomes of 24 OCLs with an average lesion size of 125 mm2 at an average of 16.2 months of follow-up. The average AOFAS score at final follow-up was 85 with 78% ankles demonstrating good or excellent scores. One partial graft delamination was reported at 16 months. The largest case series to date by Drakos et al. [86] evaluated 50 patients at a 2-year follow-up and found that clinical outcomes were similar to microfracture alone at the 2-year follow-up. The role of particulate juvenile cartilage allograft remains therefore to be determined. Longer-term outcomes may show prolongation of the cartilage phenotype when compared to BMS alone, but level I and level II studies need to be done before meaningful conclusions can be drawn.
BioCartilage (Arthrex, Inc. Nales, FL) is an extracellular matrix cartilage allograft. It contains an allogeneic extracellular matrix and includes type II collagen, proteoglycans, and cartilaginous growth factors. Fortier et al. [87] reported that BioCartilage with PRP safely improved cartilage repair compared with microfracture alone in an equine model with up to 13 months of follow-up. In clinical studies, Desai et al. [88] reported a case series of nine patients with an average lesion size of 132 mm2. At an average of 12 months of follow-up, seven of nine patients reported excellent outcomes with no functional limitations. The long-term data on this procedure is also lacking. Further well-designed comparative studies with sufficient quality and level of evidence will be required to substantiate its widespread use in the treatment of OLT.
Biologic augmentation
Platelet-rich plasma
PRP is an autologous blood product that contains at least twice the concentration of platelets above the baseline value, or >1.1 × 106 platelets per microliter. Platelets contain numerous growth factors and cytokines that play an essential role in tissue healing, such as transforming growth factor, vascular endothelial growth factor, fibroblast growth factor, and platelet-derived growth factor [89].
The current basic science evidence with in vitro and in vivo studies suggests that PRP has positive effects on cartilage repair. A systematic review by Smyth et al. [90] examined the evidence for the use of PRP for cartilage repair. The authors concluded that 18 of 21 (85.7%) basic science papers reported positive effects of PRP on cartilage repair and a proof of concept was established. Additionally, Smyth et al. [91] found in a rabbit model that application of PRP at the time of AOT improved the integration of the osteochondral graft at the cartilage interface and decreased graft degeneration.
In clinical investigations, several comparative studies have examined the use of PRP for OLT. Guney et al. [92] performed a randomized prospective controlled trial comparing the clinical and functional outcomes of 16 patients treated with BMS alone and 19 patients treated with BMS using PRP. They reported that BMS with the PRP group had better functional outcomes than the BMS-alone group. In addition, Görmeli et al. [93] compared, in a prospective randomized clinical trial, the effects of hyaluronic acid (HA) and PRP injections after BMS for OLT. It was found that PRP injection provided significantly better clinical outcomes than HA or saline injection at a mean 15.3 months of follow-up. Although few comparative studies are available in the published literature, these results suggest that the use of PRP combined with the operative treatment for OLT may improve clinical and functional outcomes.
Concentrated bone marrow aspirate
CBMA has been used to deliver mesenchymal stem cells (MSCs) to damaged cartilage to augment cartilage repair. CBMA is produced by centrifugation of the bone marrow aspirate, typically harvested from the iliac crest, at the same time of the surgery. In addition to MSCs, the bone marrow aspirate contains growth factors and cytokines that have the ability to induce chondrocyte differentiation.
The use of CBMA as an adjunct to the treatment for OLT has been investigated in both in vivo models and a clinical study. In the equine model, improved cartilage healing has been demonstrated both histologically and radiologically in groups receiving CBMA at the time of BMS, compared with groups receiving BMS alone [94]. In addition, similar results were reported in a goat model when using BMS combination with CBMA and HA [95]. Clinically, Hannon et al. [96] compared mid-term functional outcomes of patients who underwent BMS for OLT with and without CBMA. The authors reported that BMS with CBMA resulted in comparably good mid-term functional outcomes, but improved border repair tissue integration with less evidence of fissuring and fibrillation on MRI, compared to BMS alone. Kennedy et al. [10] reported significant improvement of clinical outcomes in 72 patients who underwent AOT with CBMA at a mean 28 months of follow-up. They also demonstrated that restoration of the radius of curvature and color stratification similar to that of native cartilage were found on MRI using T2 mapping. Overall, current evidence suggests that CBMA as an adjunct to the treatment for OLT can improve cartilage repair. In a recent study by Cassano et al. [97], the authors demonstrated high levels of interleukin 1 receptor antagonist protein in CBMA. This suggests that CBMA is a very potent anti-inflammatory as well as possessing chondrogenic capability. This anti-inflammatory component may have a joint trophic effect that reduces the catabolic effect on the joint as a whole.
Early return to sports is a crucial outcome measure for athletes, but the authors believe that the quality of repaired cartilage is essential in both professional and recreational athletes alike. Consequently, return to sports following the operative treatment for OLT is based on clinical symptoms, MRI evaluation, and the type of sports played. Most patients treated with either reparative procedures or replacement procedures will start training 3 months post surgery with sports-specific training at 6 months. In certain cases, patients will be back at sports at 6 months but many may require a full 12 months of rehabilitation. The prognostic factors for early return to sports have been established as younger age, lower body mass index, smaller OLT size, and coordinated physical therapy [16, 98].
Summary
The surgical treatment of OLT still remains controversial. Although reparative and replacement procedures provide good outcomes in the treatment for OLT in short- and mid-term follow-up in the athlete, outcomes in the long term lack evidence. Biologic augmentation appears to be promising and has shown to improve outcomes in OLT in the general population, which could potentially extend to athletes. However, there is a risk of bias when assessing clinical outcomes following the treatment of OLT because most studies are of a low level of evidence. The current evidence-based review of the literature demonstrated both reparative and replacement procedures for the treatment of OLT in athletes with satisfactory clinical outcomes; however, high-level evidence and well-designed clinical trials are required to establish the most effective treatment.
Compliance with ethics standards
Conflict of interest
Yoshiharu Shimozono, Youichi Yasui, and Andrew W. Ross declare that they have no conflict of interest. John G. Kennedy reports grants from Arteriocyte, Inc., The Ohnell Family Foundation, and Mr. and Mrs. Michael J. Levitt outside of the submitted work. He is a board member for the European Society of Sports Traumatology, Knee Surgery, and Arthroscopy (ESSKA) and a finance board member of the International Cartilage Repair Society (ICRS), and he is on the committee for the American Orthopaedic Foot & Ankle Society (AOFAS) Awards and Scholarships.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Foot and Ankle Sports Medicine
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot ankle Int. 2000;21(2):119–126. doi: 10.1177/107110070002100205. [DOI] [PubMed] [Google Scholar]
- 2.O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38(2):392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 3.Nelson AJ, Collins CL, Yard EE, Fields SK, Comstock RD. Ankle injuries among United States high school sports athletes, 2005-2006. J Athl Train. 2007;42(3):381–387. [PMC free article] [PubMed] [Google Scholar]
- 4.Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18(2):238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534–541. doi: 10.1177/0363546511431238. [DOI] [PubMed] [Google Scholar]
- 6.O'Loughlin PF, Hodgkins CW, Kennedy JG. Ankle sprains and instability in dancers. Clin Sports Med. 2008;27(2):247–262. doi: 10.1016/j.csm.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Handoll HH, Rowe BH, Quinn KM, de Bie R. Interventions for preventing ankle ligament injuries. Cochrane Database Syst Rev. 2001;3:CD000018. doi: 10.1002/14651858.CD000018. [DOI] [PubMed] [Google Scholar]
- 8.Chuckpaiwong B, Berkson EM, Theodore GH. Microfracture for osteochondral lesions of the ankle: outcome analysis and outcome predictors of 105 cases. Arthroscopy. 2008;24:106–112. doi: 10.1016/j.arthro.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. doi: 10.1177/0363546509335765. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy JG, Murawski CD. The treatment of osteochondral lesions of the talus with autologous osteochondral transplantation and bone marrow aspirate concentrate: surgical technique. Cartilage. 2011;2:327–336. doi: 10.1177/1947603511400726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014;42:1896–1903. doi: 10.1177/0363546514535186. [DOI] [PubMed] [Google Scholar]
- 12.Yasui Y, Ross AW, Murawski CD, Kennedy JG. Authors’ reply. Arthroscopy. 2016;32(8):1491–1493. doi: 10.1016/j.arthro.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Verhagen RA, Struijs PA, Bossuyt PM, van Dijk CN. Systematic review of treatment strategies for osteochondral defects of the talar dome. Foot Ankle Clin. 2003;8(2):233–242. doi: 10.1016/S1083-7515(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 14.van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95-A:519–525. doi: 10.2106/JBJS.L.00675. [DOI] [PubMed] [Google Scholar]
- 15.Polat G, Erşen A, Erdil ME, Kızılkurt T, Kılıçoğlu Ö, Aşık M. Long-term results of microfracture in the treatment of talus osteochondral lesions. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1299–1303. doi: 10.1007/s00167-016-3990-8. [DOI] [PubMed] [Google Scholar]
- 16.Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680–1687. doi: 10.1177/0363546507303561. [DOI] [PubMed] [Google Scholar]
- 17.Seijas R, Alvarez P, Ares O, Steinbacher G, Cuscó X, Cugat R. Osteocartilaginous lesions of the talus in soccer players. Arch Orthop Trauma Surg. 2010;130(3):329–333. doi: 10.1007/s00402-008-0783-7. [DOI] [PubMed] [Google Scholar]
- 18.Ferkel RD, Zanotti RM, Komenda GA, Sgaglione NA, Cheng MS, Applegate GR, Dopirak RM. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. doi: 10.1177/0363546508316773. [DOI] [PubMed] [Google Scholar]
- 19.Lee KB, Bai LB, Yoon TR, Jung ST, Seon JK. Second-look arthroscopic findings and clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2009;37(Suppl 1):63S–70S. doi: 10.1177/0363546509348471. [DOI] [PubMed] [Google Scholar]
- 20.Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18:656–663. doi: 10.1007/s00167-009-1036-1. [DOI] [PubMed] [Google Scholar]
- 21.Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2(4):192–201. doi: 10.5435/00124635-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–162. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Duncan H, Jundt J, Riddle JM, Pitchford W, Christopherson T. The tibial subchondral plate. A scanning electron microscopic study. J Bone Joint Surg Am. 1987;69(8):1212–1220. doi: 10.2106/00004623-198769080-00015. [DOI] [PubMed] [Google Scholar]
- 24.Pugh JW, Radin EL, Rose RM. Quantitative studies of human subchondral cancellous bone. Its relationship to the state of its overlying cartilage. J Bone Joint Surg Am. 1974;56(2):313–321. doi: 10.2106/00004623-197456020-00010. [DOI] [PubMed] [Google Scholar]
- 25.Orth P, Meyer HL, Goebel L, Eldracher M, Ong MF, Cucchiarini M, Madry H. Improved repair of chondral and osteochondral defects in the ovine trochlea compared with the medial condyle. J Orthop Res. 2013;31(11):1772–1779. doi: 10.1002/jor.22418. [DOI] [PubMed] [Google Scholar]
- 26.Reilingh ML, van Bergen CJ, Blankevoort L, Gerards RM, van Eekeren IC, Kerkhoffs GM, van Dijk CN. Computed tomography analysis of osteochondral defects of the talus after arthroscopic debridement and microfracture. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1286–1292. doi: 10.1007/s00167-015-3928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Hoemann CD, Sun J, Chevrier A, McKee MD, Shive MS, Hurtig M, Buschmann MD. Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair. J Orthop Res. 2011;29(8):1178–1184. doi: 10.1002/jor.21386. [DOI] [PubMed] [Google Scholar]
- 28.Marchand C, Chen G, Tran-Khanh N, et al. Microdrilled cartilage defects treated with thrombin solidified chitosan/blood implant regenerate a more hyaline, stable, and structurally integrated osteochondral unit compared to drilled controls. Tissue Eng Part A. 2012;18(5–6):508–519. doi: 10.1089/ten.tea.2011.0178. [DOI] [PubMed] [Google Scholar]
- 29.Eldracher M, Orth P, Cucchiarini M, Pape D, Madry H. Small subchondral drill holes improve marrow stimulation of articular cartilage defects. Am J Sports Med. 2014;42(11):2741–2750. doi: 10.1177/0363546514547029. [DOI] [PubMed] [Google Scholar]
- 30.Orth P, Duffner J, Zurakowski D, Cucchiarini M, Madry H. Small-diameter awls improve articular cartilage repair after microfracture treatment in a translational animal model. Am J Sports Med. 2016;44(1):209–219. doi: 10.1177/0363546515610507. [DOI] [PubMed] [Google Scholar]
- 31.Kok AC, Tuijthof GJ, den Dunnen S, et al. No effect of hole geometry in microfracture for talar osteochondral defects. Clin Orthop Relat Res. 2013;471(11):3653–3662. doi: 10.1007/s11999-013-3189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG. Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med. 2013;41(3):689–695. doi: 10.1177/0363546512458218. [DOI] [PubMed] [Google Scholar]
- 33.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 34.Giannini S, Battaglia M, Buda R, Cavallo M, Ruffilli A, Vannini F. Surgical treatment of osteochondral lesions of the talus by open-field autologous chondrocyte implantation: a 10-year follow-up clinical and magnetic resonance imaging T2-mapping evaluation. Am J Sports Med. 2009;37(Suppl 1):112S–118S. doi: 10.1177/0363546509349928. [DOI] [PubMed] [Google Scholar]
- 35.Nam EK, Ferkel RD, Applegate GR. Autologous chondrocyte implantation of the ankle: a 2- to 5-year follow-up. Am J Sports Med. 2009;37(2):274–284. doi: 10.1177/0363546508325670. [DOI] [PubMed] [Google Scholar]
- 36.Niemeyer P, Salzmann G, Schmal H, Mayr H, Südkamp NP. Autologous chondrocyte implantation for the treatment of chondral and osteochondral defects of the talus: a meta-analysis of available evidence. Knee Surg Sports Traumatol Arthrosc. 2012;20:1696–1703. doi: 10.1007/s00167-011-1729-0. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia M, Vannini F, Buda R, Cavallo M, Ruffilli A, Monti C, Galletti S, Giannini S. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1376–1384. doi: 10.1007/s00167-011-1509-x. [DOI] [PubMed] [Google Scholar]
- 38.Kwak SK, Kern BS, Ferkel RD, Chan KW, Kasraeian S, Applegate GR. Autologous chondrocyte implantation of the ankle: 2- to 10-year results. Am J Sports Med. 2014;42(9):2156–2164. doi: 10.1177/0363546514540587. [DOI] [PubMed] [Google Scholar]
- 39.Giza E, Sullivan M, Ocel D, Lundeen G, Mitchell ME, Veris L, et al. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 2010;31:747–753. doi: 10.3113/FAI.2010.0747. [DOI] [PubMed] [Google Scholar]
- 40.Schneider TE, Karaikudi S. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810–814. doi: 10.3113/FAI.2009.0810. [DOI] [PubMed] [Google Scholar]
- 41.Nehrer S, Domayer SE, Hirschfeld C, Stelzeneder D, Trattnig S, Dorotka R. Matrix-associated and autologous chondrocyte transplantation in the ankle: clinical and MRI follow-up after 2 to 11 years. Cartilage. 2011;2(1):81–91. doi: 10.1177/1947603510381095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, Blackney M. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 43.Magnan B, Samaila E, Bondi M, Vecchini E, Micheloni GM, Bartolozzi P. Three-dimensional matrix-induced autologous chondrocytes implantation for osteochondral lesions of the talus: midterm results. Adv Orthop. 2012;2012:942174. doi: 10.1155/2012/942174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giannini S, Buda R, Ruffilli A, Cavallo M, Pagliazzi G, Bulzamini MC, Desando G, Luciani D, Vannini F. Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1311–1319. doi: 10.1007/s00167-013-2640-7. [DOI] [PubMed] [Google Scholar]
- 45.Kubosch EJ, Erdle B, Izadpanah K, Kubosch D, Uhl M, Südkamp NP, Niemeyer P. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40(1):65–71. doi: 10.1007/s00264-015-2988-z. [DOI] [PubMed] [Google Scholar]
- 46.Wiewiorski M, Miska M, Kretzschmar M, Studler U, Bieri O, Valderrabano V. Delayed gadolinium-enhanced MRI of cartilage of the ankle joint: results after autologous matrix-induced chondrogenesis (AMIC)-aided reconstruction of osteochondral lesions of the talus. Clin Radiol. 2013;68(10):1031–1038. doi: 10.1016/j.crad.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med. 2013;41(3):519–527. doi: 10.1177/0363546513476671. [DOI] [PubMed] [Google Scholar]
- 48.Giannini S, Buda R, Vannini F, Di Caprio F, Grigolo B. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36(5):873–880. doi: 10.1177/0363546507312644. [DOI] [PubMed] [Google Scholar]
- 49.Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–3320. doi: 10.1007/s11999-009-0885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giannini S, Buda R, Cavallo M, Ruffilli A, Cenacchi A, Cavallo C, Vannini F. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41(11):1196–1203. doi: 10.1016/j.injury.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 51.Buda R, Vannini F, Cavallo M, Baldassarri M, Natali S, Castagnini F, Giannini S. One-step bone marrow-derived cell transplantation in talar osteochondral lesions: mid-term results. Joints. 2014;1(3):102–107. [PMC free article] [PubMed] [Google Scholar]
- 52.Buda R, Vannini F, Castagnini F, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Giannini S. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39(5):893–900. doi: 10.1007/s00264-015-2685-y. [DOI] [PubMed] [Google Scholar]
- 53.Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Vannini F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2013;41(3):511–518. doi: 10.1177/0363546512467622. [DOI] [PubMed] [Google Scholar]
- 54.Vannini F, Cavallo M, Ramponi L, Castagnini F, Massimi S, Giannini S, Buda R. Return to sports after bone marrow-derived cell transplantation for osteochondral lesions of the talus. Cartilage. 2016 doi: 10.1177/1947603516642574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. doi: 10.1177/0363546508328414. [DOI] [PubMed] [Google Scholar]
- 56.Mithoefer K, Hambly K, Della Villa S, Silvers H, Mandelbaum BR. Return to sports participation after articular cartilage repair in the knee: scientific evidence. Am J Sports Med. 2009;37(Suppl 1):167S–176S. doi: 10.1177/0363546509351650. [DOI] [PubMed] [Google Scholar]
- 57.Hangody L, Dobos J, Baló E, Pánics G, Hangody LR, Berkes I. Clinical experiences with autologous osteochondral mosaicplasty in an athleticpopulation: a 17-year prospective multicenter study. Am J Sports Med. 2010;38(6):1125–1133. doi: 10.1177/0363546509360405. [DOI] [PubMed] [Google Scholar]
- 58.Scranton PE, Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88(5):614–619. doi: 10.1302/0301-620X.88B5.17306. [DOI] [PubMed] [Google Scholar]
- 59.Flynn S, Ross KA, Hannon CP, Yasui Y, Newman H, Murawski CD, Deyer TW, Do HT, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus. Foot Ankle Int. 2016;37(4):363–372. doi: 10.1177/1071100715620423. [DOI] [PubMed] [Google Scholar]
- 60.Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24:1272–1279. doi: 10.1007/s00167-015-3606-8. [DOI] [PubMed] [Google Scholar]
- 61.Paul J, Sagstetter M, Lämmle L, Spang J, El-Azab H, Imhoff AB, Hinterwimmer S. Sports activity after osteochondral transplantation of the talus. Am J Sports Med. 2012;40(4):870–874. doi: 10.1177/0363546511435084. [DOI] [PubMed] [Google Scholar]
- 62.Fansa AM, Murawski CD, Imhauser CW, Nguyen JT, Kennedy JG. Autologous osteochondral transplantation of the talus partially restores contact mechanics of the ankle joint. Am J Sports Med. 2011;39(11):2457–2465. doi: 10.1177/0363546511419811. [DOI] [PubMed] [Google Scholar]
- 63.Lamb J, Murawski CD, Deyer TW, Kennedy JG. Chevron-type medial malleolar osteotomy: a functional, radiographic and quantitative T2-mapping MRI analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21:1283–1288. doi: 10.1007/s00167-012-2050-2. [DOI] [PubMed] [Google Scholar]
- 64.Henkelmann R, Schmal H, Pilz IH, Salzmann GM, Dovi-Akue D, Südkamp NP. Prospective clinical trial of patients who underwent ankle arthroscopy with articular diseases to match clinical and radiological scores with intra-articular cytokines. Int Orthop. 2015;39(8):1631–1637. doi: 10.1007/s00264-015-2797-4. [DOI] [PubMed] [Google Scholar]
- 65.Adams SB, Jr, Nettles DL, Jones LC, Miller SD, Guyton GP, Schon LC. Inflammatory cytokines and cellular metabolites as synovial fluid biomarkers of posttraumatic ankle arthritis. Foot Ankle Int. 2014;35(12):1241–1249. doi: 10.1177/1071100714550652. [DOI] [PubMed] [Google Scholar]
- 66.Adams SB, Setton LA, Bell RD, Easley ME, Huebner JL, Stabler T, Kraus VB, Leimer EM, Olson SA, Nettles DL. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot Ankle Int. 2015;36(11):1264–1271. doi: 10.1177/1071100715611176. [DOI] [PubMed] [Google Scholar]
- 67.Tibesku CO, Daniilidis K, Szuwart T, Jahn UR, Schlegel PM, Fuchs-Winkelmann S. Influence of hepatocyte growth factor on autologous osteochondral transplants in an animal model. Arch Orthop Trauma Surg. 2011;131(8):1145–1151. doi: 10.1007/s00402-011-1281-x. [DOI] [PubMed] [Google Scholar]
- 68.Woelfle JV, Reichel H, Javaheripour-Otto K, Nelitz M. Clinical outcome and magnetic resonance imaging after osteochondral autologous transplantation in osteochondritis dissecans of the talus. Foot Ankle Int. 2013;34(2):173–179. doi: 10.1177/1071100712467433. [DOI] [PubMed] [Google Scholar]
- 69.Savage-Elliott I, Smyth NA, Deyer TW, Murawski CD, Ross KA, Hannon CP, Do HT, Kennedy JG. Magnetic resonance imaging evidence of postoperative cyst formation does not appear to affect clinical outcomes after autologous osteochondral transplantation of the talus. Arthroscopy. 2016 [in press] [DOI] [PubMed]
- 70.Gül M, Çetinkaya E, Aykut ÜS, Özkul B, Saygılı MS, Akman YE, Kabukcuoglu YS. Effect of the presence of subchondral cysts on treatment results of autologous osteochondral graft transfer in osteochondral lesions of the talus. J Foot Ankle Surg. 2016 [DOI] [PubMed]
- 71.Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl):105S–111S. doi: 10.1177/0363546509351481. [DOI] [PubMed] [Google Scholar]
- 72.Fraser EJ, Savage-Elliott I, Yasui Y, Ackermann J, Watson G, Ross KA, Deyer T, Kennedy JG. Clinical and MRI donor site outcomes following autologous osteochondral transplantation for talar osteochondral lesions. Foot Ankle Int. 2016 [DOI] [PubMed]
- 73.Bisicchia S, Rosso F, Amendola A. Osteochondral allograft of the talus. Iowa Orthop J. 2014;34:30–37. [PMC free article] [PubMed] [Google Scholar]
- 74.Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22(5):385–391. doi: 10.1177/107110070102200505. [DOI] [PubMed] [Google Scholar]
- 75.Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91-A:2818–2826. doi: 10.2106/JBJS.I.00398. [DOI] [PubMed] [Google Scholar]
- 76.Görtz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for osteochondral lesions of the talus. Foot Ankle Int. 2010;31(4):283–290. doi: 10.3113/FAI.2010.0283. [DOI] [PubMed] [Google Scholar]
- 77.Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31(4):277–282. doi: 10.3113/FAI.2010.0277. [DOI] [PubMed] [Google Scholar]
- 78.Adams SB, Jr, Viens NA, Easley ME, Stinnett SS, Nunley JA., 2nd Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648–654. doi: 10.2106/JBJS.J.00141. [DOI] [PubMed] [Google Scholar]
- 79.El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93-A:1634–1640. doi: 10.2106/JBJS.J.00900. [DOI] [PubMed] [Google Scholar]
- 80.Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94:1105–1110. doi: 10.2106/JBJS.J.02010. [DOI] [PubMed] [Google Scholar]
- 81.Liu H, Zhao Z, Clarke RB, Gao J, Garrett IR, Margerrison EE. Enhanced tissue regeneration potential of juvenile articular cartilage. Am J Sports Med. 2013;41(11):2658–2667. doi: 10.1177/0363546513502945. [DOI] [PubMed] [Google Scholar]
- 82.Namba RS, Meuli M, Sullivan KM, Le AX, Adzick NS. Spontaneous repair of superficial defects in articular cartilage in a fetal lamb model. J Bone Joint Surg Am. 1998;80(1):4–10. doi: 10.2106/00004623-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 83.Adkisson HD, 4th, Martin JA, Amendola RL, Milliman C, Mauch KA, Katwal AB, Seyedin M, Amendola A, Streeter PR, Buckwalter JA. The potential of human allogeneic juvenile chondrocytes for restoration of articular cartilage. Am J Sports Med. 2010;38(7):1324–1333. doi: 10.1177/0363546510361950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bonasia DE, Martin JA, Marmotti A, Amendola RL, Buckwalter JA, Rossi R, Blonna D, Adkisson HD, 4th, Amendola A. Cocultures of adult and juvenile chondrocytes compared with adult and juvenile chondral fragments: in vitro matrix production. Am J Sports Med. 2011;39(11):2355–2361. doi: 10.1177/0363546511417172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coetzee JC, Giza E, Schon LC, Berlet GC, Neufeld S, Stone RM, Wilson EL. Treatment of osteochondral lesions of the talus with particulated juvenile cartilage. Foot Ankle Int. 2013;34(9):1205–1211. doi: 10.1177/1071100713485739. [DOI] [PubMed] [Google Scholar]
- 86.Drakos MC, Murphy CI. Particulated juvenile articular cartilage allograft transplantation with bone marrow aspirate concentrate for treatment of talus osteochondral defects. Tech Foot Ankle Surg. 2015;14(2):88–93. doi: 10.1097/BTF.0000000000000092. [DOI] [Google Scholar]
- 87.Fortier LA, Chapman HS, Pownder SL, Roller BL, Cross JA, Cook JL, Cole BJ. BioCartilage improves cartilage repair compared with microfracture alone in an equine model of full-thickness cartilage loss. Am J Sports Med. 2016;44(9):2366–2374. doi: 10.1177/0363546516648644. [DOI] [PubMed] [Google Scholar]
- 88.Desai S. Treatment of osteochondral lesions of the talus with marrow stimulation and micronized allograft cartilage matrix: an all-arthroscopic technique. Tech Foot Ankle Surg. 2014;14(3):167–173. doi: 10.1097/BTF.0000000000000056. [DOI] [Google Scholar]
- 89.Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706–2715. doi: 10.1007/s11999-011-1857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Smyth NA, Murawski CD, Fortier LA, Cole BJ, Kennedy JG. Platelet-rich plasma in the pathologic processes of cartilage: review of basic science evidence. Arthroscopy. 2013;29(8):1399–1409. doi: 10.1016/j.arthro.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 91.Smyth NA, Haleem AM, Murawski CD, Do HT, Deland JT, Kennedy JG. The effect of platelet-rich plasma on autologous osteochondral transplantation: an in vivo rabbit model. J Bone Joint Surg Am. 2013;95(24):2185–2193. doi: 10.2106/JBJS.L.01497. [DOI] [PubMed] [Google Scholar]
- 92.Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384–2389. doi: 10.1007/s00167-013-2784-5. [DOI] [PubMed] [Google Scholar]
- 93.Görmeli G, Karakaplan M, Görmeli CA, Sarıkaya B, Elmalı N, Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. 2015;36(8):891–900. doi: 10.1177/1071100715578435. [DOI] [PubMed] [Google Scholar]
- 94.Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927–1937. doi: 10.2106/JBJS.I.01284. [DOI] [PubMed] [Google Scholar]
- 95.Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, Low S, Wallin KL, Ragavanaidu K. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic acid: an experimental study in a goat model. Arthroscopy. 2009;25(12):1391–1400. doi: 10.1016/j.arthro.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 96.Hannon CP, Ross KA, Murawski CD, Deyer TW, Smyth NA, Hogan MV, Do HT, O'Malley MJ, Kennedy JG. Arthroscopic bone marrow stimulation and concentrated bone marrow aspirate for osteochondral lesions of the talus: a case-control study of functional and magnetic resonance observation of cartilage repair tissue outcomes. Arthroscopy. 2016;32(2):339–347. doi: 10.1016/j.arthro.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Cassano JM, Kennedy JG, Ross KA, Fraser EJ, Goodale MB, Fortier LA. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg Sports Traumatol Arthrosc 2016 [DOI] [PubMed]
- 98.van Eekeren IC, Reilingh ML, van Dijk CN. Rehabilitation and return-to-sports activity after debridement and bone marrow stimulation of osteochondral talar defects. Sports Med. 2012;42(10):857–870. doi: 10.1007/BF03262299. [DOI] [PubMed] [Google Scholar]


