Abstract
Adipose tissue, defined as white adipose tissue (WAT) and brown adipose tissue (BAT), is a biological caloric reservoir; in response to over-nutrition it expands and, in response to energy deficit, it releases lipids. The WAT primarily stores energy as triglycerides, whereas BAT dissipates chemical energy as heat. In mammals, the BAT is a key site for heat production and an attractive target to promote weight loss. The autonomic nervous system (ANS) exerts a direct control at the cellular and molecular levels in adiposity. The sympathetic nervous system (SNS) provides a complex homeostatic control to specifically coordinate function and crosstalk of both fat pads, as indicated by the increase of the sympathetic outflow to BAT, in response to cold and high-fat diet, but also by the increase or decrease of the sympathetic outflow to selected WAT depots, in response to different lipolytic requirements of these two conditions. More recently, a role has been attributed to the parasympathetic nervous system (PNS) in modulating both adipose tissue insulin-mediated glucose uptake and fatty free acid (FFA) metabolism in an anabolic way and its endocrine function. The regulation of adipose tissue is unlikely to be limited to the autonomic control, since a number of signaling cytokines and neuropeptides play an important role, as well. In this review, we report some experimental evidences about the role played by both the ANS and orexins into different fat pads, related to food intake and energy expenditure, with a special emphasis on body weight status and fat mass (FM) content.
Keywords: adipose tissue, autonomic nervous system, orexin, body composition, thermogenesis
Introduction
Adipose tissue, once seen as a mere passive reservoir for energy storage, today is considered a highly active endocrine and metabolic structure that react to over-nutrition with expansion and to energy deficit releasing lipids (Rutkowski et al., 2015). The primary cell type of adipose tissue are the adipocytes, which contains lipid droplets storing the excess calories, as triglycerides, without experience a lipotoxicity (Konige et al., 2014; Straub and Wolfrum, 2015). This unparalleled capacity to store and release lipids upon systemic metabolic demand links the cell biology of adipocytes and adipose tissue physiology to the whole-body metabolism. The adipose tissue can be divided into two subtypes, identified as white cells and brown cells. White adipose tissue (WAT) constitutes the typical fat cells: they are the majority of cells in both subcutaneous and visceral adipose depots. Brown adipocytes encompass smaller brown fat depots that play a role in thermogenesis in most mammalian species (Lapa et al., 2015; Rutkowski et al., 2015). The regulation of adipose tissue metabolism in vivo involves different central effector pathways, strongly influencing both energy intake and energy expenditure, and whose activity is regulated by adiposity-related signals.
Based on our experiences of studying human adipose tissue regulation in vivo, this review summarizes the complexity of said tissue function, with an emphasis on some peculiar aspects of the regulation of adiposity to disclose the integrative nature of adipose tissue function.
Adipose tissue function and regulation
The different morpho-functional characterization of the adipocytes allows distinguishing adipose tissue as WAT and BAT. White adipose tissue has an evolutionary role permitting animals to survive for long periods without meals, thanks to their properties of energy storing, mainly as triglycerides, and of releasing fatty acids along fasting periods (Monda et al., 2008a; Messina et al., 2016a). Conversely, brown adipose tissue (BAT) is the most important organ for non-shivering thermogenesis (NST). The primary function of BAT is related to heat production in response to a decrease in core body temperature, due to environmental cold exposure. For this reason, brown adipocytes are characterized by an abundance of mitochondria, in contrast to white adipose cells. The thermogenic effect of BAT is strictly related to the presence of uncoupling protein 1 (UCP1), a transmembrane protein acting as mitochondrial metabolite transporter activated in the brown fat cell by fatty acids (Zingaretti et al., 2009; Yang and Ruan, 2015). Clusters of UCP1-expressing adipocytes with thermogenic capacity also develop in WAT in response to various stimuli (Vitali et al., 2012; Esposito et al., 2016). These adipocytes have been named “induced BAT.” The activities of brown and inducible “brown-like” adipocytes reduce metabolic disease, including obesity, in mice and correlate with leanness in humans.
Morphological differences between BAT and WAT are easily observable. In fact, BAT adipocytes have a polygonal shape, multilocular lipid droplets and a great number of large mitochondria. Moreover, they are innervated by numerous sympathetic nerve fibers (Zingaretti et al., 2009; Whittle et al., 2011; Villarroya and Vidal-Puig, 2013), confirming the central control of thermogenesis. Furthermore, to allow a high heat dissipation, BAT is also highly vascularized (Wu et al., 2013).
The modulation of both sympathetic and parasympathetic outflow provides a complex homeostatic mechanism enabling to specifically regulate the functional crosstalk of organs involved in the balance between caloric intake and energy expenditure (Villarroya and Vidal-Puig, 2013). Whereas the adaptive thermogenic response to cold and high-fat diet increases the SNS outflow to BAT, the different lipolytic requirements of these two conditions are appropriately met by the increase or decrease of the sympathetic outflow to selected WAT depots (Brito et al., 2008). A strict involvement of the SNS is also observed during caloric restriction, which is characterized by a decrease in the sympathetic outflow to BAT, resulting in a reduced energy expenditure and a simultaneous increase in SNS outflow to specific WAT depots facilitating lipid mobilization.
Signals related to food intake from various origins (e.g., gut, hepatic-portal area, baroreceptors) are integrated in the brain and result in increased peripheral sympathetic outflow. It is noteworthy to emphasize the role of diet composition in sympathetic responsiveness during the day, in view of the potential role of adrenergic per-responsiveness in the pathogenesis of obesity and metabolic syndrome (van Baak, 2008). It is well-known that chronic sympathetic hyper-responsiveness is present in central obesity; recent studies also demonstrate the consequence of a high sympathetic outflow to kidneys, heart, and blood vessels. Increased sympathetic reactivity can also be involved in the decline of insulin sensitivity, determining a vicious cycle responsible for hypertension, and the development of metabolic syndrome. Albeit the reason of this hyper-responsiveness is not yet clear, it may be driven by particular adipokines (Smith and Minson, 2012). While it has been clearly established that WAT receives sympathetic innervation, whether it receives parasympathetic innervation still appears to be controversial (Kreier et al., 2002). Neuroanatomical studies have demonstrated parasympathetic innervation of WAT in rats (Bartness, 2002). In addition, parasympathetic input affects hormone synthesis in WAT as evident from the effects of selective vagotomy on mRNA expression of resistin and leptin (Kreier et al., 2002; Di Bernardo et al., 2014).
Energy homeostasis is regulated by a complex network of neuroendocrine and autonomic pathways (Messina et al., 2014a), in which hypothalamus plays a key role monitoring signals that reflect energy status, thus initiating appropriate metabolic responses and behavioral (Suzuki et al., 2012; Esposito et al., 2014). The orexins (OX-A and OX-B), also named hypocretins (Eriksson et al., 2001; Messina et al., 2014b), are neuropeptides with critical functions in energy balance and obesity, and therefore in the accumulation of adipose tissue (Tsuda et al., 2002; Monda et al., 2008a). The neurons that produce those neuropeptides are in the lateral hypothalamic area (LHA), the dorsomedial nucleus of the hypothalamus (DMH), and the perifornical hypothalamus (Sakurai et al., 1998; López et al., 2010; Messina et al., 2016b). In this line, orexins play a crucial role in energy balance and feeding (Sakurai et al., 1998; López et al., 2010), and compelling evidence derived from genetic murine models suggest a role for orexins in promoting energy expenditure through modulation of locomotor activity and BAT thermogenesis (López et al., 2010; Sakurai et al., 1998). In fact, orexins are required for BAT development, differentiation, and function (Sellayah et al., 2011; Monda et al., 2014). Moreover, lack of orexins' action compromises energy balance, as demonstrated in orexin knockout mice, which are prone to diet-induced obesity, when compared with wild type mice (Shen et al., 2008; Sellayah et al., 2011). Figure 1 shows a proposed model for action of central orexin on adipose tissue (Monda et al., 2007). A protective role in aging-decreased thermogenic capacity was also recently suggested for orexins (Sellayah and Sikder, 2014). The aging process causes an increase in body fat percent, but the mechanism remains unclear. Aging is related to defective differentiation of BAT, alongside morphologic abnormalities and thermogenic dysfunction in humans and in rodents (Monda et al., 2006b; Sellayah and Sikder, 2014). In aged mice, indeed, interscapular BAT (IBAT) is progressively populated by adipocytes, bearing white morphologic characteristics (Monda et al., 2008b).
Figure 1.
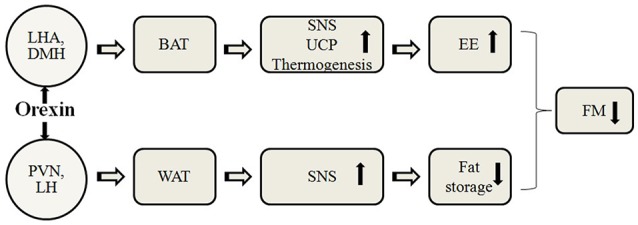
A proposed model for action of central orexin on adipose tissue. LHA, lateral hypothalamic area; DMH, dorsomedial nucleus of the hypothalamus; PVN, paraventricular nucleus of the hypothalamus; LH, lateral hypothalamus; BAT, brown adipose tissue; WAT, white adipose tissue; SNS, sympathetic nervous system; UCP, uncoupling protein; EE, energy expenditure; FM, fat mass.
The orexin neurons have a wide projection pattern within the central nervous system, which includes several brain nuclei identified by WAT retrograde tracing studies (e.g., the arcuate nucleus of the hypothalamus, the locus coeruleus, the nucleus of the solitary tract and the suprachiasmatic nucleus (Peyron et al., 1998; Adler et al., 2012). Therefore, it is conceivable that orexin neurons may influence WAT metabolism, not only through their projections to the paraventricular nucleus of the hypothalamus (PVN), but also through modulation of other brain regions, suggesting that effects of orexins on WAT do not follow an exclusive pathway, but are distributed over multiple brain regions. Finally, as the orexin neurons appear to integrate multiple sources of metabolic and neuronal information (Kampe et al., 2009), it is possible that another role of these neurons is to coordinate the response from WAT based on the brain's perception of the metabolic status (Viggiano et al., 2016; Moscatelli et al., 2016a,b,c).
Brown adipose tissue-mediated thermogenesis
The IBAT is responsible for 35–65% of the total metabolic heat increase, unrelated to shivering in rodents (Rinaldi et al., 2015). In vivo, prostaglandin E1(PGE1) reduces heat loss and increases heat production in order to raise body temperature to a new set point. The simultaneous measurements of and food intake and sympathetic firing rate may represent the most relevant demonstration of the feed-back between body temperature and food intake, since the increase in body temperature due to PGE1 can be recognized as a signal of satiety, which reduces food intake (Mantzoros et al., 1996). Alterations of food intake and IBAT activity in response to hyperthermia induced by PGE1 injection in rat cerebral ventricle are reported (Monda et al., 1999). The firing rate of the sympathetic nerves to IBAT, along with IBAT and colonic temperatures were monitored in male Sprague-Dawley rats before and after food presentation. Saline or PGE1 were injected intraventricularly immediately before food presentation. The amount of food ingested was also measured. Prostaglandin E1 injection induced elevation of body temperature and reduction of food intake. Furthermore, IBAT temperature increase was inversely proportional to food intake. Figure 2 illustrates the cumulative effects of PGE1-induced hyperthermia. Overall, these findings provide direct evidence of sympathetic discharge of nerves to IBAT after PGE1 injection, supporting the hypothesis of a functional involvement of the ventromedial hypothalamus (VMH), a key structure in the control of sympathetic activity and food intake (Thornhill and Halvorson, 1994), as a consequence of the stimulatory effect of PGE1 upon the preoptic-anterior hypothalamus (PO/AH) (Thornhill and Halvorson, 1994).
Figure 2.
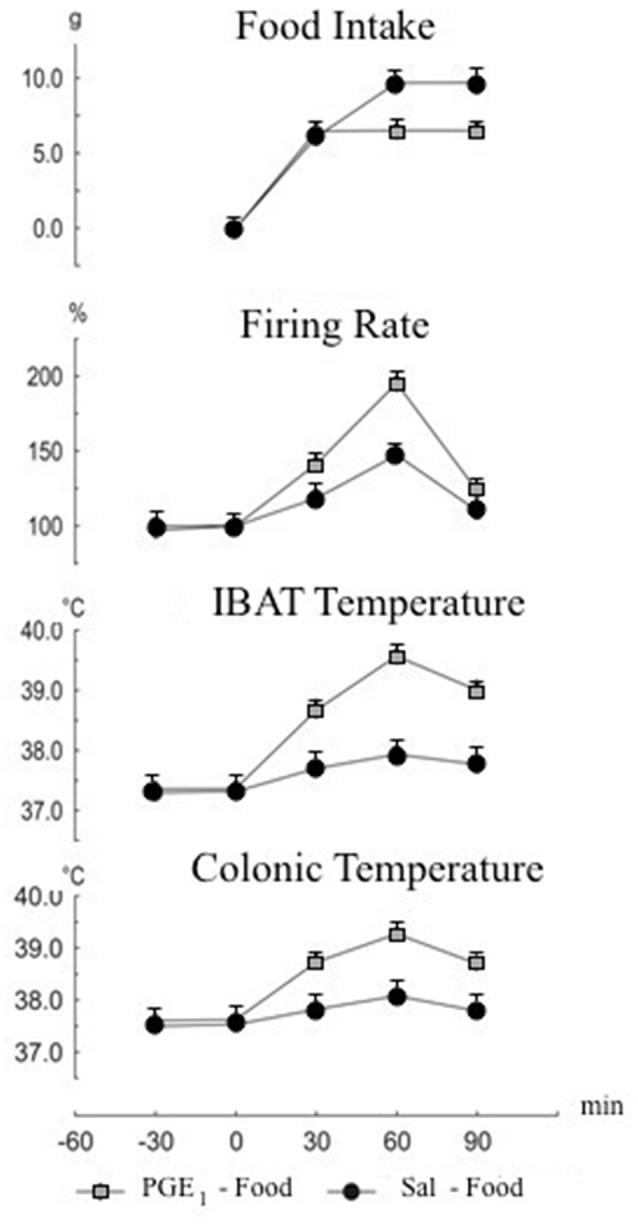
Cumulative changes in food intake, firing rate of nerves to interscapular brown adipose tissue (IBAT), IBAT and colonic temperatures. Food presentation at time 0. Intracerebroventricular injection of prostaglandin E-1 (PGE1) or saline was made at time 0. Values are expressed as mean ± standard error.
Interscapular brown adipose tissue activity and eating behavior
The PO/AH is considered the most important area deputed to control body temperature. The orexin A affects the temperature of IBAT, the most relevant effector of NST in rodents (Cannon et al., 1998), indicating that the rise in heat production is also due to a thermogenic mechanism independent of muscle contraction. Pyrogens, like PGE, influence PO/HA function inducing hyperthermia, whereas inhibitors of prostaglandins synthesis inhibit this response (Cannon et al., 1998).
The effect of the thermogenic-induced orexin A activation upon eating behavior was investigated (Monda et al., 2013). Food intake, IBAT (TIBAT) and abdominal temperature (Tab) were monitored in 24-h fasting male Sprague-Dawley rats along 12 h after food presentation. Test animals received orexin A, through injection into the lateral cerebral ventricle, 6 h before food presentation. Control animals received orexin A contemporaneously to food presentation. As shown in Figure 3, a significant reduction of food intake, TIBAT and Tab was found in rats receiving orexin A prior to food presentation. These results clearly indicate that the reduction of food intake was a function of orexin A dependent temperature rise at the time of food presentation, outlining the prevailing role of orexin A in the control of body temperature, which in turn affects hypophagic behavior. According to these findings, the prevalent role so far displayed by the orexins in controlling eating behavior requires a substantial revision, since orexin A can induce simultaneously increase of sympathetic discharge, hyperthermia and hypophagia, thus contradicting the prevalent meaning of orexin as a primary hyperphagic substance. Conversely, other hyperphagic peptides, namely neuropeptide Y or galanin, induce a reduction of the sympathetic discharge and a decrease in body temperature (Bouali et al., 1995); while primary hypophagic substances, as leptin, cause an increase in the sympathetic activity and an increase in food intake (Haque et al., 1999).
Figure 3.
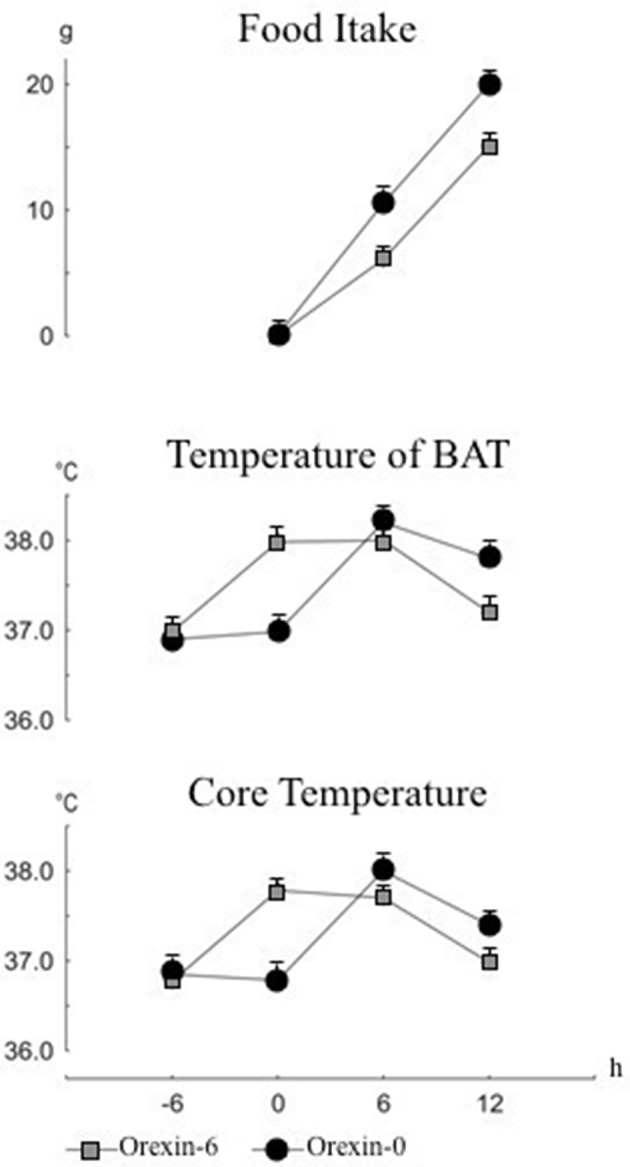
Cumulative changes in food intake, temperature of interscapular brown adipose tissue (IBAT) and in core temperature. Food presentation at time 0. Intracerebroventricular injection of orexin or saline was made at time -6 h or time 0. Values are expressed as mean ± standard error.
The relationship between heart rate variability and adiposity
Heart rate variability (HRV) power spectral analysis is widely considered a standard noninvasive method for assessing Autonomic Nervous System (ANS) function, due to its regulation of heart rate in a continuous, beat-to-beat manner (NASPE, 1996; Messina et al., 2012). Sympathetic activity is associated with the low frequency range (LF, 0.04–0.15 Hz) while parasympathetic activity is associated with the higher frequency range (HF, 0.15–0.4 Hz) of modulation frequencies of the heart rate (Messina et al., 2013). This difference in frequency ranges allows HRV analysis to separate sympathetic and parasympathetic contributions evident. Lower HRV is generally considered an indicator of poorer autonomic function (Viggiano et al., 2010; Messina et al., 2016c).
HRV was investigated in lean and obese women at premenopausal and postmenopausal age. As main findings, power spectral analysis of HRV showed a significant reduction in LF and HF components in obese than in lean subjects, both in premenopausal and postmenopausal age. These findings indicate a reduction of both the sympathetic and parasympathetic activity. The reduction of the sympathetic activity may play a key role in the weight maintenance in obese premenopausal women. Conversely, a reduction of the activity of sympathetic branch, could be linked to low energy expenditure, explaining the adipose tissue accumulation and the high body weight in premenopausal women. This is in line with the so-called “Mona Lisa Hypothesis,” acronym for “most obesities known are low in sympathetic activity” (Messina et al., 2013). Furthermore, the autonomic activity in postmenopausal women was lower than in premenopausal women, indicating that autonomic modulation changes in post menopause cannot be related to obesity. Several studies highlighted the relationship between an increase in sympathetic and thermogenic activity, and the reduction of food intake. So, it can be hypothesized that obesity can be related to the increase in food intake associated with a reduction of the sympathetic activity. On the other hand, some study pointed to a lower respiratory sinus arrhythmia, computed through the HRV power spectral analysis together with deep breathing tests, which indicated a cardiac vagal dysfunction in obese adolescents (Messina et al., 2012). Finally, a decreased parasympathetic activity may represent a final common pathway in different conditions related to higher rate of morbidity and mortality (Messina et al., 2012).
Physical training may induce several adaptive modifications, including changes in either ANS activity (Triggiani et al., 2015; Valenzano et al., 2016), or in resting energy expenditure (REE) (Kalsbeek et al., 2007). Heart rate variability power spectral analysis is an additional tool used to evaluate the autonomic hear rate control during exercise (Arai et al., 1989). The parasympathetic tone is enhanced by exercise training, so that a reduction in the heart rate, induced by vagal influence, is considered an index of training status in athletes (Kalsbeek et al., 2007). Moreover, body composition can be considered as a determinant for energy expenditure. The appraisal of the relationship among REE, tissue mass and HRV measures was carried out in adults female basketball players (Chieffi et al., 2004, 2012, 2014; Viggiano et al., 2014). Body composition, REE and HRV were measured before and after a period of 6 months in ten athletes and ten non-athletes. In athletes, physical activity induced an increase in REE and a decrease in FM, without any noticeable change in body weight. Athletes showed a significant increase in the parasympathetic activity, as revealed by the HF component of HRV. These findings showed a higher REE in athletes, than in non-athletes, despite the increased parasympathetic activity, typically related to lower energy expenditure (Viggiano et al., 2014). This is the first study examining the effects of long-term training on HRV, body composition, and REE. The relationship between physical activity, resting energy expenditure, parasympathetic nervous system (PNS) and fat mass is depicted in Figure 4. Furthermore, it is particularly relevant that exercise induced an increase of parasympathetic activity at rest, but the LF component of HRV did not show any change. Overall, the parallel increase in both parasympathetic activity and REE, found in long-term trained female athletes, can be considered an aspect of particular importance supporting the adaptive capacities of the athlete as compared to non-athlete. In fact, parasympathetic activity shows an inverse correlation with REE (Oldfield et al., 2007; Morrison et al., 2014).
Figure 4.
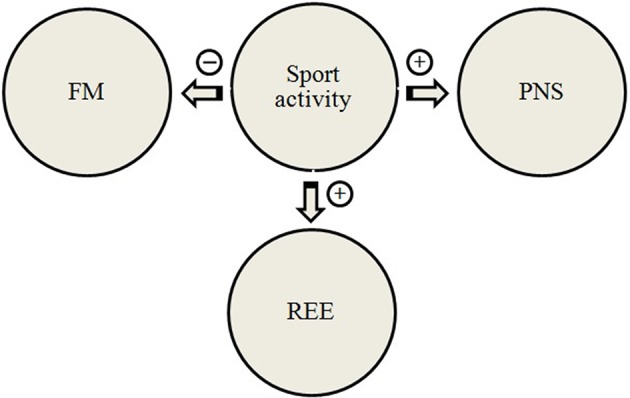
A proposed model for the relationship between physical activity, resting energy expenditure, parasympathetic nervous system, and fat mass. REE, resting energy expenditure; PNS, parasympathetic nervous system; FM, fat mass.
Different patterns of adiposity are related to the occurrence of autonomic impairment and may be correlated with the observation of a higher risk for cardiovascular disease. The relationship between HRV and body mass index (BMI) has been repeatedly investigated in obese subjects with conflicting results (Zahorska-Markiewicz et al., 1993; Karason et al., 1999; Skrapari et al., 2007). The role of adiposity, measured as FM extent, on cardiac autonomic function was recently investigated in healthy adult women, by monitoring their short-term HRV response at rest (Triggiani et al., 2015). As a major finding, a reduction in both LF and HF bands, was found in overweight/obese, while in underweight subjects there was a reduction in the sole LF band. The simultaneous reduction of either HF, or LF components of HRV in overweight/obese women was related to a possible impairment in the baroceptive reflex sensibility. Conversely, in underweight women the reduction of the sole LF indicated that the baroceptive response was normal. More interestingly, the associations between body FM extent and HRV response was demonstrated adopting a curvilinear model, which indicated that a second order regression was considerably more successful to represent HRV changes, with respect to subjects' adiposity. The inverted U-shaped association (quadratic regression) between HRV and percent fat mass is shown in Figure 5. Overall, these data indicate that the adaptive flexibility of the autonomic cardiac activity found in both underweight and overweight/obese subjects, although differently reduced, turns out to be poorer, than in normal weight subjects. The question whether such a curvilinear model may reflect also differences in visceral fat distribution, is still opened.
Figure 5.
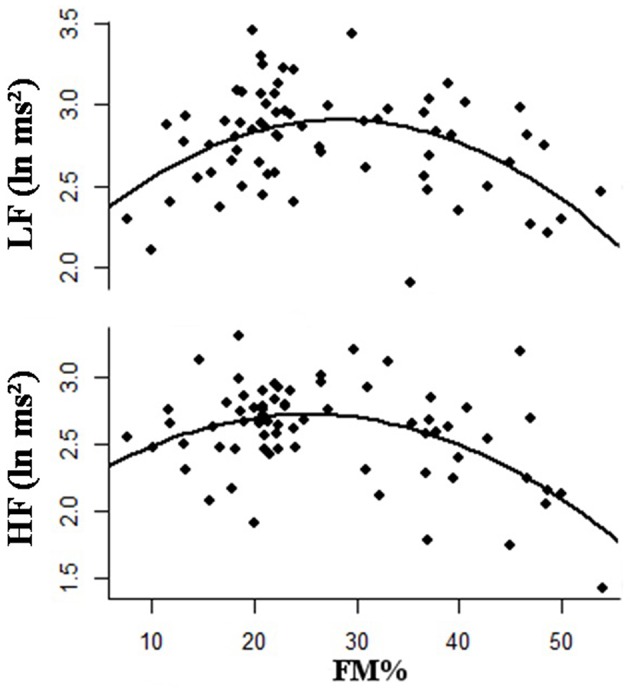
Second order polynomial regression showing an inverted U-shaped relationship between percentage of body fat mass (FM) and low frequency power (LF) and high frequency power (HF).
Discussion
The biological mechanisms underlying the precise regulation of eating behavior, energy expenditure, and energy storage in adipose tissue can be explained assuming that signals generated in proportion to body adiposity may influence the brain to modulate food intake and/or energy expenditure. Insulin has been the first signal molecule recognized to act in the brain to reduce food intake (Woods et al., 1979). Afterward, leptin was proposed as the principal responsible in this regulation process (Campfield et al., 1996). Although these two hormones probably play the major role in this regulation, an increasing number of endogenous signaling molecules, associated with neuroendocrine and autonomic control systems, are implicated as adiposity-related signals. The overall pattern of the central network innervating adipose tissue indicates sympathetic regulation of this tissue, in which orexins plays an important role. As reported in this review, the orexins are part of this network and can affect energy balance through modulation of either energy intake, or expenditure (Monda et al., 2008a). Fasting state is highly conditioning orexin receptors gene expression (Lu et al., 2000), a finding which has been confirmed by the functional interaction between orexin neurons and glucose-sensitive neurons in the hypothalamus (Shiraishi et al., 2000; Liu et al., 2001). More recently, Venner and coll. (Venner et al., 2011) proposed a role for orexin neurons as glucose sensors, since their electrical activity is dependent on intracellular energy levels changes in response to glucose concentration.
Sympathetic activation increases lipolysis and β-oxidation of fatty acids in BAT, allowing heat production, by drawing on lipids stores (Cannon and Nedergaard, 2004). Reduced thermogenesis, and thus lipid consumption, in BAT may contribute to the etiology of some forms of obesity. In fact, humans with low body temperature, maybe due to a low thermogenesis, are more inclined to obesity (van Marken Lichtenbelt and Daanen, 2003). In humans, obesity is associated with decreased BAT activity (van Marken Lichtenbelt et al., 2009).
An intra ventricular administration of orexin A induced an increase in firing rate of the sympathetic nerves to BAT, accompanied with a rise in BAT and colonic temperatures. The simultaneous increase in heart rate and body temperature after intra ventricular injection of orexin A shows a generalized activation of the SNS. Overall, the functional organization and neurochemical influences within the central nervous system networks govern the level of BAT sympathetic nerve activity to produce the thermoregulatory and metabolically driven alterations in BAT thermogenesis and energy expenditure, thus contributing to energy homeostasis (Morrison et al., 2014; Messina et al., 2015a).
Orexin A was shown able to influence both the thermogenesis and hyperphagia, so, the possibility that a previously activating thermogenic response orexin A might modify eating behavior was tested The result showed that the effects on orexin-induced food intake, depend on the time of food presentation. Such a result led us to review the functional meaning of orexin in food intake mechanism, highlighting the role of orexin A in the control of SNS activity and body temperature, which, sequentially, affects food intake (Monda et al., 2008b).
Food ingestion rise the body temperature caused by postprandial thermogenesis (Monda et al., 2008a; Messina et al., 2012). A reduced response of SNS might bring to an altered postprandial thermogenesis, becoming a crucial factor for obesity. Low postprandial sympathetic activation led subjects to a higher food intake to reach a prefixed level of body temperature. Conversely, overweight status increases the sympathetic discharge, and may contribute to induce diseases related to abnormal body weight (Lambert et al., 2010). Chronic sympathetic overactivity is well-known to play a role in central obesity, and many evidences demonstrate the consequence of a high sympathetic outflow to heart, kidneys, and blood vessels (Valenzano et al., 2016).
It has been generally assumed that obesity is characterized by the reduction of HRV reactivity, though these results are not even convergent. In previous studies, Monda et al. (2006a), demonstrated that the power spectral analysis of LF and HF components of HRV significantly changed in lean and obese women, due to the pre-/post-menopausal age. In obese pre-menopausal women, a lower sympathetic tone was found than in lean ones; while a parallel decrease in both LF and HF components appeared evident either in obese or in lean women after menopause. This unparallel effect of body fat content upon HRV variables in pre-/post-menopausal women indicates that the autonomic imbalance might rather be attributed to the age factor and the mutated sex hormonal balance, following the menopause onset. Indeed, suppression of sex hormones to postmenopausal levels reduces resting energy expenditure in young healthy women, through a reduction of autonomic nervous activity (Day et al., 2005; Messina et al., 2015b).
Heart rate variability is altered in obese subjects, but whether this is true also in underweight subjects is still under debate. In a recent study, we investigated the HRV profile in a sample of healthy adult women and its association with adiposity (Triggiani et al., 2015). The data reported in this study reflected the trend in HRV association with FM, among healthy adult women. In fact, a reduction in time and frequency domain measures was observed in overweight/obese women, when compared to normal weight subjects, which reflects sympathetic modulation of heart rate, in agreement with previously published studies. A similar HRV profile was found in underweight women, but the impairment of parasymphatetic activity was not proven. Our findings confirm that the adaptive flexibility of the autonomic cardiac activity found in both underweight and overweight/obese subjects, even though differently reduced, turned out to be poorer than in normal weight subjects. To look for associations between body FM extent and HRV indices we found that this relationship follows a parabolic trend, with lower HRV measures for either lower and higher FM values, leading to the question of whether this process might involve opposing or synergic processes that could be mediated by the two branches of the ANS. The different ways characterizing the power oscillatory RR signal reduction in underweight and overweight/obese subjects would suggest that a relationship between the autonomic bottom tone and the power of oscillatory RR signal, defined as full scale effect, may exists. Therefore, the underweight and overweight/obese status correspond to two distinct levels of tonic sympathetic activity, respectively, higher and lower than the level of tonic activity of normal weight subjects. In both cases, the expected response would be a reduced modulation of the sympathetic components in the LF band. Overall, these findings are consistent with previous studies demonstrating that the reduction of HRV in both underweight and overweight subjects may represent a risk factor for cardiovascular disease mortality (Nolan et al., 1998; Dudina et al., 2011).
Conclusion
In this review, we have briefly focused on the relationship between ANS and orexins in the control of body weight, according to the theory of the “thermoregulatory hypothesis” of food intake. In summary, BAT is the strategic organ in the control of body temperature, through heat dissipation; while, WAT primarily stores energy as triglycerides and releases fatty acid during starvation. Although the central control of adipose tissue function was mainly based on the modulation of sympathetic outflow, recent developments have demonstrated that the orexins' system is a key factor in modulating adipose tissue functions by acting on several hypothalamic nuclei. Therefore, we hope that this review stimulates the reader's thinking to extend beyond the traditionally accepted roles of neural and hormonal factors in the control of body fat levels. To include the neural circuitry involved in orexin control of adipose tissue will help to provide therapeutic targets for obesity intervention.
Author contributions
MS, TE, VM, and AM: conceived the study, participated in its design and wrote the manuscript. AVa, FM, AL, GCo, AM, AT, and SC: contributed to the conception and design. GM, AVi, GG, MM, and GCi: drafted the article and revised it critically for important intellectual content. GM: final approval of the version to be published. All authors read and approved the final manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer VP and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
References
- Adler E. S., Hollis J. H., Clarke I. J., Grattan D. R., Oldfield B. J. (2012). Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J. Neurosci. 32, 15913–15921. 10.1523/JNEUROSCI.2591-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y., Saul J. P., Albrecht P., Hartley L. H., Lilly L. S., Cohen R. J., et al. (1989). Modulation of cardiac autonomic activity during and immediately after exercise. Am. J. Physiol. 256, H132–H141. [DOI] [PubMed] [Google Scholar]
- Bartness T. J. (2002). Dual innervation of white adipose tissue: some evidence for parasympathetic nervous system involvement. J. Clin. Invest. 110, 1235–1237. 10.1172/JCI0217047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouali S. M., Fournier A., St-Pierre S., Jolicoeur F. B. (1995). Effects of NPY and NPY2-36 on body temperature and food intake following administration into hypothalamic nuclei. Brain Res. Bull. 36, 131–135. 10.1016/0361-9230(94)00177-3 [DOI] [PubMed] [Google Scholar]
- Brito N. A., Brito M. N., Bartness T. J. (2008). Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1445–R1452. 10.1152/ajpregu.00068.2008 [DOI] [PubMed] [Google Scholar]
- Campfield L. A., Smith F. J., Burn P. (1996). The OB protein (leptin) pathway–a link between adipose tissue mass and central neural networks. Horm. Metab. Res. 28, 619–632. 10.1055/s-2007-979867 [DOI] [PubMed] [Google Scholar]
- Cannon B., Houstek J., Nedergaard J. (1998). Brown adipose tissue. More than an effector of thermogenesis? Ann. N. Y. Acad. Sci. 856, 171–187. 10.1111/j.1749-6632.1998.tb08325.x [DOI] [PubMed] [Google Scholar]
- Cannon B., Nedergaard J. (2004). Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359. 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Chieffi S., Conson M., Carlomagno S. (2004). Movement velocity effects on kinaesthetic localisation of spatial positions. Exp. Brain Res. 158, 421–426. 10.1007/s00221-004-1916-z [DOI] [PubMed] [Google Scholar]
- Chieffi S., Iavarone A., Iaccarino L., La Marra M., Messina G., De Luca V., et al. (2014). Age-related differences in distractor interference on line bisection. Exp. Brain Res. 232, 3659–3664. 10.1007/s00221-014-4056-0 [DOI] [PubMed] [Google Scholar]
- Chieffi S., Iavarone A., Viggiano A., Monda M., Carlomagno S. (2012). Effect of a visual distractor on line bisection. Exp. Brain Res. 219, 489–498. 10.1007/s00221-012-3106-8 [DOI] [PubMed] [Google Scholar]
- Day D. S., Gozansky W. S., Van Pelt R. E., Schwartz R. S., Kohrt W. M. (2005). Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J. Clin. Endocrinol. Metab. 90, 3312–3317. 10.1210/jc.2004-1344 [DOI] [PubMed] [Google Scholar]
- Di Bernardo G., Messina G., Capasso S., Del Gaudio S., Cipollaro M., Peluso G., et al. (2014). Sera of overweight people promote in vitro adipocyte differentiation of bone marrow stromal cells. Stem Cell Res. Ther. 5, 4. 10.1186/scrt393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudina A., Cooney M. T., Bacquer D., De Backer G., De Ducimetière P., Jousilahti P., et al. (2011). Relationships between body mass index, cardiovascular mortality, and risk factors: a report from the SCORE investigators. Eur. J. Cardiovasc. Prev. Rehabil. 18, 731–742. 10.1177/1741826711412039 [DOI] [PubMed] [Google Scholar]
- Eriksson K. S., Sergeeva O., Brown R. E., Haas H. L. (2001). Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J. Neurosci. 21, 9273–9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M., Serpe F. P., Diletti G., Messina G., Scortichini G., La Rocca C., et al. (2014). Serum levels of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans and polychlorinated biphenyls in a population living in the Naples area, southern Italy. Chemosphere 94, 62–69. 10.1016/j.chemosphere.2013.09.013 [DOI] [PubMed] [Google Scholar]
- Esposito T., Lobaccaro J. M., Esposito M. G., Monda V., Messina A., Paolisso G., et al. (2016). Effects of low-carbohydrate diet therapy in overweight subjects with autoimmune thyroiditis: possible synergism with ChREBP. Drug Des. Devel. Ther. 10, 2939–2946. 10.2147/DDDT.S106440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque M. S., Minokoshi Y., Hamai M., Iwai M., Horiuchi M., Shimazu T. (1999). Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48, 1706–1712. 10.2337/diabetes.48.9.1706 [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Kreier F., Fliers E., Sauerwein H. P., Romijn J. A., Buijs R. M. (2007). Minireview: circadian control of metabolism by the Suprachiasmatic nuclei. Endocrinology 148, 5635–5639. 10.1210/en.2007-0776 [DOI] [PubMed] [Google Scholar]
- Kampe J., Tschöp M. H., Hollis J. H., Oldfield B. J. (2009). An anatomic basis for the communication of hypothalamic, cortical and mesolimbic circuitry in the regulation of energy balance. Eur. J. Neurosci. 30, 415–430. 10.1111/j.1460-9568.2009.06818.x [DOI] [PubMed] [Google Scholar]
- Karason K., Mølgaard H., Wikstrand J., Sjöström L. (1999). Heart rate variability in obesity and the effect of weight loss. Am. J. Cardiol. 83, 1242–1247. 10.1016/S0002-9149(99)00066-1 [DOI] [PubMed] [Google Scholar]
- Konige M., Wang H., Sztalryd C. (2014). Role of adipose specific lipid droplet proteins in maintaining whole body energy homeostasis. Biochim. Biophys. Acta 1842, 393–401. 10.1016/j.bbadis.2013.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreier F., Fliers E., Voshol P. J., Van Eden C. G., Havekes L. M., Kalsbeek A., et al. (2002). Selective parasympathetic innervation of subcutaneous and intra-abdominal fat-Functional implications. J. Clin. Invest. 110, 1243–1250. 10.1172/JCI0215736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. W., Straznicky N. E., Lambert E. A., Dixon J. B., Schlaich M. P. (2010). Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol. Ther. 126, 159–172. 10.1016/j.pharmthera.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Lapa C., Maya Y., Wagner M., Arias-Loza P., Werner R. A., Herrmann K., et al. (2015). Activation of brown adipose tissue in hypothyroidism. Ann. Med. 47, 538–545. 10.3109/07853890.2015.1085126 [DOI] [PubMed] [Google Scholar]
- Liu X. H., Morris R., Spiller D., White M., Williams G. (2001). Orexin a preferentially excites glucose-sensitive neurons in the lateral hypothalamus of the rat in vitro. Diabetes 50, 2431–2437. 10.2337/diabetes.50.11.2431 [DOI] [PubMed] [Google Scholar]
- López M., Tena-Sempere M., Diéguez C. (2010). Cross-talk between orexins (hypocretins) and the neuroendocrine axes (hypothalamic-pituitary axes). Front. Neuroendocrinol. 31, 113–127. 10.1016/j.yfrne.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Lu X. Y., Bagnol D., Burke S., Akil H., Watson S. J. (2000). Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm. Behav. 37, 335–344. 10.1006/hbeh.2000.1584 [DOI] [PubMed] [Google Scholar]
- Mantzoros C. S., Qu D., Frederich R. C., Susulic V. S., Lowell B. B., Maratos-Flier E., et al. (1996). Activation of beta(3) adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes 45, 909–914. 10.2337/diab.45.7.909 [DOI] [PubMed] [Google Scholar]
- Messina A., De Fusco C., Monda V., Esposito M., Moscatelli F., Valenzano A., et al. (2016a). Role of the orexin system on the hypothalamus-pituitary-thyroid axis. Front. Neural Circuits 10:66. 10.3389/fncir.2016.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G., Chieffi S., Viggiano A., Tafuri D., Cibelli G., Valenzano A., et al. (2016c). Parachute jumping induces more sympathetic activation than cortisol secretion in first-time parachutists. Asian J. Sports Med. 7:e26841. 10.5812/asjsm.26841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G., Dalia C., Tafuri D., Monda V., Palmieri F., Dato A., et al. (2014a). Orexin-A controls sympathetic activity and eating behavior. Front. Psychol. 5:997. 10.3389/fpsyg.2014.00997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G., De Luca V., Viggiano A., Ascione A., Iannaccone T., Chieffi S., et al. (2013). Autonomic nervous system in the control of energy balance and body weight: personal contributions. Neurol. Res. Int. 2013:639280. 10.1155/2013/639280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G., Di Bernardo G., Viggiano A., De Luca V., Monda V., Messina A., et al. (2016b). Exercise increases the level of plasma orexin A in humans. J. Basic Clin. Physiol. Pharmacol. 27, 611–616. 10.1515/jbcpp-2015-0133 [DOI] [PubMed] [Google Scholar]
- Messina G., Monda V., Moscatelli F., Valenzano A. A., Monda G., Esposito T., et al. (2015a). Role of orexin system in obesity. Biol. Med. 7:248 10.4172/0974-8369.1000248 [DOI] [Google Scholar]
- Messina G., Valenzano A., Moscatelli F., Triggiani A., Capranica L., Messina A., et al. (2015b). Effects of emotional stress on neuroendocrine and autonomic functions in skydiving. J. Psychiatry 18, 1–7. 10.4172/2378-5756.100028 [DOI] [Google Scholar]
- Messina G., Vicidomini C., Viggiano A., Tafuri D., Cozza V., Cibelli G., et al. (2012). Enhanced parasympathetic activity of sportive women is paradoxically associated to enhanced resting energy expenditure. Auton. Neurosci. Basic Clin. 169, 102–106. 10.1016/j.autneu.2012.05.003 [DOI] [PubMed] [Google Scholar]
- Messina G., Viggiano A., Tafuri D., Palmieri F., De Blasio S., Messina A., et al. (2014b). Role of orexin in obese patients in the intensive care unit. J. Anesth. Clin. Res. 5:395 10.4172/2155-6148.1000395 [DOI] [Google Scholar]
- Monda M., Messina G., Mangoni C., De Luca B. (2008a). Resting energy expenditure and fat-free mass do not decline during aging in severely obese women. Clin. Nutr. 27, 657–659. 10.1016/j.clnu.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Monda M., Messina G., Scognamiglio I., Lombardi A., Martin G. A., Sperlongano P., et al. (2014). Short-term diet and moderate exercise in young overweight men modulate cardiocyte and hepatocarcinoma survival by oxidative stress. Oxid. Med. Cell. Longev. 2014:131024. 10.1155/2014/131024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda M., Messina G., Vicidomini C., Viggiano A., Mangoni C., De Luca B. (2006a). Activity of autonomic nervous system is related to body weight in pre-menopausal, but not in post-menopausal women. Nutr. Neurosci. 9, 141–145. 10.1080/10284150600903552 [DOI] [PubMed] [Google Scholar]
- Monda M., Sullo A., Viggiano A., De Luca V. (1999). Eating behavior is associated to sympathetic activation induced by ICV injection of prostaglandin E 1 in the rat. Nutr. Neurosci. 2, 69–74. 10.1080/1028415X.1999.11747264 [DOI] [PubMed] [Google Scholar]
- Monda M., Viggiano A., Tafuri D., Viggiano E., Chieffi S., De Luca V., et al. (2013). Orexin a induces sympathetic and thermogenic activation as a thermoregulatory peptide. Curr. Top. Pept. Protein Res. 14, 41–48. 10.3389/fpsyg.2014.00997 [DOI] [Google Scholar]
- Monda M., Viggiano A., Viggiano A., Mondola R., Viggiano E., Messina G., et al. (2008b). Olanzapine blocks the sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Peptides 29, 120–126. 10.1016/j.peptides.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Monda M., Viggiano A., Viggiano A., Viggiano E., Messina G., Tafuri D., et al. (2006b). Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides 40, 357–363. 10.1016/j.npep.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Monda M., Viggiano A., Viggiano A., Viggiano E., Messina G., Tafuri D., et al. (2007). Sympathetic and hyperthermic reactions by orexin A: role of cerebral catecholaminergic neurons. Regul. Pept. 139, 39–44. 10.1016/j.regpep.2006.10.002 [DOI] [PubMed] [Google Scholar]
- Morrison S. F., Madden C. J., Tupone D. (2014). Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 19, 741–756. 10.1016/j.cmet.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli F., Messina G., Valenzano A., Monda V., Viggiano A., Messina A., et al. (2016a). Functional assessment of corticospinal system excitability in karate athletes. PLoS ONE 11:e0155998. 10.1371/journal.pone.0155998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli F., Messina G., Valenzano A., Petito A., Triggiani A. I., Messina A., et al. (2016b). Differences in corticospinal system activity and reaction response between karate athletes and non-athletes. Neurol. Sci. 37, 1947–1953. 10.1007/s10072-016-2693-8 [DOI] [PubMed] [Google Scholar]
- Moscatelli F., Valenzano A., Petito A., Triggiani A. I., Ciliberti M. A. P., Luongo L., et al. (2016c). Relationship between blood lactate and cortical excitability between taekwondo athletes and non-athletes after hand-grip exercise. Somatosens. Mot. Res. 33, 137–144. 10.1080/08990220.2016.1203305 [DOI] [PubMed] [Google Scholar]
- NASPE T. F. O. E. A. (1996). Heart rate variability : standards of measurement, physiological interpretation, and clinical use. Circulation 93, 1043–1065. 10.1161/01.CIR.93.5.1043 [DOI] [PubMed] [Google Scholar]
- Nolan J., Batin P. D., Andrews R., Lindsay S. J., Brooksby P., Mullen M., et al. (1998). Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart). Circulation 98, 1510–1516. 10.1161/01.CIR.98.15.1510 [DOI] [PubMed] [Google Scholar]
- Oldfield B. J., Allen A. M., Davern P., Giles M. E., Owens N. C. (2007). Lateral hypothalamic “command neurons” with axonal projections to regions involved in both feeding and thermogenesis. Eur. J. Neurosci. 25, 2404–2412. 10.1111/j.1460-9568.2007.05429.x [DOI] [PubMed] [Google Scholar]
- Peyron C., Tighe D. K., van den Pol A. N., de Lecea L., Heller H. C., Sutcliffe J. G., et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi B., Guida F., Furiano A., Donniacuo M., Luongo L., Gritti G., et al. (2015). Effect of prolonged moderate exercise on the changes of nonneuronal cells in early myocardial infarction. Neural Plast. 2015:265967. 10.1155/2015/265967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski J. M., Stern J. H., Scherer P. E. (2015). The cell biology of fat expansion. J. Cell Biol. 208, 501–512. 10.1083/jcb.201409063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. 10.1016/S0092-8674(00)80949-6 [DOI] [PubMed] [Google Scholar]
- Sellayah D., Bharaj P., Sikder D. (2011). Orexin is required for brown adipose tissue development, differentiation, and function. Cell Metab. 14, 478–490. 10.1016/j.cmet.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Sellayah D., Sikder D. (2014). Orexin restores aging-related brown adipose tissue dysfunction in male mice. Endocrinology 155, 485–501. 10.1210/en.2013-1629 [DOI] [PubMed] [Google Scholar]
- Shen J., Tanida M., Yao J.-F., Niijima A., Nagai K. (2008). Biphasic effects of orexin-A on autonomic nerve activity and lipolysis. Neurosci. Lett. 444, 166–171. 10.1016/j.neulet.2008.08.031 [DOI] [PubMed] [Google Scholar]
- Shiraishi T., Oomura Y., Sasaki K., Wayner M. J. (2000). Effects of leptin and orexin-A on food intake and feeding related hypothalamic neurons. Physiol. Behav. 71, 251–261. 10.1016/S0031-9384(00)00341-3 [DOI] [PubMed] [Google Scholar]
- Skrapari I., Tentolouris N., Perrea D., Bakoyiannis C., Papazafiropoulou A., Katsilambros N. (2007). Baroreflex sensitivity in obesity: relationship with cardiac autonomic nervous system activity. Obesity (Silver Spring). 15, 1685–1693. 10.1038/oby.2007.201 [DOI] [PubMed] [Google Scholar]
- Smith M. M., Minson C. T. (2012). Obesity and adipokines: effects on sympathetic overactivity. J. Physiol. 590, 1787–1801. 10.1113/jphysiol.2011.221036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub L., Wolfrum C. (2015). FGF21, energy expenditure and weight loss-How much brown fat do you need? Mol. Metab. 4, 605–609. 10.1016/j.molmet.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Jayasena C. N., Bloom S. R. (2012). Obesity and appetite control. Exp. Diabetes Res. 2012:824305. 10.1155/2012/824305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill J. A., Halvorson I. (1994). Electrical stimulation of the posterior and ventromedial hypothalamic nuclei causes specific activation of shivering and nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 72, 89–96. 10.1139/y94-014 [DOI] [PubMed] [Google Scholar]
- Triggiani A. I., Valenzano A., Ciliberti M. A., Moscatelli F., Villani S., Monda M., et al. (2015). Heart rate variability is reduced in underweight and overweight healthy adult women. Clin. Physiol. Funct. Imaging. 37, 162–167. 10.1111/cpf.12281 [DOI] [PubMed] [Google Scholar]
- Tsuda K., Yoshimatsu H., Niijima A., Chiba S., Okeda T., Sakata T. (2002). Hypothalamic histamine neurons activate lipolysis in rat adipose tissue. Exp. Biol. Med. (Maywood). 227, 208–213. [DOI] [PubMed] [Google Scholar]
- Valenzano A., Moscatelli F., Triggiani A. I., Capranica L., De Ioannon G., Piacentini M. F., et al. (2016). Heart rate changes after ultra-endurance swim from italy to albania: a case report. Int. J. Sports Physiol. Perform. 11, 407–409. 10.1123/ijspp.2015-0035 [DOI] [PubMed] [Google Scholar]
- van Baak M. A. (2008). Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiol. Behav. 94, 178–186. 10.1016/j.physbeh.2007.12.020 [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W. D., Daanen H. A M. (2003). Cold-induced metabolism. Curr. Opin. Clin. Nutr. Metab. Care 6, 469–475. 10.1097/01.mco.0000078992.96795.5f [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M. A. F. L., Kemerink G. J., Bouvy N. D., et al. (2009). Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508. 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- Venner A., Karnani M. M., Gonzalez J. A., Jensen L. T., Fugger L., Burdakov D. (2011). Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. J. Physiol. 589, 5701–5708. 10.1113/jphysiol.2011.217000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano A., Chieffi S., Tafuri D., Messina G., Monda M., De Luca B. (2014). Laterality of a second player position affects lateral deviation of basketball shooting. J. Sports Sci. 32, 46–52. 10.1080/02640414.2013.805236 [DOI] [PubMed] [Google Scholar]
- Viggiano A., Nicodemo U., Viggiano E., Messina G., Viggiano A., Monda M., et al. (2010). Mastication overload causes an increase in O2 production into the subnucleus oralis of the spinal trigeminal nucleus. Neuroscience 166, 416–421. 10.1016/j.neuroscience.2009.12.071 [DOI] [PubMed] [Google Scholar]
- Viggiano E., Monda V., Messina A., Moscatelli F., Valenzano A., Tafuri D., et al. (2016). Cortical spreading depression produces a neuroprotective effect activating mitochondrial uncoupling protein-5. Neuropsychiatr. Dis. Treat. 12, 1705–1710. 10.2147/NDT.S107074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya F., Vidal-Puig A. (2013). Beyond the sympathetic tone: the new brown fat activators. Cell Metab. 17, 638–643. 10.1016/j.cmet.2013.02.020 [DOI] [PubMed] [Google Scholar]
- Vitali A., Murano I., Zingaretti M. C., Frontini A., Ricquier D., Cinti S. (2012). The adipose organ of obesity-prone C57BL/6J mice is composed of mixed white and brown adipocytes. J. Lipid Res. 53, 619–629. 10.1194/jlr.M018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle A. J., López M., Vidal-Puig A. (2011). Using brown adipose tissue to treat obesity - the central issue. Trends Mol. Med. 17, 405–411. 10.1016/j.molmed.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Woods S. C., Lotter E. C., McKay L. D., Porte D. (1979). Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505. 10.1038/282503a0 [DOI] [PubMed] [Google Scholar]
- Wu J., Cohen P., Spiegelman B. M. (2013). Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250. 10.1101/gad.211649.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ruan H.-B. (2015). Neuronal control of adaptive thermogenesis. Front. Endocrinol. (Lausanne: ). 6:149. 10.3389/fendo.2015.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorska-Markiewicz B., Kuagowska E., Kucio C., Klin M. (1993). Heart rate variability in obesity. Int. J. Obes. Relat. Metab. Disord. 17, 21–3. [PubMed] [Google Scholar]
- Zingaretti M. C., Crosta F., Vitali A., Guerrieri M., Frontini A., Cannon B., et al. (2009). The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120. 10.1096/fj.09-133546 [DOI] [PubMed] [Google Scholar]


