Abstract
The aim of this study was to evaluate the elemental composition of black tea samples and their infusions in view of their geographical origin. In total, 14 elements were analyzed, 13 (Ca, K, Mg, Na, Mn, Fe, Zn, Cu, Cr, Ni, Co, Cd, and Pb) by flame atomic absorption spectrometry, and P by UV-Vis spectrometry, after mineralization of samples. It was found that K was the most abundant macroelement in the analyzed samples, whereas among microelements, the highest concentration was found for Mn. Based on the obtained data, the percentage of elements leached into the infusions as well as the daily elemental intake from tea were calculated. The daily intake from tea was compared to the recommended daily allowances (RDAs), and the highest percentages of the RDAs were found for Mn (15 %) and Co (10 %). To study the relations between elemental composition and country of origin of samples, factor analysis and cluster analysis were applied. These multivariate techniques proved to be efficient tools able to differentiate samples according to their provenance as well as plantation within the common regions.
Keywords: Factor analysis, Cluster analysis, RDA, PTWI, Black tea, FAAS
Introduction
According to the Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT), total tea production was estimated at 5.3 million tons in 2011, which makes it the most commonly consumed beverage around the world. Tea production in Europe reaches 513,000 tons per year [1]. The main world tea producers are China and India (1.4 and 0.92 million tons, respectively) [2], whereas in Europe, the Russian Federation and the UK (184,000 tons and 129,000 tons, respectively) are the leading producers [1]. The countries with the highest rate of tea consumption include Paraguay and Afghanistan, 7.93 and 4.55 kg/year/person, respectively [2]. According to FAOSTAT data, Malta has the largest tea consumption per capita in Europe amounting to 2.24 kg/year [1], followed by the UK and Ireland, whereas Poland is in seventh place with 0.92 kg/year [1]. According to Polish literature, the average Polish resident consumes 2–3 cups of tea per day, and 20 % of the population consumes 4–5 cups per day [3]. Many studies have reported positive effects of tea consumption on human health, such as cancer prevention, diabetes management by reducing glucose and cholesterol levels in blood, and improved immune defense [4]. Most of the tea world consumption is black tea (80 %), whereas the remaining 20 % belong to green, oolong, red, and yellow types of this beverage. The price of tea products as well as consumer interests are usually connected with the certified geographical origin [5]. Therefore, it is important to have tools that are able to ensure good quality of products, which is usually associated with their geographical provenance. There are several studies of authentication of tea origin, using multivariate chemometric techniques, such as principal component analysis (PCA), factor analysis (FA), cluster analysis (CA), or linear discriminant analysis (LDA) [5]. These techniques have been applied to elemental and organic composition data, obtained using various analytical techniques including HPLC [6–8], GC-MS [9–11], 1HNMR [12], FT-NIRS [13], ICP-MS [5, 14–16], and FAAS [17–20]. Our previous studies have showed that FA and CA are efficient tools of green, fruit, and Pu-erh tea diversification [19, 20]. These two multivariate techniques applied to green tea data enabled differentiation of samples according to their provenance [19]. Moreover, it was also possible to diversify Pu-erh tea according to its type of confection, whereas fruit teas were differentiated according to their type [20]. Such information is of great importance to consumers, who expect that price is an equivalent of good quality products.
Therefore, in this study, we aimed to verify the geographical origin of commercialized black tea samples applying factor analysis (FA) and cluster analysis (CA) to their elemental composition. These techniques were found useful to determine the country of origin of the tea as well as its provenance within a single country. In addition, the percentage of elements leached into the tea infusions was determined, and the daily elemental intake from tea was calculated and compared to the recommended daily allowances (RDAs). For Cd and Pb, the intake from tea was compared to the provisional tolerable weekly intake (PTWI).
Materials and Methods
Samples
The analyzed tea samples (loose form and tea bags) were purchased from markets and tea shops (original tea) in Poland, and analyzed for their content of 14 elements: K, Na, Ca, Mg, Mn, Zn, Cu, Fe, P, Co, Ni, Cr, Cd, and Pb. In total, 118 types of black tea from different producers were tested, i.e., 708 analytical samples of black tea leaves and their infusions were prepared. Of the 118 tea types analyzed, 43 were purchased in tea shops (12 Chinese, 17 Indian, 10 Ceylon, and 4 Kenyan teas), and 75 in markets (loose form and tea bags) (Table 1).
Table 1.
Characteristics of the analyzed products
| No. | Name of tea | Producer | Country/producer declaration | Confection |
|---|---|---|---|---|
| Original tea | ||||
| 1. | Ceylon FBOPF “Malatiyana” | Maraska | Ceylon | Loose |
| 2. | Ceylon OP “Lumbini” | Maraska | Ceylon | Loose |
| 3. | Ceylon “Kendy” | Maraska | Ceylon | Loose |
| 4. | Ceylon UVA OPI “Ivy Hills” | Maraska | Ceylon | Loose |
| 5. | Ceylon Dimbula | Five o’clock | Ceylon | Loose |
| 6. | Ceylon Raigama Korales | Five o’clock | Ceylon | Loose |
| 7. | Ceylon Pothotuwa | Five o’clock | Ceylon | Loose |
| 8. | Ceylon Sithaka FBOPFEXS W | Five o’clock | Ceylon | Loose |
| 9. | Ceylon Earl Grey | Five o’clock | Ceylon | Loose |
| 10. | Ceylon High Grown | Time to tea | Ceylon | Loose |
| 11. | Yunnan Golden OP | Maraska | China | Loose |
| 12. | Golden Monkey | Maraska | China | Loose |
| 13. | China OP Keemun | Maraska | China | Loose |
| 14. | Yunnan Special Black | Five o’clock | China | Loose |
| 15. | China Keemun Mao Feng | Five o’clock | China | Loose |
| 16. | Golden Yunnana | Five o’clock | China | Loose |
| 17. | Lapsang Souchonga | Five o’clock | China | Loose |
| 18. | China Black Golden Monkey | Five o’clock | China | Loose |
| 19. | Lapsang Souchonga | Time to tea | China | Loose |
| 20. | China Keemun Congu | Time to tea | China | Loose |
| 21. | Yunnan Black Premium | Time to tea | China | Loose |
| 22. | Golden Yunnana | Time to tea | China | Loose |
| 23. | Darjeeling FTGFOPI “Himalaya” | Maraska | India | Loose |
| 24. | Assam TGFOPI “Dikom” | Maraska | India | Loose |
| 25. | Assam TGFOP “Ambaguri” | Maraska | India | Loose |
| 26. | Assam Jamguri FTGFOP1 | Five o’clock | India | Loose |
| 27. | Assam Dagapur | Five o’clock | India | Loose |
| 28. | Darjeeling Castleton 2011 WP | Five o’clock | India | Loose |
| 29. | Assam Satishpur TGFOP W | Five o’clock | India | Loose |
| 30. | Assam Marangi FTGFOP1 | Five o’clock | India | Loose |
| 31. | Darjeeling Thurbo FTGFOP | Five o’clock | India | Loose |
| 32. | Assam Halmari GTGFOP CL W | Five o ‘clock | India | Loose |
| 33. | Darjeeling Margarets Hope | Five o’clock | India | Loose |
| 34. | Assam Dikom | Time to tea | India | Loose |
| 35. | Assam Sec. Flush | Time to tea | India | Loose |
| 36. | Assam Dekorai | Time to tea | India | Loose |
| 37. | Darjeeling Gielle | Time to tea | India | Loose |
| 38. | Darjeeling First Flush | Time to tea | India | Loose |
| 39. | Darjeeling Sec. Flush | Time to tea | India | Loose |
| 40. | Kenia GFOP “Milima | Maraska | Kenia | Loose |
| 41. | Kenia TGFOP Golden Tipped | Maraska | Kenia | Loose |
| 42. | Kenia Marinyn | Five o’clock | Kenia | Loose |
| 43. | Ruanda Rukeri | Five o’clock | Kenia | Loose |
| Marketed tea | ||||
| 1. | English Breakfasta | Ahmad Tea | Ceylon | Loose |
| 2. | Ceylon Tea | Ahmad Tea | Ceylon | Loose |
| 3. | Earl Grey Teaa | Ahmad Tea | Ceylon | Loose |
| 4. | Earl Greya | Ahmad Tea | Ceylon | Bags |
| 5. | English Breakfasta | Ahmad Tea | Ceylon | Bags |
| 6. | English No.1 | Ahmad Tea | Ceylon | Bags |
| 7. | Assama | Ahmad Tea | Ceylon | Bags |
| 8. | Ceylon | Ahmad Tea | Ceylon | Bags |
| 9. | English V.I.P Tea | Brizton | Ceylon | Bags |
| 10. | English Royal Teaa | Chelton Tea Collection | Ceylon | Loose |
| 11. | Scottish Breakfast | Chelton Tea Collection | Ceylon | Loose |
| 12. | English Royal Teaa | Chelton Tea Collection | Ceylon | Bags |
| 13. | Ceylon Supreme Tea | Dilmah | Ceylon | Loose |
| 14. | Earl Grey Teaa | Dilmah | Ceylon | Loose |
| 15. | Meda Watte | Dilmah | Ceylon | Loose |
| 16. | Ran Watte | Dilmah | Ceylon | Loose |
| 17. | Uda Watte | Dilmah | Ceylon | Loose |
| 18. | English Breakfast Tea | Dilmah | Ceylon | Loose |
| 19. | English Afternoon Tea | Dilmah | Ceylon | Bags |
| 20. | Premium Tea | Dilmah | Ceylon | Bags |
| 21. | Ceylon Gold | Dilmah | Ceylon | Bags |
| 22. | Perfect Ceylon Tea | Dilmah | Ceylon | Bags |
| 23. | Elegant Earl Grey | Dilmah | Ceylon | Bags |
| 24. | Ceylon OP | Drury | Ceylon | Loose |
| 25. | Royal Ceylan | Lipton | Ceylon | Loose |
| 26. | Yellow Label Teaa | Lipton | Ceylon | Bags |
| 27. | Gold Tea Black | Lipton | Ceylon | Bags |
| 28. | Mild Ceylon | Lipton | Ceylon | Bags |
| 29. | Earl Greya | Sir Roger | Ceylon | Bags |
| 30. | Ceylon Gold | Sir William’s | Ceylon | Bags |
| 31. | Black Tea Ceylon | Sir William’s | Ceylon | Bags |
| 32. | Super Pekoe | Tarlton | Ceylon | Loose |
| 33. | Ceylon Orange Pekoe Tea | Twinings | Ceylon | Bags |
| 34. | Yunnan | Loyd Tea | China | Loose |
| 35. | Prince of Wales | Twinings | China | Bags |
| 36. | Black Teaa | Yunnan | China | Loose |
| 37. | Darjeelinga | Ahmad Tea | India | Loose |
| 38. | Darjeelinga | Ahmad Tea | India | Loose |
| 39. | Assama | Ahmad Tea | India | Loose |
| 40. | Darjeelinga | Darvilles of Windsor | India | Loose |
| 41. | Royalty Assam | Darvilles of Windsor | India | Loose |
| 42. | Maharajah Reseve Assam | Dilmah | India | Bag |
| 43. | Rich Assam | Lipton | India | Bags |
| 44. | Darjeelinga | Premier’s Tea Limited | India | Loose |
| 45. | Earl Greya | Premier’s Tea Limited | India | Loose |
| 46. | Darjeeling SFTGFOP1 | Rich Mont | India | Loose |
| 47. | Earl Greya | Sir William’s | India | Bags |
| 48. | Intensive Tea | Tetley | India | Bags |
| 49. | Darjeeling Teaa | Twinings | India | Loose |
| 50. | Darjeeling Teaa | Twinings | India | Bags |
| 51. | English Tea No.1 | Ahmad Tea | – | Loose |
| 52. | English Breakfasta | Time to tea | – | Loose |
| 53. | Earl Greya | Time to tea | – | Loose |
| 54. | English Breakfasta | Darvilles of Windsor | – | Loose |
| 55. | Earl Greya | Darvilles of Windsor | – | Loose |
| 56. | Earl Greya | Dilmah | – | Bags |
| 57. | Daily Superior | Irving | – | Loose |
| 58. | Daily Classic | Irving | – | Bags |
| 59. | Earl Greya | Irving | – | Bags |
| 60. | Russian Earl Grey | Lipton | – | Loose |
| 61. | Yellow Label Teaa | Lipton | – | Loose |
| 62. | Earl Grey Classic | Lipton | – | Bags |
| 63. | Taste of London | Lipton | – | Bags |
| 64. | Earl Greya | Loyd Tea | – | Loose |
| 65. | Fairtrade Luxury Gold Tea | Marks & Spancer | – | Bags |
| 66. | Earl Greya | Maraska | – | Loose |
| 67. | Black Teaa | Minutka | – | Bags |
| 68. | Black Teaa | Saga | – | Loose |
| 69. | Black Teaa | Saga | – | Bags |
| 70. | Earl Greya | Saga | – | Bags |
| 71. | English Breakfasta | Twinings | – | Loose |
| 72. | Earl Greya | Twinings | – | Loose |
| 73. | Prince of Wales Tea | Twinings | – | Loose |
| 74. | Earl Greya | Twinings | – | Bags |
| 75. | Simply Tea | Twinings | – | Bags |
aVarious producer or confections of tea the same name
Preparation of Samples and Elemental Analysis
The bulk teas were homogenized and representative samples were mineralized in an electric furnace and then analyzed by flame atomic absorption spectrometry (FAAS) according to the previously published procedure by Brzezicha-Cirocka et al. [19, 20].
Method Validation
The limit of detection (LOD) and limit of quantification (LOQ) for all the analyzed elements were calculated based on the independently prepared blank samples measurements. According to the method described by Konieczka and Namieśnik [21], LODs were set to blank means +3SD, where blank mean is a result of all blank samples measurements and SD is their standard deviation; whereas LOQ was calculated by multiplying LOD by a factor of three. Data for the validation procedure are given in Table 2.
Table 2.
Results of the validation procedure of the analytical methodology
| Element | Linearity | LOD (mg/100 g) | LOQ (mg/100 g) | ||
|---|---|---|---|---|---|
| Calibration curve range (μg/mL) | Calibration curve | R 2 | |||
| Ca | 2.00–15.0 | y = 0.05801× + 0.0089 | 0.999 | 0.020 | 0.060 |
| K | 0.50–1.50 | y = 0.00048× + 0.0149 | 0.997 | 0.040 | 0.120 |
| Mg | 0.10–0.90 | y = 0.00107× + 0.0220 | 0.998 | 0.020 | 0.060 |
| Na | 0.50–1.20 | y = 0.00071× + 0.0192 | 0.996 | 0.020 | 0.060 |
| P | 0.10–1.20 | y = 0.00444× + 0.0117 | 0.999 | 0.030 | 0.090 |
| Mn | 0.15–5.00 | y = 0.00015× + 0.0058 | 0.999 | 0.020 | 0.060 |
| Fe | 1.00–10.0 | y = 0.00006× + 0.0082 | 0.996 | 0.010 | 0.030 |
| Zn | 0.20–1.50 | y = 0.00034× + 0.0079 | 0.998 | 0.020 | 0.060 |
| Cu | 0.50–4.00 | y = 0.00013× + 0.0022 | 0.999 | 0.009 | 0.027 |
| Co | 1.00–5.00 | y = 0.00008× + 0.0052 | 0.999 | 0.003 | 0.009 |
| Cd | 0.20–2.00 | y = 0.00035× + 0.0040 | 0.999 | 0.003 | 0.009 |
| Cr | 0.20–2.00 | y = 0.00005× + 0.0007 | 0.999 | 0.001 | 0.003 |
| Ni | 0.50–2.00 | y = 0.00008× + 0.0007 | 0.999 | 0.002 | 0.006 |
| Pb | 0.20–2.00 | y = 0.00004× + 0.0004 | 0.999 | 0.004 | 0.012 |
The reliability of the method was determined using certified reference materials, i.e., Oriental Basma Tobacco Leaves (INCT-OBTL-5) and Polish Virginia Tobacco Leaves (INCT-PVTL-6). They were prepared according to the same procedure as the analytical samples. Recoveries of the studied elements ranged between 87 and 113 % (RSDs between 0.02–10.3 %) of the certified values for all the elements (Table 3).
Table 3.
Element concentrations and RSD with recovery data for the certified reference materials Oriental Basma Tobacco Leaves (INCT-OBTL-5) and Polish Virginia Tobacco Leaves (INCT-PVTL-6)
| Element | Certified values (mg/100 g) | Determined values (mg/100 g) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Caa | 3859 ± 142 | 3566 ± 149 | 4.17 | 92 |
| Cab | 2297 ± 78 | 2513 ± 13.4 | 0.50 | 109 |
| Coa | 0.10 ± 0.007 | 0.09 ± 0.005 | 5.65 | 90 |
| Cua | 1.01 ± 0.04 | 1.00 ± 0.01 | 1.42 | 99 |
| Cub | 0.51 ± 0.02 | 0.54 ± 0.01 | 2.00 | 106 |
| Cda | 0.26 ± 0.01 | 0.28 ± 0.01 | 3.48 | 105 |
| Cdb | 0.22 ± 0.01 | 0.21 ± 0.001 | 0.40 | 95 |
| Cra | 0.63c | 0.56 ± 0.0001 | 0.02 | 89 |
| Crb | 0.09c | 0.09 ± 0.001 | 1.30 | 98 |
| Mga | 853 ± 34 | 845 ± 4.96 | 0.59 | 99 |
| Mgb | 241 ± 9 | 247 ± 6.01 | 2.40 | 102 |
| Mna | 18.0 ± 0.6 | 20.2 ± 0.34 | 1.67 | 112 |
| Mnb | 13.6 ± 0.5 | 15.3 ± 0.18 | 1.20 | 113 |
| Zna | 5.24 ± 0.18 | 5.59 ± 0.24 | 4.31 | 107 |
| Znb | 4.36 ± 0.1 | 4.64 ± 0.01 | 0.30 | 106 |
| Ka | 2271 ± 76 | 2449 ± 50.3 | 2.06 | 108 |
| Kb | 2640 ± 90 | 2692 ± 31.6 | 1.20 | 102 |
| Nab | 6.24c | 5.50 ± 0.03 | 0.50 | 88 |
| Pba | 0.20 ± 0.03 | 0.19 ± 0.01 | 5.60 | 95 |
| Pbb | 0.10 ± 0.01 | 0.09 ± 0.01 | 10.3 | 89 |
| Pa | 170 ± 12 | 170 ± 0.41 | 0.24 | 100 |
| Pb | 242 ± 5 | 239 ± 0.96 | 0.40 | 99 |
| Nia | 0.85 ± 0.05 | 0.80 ± 0.078 | 9.80 | 94 |
| Nib | 0.15 ± 0.01 | 0.13 ± 0.001 | 0.6 | 87 |
| Fea | 149c | 160 ± 1.01 | 0.63 | 107 |
| Feb | 25.8c | 28 ± 0.30 | 1.1 | 109 |
aOriental Basma Tobacco Leaves INCT-OBTL-5
bPolish Virginia Tobacco Leaves INCT-PVTL-6
cInformation value
Statistical Analysis
The Shapiro-Wilk test showed that the data were not normally distributed; therefore, nonparametric tests were applied [22]. Moreover, data standardization was adopted, and the correlation analysis was performed using Spearman rank analysis. Kruskal-Wallis test, factor analysis, and cluster analysis were conducted in order to obtain statistically significant information about the quality and origin of samples.
Results and Discussion
Macroelements
The highest contents of Ca and Na were found in marketed teas (264 and 89.3 mg/100 g, respectively), whereas Indian and Kenyan teas were characterized by the lowest Na level (30.7 and 21.8 mg/100 g, respectively). Products from China, India, Ceylon, and Kenya had similar amounts of Ca (153–168 mg/100 g). Comparable Ca results (215 mg/100 g) were obtained by Dambiec et al. [23], whereas similar Na levels (88 mg/100 g) were reported by Soomro et al. [24] and Yemane et al. [25]. Among all macroelements, the highest levels were found for K (2349–2981 mg/100 g). Considerable variation was found in the content of Mg, as the highest levels amounted to 822 mg/100 g in Indian teas, and the lowest to 518 mg/100 g in marketed teas (Table 4). The latter value is higher than the one obtained by Gerbresadik and Chandravanshi [26] (354 mg/100 g). Indian, Ceylon, and Kenyan products contained similar amounts of P (305–359 mg/100 g), and Chinese tea had the highest P concentration (408 mg/100 g). Malik et al. [27] published comparable results for P (366 mg/100 g). Dambiec et al. [23] estimated a percentage of Na leaching (45.3 %) similar to ours (40.0 %), while Szymczycha-Madeja et al. [17] classified this macroelement as highly extractable (>55 %), which was confirmed in the case of teas from Kenya (62.0 %). The percentages of Ca leaching were the highest in Chinese teas (17.2 %) and the lowest in Kenyan teas (5.89 %). The lowest percentage of Mg leaching was found in Chinese teas (29.6 %), which is comparable to findings reported by Dambiec et al. [23]. Magnesium and P leaching percentages ranged between 29.6–39.7 and 26.8–38.2 %, respectively. The average percentage of Mg extraction to infusions (35.3 %) obtained by Dambiec et al. [23] and Gallaher et al. [28] is comparable to our results.
Table 4.
Concentration of bioelements and toxic metals in dry tea samples in milligrams/100 g ( ± SD range) and percentage of leaching
| Elements | China | India | Ceylon | Kenya | Marketed |
|---|---|---|---|---|---|
| n | 12 × 3 | 17 × 3 | 10 × 3 | 4 × 3 | 75 × 3 |
| Ca | 161 ± 101 | 153 ± 35 | 168 ± 51 | 155 ± 33 | 264 ± 52 |
| (22–449) | (105–220) | (87–250) | (105–193) | (133–420) | |
| 17.2 ± 12.0 % | 11.1 ± 8.22 % | 9.54 ± 6.37 % | 5.89 ± 1.60 % | 11.1 ± 7.06 % | |
| K | 2666 ± 223 | 2803 ± 161 | 2981 ± 153 | 2738 ± 119 | 2349 ± 271 |
| (2322–2992) | (2474–3084) | (2656–3207) | (2536–2838) | (1845–3057) | |
| 28.3 ± 8.03 % | 33.6 ± 4.65 % | 30.6 ± 6.25 % | 28.4 ± 4.27 % | 23.1 ± 4.08 % | |
| Mg | 764 ± 77 | 822 ± 103 | 769 ± 41.7 | 774 ± 63.1 | 518 ± 253 |
| (669–936) | (601–1052) | (704–829) | (699–873) | (164–901) | |
| 29.6 ± 8.26 % | 36.5 ± 3.07 % | 35.6 ± 3.59 % | 39.7 ± 4.37 % | 35.1 ± 11.8 % | |
| Na | 67.3 ± 61.4 | 30.7 ± 11.5 | 55.5 ± 31.0 | 21.8 ± 6.06 | 89.3 ± 126 |
| (24.2–267) | (17.5–60.3) | (23.9–122) | (13.4–30.5) | (10.4–728) | |
| 29.7 ± 11.4 % | 47.8 ± 18.2 % | 32.6 ± 12.2 % | 62.0 ± 12.7 % | 27.7 ± 20.7 % | |
| Mn | 30.9 ± 11.8 | 30.4 ± 10.5 | 27.7 ± 10.3 | 53.2 ± 12.6 | 56.3 ± 28.4 |
| (11.7–59.1) | (12.8–49.0) | (16.4–50.0) | (33.2–68.2) | (16.1–143) | |
| 41.4 ± 14.4 % | 44.8 ± 11.3 % | 39.1 ± 8.53 % | 44.3 ± 9.51 % | 25.1 ± 7.00 % | |
| P | 408 ± 58.0 | 359 ± 39.9 | 305 ± 52.8 | 344 ± 25.3 | 296 ± 66 |
| (317–482) | (309–460) | (236–434) | (310–380) | (198–490) | |
| 34.1 ± 9.50 % | 34.7 ± 8.66 % | 26.8 ± 8.53 % | 31.6 ± 5.35 % | 38.2 ± 7.66 % | |
| Fe | 0.90 ± 0.26 | 0.57 ± 0.29 | 0.43 ± 0.09 | 0.53 ± 0.01 | 0.73 ± 0.37 |
| (0.37–1.35) | (0.30–1.22) | (0.30–0.63) | (0.51–0.54) | (0.23–2.50) | |
| 38.7 ± 17.9 % | 38.0 ± 26.1 % | 36.7 ± 22.0 % | 29.1 ± 9.62 % | 37.5 ± 23.8 % | |
| Zn | 4.25 ± 0.63 | 3.70 ± 0.60 | 2.70 ± 0.52 | 2.74 ± 0.22 | 3.29 ± 0.67 |
| (3.26–5.72) | (2.92–5.05) | (2.11–3.91) | (2.50–2.99) | (2.04–5.22) | |
| 34.6 ± 11.2 % | 36.2 ± 10.6 % | 41.3 ± 10.6 % | 45.1 ± 4.11 % | 33.9 ± 11.3 % | |
| Cu | 2.25 ± 0.42 | 2.39 ± 0.50 | 1.83 ± 0.14 | 1.85 ± 0.17 | 2.28 ± 0.57 |
| (1.55–2.91) | (1.67–3.34) | (1.62–2.07) | (1.67–2.07) | (1.26–3.98) | |
| 15.4 ± 3.42 % | 16.9 ± 2.57 % | 15.0 ± 1.75 % | 14.1 ± 1.91 % | 18.9 ± 5.34 % | |
| Co | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| (0.01–0.04) | (0.01–0.04) | (0.01–0.05) | (0.01–0.04) | (0.01–0.05) | |
| 39.4 ± 21.3 % | 40.0 ± 25.1 % | 43.6 ± 20.0 % | 33.9 ± 4.41 % | 33.4 ± 18.7 % | |
| Cd | 0.005 ± 0.001 | ||||
| (<LOD-0.007) | <LOD | <LOD | <LOD | <LOD | |
| <LOD | |||||
| Cr | 0.08 ± 0.02 | 0.07 ± 0.03 | 0.05 ± 0.01 | 0.11 ± 0.06 | 0.17 ± 0.16 |
| (0.04–0.11) | (0.04–0.12) | (0.04–0.06) | (0.04–0.18) | (0.04–1.28) | |
| 24.1 ± 8.48 % | 34.1 ± 13.4 % | 28.2 ± 9.34 % | 31.7 ± 6.95 % | 28.1 ± 13.3 % | |
| Ni | 0.50 ± 0.21 | 0.58 ± 0.13 | 0.35 ± 0.10 | 0.36 ± 0.05 | 0.42 ± 0.15 |
| (0.22–1.01) | (0.28–0.85) | (0.22–0.63) | (0.29–0.44) | (0.10–0.93) | |
| 56.2 ± 18.6 % | 67.7 ± 11.3 % | 68.8 ± 17.2 % | 75.8 ± 17.0 % | 64.0 ± 24.4 % | |
| Pb | 0.05 ± 0.04 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.013 ± 0.003 | 0.03 ± 0.04 |
| (0.01–0.15) | (<LOD-0.06) | (0.01–0.04) | (0.01–0.02) | (<LOD-0.32) | |
| 33.9 ± 21.3 % | 18.9 ± 22.2 % | 28.7 ± 28.6 % | 45.4 ± 22.4 % | 27.4 ± 26.4 % |
LOD for Cd = 0.003 mg/100 g; LOD for Pb = 0.004 mg/100 g
n number of samples multiplied by number of analytical subsample
Microelements
Manganese was the microelement found in the highest concentrations in our study, and the highest levels were determined in Kenyan and marketed teas (53.2 and 56.3 mg/100 g, respectively). Shaltout and Abd-Elkader [29] reported a slightly higher average Mn content (61.8 mg/100 g). Chinese samples had the highest Fe and Zn content (0.90 and 4.25 mg/100 g, respectively). According to Mupenzi et al. [30], low Fe concentration can be induced by high Mn levels. What is more, high Fe levels can cause Mn deficiency in tea plants. The determined amounts of Zn are comparable to those obtained by Al-Oud [31] for Chinese and Indian teas (2.67–5.39 mg/100 g). Copper levels in samples from Ceylon and Kenya (1.83 and 1.85 mg/100 g) were comparable and slightly lower than in Chinese, Indian, and marketed teas (2.25, 2.39, and 2.28 mg/100 g, respectively). Similar Cu results were reported by McKenzie et al. [32] (1.70 mg/100 g). Cobalt levels, which amounted to 0.02 mg/100 g, were comparable to those reported by Shaltout and Abd-Elkader [29]. Chinese and Indian samples had similar amounts of Cr (0.08 and 0.07 mg/100 g) and Ni (0.50 and 0.58 mg/100 g). Similar results were obtained by Shaltout and Abd-Elkader [29] for Ni (0.61 mg/100 g) and by Barone et al. [4] for Cr (0.04 mg/100 g). Barone et al. [4] reported in their study that Chinese tea samples generally had higher levels of elements than those originating from India.
The lowest percentage of leaching of Mn (25.1 %) was found in marketed teas and the highest (44.8 %) in Indian teas. For Fe and Zn, percentages of leaching showed similar levels in all the analyzed tea samples (29.1–38.7 and 33.9–45.1 %, respectively). Among all the analyzed elements, Ni showed the highest percentage of leaching, which ranged from 56.2 % (Chinese tea) to 75.8 % (Kenyan tea).
Many factors influence the contents of trace metals in tea leaves. According to Milani et al. [33], variations in mineral composition of tea leaves can be explained by the age of leaves used in the production (old or young), soil composition, rainfall amount, and growing conditions in general. Unfortunately, commercial teas often have unknown geographical origin, as they are a mixture of leaves from different locations [33]. Black tea is produced through leaf fermentation in contrast to green tea, which results in higher levels of certain trace elements [34]. Regulations have been established for many vegetable products in many countries, but the European Union has no specified regulations about the acceptable metal content in tea.
Toxic Metals
In general, the analyzed samples contained more Pb than Cd. The levels of Cd varied little, i.e., 0.003–0.005 mg/100 g. Similar results for Cd were obtained by Milani et al. [33] (0.001–0.002 mg/100 g) and significantly lower ones by Barone et al. [4] (0.0004 mg Cd/100 g). Chinese teas had the highest Pb content (0.05 mg/100 g), which is comparable with the results reported by Barone et al. [4] (0.05 mg Pb/100 g). The percentage of leaching of Pb to infusions varied considerably, as the highest was found for Kenyan teas (45.4 %) and the lowest for Indian ones (18.9 %).
The contents of heavy metals in tea leaves may be a result of contamination that can be caused by many factors such as use of various manufacturing and agronomic processes and of fertilizers [29, 35, 36]. Among the main sources of Pb in the environment are leaded fuel, waste incineration, and industry [37]. Lead pollution is correlated with urbanization and population density. Moreover, higher levels of this heavy metal in tea samples can be attributed to contamination during the process of tea production and its packing [37]. Cadmium found in tea leaves might be a result of phosphate and zinc fertilizers usage [38]. It is estimated that Cd from phosphate fertilizers constitutes >50 % of the total input to agricultural land not heavily polluted or heavily industrialized [39, 40]. Sarma et al. [41] reported that heavy metal contaminations of tea leaves might be explained by the position of the tea cultivation area. In that study, an Assam plantation was situated in the vicinity of the oldest crude oil exploration station, and accidental spillage during drilling and transportation could be the main sources of tea field contamination. Moreover, it has been established that heavy metals enter into plant bodies in acidic soil, in which tea usually grows [42].
Correlation
Nonparametric Spearman’s rank test was performed at three levels of significance (p < 0.05, p < 0.01, p < 0.001), and both positive and negative correlations were found between the analyzed elements. The most significant positive correlations (p < 0.001) were found for P-Zn, Mn-Co, and Mn-Cr for all the countries. There were also recorded important interelement correlations among black tea samples from Indian and Ceylon plantations. Strong interdependences (p < 0.01) were also found between Ca-Mn, P-Zn, Mn-Co, Zn-Ni, and Cu-Co.
Kruskal-Wallis Test
Through the Kruskal-Wallis test, it was possible to determine statistically significant differences in the analyzed database. Relationships were found between the geographical provenance of tea and concentrations of elements including the following: Na (H = 18.596; p = 0.001), P (H = 14.533; p = 0.001), Mn (H = 9388; p = 0.025), Fe (H = 12.002; p = 0.007), Zn (H = 26.166; p = 0.000), Cu (H = 12.861; p = 0.005), and Ni (H = 11.929; p = 0.008). Dunn’s test was also performed, confirming the outcome of the Kruskal-Wallis test. The results of Dunn’s test are shown in Table 5. Kruskal-Wallis test and Dunn’s test (Table 6) were also performed for tea samples from Asia, i.e., India (plantation Assam and Darjeeling) and the island of Ceylon. Relationships were found between provenance of tea and concentrations of several elements: Na (H = 10.739; p = 0.005), P (H = 10.992; p = 0.004), Mn (H = 7.218; p = 0.027), Fe (H = 11.833; p = 0.003), Zn (H = 14.679; p = 0.001), Cu (H = 13.443; p = 0.001), Cr (H = 7.382; p = 0.025) and Ni (H = 12.232; p = 0.002).
Table 5.
Results of the post hoc Dunn’s test conducted for the analyzed data matrix for tea samples from Ceylon, China, India, and Kenya. There are only given elements for which p < 0.05
| Ceylon | China | India | Kenya | |
|---|---|---|---|---|
| Ceylon | – | P, Zn, Fe | Cu, Na, Ni, Zn | Na |
| China | P, Zn, Fe | – | Na, Fe | Na, Zn |
| India | Cu, Na, Ni, Zn | Na, Fe | – | |
| Kenya | Na | Na, Zn | – |
Table 6.
Results of the post hoc Dunn’s test conducted for the analyzed data matrix for tea samples from India and Ceylon. There are only given elements for which p < 0.05
| Ceylon | Assam | Darjeeling | |
|---|---|---|---|
| Ceylon | – | Na, Cu, Cr, Ni | P, Fe, Zn, Ni |
| Assam | Na, Cu, Cr, Ni | – | Mn, Fe |
| Darjeeling | P, Fe, Zn, Ni | Mn, Fe | – |
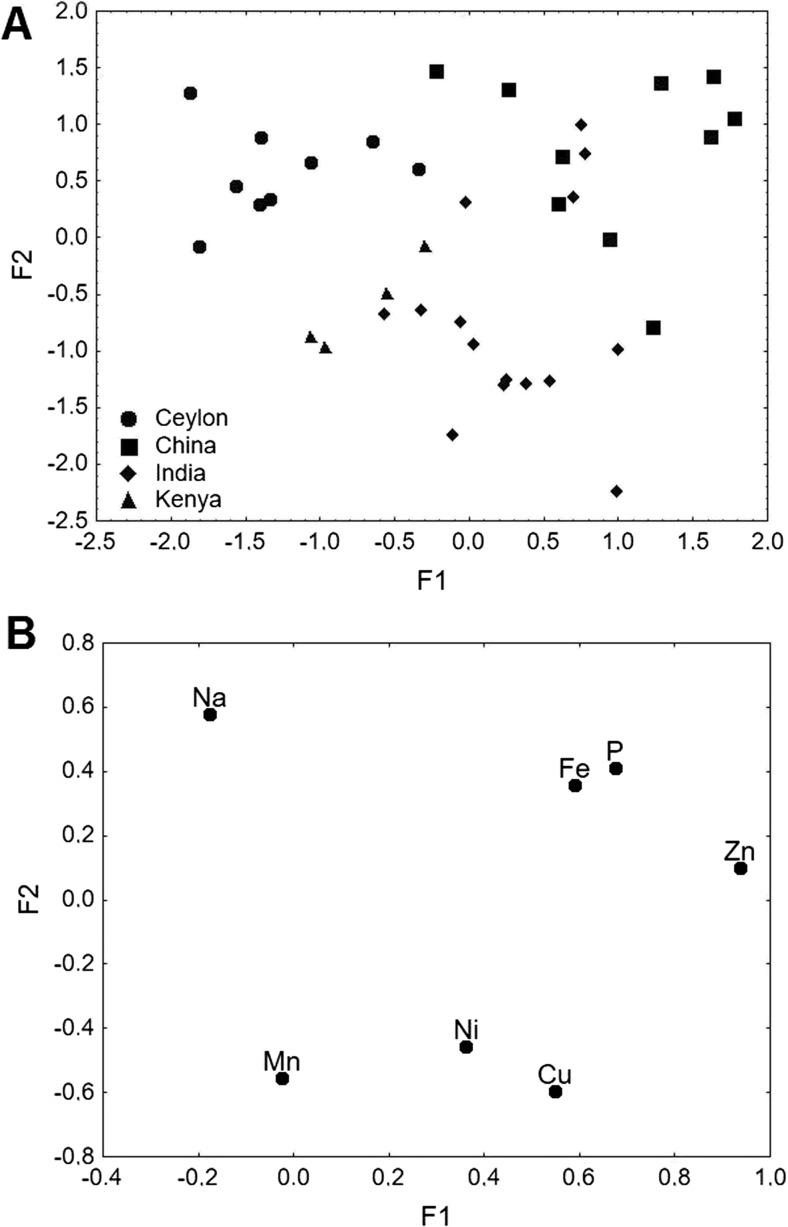
Factor Analysis
The results of the factor analysis (FA), conducted on raw data sets of black tea obtained from tea shops, are shown in Fig. 1a and b. Factor analysis performed with all the analyzed metals did not give clear output, and thus, we decided to narrow the data set to the elements Na, Mn, Ni, Cu, Fe, P, and Zn. Factor analysis was applied to data of elements which were found significant in the Kruskal-Wallis test. The final choice of descriptors was done by a series of factor analyses, which were performed in order to verify the clarity of the outcome. As a result, two factors were obtained, i.e., F1 (35.7 % of the total variance) and F2 (23.5 % of the total variance). Both factors cumulatively explain 59.2 % of the total variance, whereas the eigenvalues for F1 and F2 are 2.14 and 1.41, respectively. As can be seen in Fig. 1a, samples from all the analyzed regions can be distinguished, i.e., Ceylon, China, India, and Kenya. The scatter plot of loadings was drawn for F1–F2 in order to identify elements responsible for the grouping of objects (Fig. 1b). Higher values of F1 correspond to Indian and Chinese samples, which were described by Ni, Cu, Fe, P, and Zn. Phosphorus, Fe, and Zn were responsible for differentiation of Chinese tea, whereas the highest amounts of Ni and Cu were noted in Indian samples. Such high levels of these metals in samples of these two origins could possibly be explained by product contamination during its manufacture or area pollution. Simultaneously, high amounts of P in Chinese samples may be related to the increasing use of fertilizers, which was confirmed by research conducted by Mupenzi et al. [30].
Fig. 1.
a Scatter plot of objects samples of two factors of the all tea samples from Ceylon, China, India, and Kenya. b Scatter plot of loading for elements in all the analyzed tea samples from Ceylon, China, India, and Kenya
Lower values of F1 characterize Kenyan and Ceylon tea samples which are described by Mn and Na (Fig. 1a, b). Kenyan tea was differentiated by Mn as significant differences were found in Mn concentrations between these two groups of tea. Sodium was found to be a descriptor of Ceylon teas, which may be associated with geographical location of the plantation, as Ceylon is an island surrounded by the waters of the Indian Ocean. Higher values of F2 described Ceylon, Chinese, and partly Indian tea samples, which can be identified by Na, Fe, P, and Zn. Lower values of F2 were associated with Indian and Kenyan samples, which were differentiated by Mn, Ni, and Cu (Fig. 1a, b).
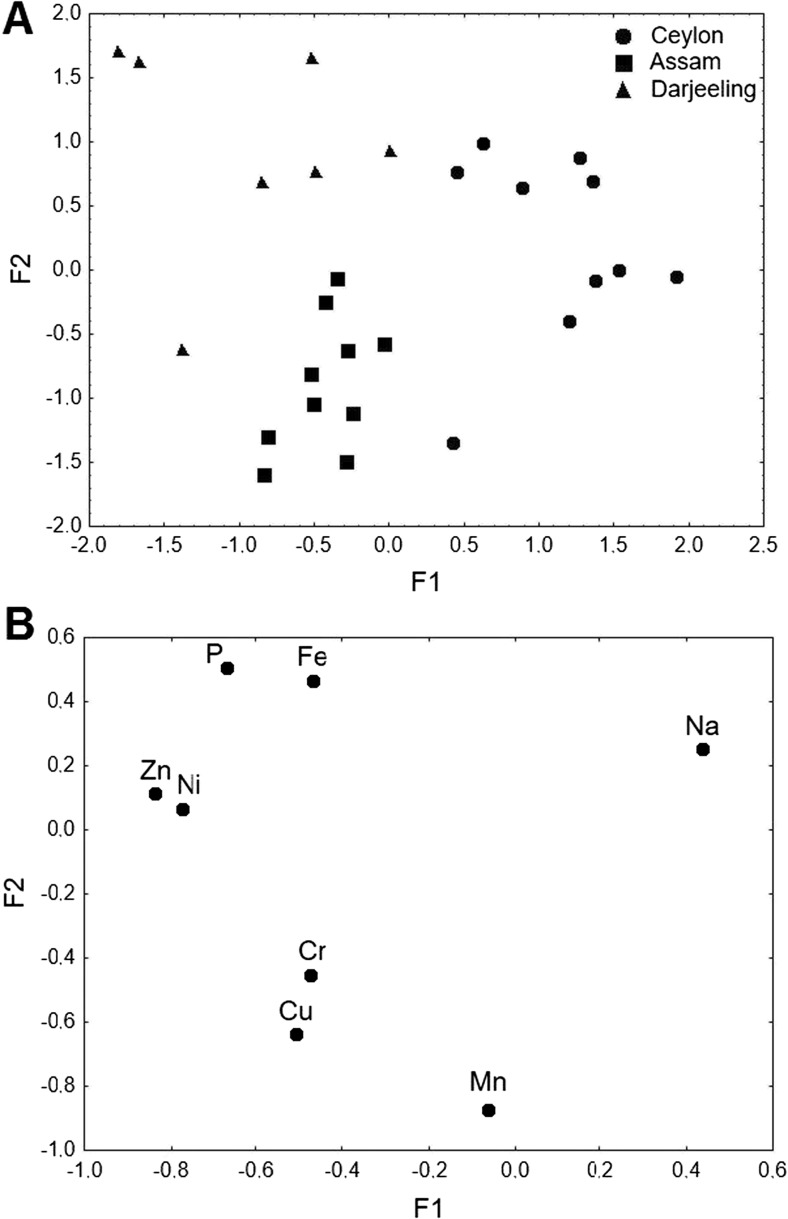
Factor analysis was also performed for Indian plantations, i.e., Assam and Darjeeling, and Ceylon samples in order to differentiate samples within plantations (Fig. 2a, b). It was found that 57.2 % of the total variance is explained by F1 (33.1 %) and F2 (24.1 %). The eigenvalues were 2.64 for F1 and 1.93 for F2. The factor analysis clearly differentiated between tea samples originating from different plantations. Ceylon samples were described by higher F1 values and Na, which might be associated with its significant content in soils of this country (Fig. 2a, b). Lower values of F1 corresponded to Assam and Darjeeling samples, which were described by P, Fe, Zn, Ni, Cr, Cu, and Mn. Tea samples from Darjeeling were differentiated by P, Fe, and Zn, whereas Assam tea samples were significantly correlated with Cr, Cu, and Mn. Factor 2 differentiated samples from India (Assam and Darjeeling) as its higher values corresponded to Darjeeling plantations and lower values to Assam ones. Although Ni is characterized with positive F2 loading, there were no significant variations in its content in Assam and Darjeeling samples.
Fig. 2.
a Scatter plot of objects samples of two factors of the all tea samples from India plantations and Ceylon. b Scatter plot of loadings for elements in all the analyzed tea samples from India plantations and Ceylon
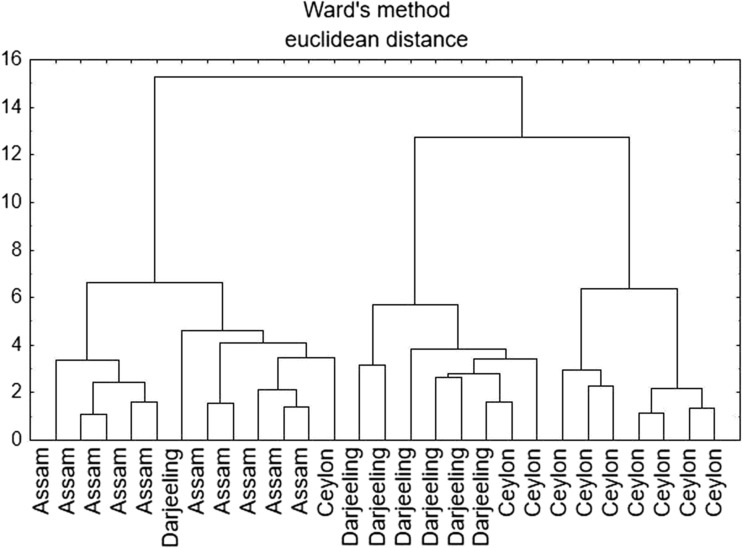
Cluster Analysis
The cluster analysis (CA) was based on Ward’s method with the usage of the Euclidean distance. Application of CA made it possible to differentiate samples according to their origin, i.e., Ceylon and Indian plantations (Fig. 3). Assam plantations were discriminated by Cr, Cu, and Mn, and Darjeeling ones by Fe, Zn, and P. Ceylon tea samples corresponded to Na, which was already confirmed to be a discriminative element for this region. However, only few samples were assigned to the improper cluster, which might be due to the similarity between samples, especially in view of their contamination with heavy metals. What is more interesting, it was noted that Darjeeling samples are more similar to Ceylon samples than to the other Indian plantation (Assam) (Fig. 3).
Fig. 3.
Hierarchical dendrogram for tea samples from India and Ceylon plantations
Thus, it can be concluded that CA similarly to FA is able to distinguish tea samples in view of their geographical origin, which might be helpful when authenticity assessment and fraud detection is necessary.
Recommended Dietary Intake
The daily intake of bioelements from tea was evaluated in view of the latest available Polish [43] and American recommended dietary intakes (RDA) [44]. Consumption of one cup of black tea (200 mL) results in intakes of Ca, K, Na, Mg, and P in the range of 0.02–1.30 % of their respective RDAs. Thus, black tea is not a rich source of these macroelements, despite the fact that their concentrations in tea leaves are the highest among all the analyzed elements (Table 7). Realization of the recommended daily intakes for microelements such as Fe, Zn, Cu, and Ni is less than 1.0 %. For Cr, intake of 200 mL of tea infusion supplies 2.8 and 4.0 % of the RDA for men and women, respectively. Black tea is a significant source of Mn and Co, one cup daily provides 15 % of the RDA for Mn and 10 % for Co. However, Mn bioavailability amounts up to 40 % [45]. Therefore, only 6 % of Mn present in one cup of black tea will be absorbed. Moreover, according to the World Health Organization [46] there is no quantitative information available to indicate toxic levels of manganese in the human diet. High Co content in tea leaves may possibly be explained by the use of Co-containing fertilizers. There is evidence that Co in higher plants promotes the formation of chlorophyll and plant growth [47]. According to Kabata-Pendias and Szteke [47], approximately 50 % of Co will be absorbed in the gastrointestinal tract. Furthermore, Co absorption can be increased among individuals who are Fe deficient.
Table 7.
Comparison of Recommended Dietary Allowance (for a person weighing 70 kg through consumption of one cup (200 mL) of black tea beverage) to the daily intake from tea with consideration of PTWI for Pb
| Element | Recommended daily allowance (RDA) (mg/day/person) | Average content in 200 mL of infusion (mg/200 mL) | Percentage of RDA | ||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| (31–50 years) | (31–50 years) | (31–50 years) | (31–50 years) | ||
| Ca | 1000 | 1000 | 0.49 ± 0.38 | 0.05 | 0.05 |
| 0.03–1.83 | |||||
| K | 4700 | 4700 | 13.2 ± 4.27 | 0.3 | 0.3 |
| 4.19–24.5 | |||||
| Mg | 420 | 320 | 4.21 ± 1.99 | 1.0 | 1.3 |
| 0.46–8.34 | |||||
| Na | 1500 | 1500 | 0.27 ± 0.12 | 0.02 | 0.02 |
| 0.07–0.67 | |||||
| Mna | 2.3 | 1.8 | 0.27 ± 0.13 | 11.7 | 15 |
| 0.07–0.84 | |||||
| Feb | 10 | 18 | 0.005 ± 0.003 | 0.05 | 0.03 |
| 0.0002–0.01 | |||||
| P | 700 | 700 | 2.29 ± 0.69 | 0.3 | 0.3 |
| 0.98–4.54 | |||||
| Zn | 11 | 8 | 0.02 ± 0.009 | 0.2 | 0.2 |
| 0.001–0.056 | |||||
| Cu | 0.9 | 0.9 | 0.01 ± 0.003 | 1.1 | 1.1 |
| 0.003–0.017 | |||||
| Coc | 0.002 | 0.002 | 0.0002 ± 0.0001 | 10 | 10 |
| <LOD-0.0005 | |||||
| Cra | 0.035 | 0.025 | 0.001 ± 0.0004 | 2.8 | 4.0 |
| 0.00005–0.002 | |||||
| Nia | 1 | 1 | 0.006 ± 0.003 | 0.6 | 0.6 |
| <LOD-0.01 | |||||
| Element | PTWI | PTWI for a person weighing 70 kg | The average content in 200 mL beverage (mg/200 mL) | Realization of PTWI through consumption of one cup daily per week of 200 mL of the product for a person weighing 70 kg (%) | |
| Pb | 25 μg/kg | 1750 | 0.0002 ± 0.0001 | 0.01 | |
| <LOD-0.0007 | |||||
There were also assessed levels of heavy metals such as Pb (Table 7) and Cd in the infusions, but the latter was under the limit of detection of the method applied. Thus, there could not have been estimated its provisional tolerable weekly intake (PTWI) realization. Former PTWI dose for Pb as recommended by the WHO/FAO [48] should not exceed 25 μg/kg, but it was withdrawn by the 73rd report of the Joint FAO/WHO Expert Committee of Food Additives [49]. It was found that it is not possible to establish a new dose PTWI that would be health protective. The PTWI’s analyses were based on the earlier guidelines of WHO/FAO [48] for a person weighing 70 kg. The average Pb levels in 200 mL beverage amounted to 0.0002 mg. Therefore, the consumption of one cup daily per week of 200 mL tea results in the realization of PTWI for Pb in 0.01 %. It can be concluded that drinking black tea does not result in exceeding PTWIs; thus, it poses no health risk for human.
Conclusions
Although specified regulations concerning tea quality are not established in the European Union, it is important to control it as this beverage is one of the most commonly consumed. Therefore, we determined the elemental composition of various black tea samples originating from China, India, Ceylon, and Kenya. We conclude that there is no significant health risk associated with consumption of the analyzed tea samples, but that tea can constitute a valuable source of manganese in the human diet. Based on the obtained elemental data, the percentage of leaching as well as daily intake realization were assessed. The highest level of RDAs’ realization was noted for Mn (15 %) and Co (10 %). Verification of the interdependences between elemental composition and country of origin of samples was done by multivariate techniques such as factor analysis and cluster analysis. They allowed on differentiation of teas according to the country of origin, i.e., China, India, Ceylon, and Kenya. Moreover, they were found helpful in diversification of teas originating from various plantations within a single country. Thus, they proved to be good tools able to differentiate samples in view of their provenance as well as plantation within the common region.
Compliance with Ethical Standards
The manuscript does not contain clinical studies or patient data.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This project was supported by the Ministry of Science and Higher Education of the Republic of Poland, from the quality promoting subsidy under the Leading National Research Centre (KNOW) programme for the years 2012–2017.
Recipient
Justyna Brzezicha-Cirocka, MSc.
References
- 1.FAO (Food and Agriculture Organization) (2011) FAOSTAT. http://faostat3.fao.org/download/FB/CC/E. Accessed: May 2016
- 2.FAO (Food and Agriculture Organization) (2012) FAOSTAT. http://faostat3.fao.org/download/FB/CC/E. Accessed: May 2016
- 3.Wojciechowska-Mazurek M, Starska K, Mania M, Rebeniak M, Karłowski K. Pierwiastki szkodliwe dla zdrowia w herbacie – ocena zagrożenia dla zdrowia. Bromat Chem Toksykol. 2010;43:233–239. [Google Scholar]
- 4.Barone G, Giacominelli-Stuffler R, Storelli MM. Evaluation of trace metal and polychlorinated biphenyl levels in tea brands of different origin commercialized in Italy. Food Chem Toxicol. 2016;87:113–119. doi: 10.1016/j.fct.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Ma G, Zhang Y, Zhang J, Wang G, Chen L, Zhang M, Liu T, Liu X, Lu C. Determining the geographical origin of Chinese green tea by linear discriminant analysis of trace metals and rare earth elements: taking Dongting Biluochun as an example. Food Control. 2016;59:714–720. doi: 10.1016/j.foodcont.2015.06.037. [DOI] [Google Scholar]
- 6.Fernández PL, Pablos F, Martin MJ, González AG. Multi-element analysis of tea beverages by inductively coupled plasma atomic emission spectrometry. Food Chem. 2002;76:483–489. doi: 10.1016/S0308-8146(01)00312-0. [DOI] [Google Scholar]
- 7.Wang L, Wei K, Cheng H, He W, Li X, Gong W. Geographical tracing of Xihu Longjing tea using high performance liquid chromatography. Food Chem. 2014;146:98–103. doi: 10.1016/j.foodchem.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Wu QJ, Dong QH, Sun WJ, Huang Y, Wang QQ, Zhou WL. Discrimination of Chinese teas with different fermentation degrees by stepwise linear discriminant analysis (S-LDA) of the chemical compounds. J Agr Food Chem. 2014;62:9336–9344. doi: 10.1021/jf5025483. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Zhang P, Pan Z, Xu H, Luo Y, Wang X. Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS-SPME/GC-MS. Food Chem. 2013;141:259–265. doi: 10.1016/j.foodchem.2013.02.128. [DOI] [PubMed] [Google Scholar]
- 10.Qin Z, Pang X, Chen D, Cheng H, Hu X, Wu J. Evaluation of Chinese tea by the electronic nose and gas chromatography-mass spectrometry: correlation with sensory properties and classification according to grade level. Food Res Int. 2013;53:864–874. doi: 10.1016/j.foodres.2013.02.005. [DOI] [Google Scholar]
- 11.Ye N, Zhang L, Gu X. Discrimination of green teas from different geographical origins by using HS-SPME/GC-MS and pattern recognition methods. Food Anal Method. 2012;5:856–860. doi: 10.1007/s12161-011-9319-9. [DOI] [Google Scholar]
- 12.Lee JE, Lee BJ, Chung JO, Hwang JA, Lee SJ, Lee CH. Geographical and climatic dependencies of green tea (Camellia sinensis) metabolites: a H1NMR based metabolomics study. J Agr Food Chem. 2010;58:10582–10589. doi: 10.1021/jf102415m. [DOI] [PubMed] [Google Scholar]
- 13.Ren G, Wang S, Ning J, Xu R, Wang Y, Xing Z. Quantitative analysis and geographical traceability of black tea using fourier transform near-infrared spectroscopy (FT-NIRS. Food Res Int. 2013;53:822–826. doi: 10.1016/j.foodres.2012.10.032. [DOI] [Google Scholar]
- 14.Fernández-Cáceres PL, Martín MJ, Pablos F, Gonzalez AG. Differentiation of tea (Camellia sinensis) varieties and their geographical origin according to their metal content. J Agr Food Chem. 2001;49:4775–4779. doi: 10.1021/jf0106143. [DOI] [PubMed] [Google Scholar]
- 15.Herrador MA, González AG. Pattern recognition procedures for differentiation of green, black and oolong teas according to their metal content from inductively coupled plasma atomic emission spectrometry. Talanta. 2001;53:1249–1257. doi: 10.1016/S0039-9140(00)00619-6. [DOI] [PubMed] [Google Scholar]
- 16.Moreda-Piñeiro A, Fisher A, Hill SJ. The classification of tea according to region of origin using pattern recognition techniques and trace metal data. J Food Compos Anal. 2003;16:195–211. doi: 10.1016/S0889-1575(02)00163-1. [DOI] [Google Scholar]
- 17.Szymczycha-Madeja A, Welna M, Pohl P. Elemental analysis of teas and their infusions by spectrometric methods. Trends Anal Chem. 2012;35:165–181. doi: 10.1016/j.trac.2011.12.005. [DOI] [Google Scholar]
- 18.Paz-Rodríguez B, Domínguez-González MR, Aboal-Somoza M, Bermejo-Barrera P. Application of high resolution-continuum source flame atomic absorption spectrometry (HR-CS FAAS): determination of trace elements in tea and tisanes. Food Chem. 2015;170:492–500. doi: 10.1016/j.foodchem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Brzezicha-Cirocka J, Grembecka M, Szefer P. Monitoring of essential and heavy metals in green tea from different geographical origins. Environ Monit Assess. 2016;188:1–11. doi: 10.1007/s10661-016-5157-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzezicha-Cirocka J, Grembecka M, Szefer P. Analytical assessment of bio- and toxic elements distribution in Pu-erh and fruit teas in view of chemometric approach. Biol Trace Elem Res. 2016 doi: 10.1007/s12011-016-0669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Konieczka P, Namieśnik J. Quality assurance and quality control in the analytical chemical laboratory: a practical approach. Boca: CRC Press - Taylor & Francis Group; 2009. [DOI] [PubMed] [Google Scholar]
- 22.Szefer P. Chemometric techniques in analytical evaluation of food quality. In: Szefer P, Nriagu JO, editors. Mineral components in foods. Boca Raton: CRC Press - Taylor & Francis Group; 2007. [Google Scholar]
- 23.Dambiec M, Polechonska L, Klink A. Levels of essentials and non-essential elements in black teas commercialized in Poland and their transfer to tea infusion. J Food Compos Anal. 2013;31:62–66. doi: 10.1016/j.jfca.2013.03.006. [DOI] [Google Scholar]
- 24.Soomro MT, Zahir E, Mohiuddin S, Khan AN, Naqvi II. Quantitative assessment of metals in local brands of tea in Pakistan. Pak J Biol Sci. 2008;11:285–289. doi: 10.3923/pjbs.2008.285.289. [DOI] [PubMed] [Google Scholar]
- 25.Yemane M, Chandravanshi BS, Wondimu T. Levels of essential and non-essential metals in leaves of the tea plant (Camellia sinensis L.) and soil of Wushwush farms, Ethiopia. Food Chem. 2008;107:1236–1243. [Google Scholar]
- 26.Gebretsadik DW, Chandravanshi BS. Levels of metals in commercially available Ethiopian black teas and their infusions. Bull Chem Soc Ethiop. 2010;24:339–349. doi: 10.4314/bcse.v24i3.60664. [DOI] [Google Scholar]
- 27.Malik J, Szakova J, Drabek O, Balik J, Kokoska L. Determination of certain micro and macroelements in plant stimulants and their infusions. Food Chem. 2008;111:520–525. doi: 10.1016/j.foodchem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Gallaher RN, Gallaher K, Marshall AJ, Marshall AC. Mineral analysis of ten types of commercially available tea. J Food Compos Anal. 2006;19:53–57. doi: 10.1016/j.jfca.2006.02.006. [DOI] [Google Scholar]
- 29.Shaltout AA, Abd-Elkader OH. Levels of trace elements in black teas commercialized in Saudi Arabia using inductively coupled plasma mass spectrometry. Biol Trace Elem Res. 2016 doi: 10.1007/s12011-016-0728-x. [DOI] [PubMed] [Google Scholar]
- 30.Maupenzi JP, Li L, Ge J, Varenyam A, Habiyaremye G, Theoneste N, Emmanuel K. Assessment of soil degradation and chemical compositions in Rwandan tea-growing areas. Geosci Front. 2011;2:599–607. doi: 10.1016/j.gsf.2011.05.003. [DOI] [Google Scholar]
- 31.Al-Oud SS. Heavy metal contents in tea and herb leaves, Pakistan. J Biol Sci. 2003;6:208–212. [Google Scholar]
- 32.McKenzie JS, Jurado JM, Pablos F. Characterisation of tea leaves according to their total mineral content by means of probabilistic neural networks. Food Chem. 2010;123:859–864. doi: 10.1016/j.foodchem.2010.05.007. [DOI] [Google Scholar]
- 33.Milani RF, Morgano MA, Cadore S. Trace elements in Camelia sinensis marketed in southern Brazil: extraction from tea leaves to beverages and dietary exposure. LWT-Food Sci Technol. 2016;68:491–498. doi: 10.1016/j.lwt.2015.12.041. [DOI] [Google Scholar]
- 34.Wong MH, Hang ZQ, Wong JWC, Lan CY. Trace metal contents (Al, Cu and Zn) of tea: tea and soil from two tea plantations, and tea products from different provinces of China. Environ Geochem Health. 1998;20:87–94. doi: 10.1023/A:1006545825302. [DOI] [Google Scholar]
- 35.Han WY, Zhao FJ, Shi YZ, Ma LF, Ruan JY. Scale and causes of lead contamination in Chinese tea. Environ Pollut. 2006;139:125–132. doi: 10.1016/j.envpol.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Seenivasan S, Manikandan N, Muraleedharan NN, Selvasundaram R. Heavy metal content of black teas from south India. Food Control. 2008;19:746–749. doi: 10.1016/j.foodcont.2007.07.012. [DOI] [Google Scholar]
- 37.Görür FK, Keser R, Akçay N, Dizman S, Okumuşoğlu NT. Radionuclides and heavy metals concentrations in Turkish market tea. Food Control. 2011;22:2065–2070. doi: 10.1016/j.foodcont.2011.06.005. [DOI] [Google Scholar]
- 38.Franklin RE, Duis L, Brown R, Kemp T. Trace element content of selected fertilizers and micronutrient source materials. Commun Soil Sci Plan. 2005;36:1591–1609. doi: 10.1081/CSS-200059091. [DOI] [Google Scholar]
- 39.De Meeȗs C, Eduljee GH, Hutton M. Assessment and management of risk arising from exposure to cadmium in fertilisers. Sci Total Environ. 2002;291:167–187. doi: 10.1016/S0048-9697(01)01098-1. [DOI] [PubMed] [Google Scholar]
- 40.Lv HP, Zhang YJ, Lin Z, Liang YR. Processing and chemical constituents of Pu-erh tea: a review. Food Res Intern. 2013;53:608–618. doi: 10.1016/j.foodres.2013.02.043. [DOI] [Google Scholar]
- 41.Sarma H, Islam NF, Borgohain P, Sarma A, Prasad MNV. Localization of polycyclic aromatic hydrocarbons and heavy metals in surface soil of Asia’s oldest oil and gas drilling site in Assam, north-east India: implications for the bio-economy. Emerging Contaminants. 2016 [Google Scholar]
- 42.Hua D, Ming-Shun L, Yu-Chan Z. Soil metal contamination and fractionation of tea plantations: case studies in a normal tea garden and in a restored mineland tea stand. Pol J Environ Stud. 2012;21:1223–1228. [Google Scholar]
- 43.Jarosz M. Normy żywienia dla populacji polskiej – nowelizacja. Warsaw: IŻŻ; 2012. [Google Scholar]
- 44.American recommendations (2011) Food and Nutrition Board, Institute of Medicine, National Academies Press. URL https://fnic.nal.usda.gov/sites/fnic.nal.usda.gov/files/uploads/recommended_intakes_individuals.pdf. Accessed May 2016
- 45.Powell JJ, Trevor JB, Thompson RPH. In vitro mineral availability from digested tea: a rich dietary source of manganese. Analyst. 1998;123:1721–1724. doi: 10.1039/a802131g. [DOI] [PubMed] [Google Scholar]
- 46.WHO (2011a) Manganese in drinking-water, background document for preparation of WHO guidelines for drinking water quality. World Health Organization, Geneva. URL http://www.who.int/ipcs/publications/cicad/cicad69%20.pdf. Accessed 3 Apr 2016
- 47.Kabata-Pendias A, Szteke B. Trace elements in abiotic and biotic environments. Boca Raton: CRC Press - Taylor & Francis Group; 2015. [Google Scholar]
- 48.WHO (2010) Lead in drinking-water, background document for preparation of WHO Guidelines for drinking-water quality. World Health Organization, Geneva. URL http://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf. Accessed 3 Apr 2016
- 49.WHO (2011b) Seventy-third report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization, Geneva. URL http://whqlibdoc.who.int/trs/WHO_TRS_960_eng.pdf. Accessed 3 Apr 2016