Abstract
Objective: To evaluate the effects of ticagrelor and high-dose clopidogrel on the platelet functions in patients with inadequate response to clopidogrel. Methods: In this prospective, randomized and controlled study, patients who had been diagnosed as acute coronary syndrome (ACS) with inadequate response to clopidogrel in the Second Hospital of Hebei Medical University from July 2015 to June 2016 were enrolled. Inadequate response to clopidogrel was defined as absolute reduction of platelet aggregation rate (PAR) <30% or PAR >70%. Eligible patients were randomly assigned to two groups, the high-dose group and the ticagrelor group. Clinical information and intervention protocols were compared. The PAR of the two groups were measured at the time of baseline, the 24th hour, 72nd hour, and the 7th day after treatments, the other platelet-related parameters were measured including platelet count (PLT), mean platelet volume (MPV), platelet distribution width (PDW) at the same time points. Besides, the markers of platelet activation human P-selectin (CD62P) and thromboxane A2 (TXA2) were also recorded to compare. The incidence of major adverse cardiac events (MACE) and the side effects between two groups were followed up for three months. Results: A total of 74 patients were finally enrolled, 38 of whom were assigned to the ticagrelor group and the rest of them to the high-dose clopidogrel group. The baseline clinical and procedural characterists were similar. There were no significant differences in baseline levels of PAR between the two groups [(79.38±11.20)% vs. (73.97±12.74)%, P>0.05]. For both groups, the levels of PAR significantly decreased at each time point (P<0.001). Besides, the levels of PAR in ticagrelor group were lower than those in high-dose clopidogrel group at the 24th hour, 72nd hour and 7th day after treatments: [(25.92±10.31)% vs. (37.95±11.63)%, P<0.001], [(28.02±14.61)% vs. (30.64±10.73)%, P<0.001], [(37.17±11.11)% vs. (36.80±7.26)%, P<0.001]. The baseline levels of platelet related parameters were similar between the two groups (P>0.05), and there were no significant differences in the levels of PLT, PDW, and MPV at the 24th hour, 72nd hour and 7th day. It was lower in ticagrelor group than that in clopidogrel group at the 24th hour [(5.47±1.03) ng/ml vs. (8.02±1.45) ng/ml, P<0.001] while the CD62P concentrations in the two groups significantly decreased comparing to the baseline levels (P<0.001). During 3-month follow-up, the incidences of MACE and side effects were not significantly different between the two group. Conclusions: ticagrelor could further decrease levels of platelet aggression rate and CD62p compared with high-dose clopidogrel, without serious side effects.
Keywords: Ticagrelor, acute coronary syndrome, clopidogrel resistance, human P-selectin, thromboxane A2
Introduction
Acute coronary syndrome (ACS) is one of the most severe types of heart attack which usually occurs when thrombus forms on a ruptured atheromatous plaque and occludes an epicardial coronary artery. Clopidogrel, in combination with aspirin, reduces the risk of cardiovascular events in patients with ACS and in those undergoing percutaneous coronary intervention (PCI) [1-3]. However, a high proportion of patients had a suboptimal response to a standard dose of clopidogrel, which is associated with an increased risk of ischemic events in patients with ACS undergoing PCI [4]. Although a high maintenance dose of clopidogrel (150 mg per day) enhanced platelet inhibition in patients with elevated platelet reactivity while on standard treatment regimens, a considerable proportion of the population maintained persistently elevated platelet function despite the more aggressive P2Y12 receptor blockade [5]. These results demonstrate the need for alternative platelet inhibition strategies for this high-risk patient population.
Ticagrelor, an oral reversible P2Y12 receptor inhibitor, has been demonstrated to decrease the atherosclerotic thrombosis by inhibiting the formation of new blood clots [6]. Moreover, in the PLATO trial, ticagrelor has been found to be superior to clopidogrel in reducing cardiac events without causing a significant increase in the incidence of bleeding. However, few studys have focused on the clinical effects and platelet functions of ticagrelor and high-dose clopidogrel in ACS patients with inadequate response to clopidogrel. The aim of the study was to evaluate the effects of ticagrelor and high-dose clopidogrel on the platelet functions and clinical outcomes in ACS patients with inadequate response to clopidogrel.
Methods
Study population
From July 2015 to June 2016, all consecutive non-ST elevation ACS patients with inadequate response to clopidogrel in the Department of Cardiology of the Second Hospital of Hebei Medical University were enrolled in our study.
The inclusion criteria were as follows: (1) Patients were included with the diagnosis of ACS made according to the ACC/AHA guideline [7]; (2) Laboratory examinations indicated inadequate response to clopidogrel. In this study, the platelet aggregation rate was measured by turbidimetric method. Inadequate response to clopidogrel was defined as absolute reduction of platelet aggregation rate (PAR) <30% or PAR >70%.
Exclusion criteria were: 1) history of old myocardial infarction; 2) contraindication for aspirin, clopidrogrel, ticagrelor or heparin; 3) renal failure [indicated by a serum creatinine concentration above 2.5 mg/dL (221 mmol/L)]; 4) history of neutropenia, thrombocytopenia, or hepatic dysfunction; 5) participated in other clinical trials. Patients with bleeding diathesis, thrombocytopenia, recent stroke, severe hepatic dysfunction, women with pregnancy and lactation, and received GP II b/III a receptor antagonist therapy were also excluded.
The study protocol was approved by the Ethical Committee of the Second Hospital of Hebei Medical University, and the written informed consent was given by all patients before cardiac catheterization procedures were performed.
Study protocols
Eligible patients were randomly assigned to the high-dose clopidogrel group and the ticagrelor group, and recieved high-dose clopidogrel (600 mg loading dose followed by a 150-mg MD) or ticagrelor (180 mg loading dose followed by a 90-mg BID). The other medications were administered to the patients (including diuretics, intravenous vasodilator, lipid-lowering, beta-blockade, and angiotensin-converting enzyme inhibitors) according to current best practice. Coronary angiography (CAG) and percutaneous coronary intervention (PCI) were performed via transradial artery approach for patients in both groups utilizing the standard technique. According to the results of angiography, PCI was performed unless the blood flow of IRA achieved TIMI flow grade 3 without significant stenosis.
Clinical information were collected including: age, gender, risk factors, Body Mass Index (BMI), pathological changes of coronary artery and the basic medical treatment. Platelet aggregation rate of the two groups were measured at the time of baseline, the 24th hour, the 72nd hour, and the 7th day after treatments, the other platelet-related parameters were measured including platelet count (PLT), mean platelet volume (MPV), and platelet distribution width (PDW) at the same time points. Besides, the markers of platelet activation Human P-selectin (CD62P) and thromboxane A2 (TXA2) at baseline and 72nd hour after treatments were also measured.
The incidence of major adverse cardiac events (MACE) and the adverse events between two groups were followed up for three months, including cardiac death, heart failure, remyocardial, targeted vessel reconstruction, cardiac readmission, bleeding complications, dyspnea, and bradycardia.
Endpoints and definitions
Endpoints were recorded from the admission to discharge from hospital. The primary endpoint of this study was PAR measured at 24th hour and 72nd hour after the administration of the study drugs. Secondary endpoint was the incidences of MACE and side effects.
Statistical analyses
All clinical datas were analyzed with SPSS 20.0. Absolute numbers and percentages were computed to describe the patient population. Continuous variables are expressed as mean ± SD and are compared using the unpaired t test for normal distributions and Mann-Whitney U test for non-normally distributed variables. Categorical variables are expressed as absolute or relative frequencies and are compared using chi-square analyses or the Fisher’s exact test, as appropriate to the cell frequencies. Values of P<0.05 were considered statistically significant.
Results
Among the overall 81 ACS patients with inadequate response to clopidogrel, 7 of them were excluded: 2 patients with renal or liver dysfunctions, 3 patient was used tirofiban which was a GP II b/III a receptor antagonist, 1 patient with contraindication of ticagrelor, and 1 patient with refusal of study. A total of 74 patients were finally enrolled, 38 of whom were assigned to the ticagrelor group and the rest of them to the high-dose clopidogrel group.
Comparisons of baseline clinical characteristics
There were no significant differences between both groups in baseline characteristics, including gender distribution, age, risk factors (hypertension, diabetes mellitus, hyperlipidermia, smoking history), BMI, GRACE score, CRUSADE score, basic medication, pathological changes of coronary artery, angiographic and procedural characteristics (P>0.05) (Table 1).
Table 1.
Comparisons of baseline clinical characteristics
| Ticagrelor group (n=38) | Clopidogrel group (n=36) | P value | |
|---|---|---|---|
| Age (yrs) | 58.53±9.43 | 60.15±9.14 | 0.456 |
| Male-n. (%) | 30 (78.9) | 27 (75.0) | 0.899 |
| BMI | 23.48±2.68 | 23.76±2.46 | 0.642 |
| Smoking-n. (%) | 25 (65.8) | 21 (58.31) | 0.674 |
| Hypertension-n. (%) | 25 (65.8) | 24 (66.7) | 0.868 |
| Diabetes-n. (%) | 10 (26.3) | 11 (30.6) | 0.884 |
| Dyslipidemia-n. (%) | 13 (34.2) | 9 (25.0) | 0.541 |
| Family history of CAD-n. (%) | 8 (21.1) | 7 (19.4) | 0.907 |
| GRACE Score | 115.49±41.53 | 105.02±29.86 | 0.219 |
| CRUSADE Score | 27.67±11.35 | 25.36±10.04 | 0.358 |
| Ejection fraction-n. (%) | 54.19±6.17 | 55.61±4.42 | 0.261 |
| UA-n. (%) | 29 (76.3) | 26 (72.2) | 0.891 |
| NSTEMI-n. (%) | 9 (23.7) | 10 (27.8) | 0.891 |
| Beta-blockers-n. (%) | 32 (84.2) | 31 (86.1) | 0.923 |
| ACE inhibitors-n. (%) | 29 (76.3) | 24 (83.3) | 0.645 |
| Calcium blockers-n. (%) | 15 (39.5) | 18 (50.0) | 0.499 |
| Statins-n. (%) | 36 (94.7) | 35 (97.2) | 1.000 |
| Nitrate agent-n. (%) | 27 (71.1) | 24 (66.7) | 0.604 |
NOTE: BMI: Body Mass Index; CAD: coronary artery disease; NSTEMI: Non-ST-Segment Elevation Myocardial infarction; UA: Unstable angina.
CAG and PCI procedural characteristics
CAG and PCI procedural characteristics were shown in Table 2. All cases received CAG and PCI successfully. There were no significant differences in distributions of culprit vessels, number of stent(s), and coronary flow of culprit vessels after PCI procedurals between the two groups (all P<0.05) (Table 2).
Table 2.
Angiographic and PCI procedural characteristics
| Ticagrelor group (n=38) | Clopidogrel group (n=36) | P value | |
|---|---|---|---|
| Multivessel-n. (%) | 15 (39.5) | 11 (30.6) | 0.576 |
| CTO-n. (%) | 3 (7.9) | 4 (11.1) | 0.707 |
| Culprit vessel-n. (%) | |||
| LAD | 16 (42.1) | 17 (47.2) | 0.835 |
| LCX | 9 (23.7) | 8 (22.2) | 0.899 |
| RCA | 12 (31.6) | 9 (25.0) | 0.712 |
| LM | 1 (2.6) | 2 (5.6) | 0.604 |
| PCI-n. (%) | 34 (89.5) | 31 (86.1) | 0.732 |
| No. of stents per patient | 1.2±0.6 | 1.3±0.5 | 0.440 |
| TIMI grade after PCI | 1.000 | ||
| TIMI grade <3-n. (%) | 2 (5.3) | 2 (5.6) | |
| TIMI grade 3-n. (%) | 36 (94.7) | 34 (94.4) | |
| CTFC | 26.73±3.98 | 27.18±3.62 | 0.613 |
| TMPG after PCI | 0.907 | ||
| TMPG <3-n. (%) | 7 (18.4) | 8 (22.2) | |
| TMPG 3-n. (%) | 31 (81.6) | 28 (77.8) |
NOTE: CTO: chronic total occlusion; LAD: Left Artery Descending; LCX: Left Circumflex Artery; RCA: Right Coronary Artery; PCI: percutaneous coronary intervention; CTFC: corrected TIMI frame count; TMPG: TIMI myocardial perfusion grade.
Comparison of PAR between the two groups
There were no significant differences in baseline levels of PAR between the two groups [(79.38±11.20)% vs. (73.97±12.74)%, P>0.05]. For both groups, the levels of PAR significantly decreased at each time point (P<0.001). Besides, the levels of PAR in ticagrel group were lower than those in high-dose clopidogrel group at the 24th hour, 72nd hour and 7th day after treatments: [(25.92±10.31)% vs. (37.95±11.63)%, P<0.001], [(28.02±14.61)% vs. (30.64±10.73)%, P<0.001], [(37.17±11.11)% vs. (36.80±7.26)%, P<0.001] (Figure 1).
Figure 1.
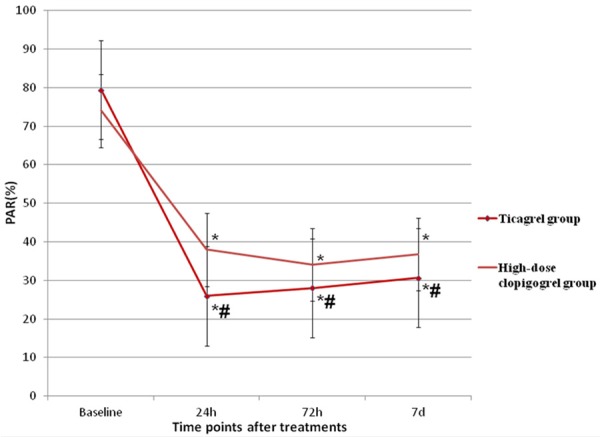
Comparison of platelet aggregation rates between the two groups. Notes: *: compared with baseline level. #: compared with high-dose clopidogrel group.
Comparison of levels of platelet related parameters between the two groups
The baseline levels of PLT, PDW, and MPV were similar between the two groups (P>0.05), and there were no significant differences in the levels of PLT, PDW, and MPV at the 24th hour, 72nd hour and 7th day. Besides, Compared to high-dose clopidogrel group, the values of PLT and PDW at different time points were also not significantly different (P>0.05), but the level of MPV in ticargrel group had a tendency to decrease (Figures 2, 3 and 4). There were also no significant differences of the platelet activation markers CD62p (P=0.825) and TXA2 (P=0.233) between the two groups. However, it was lower in ticagrelor group than that in clopidogrel group at the 24th hour [(5.47±1.03) ng/ml vs. (8.02±1.45) ng/ml, P<0.001] while the CD62P concentrations of the two groups significantly decrease to the baseline levels (P<0.001). However, there were no significant differences in TXA2 concentrations between the two groups or the same group at each time point (Table 3).
Figure 2.
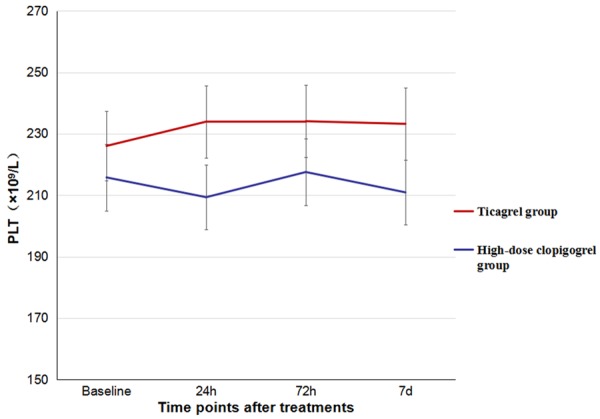
Comparison of levels of PLT counts between the two groups.
Figure 3.
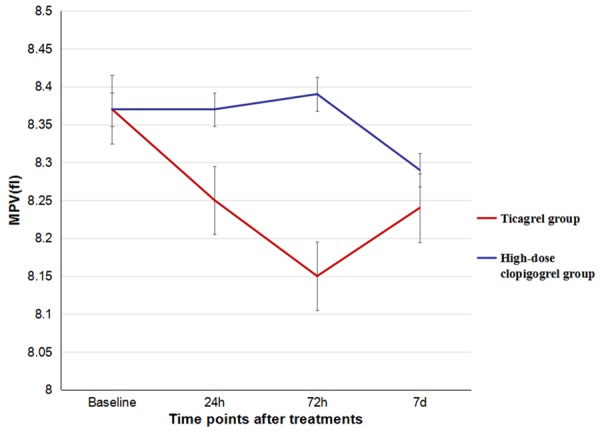
Comparison of levels of MPV between the two groups.
Figure 4.
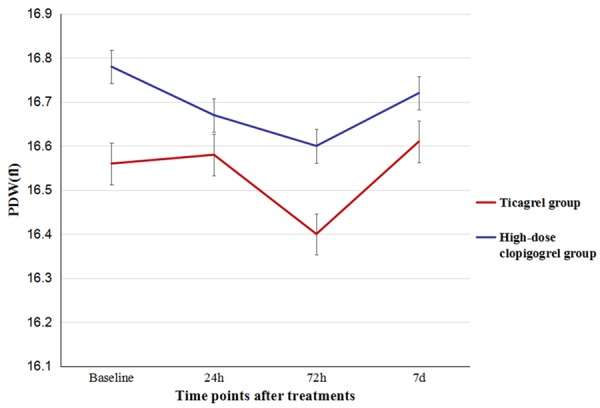
Comparison of levels of PDW between the two groups.
Table 3.
Platelet-related parameters between the two groups
| Ticagrelor group (n=38) | Clopidogrel group (n=36) | P | |
|---|---|---|---|
| CD62P (0) | 10.87±1.67 | 10.79±1.31 | 0.820 |
| CD62P (1) | 5.47±1.03# | 8.02±1.45# | <0.001 |
| TXA2 (0) | 52.07±8.26 | 54.43±8.94 | 0.242 |
| TXA2 (1) | 49.29±6.72 | 48.77±8.64 | 0.773 |
NOTE: CD62P (0): CD 62P at baseline; CD62P (1): CD 62P 72nd hour after treatments; TXA2 (0): TXA2 at baseline; TXA2 (1): TXA2 72nd hour after treatments;
P<0.001 Compared with baseline level.
Incidences of MACE and side effects during 3-month follow-up
During 3-month follow-up, there were 9 cases of cardiovascular adverse events, and 3 cases of Minor bleeding in high-dose clopidogrel group; there were 12 cases of cardiovascular adverse events, 4 cases of minor bleeding, 4 cases of dyspnea and 1 case of bradycardia in ticagrelor group, not resulting in a statistically difference (P>0.05) (Table 4).
Table 4.
Major adverse cardiac events and side effects during follow-up
| Ticagrelor group (n=38) | Clopidogrel group (n=36) | P | |
|---|---|---|---|
| Cardiac death-n. (%) | 0 (0) | (0) | - |
| Heart Failure-n. (%) | 0 (0) | (0) | - |
| Remyocardial Infarction-n. (%) | 0 (0) | 1 (2.7) | 0.486 |
| Targeted Vessel Reconstruction-n. (%) | 0 (0) | (0) | - |
| Cardiac Readmission-n. (%) | 2 (5.3) | 4 (11.1) | 0.418 |
| TIMI bleeding-n. (%) | |||
| Major bleeding | 0 (0) | (0) | - |
| Minor bleeding | 4 (10.5) | 3 (8.3) | 1.000 |
| Minimal bleeding | 1 (2.6) | 1 (2.7) | 1.000 |
| Dyspnea-n. (%) | 4 (10.5) | 0 (0) | 0.116 |
| Bradycardia-n. (%) | 1 (2.6) | 0 (0) | 1.000 |
Discussion
Acute coronary syndrome (ACS) has evolved as an operational term that refers to a spectrum of conditions compatible with acute myocardial ischemia and/or infarction due to an abrupt reduction in coronary blood flow [8]. The main pathophysiological mechanism of ACS involves the development of acute thrombosis subsequent to atherosclerotic plaque rupture, with activated platelets serving a key role in this process [9]. Consequently, anti-platelet therapies for ACS is of high importance. Thus far, clopidogrel plus aspirin has been used as the standard treatment to prevent the recurrence of cardiovascular diseases [10]. Although the efficacy of clopidogrel has been well established, it exhibits pharmacodynamic variability in diverse conditions which can be due to the loss-of-function CYP2C19 allele, drug-drug interactions or clinical factors [11]. Inadequate response to clopidogrel was defined as absolute reduction of platelet aggregation rate <30% or platelet aggregation rate >70% [12-14]. The use of double doses of clopidogrel and the more potent thienopyridine prasugrel to reduce PAR and subsequent cardiovascular events have not been successful, partially due to the studies being conducted with low event rates and low-risk patients [15,16]. As a new developed P2Y12 receptor antagonist, ticagrelor is chosen to perform the optimization of DAPT due to its direct and reversible role [8,17,18]. Because ticagrelor does not need metabolic activation and has less interindividual variation in drug action, it is a more potent antiplatelet drug in comparison with clopidogrel. In the global Phase III PLATelet inhibition and patient Outcomes (PLATO) trial, ticagrelor exceeded clopidogrel in reducing cardiovascular (CV) death [6]. However, few studies have focused on the clinical effects and platelet functions of ticagrelor and high-dose clopidogrel in ACS patients inadequate response to clopidogrel. The aim of the study was to comparison of ticagrelor and high-dose clopidogrel on the platelet functions in ACS patients with inadequate response to clopidogrel. The results of this study indicated that routine dose ticagrelor and high-dose clopidogrel could obviously decrease platelet reactivity, and the effect of ticagrelor was much more significantly. Ticagrelor could decrease the expression of CD62P and strongly inhibit the activation of platelet more significantly than high-dose of clopidogrel. Comparing with high-dose clopidogrel, there was a tendency that ticagrelor could reduce the incidence of MACE, without increase of hemorrhage events.
In this study, the platelet aggregation rate was measured by turbidimetric method. Inadequate response to clopidogrel was defined as absolute reduction of PAR <30% or PAR >70% with 5 μmol/l ADP. In this study, both ticagrelor and high-dose clopidogrel could decrease the incidences of high platelet reactivity. Percent inhibitions of 5 μmol/l ADP-induced PAR were significantly lower in the ticagrelor group versus high-dose clopidogrel group. This may indicated that ticagrelor may be more officious and benefit for the treatments of ACS.
MPV and PDW are simple platelet indices, which increase during platelet activation. Platelet size measured as mean platelet volume (MPV) possibly is a simple and accurate way to estimate platelet activity [19]. Previous studies showed that increased MPV was associated with increased risk of myocardial infarction independent of known cardiovascular risk factors. Increased PDW probably reflects a dysregulation in thrombopoiesis, which is also translated by the multiple abnormalities of platelet reactivity and the change in platelet membrane and adenine nucleotide content that have been previously described [20,21]. No differences were found during the treatments of ticagrelor and high-dose clopidogrel on the levels of PLT counts, MPV and PDW. The main reasons of this results were due to the mechanisms of ticagrelor and clopidogrel, which worked through CYP pathways on the antiplatelet response. Other names for CD62P includes P-selectin [22], and functions as a cell adhesion molecule on the surfaces of activated endothelial cells, which line the inner surface of blood vessels, and activated platelets. TXA2 is one of the secondary agonists which play an important role as cofactors when platelets are activated by agonists such as collagen or thrombin [23]. We found that it was lower in ticagrelor group than that in high-dose clopidogrel group at the 24th hour [(5.47±1.03) ng/ml vs. (8.02±1.45) ng/ml, P<0.001] while the CD62P concentrations of the two groups significantly decrease to the baseline levels (P<0.001). This effect of ticagrelor may contribute to further inhibition on platelet activation in ACS patients.
In this study, no differences of the incidences of MACE were found between the two groups, although the incidences of minor bleeding and dyspnea were a little higher in ticagrelor group. It was thought be considered safety of ticagrelor in the use of the treatments in patients with ACS.
Limitation of the study: This is a small scale study. Therefore, further adequately powered studies will be required to determine whether these physiological observations can be translated to improve patient outcomes and to assess the safety of this strategy.
This is the first study to evaluate the effects of ticagrelor and high-dose clopidogrel on the platelet functions in patients with inadequate response to clopidogrel. We found that ticagrelor could further decrease levels of platelet aggression rate and CD62p compared with high-dose clopidogrel, without serious side effects.
Disclosure of conflict of interest
None.
References
- 1.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 2.Steinhubl SR, Berger PB, Mann JT 3rd, Fry ET, DeLago A, Wilmer C, Topol EJ CREDO Investigators. Clopidogrel for the reduction of events during observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 3.Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, Lewis BS, Murphy SA, McCabe CH, Braunwald E Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 Investigators. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 4.Ahn SG, Lee SH, Yoon JH, Kim WT, Lee JW, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Choe KH. Different prognostic significance of high on-treatment platelet reactivity as assessed by the VerifyNow P2Y12 assay after coronary stenting in patients with and without acute myocardial infarction. JACC Cardiovasc Interv. 2012;5:259–267. doi: 10.1016/j.jcin.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Angiolillo DJ, Shoemaker SB, Desai B, Yuan H, Charlton RK, Bernardo E, Zenni MM, Guzman LA, Bass TA, Costa MA. Randomized comparison of a high clopidogrel maintenance dose in patients with diabetes mellitus and coronary artery disease: results of the optimizing antiplatelet therapy in diabetes mellitus (OPTIMUS) study. Circulation. 2007;115:708–716. doi: 10.1161/CIRCULATIONAHA.106.667741. [DOI] [PubMed] [Google Scholar]
- 6.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 7.Hauk L. Management of non-ST elevation acute coronary syndrome: a guideline from the AHA and ACC. Am Fam Physician. 2015;92:151–153. [PubMed] [Google Scholar]
- 8.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2013;61:e179–347. doi: 10.1016/j.jacc.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SD, Shah NK, Yim J, Epstein BJ. Efficacy and safety of ticagrelor: a reversible P2Y12 receptor antagonist. Ann Pharmacother. 2010;44:524–537. doi: 10.1345/aph.1M548. [DOI] [PubMed] [Google Scholar]
- 10.Kushner FG, Hand M, Smith SC Jr, King SB 3rd, Anderson JL, Antman EM, Bailey SR, Bates ER, Blankenship JC, Casey DE Jr, Green LA, Hochman JS, Jacobs AK, Krumholz HM, Morrison DA, Ornato JP, Pearle DL, Peterson ED, Sloan MA, Whitlow PL, Williams DO. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Am Coll Cardiol. 2009;54:2205–2241. doi: 10.1016/j.jacc.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. 2003;107:2908–2913. doi: 10.1161/01.CIR.0000072771.11429.83. [DOI] [PubMed] [Google Scholar]
- 12.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. N Engl J Med. 2009;119:237–242. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 14.Sibbing D, Braun S, Morath T, Mehilli J, Vogt W, Schömig A, Kastrati A, von Beckerath N. Platelet reactivity after clopidogrel treatment assessed with point-of-care analysis and early drug-eluting stent thrombosis. J Am Coll Cardiol. 2009;53:849–856. doi: 10.1016/j.jacc.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, Richardt G, Jakubowski JA, Neumann FJ. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing platelet reactivity in patients undergoing elective stent placement on clopidogrel to guide alternative therapy with prasugrel) study. J Am Coll Cardiol. 2012;59:2159–2164. doi: 10.1016/j.jacc.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, Tanguay JF, Cannon CP, Topol EJ. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: impact on thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124:1132–1137. doi: 10.1161/CIRCULATIONAHA.111.029165. [DOI] [PubMed] [Google Scholar]
- 17.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D ESC Committee for Practice Guidelines. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 18.Wallentin L. P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. Eur Heart J. 2009;30:1964–1977. doi: 10.1093/eurheartj/ehp296. [DOI] [PubMed] [Google Scholar]
- 19.Klovaite J, Benn M, Yazdanyar S, Nordestgaard BG. High platelet volume and increased risk of myocardial infarction: 39,531 participants from the general population. J Thromb Haemost. 2011;9:49–56. doi: 10.1111/j.1538-7836.2010.04110.x. [DOI] [PubMed] [Google Scholar]
- 20.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events. Stroke. 2004;35:1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 21.Martin JF, Bath PM, Burr ML. Influence of platelet size on outcome after myocardial infarction. Lancet. 1991;3383:1409–1411. doi: 10.1016/0140-6736(91)92719-i. [DOI] [PubMed] [Google Scholar]
- 22.George R, Bhatt A, Narayani J, Thulaseedharan JV, Sivadasanpillai H, Tharakan JA. Enhanced P-selectin expression on platelet-a marker of platelet activation, in young patients with angiographically proven coronary artery disease. Mol Cell Biochem. 2016;419:125–133. doi: 10.1007/s11010-016-2756-4. [DOI] [PubMed] [Google Scholar]
- 23.Mangin P, Ohlmann P, Eckly A, Cazenave JP, Lanza F, Gachet C. The P2Y1 receptor plays an essential role in the platelet shape change induced by collagen when TxA2 formation is prevented. J Thromb Haemost. 2004;2:969–77. doi: 10.1111/j.1538-7836.2004.00722.x. [DOI] [PubMed] [Google Scholar]


