Abstract
Background
Prenatal exposure to air pollutants can increase the risk of adverse birth outcomes and susceptibility to a number of complex disorders later in life. Despite this general understanding, the molecular and cellular responses to air pollution exposure during early life are not completely clear.
Objective
The aims of this study are to test the association between air pollution and adverse pregnancy outcomes, and to determine whether the levels of maternal and cord blood and of placental DNA methylation during pregnancy predict adverse birth outcomes in polluted areas.
Methods
This is a birth cohort study. We will enroll pregnant healthy women attending prenatal care clinics in Tehran, Iran, who are resident in selected polluted and unpolluted regions before the 14th week of pregnancy. We will calculate the regional background levels of fine particulate matter (particles with a diameter between 2.5 and 10 μm) and nitrogen dioxide for all regions of by using data from the Tehran Air Quality Control Company. Then, we will select 2 regions as the polluted and unpolluted areas of interest. Healthy mothers living in the selected polluted and non polluted regions will be enrolled in this study. A maternal health history questionnaire will be completed at each trimester. During the first and second trimester, we will draw mothers’ blood for biochemical and DNA methylation analyses. At the time of delivery time, we will collect maternal and cord blood for biochemical, gene expression, and DNA methylation analyses. We will also record birth outcomes (the newborn’s sex, birth date, birth weight and length, gestational age, Apgar score, and level of neonatal care required).
Results
The project was funded in March 2016 and enrollment will be completed in August 2017. Data analysis is under way, and the first results are expected to be submitted for publication in November 2017.
Conclusions
We supposed that prenatal exposures to air pollutants can influence fetal reprogramming by epigenetic modifications such as DNA methylation. This could explain the association between air pollution and adverse pregnancy outcomes.
Keywords: epigenomics, DNA methylation, air pollutants, pregnancy, adverse birth outcomes, placenta
Introduction
Air pollution, the most pervasive environmental concern, is estimated to cause around 800,000 deaths every year worldwide. Among air pollutants, fine particulate matter is known as a possible cause or exacerbator of diseases [1]. Previous studies showed significant associations between fine particulate matter (particles with a diameter between 2.5 [PM2.5] and 10 μm [PM10]) and mortality from complex disorders such as cardiovascular disease, cardiopulmonary disease, and lung cancer [2].
The highest number of estimated annual premature deaths due to fine particulate matter occurs in the developing countries of Asia [3]. In most Asian cities, sulfur dioxide, nitrogen dioxide, PM2.5 and PM10 levels are above the World Health Organization (WHO) guidelines [3]. Although Tehran, Iran, is rated as one of the world’s most polluted cities, there are few reports on this matter [4,5]. Naddafi et al reported an annual average of 71 μg/m3, which is 4.5 times the values recommended by the WHO [4]. Based on a WHO estimation, the average urban PM10 concentration in Iran is 68 µg/m3, about 3.4 times higher than the WHO’s air quality guidelines, which is estimated to cause about 9100 deaths per year [6].
Evidence shows that the elderly, children, and pregnant women appear to be more susceptible than the general population to the adverse health effects of air pollution, although people of all age groups are affected by air pollution [7-12]. In addition, growing evidence has been reported of the impact of environmental pollution on adverse birth outcomes [13-15]. Birth outcomes are important for public health policy, because health in early life is crucial for health later in life. Based on a life course approach, most epidemiologic research on chronic diseases has demonstrated that intrauterine and early life conditions significantly affect the occurrence of complex disorders that are of interest to public health [16,17].
Low birth weight, intrauterine growth retardation, and impaired growth in the early years of life are known to increase mortality and morbidity in childhood and the susceptibility of an individual to several complex disorders later in life, such as hypertension, coronary heart disease, and diabetes [15,18]. Despite this general understanding, the biological interactions responsible for impaired development and adverse birth outcomes are not completely clear.
The underlying mechanisms by which air pollutants may induce adverse birth outcomes are not clear. The hypothesis of fetal programming could explain some part of this interaction [19]. This hypothesis states that exposure to endogenous or exogenous factors during a sensitive period can lead to responses at a molecular and cellular level. However, environmental influences on metabolism could persist even under normal conditions or in the absence of stimulating factors [19]. Furthermore, long-term or permanent alterations in the function of target cells can lead to an increased risk for adult-onset diseases such as type 2 diabetes mellitus, hypertension, cardiovascular disease, and cancer [20-22]. The underlying biological mechanisms of fetal programming can be explained by epigenetic modifications such as DNA methylation [23-25], which is one important regulatory mechanism in cell development and differentiation [26,27]. It seems that maternal exposure to air pollutants is associated with an epigenetic modification such as DNA methylation. DNA methylation of cytosine residues is a heritable epigenetic modification that can maintain specific gene expression patterns in different cell types. Alterations in DNA methylation due to metabolic exposure during gestation or after birth may increase the susceptibility of an individual to complex disorders such as cancer and metabolic disorders later in life [28-30].
We systematically searched PubMed and SCOPUS up to December 1, 2016 for literature addressing adverse pregnancy outcomes (infant mortality, postneonatal mortality, birth weight, intrauterine growth retardation, premature birth, birth outcomes, and fetal development), pollution, and DNA methylation. This systematic search identified a few studies that assessed air pollution’s effect on global and gene-specific methylation [31-36]; 2 studies focused on the association of DNA methylation of repetitive elements and global DNA methylation of placental tissue with air pollution in early life [32,36].
An environmental inputs birth cohort study [31] showed that “epigenetic modifications in the mitochondrial genome, especially in the MT-RNR1 region, substantially mediate the association between PM2.5 exposure during gestation and placental mtDNA content, which could reflect signs of mitophagy and mitochondrial death.”
Another study [32] showed “a lower degree of placental global DNA methylation in association with exposure to particulate air pollution during early pregnancy”. It seems that exposure to particulate matter during fetal development can lead to alterations in genomic DNA methylation and affect gene-specific DNA methylation and gene expression patterns during this crucial time. Consistent with this hypothesis, recent evidence from both human subjects and animal models has indicated that exposure to airborne particulate matter is associated with changes in DNA methylation patterns. Alterations of DNA methylation patterns are postulated to modulate immune responses and regulate inflammatory genes in response to inhalation of particulate matter.
Among intracellular pathways, the glutathione pathway’s role in the lung is to defend the airway epithelium from damage in response to oxidants and inflammation [37]. Glutathione is a tripeptide, γ-glutamyl-cysteinyl-glycine (GSH), and is defined as the “body’s master antioxidant” [37]. At the molecular level, nucleotide variation in the glutathione gene has been associated with differences in susceptibility to adverse effects of air pollutants on lung function and growth [38]. Emerging evidence has shown that S-adenosylmethionine (SAMe) increases cellular glutathione content and has an important role in the methylation cycle [39,40]. SAMe is the main cosubstrate involved in methyl group transfers in the methylation cycle.
However, it is important to assess whether there is an association between air pollution and adverse birth outcomes, how it is modulated by alteration of genomic DNA methylation in the fetus and placental tissue, and how the adverse effects of air pollution on birth outcomes can be reduced by intervention strategies.
Study Objective
The primary objective of the study is to compare the incidence of adverse birth outcomes in a polluted urban area with that in an unpolluted urban area.
The secondary objective is to investigate the association between adverse birth outcomes and global changes in fetal and maternal DNA methylation.
In addition, we aim to determine the association between gene expression of GSH and alteration of global DNA methylation.
Methods
Study Design
This is a birth cohort study designed by the Endocrinology and Metabolism Research Institute of Tehran University of Medical Sciences. The research has been supported by the National Institute for Medical Research Development of Iran (grant no. 940173).
Study Population
In our birth cohort study, we will enroll pregnant women attending prenatal care clinics in two regions in Tehran, Iran: the most polluted and the least polluted. The study population is the group of eligible mothers living in these two regions who agree to participate in this study. The inclusion criteria are as follows: (1) resident in the selected polluted and unpolluted regions, (2) stay-at-home mothers (3) presenting to the clinics for prenatal care (4) before the 14th week of pregnancy who are (5) healthy, (6) able to read, write, and understand Persian at a middle school level and (7) willing to participate in follow-up visits. The exclusion criteria are as follows: (1) smoking mothers or living with a smoker, (2) having a history of chronic disorders, including heart disorders, hypertension, diabetes, hyperthyroidism, cancer, or autoimmune disorders such as lupus, (3) taking any kind of corticosteroids, or hyperglycemia or hypertension medications, (4) regularly taking methyl donors such as folate or vitamin B12 before pregnancy, or (5) having a multiple pregnancy. If a mother moves out the selected region, is employed, or travels between polluted and unpolluted regions regularly, she will be excluded from the study. The participants in the two regions (polluted and unpolluted) will be matched by age, pregestational body mass index, and parity.
Written informed consent will be obtained from all study participants in accordance with procedures approved by the Ethical Committee of the National Institute for Medical Research Development (IR.NIMAD.REC.1394.018) of Iran. Consent is attained by a research midwife or a doctor who is not directly involved in the routine perinatal care of the women. A participant may subsequently decide to withdraw from the study at any time without prejudice to their future care. In this study, 40 participants would be required in each group to have 90% power to detect a difference of 4% at global DNA methylation levels between the 2 groups. We anticipate recruiting 80 participants to investigate the role of indoor and outdoor air pollution on global DNA methylation levels of maternal and cord blood and placental tissue. We estimate that the dropout rate during the study will be 10%, so a total of 90 pregnant women will be needed for the duration of the study with a minimum of 45 participants in each subgroup (Figure 1).
Figure 1.
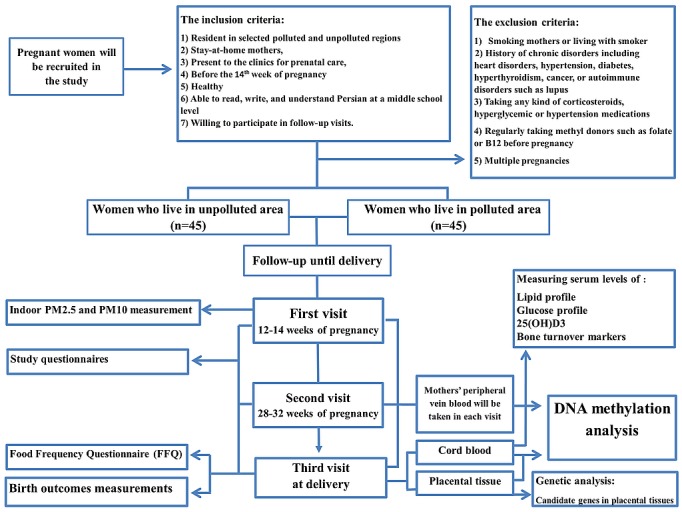
Study design. Flow diagram of selection of pregnant women living in polluted and unpolluted regions of Tehran. PM2.5: particulate matter with particle diameter 2.5 μm; PM10: particulate matter with particle diameter 10 μm.
Exposure Measurement
We will calculate the regional background levels of PM10, PM2.5, and nitrogen dioxide for each mother’s home address. The values of air pollutants will be obtained from the Tehran Air Quality Control Company in 4×4 km grids.
To explore the potential effect of exposures during pregnancy, we will calculate regional PM10 and PM2.5 concentrations (micrograms per cubic meter) during various times: the mean levels at 1 week before delivery, during the last month of pregnancy, and for each of the 3 trimesters of pregnancy. We will also calculate the exposure during the whole pregnancy.
To reduce bias due to exposure misclassification, we plan to measure the PM2.5 and PM10, as individual levels, manually by using Dylos DC1100 air quality monitors (Dylos Corporation, Riverside, CA, USA) in each participant’s address (indoor). Also, we will collect drinking water to measure contaminants including hardness, nitrite, and nitrate.
Data Collection in Each Area
First and Second Visits
At the first visit (12-14 weeks of pregnancy), we will complete study questionnaires with the participants to provide detailed information on place of residence, socioeconomic status, sleep habits, smoking status, health status, medical history, and previous pregnancy history. At the second visit (28-32 weeks of pregnancy), a maternal health history questionnaire will be completed to record what medicines, herbs, or vitamins the mother is taking and any adverse events and health problems experienced. We will take the mothers’ peripheral venous blood after an overnight fast of 10-14 hours for biochemical, DNA methylation, and gene expression analyses at the first and second visits.
Third Visit (Delivery Time)
We will follow-up participants monthly until delivery. A food frequency questionnaire will be completed to calculate nutrient elements taken in. At the delivery time, we will collect maternal and cord blood for biochemical, gene expression, and DNA methylation analyses. We will also obtain samples of placental tissue for gene expression and DNA methylation analyses.
Perinatal Outcomes
After delivery, we will record neonatal birth parameters such as the newborn’s sex, birth date, birth weight and length, gestational age, Apgar score, and level of neonatal care required (normal newborn nursery, level 2 or level 3 intensive care). All neonates will be assessed for congenital anomalies immediately after birth. We will condense birth dates into a seasonal scale, classified as cold periods (October to March) and warm periods (April to September).
Biochemical and Genetic Analyses
Blood Sampling
At each visit, we will collect blood samples for epigenetic and genetic analyses and for biochemical analysis. The serum will be divided into 2 aliquots: for routine prenatal tests (fetal blood sampling, cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, and insulin), and for measuring 25-hydroxyvitamin D3 and bone markers (procollagen I aminoterminal propeptide, osteocalcin, and C-terminal cross-linked telopeptide of type I collagen). The separated sera will be kept at –80°C until analysis.
Tissue Biopsy
The tissue biopsy will be taken from the fetal side, 1-1.5 cm below the chorioamniotic membrane at a fixed location in relation to the umbilical cord.
Global DNA Methylation Analysis
Genomic DNA will be isolated from placental tissue and the mother’s peripheral venous blood using the standard method.
Briefly, DNA is extracted by the phenol method from whole blood and homogenized placental tissues. We will determine global DNA methylation as previously published [41,42]. Global DNA methylation will be expressed as the percentage of 5-methyldeoxycytidine (5-mdC) versus the sum of 5-mdC and deoxycytidine (dC): [5-mdC/(5-mdC + dC)]%.
Gene Expression Analysis
We will extract RNA from peripheral venous blood using a Qiagen kit (QIAGEN NV, Venlo, the Netherlands). Gene expression will be analyzed by using real time polymerase chain reaction after complementary DNA synthesis. Candidate genes include GSH, DNA (cytosine-5)-methyltransferase-1-alpha, SAMe, brain-derived neurotrophic factor, synapsin I, AKT serine/threonine kinase 2, SOS Ras/Rac guanine nucleotide exchange factor 1, SOS Ras/Rac guanine nucleotide exchange factor 2, and phospholipase C gamma 2) in maternal and placental tissues.
Statistical Analysis
We will present categorical data as frequencies (%) and numbers, and continuous data as mean and standard deviation. We will use chi-square test to compare the prevalence of adverse birth outcomes in the two regions. Student t test will compare the differences in global DNA methylation levels in pregnant women in the two regions (polluted and unpolluted) at each trimester.
We will use Spearman correlation coefficients and linear regression to assess the association of global DNA methylation from blood and placental tissue with nitrogen dioxide, PM10, and PM2.5 (data will be obtained from the Air Quality Control Company).
We will construct a stepwise logistic regression model to determine the independent effect sizes of nitrogen dioxide, PM10, and PM2.5 exposures during pregnancy on global methylation. An appropriate cutoff point will be determined for DNA methylation levels, and then the levels will be defined as a dichotomous variable. We will consider covariates for entry into the model, including the newborn’s sex, maternal age (years), gestational age (weeks), parity (1, 2, or 3), sleep duration, dietary intakes of vitamin B12 and folate, regional temperature, and season at conception.
The 2(∆∆Ct) formula will be used to calculate relative transcript abundance. Student t test will be used to compare gene expression differences of all included genes in blood and placental tissue between the two groups, that is, pregnant women who live in polluted and unpolluted regions. For multiple testing corrections, we will use the false discovery rate [43,44]. We will consider 2-tailed P values <.05 as statistically significant. The same analyses will be performed in 2 subgroups from each polluted region: participants with and without classroom education.
Results
The project was funded in March 2016 and enrollment will be completed in August 2017. Data analysis is under way, and the first results are expected to be submitted for publication in November 2017.
Discussion
To our knowledge, this is the first birth cohort study in Tehran, which is rated as one of the world’s most polluted cities, to measure global DNA methylation in pregnant women who live in polluted and unpolluted regions and to investigate the interaction between adverse pregnancy outcomes and air pollution as an environmental factor. In addition, we plan to improve women’s knowledge about how to reduce prenatal exposure to air pollution and prevent adverse pregnancy outcomes attributable to air pollutants.
Developmental adaptations due to epigenetic modification may permanently “program” the fetus and may lead to adverse pregnancy outcomes that form the origin of diseases that may arise in adult life.
Based on the evidence, we supposed that prenatal exposures to air pollutants can influence fetal reprogramming by epigenetic modifications such as DNA methylation. This could explain the association between air pollution and adverse pregnancy outcomes.
Of note, there are some potential problems and limitations in our study. Primarily, some confounding factors could have a possible effect on blood and tissue DNA methylation, such as some lifestyle-related factors, environmental tobacco smoke, the season, and environmental temperature. To minimize the impact of lifestyle and regional differences in methylation patterns, we will adjust for the mother’s socioeconomic status, maternal diet, and maternal sleep habits in our analysis. Also, we will consider exposures to other air pollutants such as second-hand smoke and indoor air pollution. We will exclude mothers who smoke or live with a smoker. We will obtain the temperature of each region from Air Quality Control Company data. In addition, the differences in effect estimates of air pollutants on DNA methylation could be further related to differences in maternal nutritional status. To control for the impact of nutritional status, a food frequency questionnaire will be filled out for all participants to measure special nutrients associated with DNA methylation, such as folate and vitamin B12. However, we can’t control for some unknown factors that are associated with blood and tissue DNA methylation, as well as levels of air pollutants.
Acknowledgments
This work is supported by the National Institute for Medical Research Development of Iran through grant number IR.NIMAD.REC.1394.018.
Abbreviations
- GSH
γ-glutamyl-cysteinyl-glycine
- PM2.5
particulate matter with particle diameter 2.5 μm
- PM10
particulate matter with particle diameter 10 μm
- SAMe
S-adenosylmethionine
- WHO
World Health Organization
Footnotes
Authors' Contributions: ZM, AH, MR, and SM conceived the study design; ZM and AH contributed to design data collection tools and wrote the statistical analysis plan. ZM and AH will monitor data collection for the whole trial and analysis of the data. ZM and AH drafted the manuscript, and all authors reviewed the draft of the manuscript and revised it. All authors approved the final manuscript to be published.
Conflicts of Interest: None declared.
References
- 1.Bell ML, Davis DL, Fletcher T. A retrospective assessment of mortality from the London smog episode of 1952: the role of influenza and pollution. Environ Health Perspect. 2004 Jan;112(1):6–8. doi: 10.1289/ehp.6539. http://europepmc.org/abstract/MED/14698923 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope CA, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006 Jun;56(6):709–42. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 3.Romieu IM. Air pollution and health in developing countries: a review of epidemiological evidence. In: McGranahan G, Murray F, editors. Air Pollution and Health in Rapidly Developing Countries. Second edition. London, UK: Earthscan Publications Ltd; 2012. pp. 49–67. [Google Scholar]
- 4.Naddafi K, Hassanvand MS, Yunesian M, Momeniha F, Nabizadeh R, Faridi S, Gholampour A. Health impact assessment of air pollution in megacity of Tehran, Iran. Iranian J Environ Health Sci Eng. 2012 Dec 17;9(1):28. doi: 10.1186/1735-2746-9-28. https://www.ijehse.com/content/9/9/28 .1735-2746-9-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brajer V, Hall J, Rahmatian M. Air pollution, its mortality risk, and economic impacts in tehran, iran. Iran J Public Health. 2012;41(5):31–8. http://europepmc.org/abstract/MED/23113175 . [PMC free article] [PubMed] [Google Scholar]
- 6.Public Health and the Environment . Country profile of environmental burden of disease: Iran, Islamic Republic of. Geneva, Switzerland: World Health Organization; 2009. [2017-02-15]. http://www.who.int/quantifying_ehimpacts/national/countryprofile/iran.pdf?ua=1 . [Google Scholar]
- 7.Ballester F, Estarlich M, Iñiguez C, Llop S, Ramón R, Esplugues A, Lacasaña M, Rebagliato M. Air pollution exposure during pregnancy and reduced birth size: a prospective birth cohort study in Valencia, Spain. Environ Health. 2010 Jan 29;9:6. doi: 10.1186/1476-069X-9-6. https://ehjournal.biomedcentral.com/articles/10.1186/1476-069X-9-6 .1476-069X-9-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dadvand P, Parker J, Bell ML, Bonzini M, Brauer M, Darrow LA, Gehring U, Glinianaia SV, Gouveia N, Ha E, Leem JH, van den Hooven E, Jalaludin B, Jesdale BM, Lepeule J, Morello-Frosch R, Morgan GG, Pesatori AC, Pierik FH, Pless-Mulloli T, Rich DQ, Sathyanarayana S, Seo J, Slama R, Strickland M, Tamburic L, Wartenberg D, Nieuwenhuijsen MJ, Woodruff TJ. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013 Mar;121(3):267–373. doi: 10.1289/ehp.1205575. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheers H, Mwalili SM, Faes C, Fierens F, Nemery B, Nawrot TS. Does air pollution trigger infant mortality in Western Europe? A case-crossover study. Environ Health Perspect. 2011 Jul;119(7):1017–22. doi: 10.1289/ehp.1002913. doi: 10.1289/ehp.1002913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz B, Wilhelm M, Zhao Y. Air pollution and infant death in southern California, 1989-2000. Pediatrics. 2006 Aug;118(2):493–502. doi: 10.1542/peds.2006-0027. http://europepmc.org/abstract/MED/16882800 .118/2/493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Pinkerton KE. Air pollutant effects on fetal and early postnatal development. Birth Defects Res C Embryo Today. 2007 Sep;81(3):144–54. doi: 10.1002/bdrc.20097. [DOI] [PubMed] [Google Scholar]
- 12.Woodruff TJ, Darrow LA, Parker JD. Air pollution and postneonatal infant mortality in the United States, 1999-2002. Environ Health Perspect. 2008 Jan;116(1):110–5. doi: 10.1289/ehp.10370. doi: 10.1289/ehp.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, Spengler JD, Koutrakis P, Speizer FE, Ferris BG. Acute effects of summer air pollution on respiratory symptom reporting in children. Am J Respir Crit Care Med. 1994 Nov;150(5 Pt 1):1234–42. doi: 10.1164/ajrccm.150.5.7952546. [DOI] [PubMed] [Google Scholar]
- 14.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology. 2004 Jan;15(1):36–45. doi: 10.1097/01.ede.0000101023.41844.ac. [DOI] [PubMed] [Google Scholar]
- 15.Maisonet M, Correa A, Misra D, Jaakkola JJ. A review of the literature on the effects of ambient air pollution on fetal growth. Environ Res. 2004 May;95(1):106–15. doi: 10.1016/j.envres.2004.01.001.S001393510400009X [DOI] [PubMed] [Google Scholar]
- 16.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002 Apr;31(2):285–93. http://ije.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11980781 . [PubMed] [Google Scholar]
- 17.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001 Feb;30(1):15–23. doi: 10.1093/ije/30.1.15. http://ije.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=11171842 . [DOI] [PubMed] [Google Scholar]
- 18.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000 Jun;108 Suppl 3:545–53. doi: 10.1289/ehp.00108s3545. http://europepmc.org/abstract/MED/10852853 .sc271_5_1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lucas A. Programming by early nutrition in man. Ciba Found Symp. 1991;156:38–50; discussion 50. [PubMed] [Google Scholar]
- 20.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. 1992. Int J Epidemiol. 2013 Oct;42(5):1215–22. doi: 10.1093/ije/dyt133. http://ije.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=24159065 .dyt133 [DOI] [PubMed] [Google Scholar]
- 21.Barker DJ. The fetal origins of coronary heart disease. Acta Paediatr Suppl. 1997 Jul;422:78–82. doi: 10.1111/j.1651-2227.1997.tb18351.x. [DOI] [PubMed] [Google Scholar]
- 22.Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993 Jan;36(1):62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 23.Canovas S, Ross PJ. Epigenetics in preimplantation mammalian development. Theriogenology. 2016 Jul 01;86(1):69–79. doi: 10.1016/j.theriogenology.2016.04.020.S0093-691X(16)30051-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liang P, Song F, Ghosh S, Morien E, Qin M, Mahmood S, Fujiwara K, Igarashi J, Nagase H, Held WA. Genome-wide survey reveals dynamic widespread tissue-specific changes in DNA methylation during development. BMC Genomics. 2011 May 11;12(1):231. doi: 10.1186/1471-2164-12-231. https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-12-231 .1471-2164-12-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chmurzynska A. Fetal programming: link between early nutrition, DNA methylation, and complex diseases. Nutr Rev. 2010 Feb;68(2):87–98. doi: 10.1111/j.1753-4887.2009.00265.x.NURE265 [DOI] [PubMed] [Google Scholar]
- 26.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002 Jan 01;16(1):6–21. doi: 10.1101/gad.947102. http://www.genesdev.org/cgi/pmidlookup?view=long&pmid=11782440 . [DOI] [PubMed] [Google Scholar]
- 27.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999 Oct 29;99(3):247–57. doi: 10.1016/s0092-8674(00)81656-6. https://linkinghub.elsevier.com/retrieve/pii/S0092-8674(00)81656-6 .S0092-8674(00)81656-6 [DOI] [PubMed] [Google Scholar]
- 28.Meissner A, Mikkelsen TS, Gu H, Wernig M, Hanna J, Sivachenko A, Zhang X, Bernstein BE, Nusbaum C, Jaffe DB, Gnirke A, Jaenisch R, Lander ES. Genome-scale DNA methylation maps of pluripotent and differentiated cells. Nature. 2008 Aug 07;454(7205):766–70. doi: 10.1038/nature07107. http://europepmc.org/abstract/MED/18600261 .nature07107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehnen H, Zechner U, Haaf T. Epigenetics of gestational diabetes mellitus and offspring health: the time for action is in early stages of life. Mol Hum Reprod. 2013 Jul;19(7):415–22. doi: 10.1093/molehr/gat020. http://molehr.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=23515667 .gat020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dyer JS, Rosenfeld CR. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin Reprod Med. 2011 May;29(3):266–76. doi: 10.1055/s-0031-1275521. [DOI] [PubMed] [Google Scholar]
- 31.Janssen BG, Byun H, Gyselaers W, Lefebvre W, Baccarelli AA, Nawrot TS. Placental mitochondrial methylation and exposure to airborne particulate matter in the early life environment: An ENVIRONAGE birth cohort study. Epigenetics. 2015;10(6):536–44. doi: 10.1080/15592294.2015.1048412. http://europepmc.org/abstract/MED/25996590 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janssen BG, Godderis L, Pieters N, Poels K, Kiciński M, Cuypers A, Fierens F, Penders J, Plusquin M, Gyselaers W, Nawrot TS. Placental DNA hypomethylation in association with particulate air pollution in early life. Part Fibre Toxicol. 2013 Jun 07;10:22. doi: 10.1186/1743-8977-10-22. https://particleandfibretoxicology.biomedcentral.com/articles/10.1186/1743-8977-10-22 .1743-8977-10-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, Gagne LA, Banister CE, Padbury JF, Marsit CJ. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012 Feb;120(2):296–302. doi: 10.1289/ehp.1103927. doi: 10.1289/ehp.1103927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossnerova A, Tulupova E, Tabashidze N, Schmuczerova J, Dostal M, Rossner P, Gmuender H, Sram RJ. Factors affecting the 27K DNA methylation pattern in asthmatic and healthy children from locations with various environments. Mutat Res. 2013;741-742:18–26. doi: 10.1016/j.mrfmmm.2013.02.003.S0027-5107(13)00016-X [DOI] [PubMed] [Google Scholar]
- 35.Saenen ND, Vrijens K, Janssen BG, Roels HA, Neven KY, Vanden BW, Gyselaers W, Vanpoucke C, Lefebvre W, De Boever P, Nawrot TS. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE cohort. Environ Health Perspect. 2016 Sep 13; doi: 10.1289/EHP38. doi: 10.1289/EHP38.EHP38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kingsley SL, Eliot MN, Whitsel EA, Huang Y, Kelsey KT, Marsit CJ, Wellenius GA. Maternal residential proximity to major roadways, birth weight, and placental DNA methylation. Environ Int. 2016;92-93:43–9. doi: 10.1016/j.envint.2016.03.020.S0160-4120(16)30100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas SK, Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol Aspects Med. 2009;30(1-2):60–76. doi: 10.1016/j.mam.2008.07.001. http://europepmc.org/abstract/MED/18760298 .S0098-2997(08)00052-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman I. Regulation of glutathione in inflammation and chronic lung diseases. Mutat Res. 2005 Nov 11;579(1-2):58–80. doi: 10.1016/j.mrfmmm.2005.02.025.S0027-5107(05)00251-4 [DOI] [PubMed] [Google Scholar]
- 39.Lieber CS. S-adenosyl-L-methionine: its role in the treatment of liver disorders. Am J Clin Nutr. 2002 Nov;76(5):1183S–7S. doi: 10.1093/ajcn/76/5.1183S. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=12418503 . [DOI] [PubMed] [Google Scholar]
- 40.Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, Carrieri V, Albano O. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989 May;24(4):407–15. doi: 10.3109/00365528909093067. [DOI] [PubMed] [Google Scholar]
- 41.Maghbooli Z, Hossein-Nezhad A, Larijani B, Pasalar P, Keshtkar AA. Association between alterations in global DNA methylation and predisposing factors in diabetes: a high pressure liquid chromatography based study. Minerva Med. 2015 Aug;106(4):221–31.R10Y9999N00A140026 [PubMed] [Google Scholar]
- 42.Maghbooli Z, Hossein-nezhad A, Larijani B, Amini M, Keshtkar A. Global DNA methylation as a possible biomarker for diabetic retinopathy. Diabetes Metab Res Rev. 2015 Feb;31(2):183–9. doi: 10.1002/dmrr.2584. [DOI] [PubMed] [Google Scholar]
- 43.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003 Feb 12;19(3):368–75. doi: 10.1093/bioinformatics/btf877. http://bioinformatics.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=12584122 . [DOI] [PubMed] [Google Scholar]
- 44.Maghbooli Z, Hossein-Nezhad A. Transcriptome and molecular endocrinology aspects of epicardial adipose tissue in cardiovascular diseases: a systematic review and meta-analysis of observational studies. Biomed Res Int. 2015;2015:926567. doi: 10.1155/2015/926567. doi: 10.1155/2015/926567. [DOI] [PMC free article] [PubMed] [Google Scholar]


