Abstract
Severe Plasmodium falciparum malaria anemia (SMA) is a major cause of mortality in pediatric wards. Variations in inflammatory mediator production play an essential role in disease outcomes. Indeed, several studies have shown the involvement of pro- and anti-inflammatory cytokines such as IFN-γ, IL-6, TNF-α and IL-10 in malaria immunopathology. In other hand the exact role of Th17 cytokines such as IL-17, IL-22 and IL-21 in malaria remains poorly documented. Here, we investigated IFN-γ, TNF-α, IL-6, IL-12, IL-10, IL-4, IL-13, IL-17, IL-22 and IL-21 circulating levels and their association with malaria anemia and parasitemia in Gabonese children. Levels of IFN-γ (500 ± 100.2 pg/ml), IL-6 (64 ± 14.2 pg/ml), IL-10 (505 ± 35 pg/ml), IL-13 (30.6 ± 5.6 pg/ml) were significantly higher (P < 0.03) in infected children than in uninfected controls (210 ± 20 pg/ml, 17.5 pg/ml, 50 ± 25.9, pg/ml, 17.48 pg/ml, respectively). IFN-γ levels were significantly lower (P = 0.04) in children with SMA (400 ± 200 pg/ml) than in those with uncomplicated malaria (900 ± 450 pg/ml) and higher in those with parasitemia (P = 0.019). Levels of IL-6 and IL-10 were significantly higher in children with malarial anemia (P < 0.001) and hyperparasitemia (P < 0.0001). A significant association between IL-10 levels and parasite density was observed (P < 0.00001). IL-22 levels were significantly higher (P = 0.01) in infected children (72.57 ± 7.5 pg/ml) than in the controls (54.96 ± 1.93 pg/ml). IL-21 levels (44.46 ± 17.27 pg/ml) decreased with the severity of anemia (P < 0.05), whereas IL-17 levels increased in children with SMA (12.25 ± 1.25 pg/ml) than in those with mild malaria anemia (MMA: 6.2 ± 5.25 pg/ml, P = 0.002). Data suggest possible role of IFN-γ in the protection against SMA and parasite clearance. However, IL-6 and IL-10 could play a role in inflammatory response and pathophysiology of severe malaria anemia. Also, the role of IL-22 and IL-17 in P. falciparum malaria infection should be investigated.
Keywords: Severe Plasmodium falciparum malaria anemia (SMA), pro- and anti-inflammatory cytokines, children
Introduction
Plasmodium falciparum malaria infection remains a major public health problem worldwide mostly in African regions [1]. Children aged under 5 years are the most susceptible population with anemia as the main severe complication [2,3]. In Gabon, P. falciparum malaria is the main cause of neurological, hematological and infectious emergencies in healthcare structure [2,4,5]. In Franceville, southeast Gabon, where malaria is hyperendemic [6], a recrudescence of malaria infection has been observed between 2008 and 2012, accompanied by epidemiological modifications [7], and higher incidence rates in older subjects, suggesting a decrease in acquired protective immunity [8,9].
The pro-inflammatory cytokines seem to play an important role in malaria protection and parasite clearance. Also, the relative levels of pro- and anti-inflammatory cytokines are important mediators of development and outcomes of malarial anemia. Early production of pro-inflammatory T helper 1 (Th1) cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-12 and interferon (IFN)-gamma may limit progression from uncomplicated malaria to severe complications [10,11]. Indeed, they inhibit parasite growth and stimulate monocyte phagocytosis to enhance clearance of parasitized erythrocytes. Whereas, IL-6 cytokine is a major mediator of the acute phase response. Other pro-inflammatory cytokines such as IL-17 and IL-22, produced by other cell subtypes, including Th17 cells, are also involved in the immune response against Plasmodium. They contribute to inflammation by recruitment of neutrophils and induction of secretion of several pro-inflammatory cytokines. An increase in IL-17-producing CD4+ T cells in peripheral blood has been reported during P. vivax infection, along with the production of the pro-inflammatory cytokines IFN-γ, IL-10 and transforming growth factor (TGF)-beta [12]. Induction of IL-22 during murine infection protects against liver damage [13]. However, if these pro-inflammatory responses are not properly regulated during the acute infection, severe complications of malaria may ensue [14,15]. Hence, the need for anti-inflammatory responses to control the production and possible cytopathic effects of pro-inflammatory cytokines. Regulatory cytokines such as IL-10 play an important role in Plasmodium infection, neutralizing excessive production of inflammatory Th1 cytokines [16,17]. Anti-inflammatory Th2 cytokines including IL-4 and IL-13, regulate the humoral immune response, contributing to parasite clearance and inhibiting Th1 cytokine production [18,19].
Despite the importance of the pro and anti-inflammatory cytokines production in human immune responses to Plasmodium falciparum malaria infection, that is not well documented in Gabonese children. Hence, in this study, we investigate pro- and anti-inflammatory responses in Gabonese children according to their malarial disease status, by measuring selected cytokines in plasma from these children.
Patients and methods
Study area and population
This study was conducted at Amissa Bongo Regional Hospital, Franceville, southeastern Gabon. Between 2011 and 2014, children presenting to the pediatric ward with a febrile syndrome or a history of fever during the previous 48 h were included. The children were classified according to their hemoglobin (Hb) level: no malarial anemia (UMA ≥ 11.0 g/dl), mild malaria anemia (MMA: 5.0 to 10.9 g/dl) or severe malaria anemia (SMA: < 5.0 g/dl). P. falciparum-exposed children with negative thick blood smears and rapid P. falciparum detection kit results (Optimal-IT, Biorad, France) served as uninfected controls. The parents or legal guardians gave their written informed consent before each child’s enrollment in the study, which was approved by the Gabonese National Research Ethics Committee (N°00370/MSP/CABMD).
Blood samples
Venous blood (2.0-5.0 ml) was collected in EDTA tubes. Blood smears were stained with Giemsa according to Lambaréné method for microscopic P. falciparum identification and quantification [20]. All slides were examined by two well-trained microscopists from the International Medical Research Center in Franceville, Gabon. Hemoglobin, red blood cells, white blood cells and platelets were measured with an automated device (Beckman Coulter AcT diff2, Beckman Coulter Corporation, Miami, FL).
Cytokine assays
Blood samples were immediately centrifuged, and plasma was aliquoted and stored at -80°C until use. Circulating cytokine levels were determined with indirect enzyme-linked immunosorbent assays (ELISA) using the Ready-SET-Go!® kit (eBioscience®), according to the manufacturer’s instructions. Optical densities (OD) were measured at 450 nm with a reference at 620 nm in an ELISA plate reader (Stat Fax 3200®, Bioblock Scientific; Fisher). The detection limits were as follows: 2 pg/ml for IL-6, IL-10 and IL-4; 4 pg/ml for IFN-γ, TNF-α, IL-13 and IL-17; 8 pg/ml for IL-22 and IL-21; and 31 pg/ml for IL-12p40. All samples were tested in duplicate, and the mean of the two OD values was used for analyses.
Statistical analysis
Variables were compared between the clinical subgroups (uninfected, UAM, MMA and SMA) by using the non-parametric measures. Differences between subgroups were analyzed for statistical significance using the Kruskal-Wallis non-parametric test. If significant differences were detected, the Mann-Whitney U non-parametric test was used for pairwise comparisons. Possible correlations between levels of cytokines and parasite density were identified using Spearman’s rho test. All statistical tests used SPSS version 17.0 for Windows (SPSS Inc., Chicago, USA). P values below 0.05 were considered statistically significant.
Results
Clinical and biological characteristics (Table 1)
(Table 1) A total of 250 children aged between 6 to 168 months were included, of whom 122 had P. falciparum infection (21 with uncomplicated malaria, 86 with mild malarial anemia and 15 with severe malarial anemia) and 128 were free of malaria parasites. Mean parasitemia was 46277 ± 8188 parasites/µl. The mean age of the infected and uninfected children was 64.2 ± 2.8 and 60.5 ± 3.6 months, respectively (no significant difference). Infected children had significantly lower hemoglobin levels (8.2 ± 0.2 g/dL) than uninfected children (8.9 ± 0.3 g/dL; P = 0.04). Red blood cells (3.1 ± 0.1 × 106 cells/mm3) and platelets (1.5 ± 0.1 × 105 cells/mm3) counts were also significantly lower in infected children than in uninfected children (4.8 ± 0.4 × 106 cells/mm3 and 2.8 ± 0.1 × 105 cells/mm3, P = 0.0001 and P = 0.00001 respectively). White blood cells counts were lower in infected children (7.7 ± 0.4 × 103 cells/mm3) than in uninfected children (8.4 ± 0.5 × 103 cells/mm3) but the difference was not statistically significant.
Table 1.
Demographical and hematological parameters of included patients (mean ± SD)
| Children | ||
|---|---|---|
|
| ||
| Malaria-infected (n = 122) | Malaria-uninfected (n = 128) | |
| Age mean (months) | 64.2 ± 2.8 | 60.5 ± 3.6 |
| Hemoglobin (g/dL) | 8.2 ± 0.2* | 8.9 ± 0.3 |
| Platelets (cells/mm3) | 150,000 ± 10,000*** | 280,000 ± 10,000 |
| Red blood cells count (cells/mm3) | 3,100,000 ± 100,000** | 4,800,000 ± 400,000 |
| White blood cells count (cells/mm3) | 7700 ± 400 | 8400 ± 500 |
| Parasitemia (parasites/µL) | 46277 ± 8188 | 0 |
Age, leucocytes counts, hemoglobin concentrations and parasite densities in uninfected and infected children. Plasmodium falciparum-exposed children negative for parasites in thick blood smears and negative in rapid detection test kits for P. falciparum were defined as uninfected children.
P < 0.05;
P < 0.0001;
P < 0.000001.
SD = Standard deviation.
Increased plasma levels of pro- (IFN-γ and IL-6) and anti-inflammatory (IL-10 and IL-13) cytokines in infected children
Levels of pro- (IFN-γ, IL-6) and anti-inflammatory (IL-13 and IL-10) cytokines were significantly higher in P. falciparum-infected children than in uninfected children (Figure 1). The mean IFN-γ concentration was respectively 500 ± 100 and 210 ± 20 pg/ml (P = 0.02, Figure 1A), and the mean IL-6 concentration was respectively 64 ± 14.2 and 17.5 ± 2.5 pg/ml (P < 0.00001, Figure 1B). The mean IL-10 concentration was respectively 505 ± 35 and 50 ± 25.9 pg/ml (P < 0.00001, Figure 1C) and the mean IL-13 concentrations was respectively 30.6 ± 5.6 and 17.48 ± 1.58 pg/ml (P = 0.03, Figure 1D). The TNF-α concentration was significantly lower in infected children than in uninfected children (P = 0.002, data not shown). No significant difference in the mean IL-12p40 concentration was observed (384 ± 39.2 and 344.8 ± 29.8 pg/ml in infected and uninfected children, respectively). IL-4 levels were usually under the detection limit and were therefore excluded from the analysis.
Figure 1.
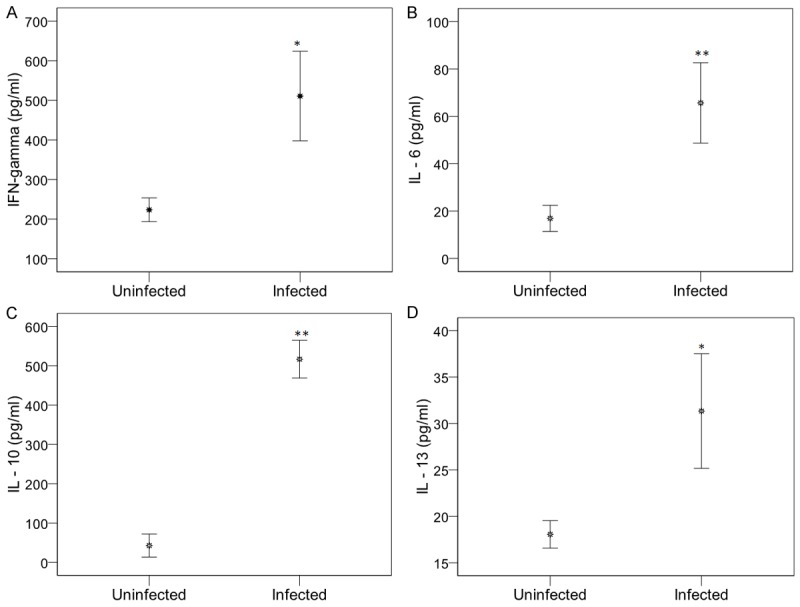
Levels of pro- and anti-inflammatory cytokines in plasma from uninfected and infected children. Plasma concentrations of cytokines IFN-γ (A), IL-6 (B), IL-10 (C) and IL-13 (D) were quantified in Plasmodium falciparum infected children and uninfected children. Data are represented with arithmetic means of cytokines concentrations in pg/ml, with the standard error. P. falciparum-exposed children negative for parasites in blood smears and in rapid detection test kits were uninfected children. Significant differences between uninfected and infected children are indicated, *P < 0.05, **P < 0.00001 compared to uninfected children.
IL-22 levels highest in malaria children (Figure 2)
The mean plasma IL-22 concentration was 72.57 ± 7.5 pg/ml and 54.96 ± 1.93 pg/ml in children with and without P. falciparum infection (P = 0.01, Figure 2A). IL-17 and IL-21 levels were not significantly different (Figure 2B, 2C).
Figure 2.
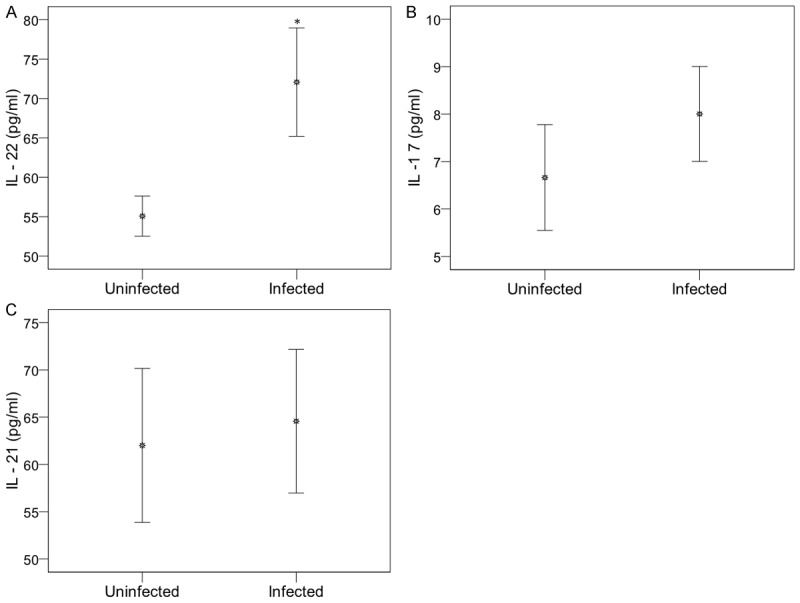
Th17-cytokine circulating levels in plasma from uninfected and infected children. Plasma concentrations of IL-22 (A), IL-17A (B) and IL-21 (C) were quantified in P. falciparum infected and uninfected children. Data are represented in arithmetic means of cytokines concentrations in pg/ml, with the standard error of mean. P. falciparum-exposed infants negative for parasites in blood smears and in rapid detection test kits for P. falciparum were uninfected children. Significant differences between uninfected and infected children are indicated, *P < 0.05 compared to uninfected children.
Plasmodium falciparum parasitemia is strongly associated with IL-10 levels (Figure 3)
Infected children were divided into three groups according to mean P. falciparum density: low (≤ 1000), medium (]1000-10000]) and high (> 10000). IL-10 levels increased significantly with the degree of parasitemia (49.6 ± 5.3 pg/ml, 298.4 ± 22.4 pg/ml and 720.0 ± 50.0 pg/ml for low, medium and high parasitemia; P < 0.0001, Figure 3A). Likewise, IL-6 levels were significantly higher in children with medium and high parasite density than in those with low parasite density (36.3 ± 15.9 and 62.7 ± 20.7 pg/ml versus 16.5 ± 13.4 pg/ml; P = 0.05; Figure 3B). IL-22 and IFN-γ concentrations were higher in children with medium parasite density (76.82 ± 15.1 pg/ml and 509.5 ± 300 pg/ml for IL-22 and IFN-γ) than in those with low parasite density (51.5 ± 9.7 pg/ml and 113.37 ± 25.3 pg/ml) or high parasite density (69.5 ± 8.9 pg/ml and 334.4 ± 63.9 pg/ml), although the difference was only significant for IFN-γ (< 1000 vs > 10000 parasites/µl, P = 0.03, Figure 3C). By contrast, IL-13 levels decreased as parasitemia increased, although the difference was not significant (P > 0.05, data not shown).
Figure 3.
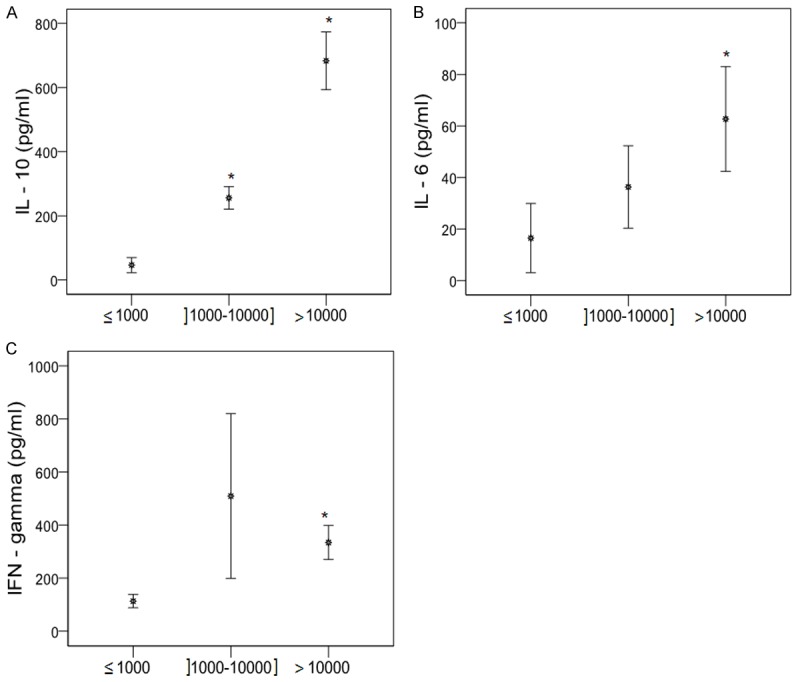
Distribution of cytokines (IFN-γ, IL-10 and IL-6) concentrations according to the parasitemia. Plasma concentrations of pro- and anti-inflammatory cytokines were quantified in P. falciparum-infected children. P. falciparum-infected children were subdivided into three groups based upon the parasite density. Low density group was characterized by parasite density ≤ 1000 parasites/µl of blood. Medium and high density groups were defined by parasite density included between 1000 and 10000 parasites/µl and > 10000 parasites/µl of blood, respectively. Significant differences between the groups were done by Mann-Whitney test. *P < 0.05 compared to low density group (≤ 1000 parasites/µl).
Thus, IL-10 (rho = 0.49, P ≤ 0.000001), IFN-γ (rho = 0.23, P = 0.019) and IL-6 (rho = 0.24, P = 0.05) levels correlated with parasite density. The strongest association with parasite density was with IL-10 (Table 2).
Table 2.
Correlation between plasma levels of inflammatory cytokines and parasitemia
| P. falciparum infected children | ||
|---|---|---|
|
|
||
| Cytokines | rho | p |
| IFN-γ | 0.231 | 0.019 |
| TNF-α | 0.017 | 0.868 |
| IL-6 | 0.242 | 0.05 |
| IL12p40 | 0.023 | 0.897 |
| IL-17 | 0.081 | 0.515 |
| IL-22 | 0.032 | 0.784 |
| IL-21 | 0.099 | 0.459 |
| IL-10 | 0.412 | 0.00001 |
| IL-4 | 0.055 | 0.714 |
| IL-13 | 0.082 | 0.546 |
Spearman Rank Correlation analysis was carried between cytokines and parasitemia in P. falciparum-infected children. Correlation coefficient (rho) is given. Correlation is significant at P < 0.05.
Cytokine levels according to anemia
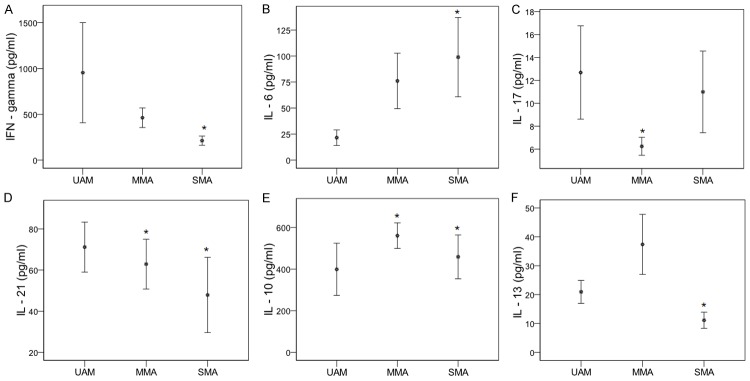
We then analyzed pro- and anti-inflammatory cytokine profiles in children with unanemic malaria (UAM), mild malarial anemia (MMA), and severe malarial anemia (SMA). As shown in Figure 4, IFN-γ, IL-17 and IL-6 levels differed significantly across the three subgroups. IFN-γ levels decreased with increasing anemia severity, with mean concentrations of 954 ± 546 pg/ml in the UAM group, 442.12 ± 100 pg/ml in the MMA group and 200 ± 49.15 pg/ml in the SMA group (P = 0.05, Figure 4A). In contrast, IL-6 levels increased with increasing anemia severity: respectively 18.3 ± 6.7 pg/ml, 75.2 ± 20.2 pg/ml and 98.9 ± 38.1 pg/ml (P = 0.03, Figure 4B). IL-17 concentrations were highest in the UAM and SMA groups (12.7 ± 4 pg/ml and 11 ± 3.6 pg/ml, respectively), and lowest in the MMA group (6.2 ± 0.8 pg/ml), (P = 0.012, Figure 4C). IL-21 levels fell significantly with increasing anemia severity: 71.2 ± 12.2, 55.7 ± 11.2 and 44.5 ± 17.3 pg/ml respectively; with significant differences between the UAM and MMA groups (P = 0.05) and between the UAM and SMA groups (P = 0.028) (Figure 4D). IL-10 levels differed significantly across the groups (P < 0.01, Figure 4E), being highest in the MMA group (580 ± 80 pg/ml), followed by the SMA (430 ± 60 pg/ml) and UAM groups (400 ± 250 pg/ml). IL-13 levels were significantly higher in the MMA group (37.37 ± 10.4 pg/ml) than in the UAM (21 ± 4 pg/ml) and SMA groups (11.2 ± 2.8 pg/ml), (P = 0.03; Figure 4F).
Figure 4.
Pro- and anti-inflammatory cytokines profiles according to malaria anaemia statue. Plasma concentrations of cytokines IFN-γ (A) and interleukin (IL)-6 (B), IL-17A (C), IL-21 (D), IL-10 (E) and IL-13 (F) were quantified in P. falciparum infected and uninfected children. Results represent the arithmetic means of cytokines concentrations in pg/ml, with the standard error of mean. Children were subdivided into severe malaria anaemia (SMA: Hb < 5.0 g/dL), mild malaria anaemia (MMA: 5 ≤ Hb < 11 g/dL) and unanaemia malaria (UAM: Hb ≥ 11 g/dL). Kruskall-Wallis tests and pairwise comparisons using Mann-Whitney U tests were used. Differences were significant at p-value < 0.05. *P < 0.05 compared to unanaemia malaria (UAM).
Discussion
We analyzed differences in cytokine responses between P. falciparum-infected and uninfected children living in the same area with similar socioeconomic and environmental conditions. All the infected children were free of cerebral malaria, anemia being the most frequent clinical manifestation in children under the age of 5 years [2]. We found that infected children had significantly higher IFN-γ levels than uninfected children, suggesting IFN-γ induction by P. falciparum. IFN-γ levels also fell significantly with the severity of malarial anemia, suggesting a protective role. This is consistent with previous data showing that IFN-γ controls parasite multiplication, promotes parasite clearance and protects against severe malaria [21-24]. Previous studies have shown a protective effect of IFN-γ against clinical malarial anemia among young children living in a holoendemic area [25]. IFN-γ production in response to pre-erythrocytic antigens was also associated with high hemoglobin concentrations and a reduced prevalence of malarial anemia in these children. Our data thus confirm the role of IFN-γ as a key mediator of the protective immune response against P. falciparum and its role in the pathophysiology of severe malarial anemia.
Proinflammatory cytokines play an important role in immune responses to P. falciparum, and IL-6 was significantly produced in our infected children. Increased levels of IL-6 were also been detected in plasma from children with severe malarial anemia, in keeping with data on Mozambican children showing that severe malaria is associated with high IL-6 levels [26]. Our data demonstrate an association between high IL-6 levels and a high parasite burden. IL-6 does not protect against parasite multiplication. Other studies have shown an association between low IL-6 and hyperparasitemia [27]. These results suggest that strong IL-6 production could contribute to the pathogenesis of severe malarial anemia. A study of Malawian children showed that chronic iron deficiency was strongly associated with higher production of IL-6 [28]. IL-6 induces an increase in transferrin receptor density on hepatocytes [29,30], an increase in ferritin synthesis, and a decrease of transferrin synthesis [29]. This pro-inflammatory response is modulated by IL-10. Indeed, initial production of IL-12 and TFN-α enhanced IL-6 production, followed by an increase in IL-10 production as a counter-regulatory mechanism inhibiting the production of pro-inflammatory cytokines such as TNF-α and IL-12. Although high levels of TFN-α have been shown to correlate with malaria severity in several studies [16,27,31], low TNF-α levels were observed in our study. This could be explained by the negative action of IL-10 on pro-inflammatory responses. IL-10 completely abolishes TNF-α production in response to malarial antigens [32]. IL-12 plays an important role in the adaptive immune response to malaria. Also in this study, plasma levels of IL12p40, the common subunit of IL12p70 and IL-23, were detected in both infected and uninfected children, with no significant difference. This could be explained by the high production of IL-10 in infected children, with a suppressive effect on IL-12 production. Indeed IL-12 levels were paradoxically lower in African children with severe malaria [33-35]. Likewise, a study of Kenyan children showed that those with malarial anemia had low circulating levels of IL-12p70 and down-regulated IL-12p40 mRNA levels, caused by phagocytosis of malarial pigment by monocytes and induction of the IL-10 overproduction by malarial pigment [36].
IL-10 is an important anti-inflammatory cytokine [37,38]. We found increased plasma levels of IL-10 in infected children compared with uninfected children, especially in those with malarial anemia. We also found that IL-10 levels increased with increasing parasitemia. These results are consistent with a study of Kenyan children in whom anemia severity and elevated parasitemia were associated with increased IL-10 concentrations [39]. This suggests that P. falciparum stimulates IL-10 production in dose-dependent manner. IL-10 is used by regulatory T cells to control the immune response. Indeed, P. falciparum-infected erythrocytes induce regulatory T cell expansion, followed by increased IL-10 and IL-6 production [22,40]. In addition to a direct correlation between T regulatory cell numbers and IL-10 plasma levels in infected patients [41,42], it has been shown that CD4+CD25+Foxp3+ T cells induced during malaria upregulate their IL-10 expression [42,43]. Other authors also report that regulatory T cells are associated with higher rates of parasite growth, suggesting that P. falciparum-mediated induction of regulatory T cells might be a virulence factor [44].
In protozoan infections, a role of Th17 cytokines has been reported in inflammatory responses and host defense mechanisms. Indeed, IL-17 is important for the resolution of Trypanosoma cruzi infection [45] and may mediate protection from Toxoplasma gondii infection [46,47]. On the other hand, T. gondii infection enhances IL-17 expression, which contributes to the inflammatory response, and IL-17 promotes fatal T. gondii infection [48] and the inflammatory reaction in human leishmaniasis [49]. Patients with acute P. vivax infection showed a significant increase in circulating Tregs producing anti-inflammatory as well as pro-inflammatory cytokines such as IL-17 [43,50]. In our study, IL-17 levels were similar in infected and uninfected children. Analysis of cytokine production according to anemic status showed significantly higher IL-17 production in children with severe malarial anemia than in those with mild malarial anemia. In contrast, Ong’echa and collaborators noted significantly low levels of IL-17 in subjects with uncomplicated malaria and severe malarial anemia in western Kenya [51]. IL-17 might play a dual role in malaria. In the early phase of infection, high IL-17 production may prevent severe malaria. However, in acute malaria, the overproduction of IL-17 induces SMA. IL-17 levels increase significantly in patients with autoimmune hemolytic anemia and correlated with the degree of anemia [52]. Also IL-17 level was more important in patients with autoimmune hemolytic anemia to red blood cells and significantly associated with more severe anemia [52]. Unlike IL-17, IL-22 levels were elevated. This is consistent with work from Mastelic and collaborators who showed a role of IL-22 in protection against liver damage during P. chabaudi infection [13]. The high production of IL-22 observed here suggests the involvement of this cytokine in protective immunity to P. falciparum. In addition, induction of IL-22 depends on IL-6, IL-23 and IL-1β. Also IL-6 production in infected children could suggest that IL-22 could be produce by Th17 cells in P. falciparum malaria infection. However, other cell subtypes such as Th22, NK and NKT are also known to produce IL-22 [53-55]. Further studies are therefore needed to determine the cellular source and role of IL-22 in protection or pathology induced during P. falciparum malaria infection.
IL-21 levels, although not significantly different between infected and uninfected subjects, fell significantly with the severity of anemia, suggesting that IL-21 plays a role in the protective immune response against P. falciparum. Our results are in agreement with data on young Gabonese children infected with P. falciparum, which showed a strong correlation of IL-21 levels with anti-EBA-175 peptide 4 IgG1 levels. Furthermore, IL-21 production is associated with low parasite burdens and normal hemoglobin levels [56].
Given the importance of the antibody response in protective immunity to malaria, we evaluated the production of Th2 cytokines, which promote immunoglobulin production and class switching. IL-4 production was detected in few of the infected children. A significant difference in IL-13 levels was observed between infected and uninfected children. Levels of IL-13 were higher in children with mild malarial anemia than in those with severe malarial anemia, which could indicate a protective role of IL-13 in severe malarial anemia. In addition, IL-13 production decreased with increasing parasitemia. The presence of IL-13 in infected children could suggest a Th2 (anti-inflammatory) response. Indeed, Th2 cytokines stimulate B cell activation producing P. falciparum-specific antibodies and downregulate the Th1 cytokine response. The absence of IL-4 did not prevent B cell responses in our subjects. Indeed, during malaria, the frequency of IL-4-producing CD4+ T cells decreases while the frequency other CD4+ T-cells able to help B-cells to produce Plasmodium-specific antibodies is high [57,58]. Moreover, control of infection and specific IgG antibody responses is possible in the complete absence of IL-4 [59]. Recent studies describe a subset of T helper cell, Tfh cells, producing IL-21 and other cytokines such as IFN-γ and IL-4, which are important for B cell help and activation of protective B cell responses against malaria [60,61]. Our results do not support the direct involvement of IL-4 in parasite clearance.
In conclusion, our findings show that malaria infected children present the significant increased levels as well as of pro- and anti-inflammatory cytokines. These children have shown especially high levels of INF-γ, which decreases with increased malarial anemia severity and high parasite density. These results suggest that INF-γ cytokine may contribute to protection against severe malaria anemia and parasite clearance. In addition, the results showed that the infected children had higher levels of IL-6 cytokine which increases with increased malarial anemia severity and high parasite density. Also increased IL-6 levels in P. falciparum infection could be associated with pathogenesis of malaria anemia. In other hand, the infected children were shown to have higher levels of IL-10 and IL-13, which decreases in children with severe malaria anemia, furthermore IL-10 levels correlated with parasite density. These findings suggest that higher levels of anti-inflammatory cytokines, especially IL-10 levels may contribute to pathogenesis of complicated malaria by inhibiting the INF-γ production. The findings presented here showed the increased IL-22 levels in infected children, the increased IL-17 levels in unanemic malaria and severe malaria anemia. In contrast, decreased IL-21 levels were associated with increased malaria anemia severity. Further studies are needed to evaluate the implication of IL-17, IL-22 and Il-21 cytokines in protection and/or pathogenesis of malaria.
Acknowledgements
We thank the children, parents and guardians from Haut-Ogooué province for their participation in this study. We also thank the staff of Amissa Bongo Hospital (Dr Ekaghba, Mr Patricien and the nurses and medical technicians) for their assistance. Thanks also to Amissa Bongo Hospital of Franceville and the staff of the International Center for Medical Research of Franceville (CIRMF) for their support during this study. This research was funded by the Ecole Doctorale Régionale d’Afrique Centrale en Infectiologie Tropicale de Franceville directed by Professor Jacques LEBIBI and Centre International de Recherche Médical de Franceville, which is supported by Total Gabon.
Disclosure of conflict of interest
None.
Authors’ contribution
SLOL conceived, designed and coordinated the study, performed statistical analysis and wrote the paper. BTAG participated in sample collection and carried out the immunoassays. HN, LCK, SMN and IPM collected clinical samples and provided parasitological and hematological data. NTM participated in immunoassays. JBLD designed and coordinated the study, performed statistical analysis, and participated in writing the paper. All the authors read and approved the final manuscript.
References
- 1.Organisation WH. World malaria report. 2014. [Google Scholar]
- 2.Dzeing-Ella A, Nze Obiang PC, Tchoua R, Planche T, Mboza B, Mbounja M, Muller-Roemer U, Jarvis J, Kendjo E, Ngou-Milama E, Kremsner PG, Krishna S, Kombila M. Severe falciparum malaria in Gabonese children: clinical and laboratory features. Malar J. 2005;4:1–8. doi: 10.1186/1475-2875-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–94. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- 4.Abdou RO Guikoumbi J, Ndinga JP, Josseaume A, Tchoua R. Les urgences pédiatriques au centre hospitalier de libreville. Médecine Tropicale. 2002;62:475–80. [Google Scholar]
- 5.Richard-Lenoble D, Kombila M, Chandenier J, Engohan E, Gannier M, Dubourg C. Malaria in Gabon. Study of 500 children with fever in Libreville. Bull Soc Pathol Exot Filiales. 1986;79:284–7. [PubMed] [Google Scholar]
- 6.Elissa N, Karch S, Bureau P, Ollomo B, Lawoko M, Yangari P, Ebang B, Georges AJ. Malaria transmission in a region of savanna-forest mosaic, Haut-Ogooué, Gabon. J Am Mosq Control Assoc. 1999;15:15–23. [PubMed] [Google Scholar]
- 7.Bitéghé Bi Essone JC, Iroungou BA, Lékana-Douki JB, Touré Ndouo FS, Onanga R, Ollomo B. Submicroscopic infection from uncomplicated Plasmodium falciparum malaria of Franceville, southeastern Gabon. Int J Adv Res. 2014;2:117–23. [Google Scholar]
- 8.Trape JF, Tall A, Sokhna C, Ly AB, Diagne N, Ndiath O, Mazenot C, Richard V, Badiane A, Dieye-Ba F, Faye J, Ndiaye G, Diene Sarr F, Roucher C, Bouganali C, Bassène H, Touré-Baldé A, Roussilhon C, Perraut R, Spiegel A, Sarthou JL, da Silva LP, Mercereau-Puijalon O, Druilhe P, Rogier C. The rise and fall of malaria in a West African rural community, Dielmo, Senegal, from 1990 to 2012: a 22 year longitudinal study. Lancet Infect Dis. 2014;14:476–88. doi: 10.1016/S1473-3099(14)70712-1. [DOI] [PubMed] [Google Scholar]
- 9.Snow RW, Marsh K. The consequences of reducing transmission of plasmodium falciparum in Africa. Adv Parasitol. 2002;52:235–64. doi: 10.1016/s0065-308x(02)52013-3. [DOI] [PubMed] [Google Scholar]
- 10.Perlmann P, Troye-Blomberg M. Malaria blood-stage infection and its control by the immune system. Folia Biol (Praha) 2000;46:210–8. [PubMed] [Google Scholar]
- 11.Perlmann P, Perlmann H, ElGhazali G, Troye Blomberg M. IgE and tumor necrosis factor in malaria infection. Immunol Lett. 1999;65:29–33. doi: 10.1016/s0165-2478(98)00120-5. [DOI] [PubMed] [Google Scholar]
- 12.Bueno LL, Marais CG, Lacerda MV, Fujiwara RT, Braga EM. Interleukin-17 producing T helper cells are increased during natural plasmodium vivax infection. Acta trop. 2012;123:53–7. doi: 10.1016/j.actatropica.2012.02.071. [DOI] [PubMed] [Google Scholar]
- 13.Mastelic B, Freitas do Rosario AP, Veldhoen M, Renauld JC, Jarra W, Sponaas AM, Roetynck S, Stockinger B, Langhorne J. IL-22 protects against liver pathology and lethality of an experimental blood-stage malaria infection. Front Immunol. 2012;3:85. doi: 10.3389/fimmu.2012.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips S. Effector mechanisms against asexual erythrocytic stages of plasmodium. Immunol Lett. 1994;41:109–14. doi: 10.1016/0165-2478(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Su, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage plasmodium chabaudi as infection. Infect Immun. 2000;68:4399–406. doi: 10.1128/iai.68.8.4399-4406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180:1288–97. doi: 10.1086/315016. [DOI] [PubMed] [Google Scholar]
- 17.Couper NK, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 18.Riley EM, Wahl S, Perkins DJ, Sch Ofield L. Regulating immunity to malaria. Parasite Immunol. 2006;28:35–49. doi: 10.1111/j.1365-3024.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 19.Foulds KE, Wu CY, Seder RA. Th1 memory: implications for vaccine development. Immunol Rev. 2006;211:58–66. doi: 10.1111/j.0105-2896.2006.00400.x. [DOI] [PubMed] [Google Scholar]
- 20.Planche T, Krisshna S, Kombila M, Engel K, Faucher JF, Ngou-Milama E, Kremsner PG. Comparison of methods for the rapid laboratory assessment of children with malaria. Am J Trop Med Hyg. 2001;65:599–602. doi: 10.4269/ajtmh.2001.65.599. [DOI] [PubMed] [Google Scholar]
- 21.Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to plasmodium falciparum malaria. J Infect Dis. 2002;185:971–9. doi: 10.1086/339408. [DOI] [PubMed] [Google Scholar]
- 22.Beeson JG, Osier FH, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–84. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 23.D’Ombrain MC, Robinson LJ, Stanisic Di, Taraika J, Nicholas B, Michon P, IMueller I, Schofield L. Association of early interferon-g production with immunity to clinical malaria: a longitudinal study among papua new guinean children. Clin Infect Dis. 2008;47:1380–7. doi: 10.1086/592971. [DOI] [PubMed] [Google Scholar]
- 24.McCall MB, Hopman J, Daou M, Maiga B, Dara V, Ploemen I, Nganou-Makamdop K, Niangaly A, Tolo Y, Arama C, Bousema JT, Van der Meer JW, van der Ven AJ, Troye-Blomberg M, Dolo A, Doumbo OK, Sauerwein RW. Early interferon-g response against plasmodium falciparum correlates with interethnic differences in susceptibility to parasitemia between sympatric fulani and dogon in mali. J Infect Dis. 2010;201:142–52. doi: 10.1086/648596. [DOI] [PubMed] [Google Scholar]
- 25.Ong’echa JM, Laal AA, Terlouw DJ, Ter Kuile FO, Kariuki SK, Udhayakumar V, Orago AS, Hightower AW, Nahlen BL, Shi YP. Association of interferon-g responses to pre-erythrocytic stage vaccine candidate antigens of plasmodium falciparum in young kenyan children with improved hemoglobin levels: XV. asembo bay cohort project. Am J Trop Med Hyg. 2003;68:590–7. doi: 10.4269/ajtmh.2003.68.590. [DOI] [PubMed] [Google Scholar]
- 26.Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, Machevo S, Puyol L, Quintó C, Menéndez C, Chitnis CE, Alonso PL, Dobaño C, Mayor A. Low antibodies against plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case-control study. Malar J. 2012;11:181. doi: 10.1186/1475-2875-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, Kone A, Harley R, Plowe CV, Doumbo OK, Sztein MB. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–7. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jason J, Archibald LK, Nwanyanwu OC, Bell M, Jensen RJ, Gunter E, Buchanan I, Larned J, Kazembe PN, Dobbie H, Jarvis WR. The effects of iron deficiency on lymphocyte cytokine production and activation: preservation of hepatic iron but not at all cost. Clin Exp Immunol. 2001;126:166–473. doi: 10.1046/j.1365-2249.2001.01707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobune M, Kohgo Y, Kato J, Miyazaki E, Niitsu Y. Interleukin-6 enhances hepatic transferrin uptake and ferritin expression in rats. Hepatology. 1994;19:1468–75. [PubMed] [Google Scholar]
- 30.Hirayama M, Kohgo Y, Kondo H, Shintani N, Fujikawa K, Sasaki K, Kato J, Niitsu Y. Regulation of iron metabolism in HepG2 cells: a possible role for cytokines in the hepatic deposition of iron. Hepatology. 1993;18:874–80. doi: 10.1002/hep.1840180420. [DOI] [PubMed] [Google Scholar]
- 31.Grau GE, Taylor TE, Molyneux ME, Wirima JJ, Vassalli P, Hommel M, Lambert PH. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–91. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 32.Ho M, Sexton MM, Tongtawe P, Looareesuwan S, Suntharasamai P, Webster HK. Interleukin-10 inhibits tumor necrosis factor production but not antigen-specific lymphoproliferation in acute plasmodium falciparum malaria. J Infect Dis. 1995;172:838–44. doi: 10.1093/infdis/172.3.838. [DOI] [PubMed] [Google Scholar]
- 33.Malaguarnera L, Musumeci S. The immune response to plasmodium falciparum malaria. Lancet Infect Dis. 2002;2:472–78. doi: 10.1016/s1473-3099(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 34.Malaguarnera L, Pignatelli S, Musumeci M, Simporè J, Musumeci S. Plasma levels of interleukin-18 and interleukin-12 in plasmodium falciparum malaria. Parasite Immunol. 2002;24:489–92. doi: 10.1046/j.1365-3024.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 35.Perkins DJ, Weinberg JB, Kremsner PG. Reduced interleukin-12 and transforming growth factor-b1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis. 2000;182:988–92. doi: 10.1086/315762. [DOI] [PubMed] [Google Scholar]
- 36.Keller CC, Yamo O, Ouma C, Ong’echa JM, Ounah D, Hittner JB, Vulule JM, Perkins DJ. Acquisition of hemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anemia. Infect Immun. 2006;74:5249–60. doi: 10.1128/IAI.00843-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawrylowicz CM, O’Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5:271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 38.O’Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunol Rev. 2008;223:114–31. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- 39.Ong’echa JM, Remo AM, Kristoff J, Hittner JB, Were T, Ouma C, Otieno RO, Vulule JM, Keller CC, Awandare GA, Perkins DJ. Increased circulating interleukin (IL)-23 in children with malarial anemia: In vivo and in vitro relationship with co-regulatory cytokines IL-12 and IL-10. Clin Immunol. 2008;126:211–21. doi: 10.1016/j.clim.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-βold beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–40. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 41.Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Tungpradabkul S, Hisaeda H, Troye-Blomberg M, Cui L, Udomsangpetch R. Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol. 2008;38:2697–705. doi: 10.1002/eji.200838186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Minigo G, Woodberrry T, Piera KA, Salwati E, Tjitra E, Kenangalem E, Price RN, Engwerda CR, Anstey NM, Plebanski M. Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog. 2009;5:e1000402. doi: 10.1371/journal.ppat.1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bueno LL, Morais CG, Araujo FF, Gomes JA, Corrêa-Oliveira R, Soares IS, Lacerda MV, Fujiwara RT, Braga EM. Plasmodium vivax: induction of CD4+CD25+FoxP3+ regulatory T cells during infection are directly associated with level of circulating parasites. PLoS One. 2010;5:e9623. doi: 10.1371/journal.pone.0009623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, Edele F, de Souza JB, Sinden RE, Gilbert SC, Riley EM, Hill AV. Upregulation of TGF-β, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–96. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki Y, Hamano S, Wang S, Shimanoe Y, Iwakura Y, Yoshida H. IL-17 is necessary for host protection against acute-phase trypanosoma cruzi infection. J Immunol. 2010;185:1150–7. doi: 10.4049/jimmunol.0900047. [DOI] [PubMed] [Google Scholar]
- 46.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against toxoplasma gondii infection. Infect Immun. 2005;73:617–21. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunte CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 48.Guiton R, Vasseur V, Charron S, Arias MT, Van Langendonck N, Buzoni-Gatel D, Ryffel B, Dimier-Poisson I. Interleukin 17 receptor signaling is deleterious during toxoplasma gondii infection in susceptible BL6 mice. J Infect Dis. 2010;202:427–35. doi: 10.1086/653738. [DOI] [PubMed] [Google Scholar]
- 49.Bacellar O, Faria D, Nascimento M, Cardoso TM, Gollob KJ, Dutra WO, Scott P, Carvalho EM. IL-17 production in patients with american cutaneous leishmaniasis. J Infect Dis. 2009;200:75–8. doi: 10.1086/599380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholzen A, Mittag D, Rogerson SJ, Cooke BM, Plebanski M. Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog. 2009;5:e100543. doi: 10.1371/journal.ppat.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ong’echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. Identification of inflammatory biomarkers for pediatric malarial anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–80. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall AM, Zamzami OM, Whibley N, Hampsey DP, Haggart AM, Vickers MA, Barker RN. Production of the effector cytokine interleukin-17, rather than interferon-gamma, is more strongly associated with autoimmune hemolytic anemia. Haematologica. 2012;97:1494–500. doi: 10.3324/haematol.2011.060822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21:241–54. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Ouyang W, Kolls JK, Zheng Y. The biological functions of t helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 56.Mewono L, Matondo Maya DW, Matsiegui PB, Agnandji ST, Kendjo E, Barondi F, Issifou S, Kremsner PG, Mavoungou E. Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of plasmodium falciparum in Gabonese children with acute falciparum malaria. Eur Cytokine Netw. 2008;19:30–6. doi: 10.1684/ecn.2008.0114. [DOI] [PubMed] [Google Scholar]
- 57.Winkler S, Willheim M, Baier K, Schmid D, Aichelburg A, Graninger W, Kremsner PG. Reciprocal regulation of Th1- and Th2-cytokine-producing T cells during clearance of parasitemia in plasmodium falciparum malaria. Infect Immun. 1998;66:6040–4. doi: 10.1128/iai.66.12.6040-6044.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langhorne J, Gillard S, Simon B, Slade S, Eichmann K. Frequencies of CD4+ T cells reactive with plasmodium chabaudi chabaudi: distinct response kinetics for cells with Th1 and Th2 characteristics during infection. Int Immunol. 1989;1:416–24. doi: 10.1093/intimm/1.4.416. [DOI] [PubMed] [Google Scholar]
- 59.von der Weid T, Kopf M, Köhler G, Langhorne J. The immune response to plasmodium chabaudi malaria in interleukin-4-deficient mice. Eur J Immunol. 1994;24:2285–93. doi: 10.1002/eji.1830241004. [DOI] [PubMed] [Google Scholar]
- 60.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 61.Pérez-Mazliah D, Ng DH, Freitas do Rosário AP, McLaughlin S, Mastellic-Gavillet B, Sodenkamp J, Kushinga G, Langhorne J. Disruption of IL-21 signaling affects T cell-B cell interactions and abrogates protective humoral immunity to malaria. PLoS Pathog. 2015;11:e1004715. doi: 10.1371/journal.ppat.1004715. [DOI] [PMC free article] [PubMed] [Google Scholar]