Abstract
There is a growing concern about consciousness loss during epileptic seizures. Understanding neural mechanisms could lead to a better comprehension of cerebral circuit function in the control of consciousness loss in intractable epilepsy. We propose that ventrolateral preoptic area (VLPO)- PnO (nucleus pontis oralis) circuits may serve a major role in the loss of consciousness in drug-refractory epilepsy. Future behavioural and neuroimaging studies are clearly needed to understand the functional connectivity between the VLPO and PnO during loss of consciousness in drug-refractory epilepsy, to greatly prevent unconsciousness in this disorder and improve the quality of life in patients with intractable epilepsy.
Keywords: Consciousness loss, drug-refractory epilepsy, ventrolateral preoptic area, nucleus pontis oralis
Introduction
It is widely perceived that seizures may produce reversible loss of consciousness [1-3]. There is a growing concern about loss of consciousness during epileptic seizures [4-9]. Some patients with epileptic seizures have experienced frequent episodes of consciousness loss, with eyes staring, often during swallowing or chewing. Many studies have found that exposure to frequent seizures can result in widespread neuronal degeneration and cognitive impairment [10-13]. Studying neural circuits of sleep-wake regulation can enhance our understanding of the pathophysiology of loss of consciousness during epileptic seizures, and provide optimal therapies for loss of consciousness during epileptic seizures.
Sleep-wake regulation in the VLPO-PnO circuits
The rapidly growing field of neural circuits provides a wealth of biological information on sleep-wake regulation. Utilizing the Cre-dependent AAV encoding hrGFP as a tracer in the projections of A2AR neurons in nucleus accumbens (NAc) mediating sleep-wake regulation, Zhang et al. found that there existed the NAc-preoptic area-pontine reticular formation pathway [14].
It is known that the ventrolateral preoptic area (VLPO) is an important sleep-promoting nucleus [15,16]. Wang et al. and colleagues previously evaluated the effects of morphine on sleep-wake profiles after administration of a mu receptor antagonist D-Phe-Cys-Thr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) or kappa opioid receptor antagonist nor-BIN to the VLPO using electroencephalogram (EEG) and electromyogram recordings in freely moving rats, and found that morphine could inhibit the activity of sleep-promoting neurons in the VLPO by affecting mu receptors and induced arousal in a dose-dependent manner [17]. Some data reported that GABA agonist Magnolol (a major bioactive constituent of the bark of Magnolia officinalis) and GABA transporter-1 inhibitor NO-711 increased c-Fos expression in the VLPO whereas they decreased c-Fos expression in the wake-promoting nuclei (e.g., lateral hypothalamus) [15,18,19].
The hypocretin (orexin), a hypothalamus-specific peptide with neuroexcitatory activity, is involved in the regulation of waking and sleep states [20,21]. The oral part of the pontine reticular formation (nucleus pontis oralis, PnO) has been shown to have an important somnogenic role and receives hypocretinergic input [22-25]. It is reported that wakefulness occurs following injections of hypocretin-1 (orexin A) into the PnO [26]. Watson and colleagues previously showed that wakefulness is either increased or decreased, respectively, by PnO administration of nipecotic acid (a GABA uptake inhibitor that increases extracellular GABA levels) or 3-mercaptopropionic acid (a GABA synthesis inhibitor that decreases extracellular GABA levels; 3-MPA), and indicated that PnO administration of hypocretin-1 increased PnO GABA levels and increases wakefulness [27]. A study by Watson et al. provided evidence that mu-opioid receptor agonist morphine disrupted sleep and obtunded wakefulness by decreasing GABAergic transmission in the brain regions regulating arousal, including the pontine reticular nucleus, oral part [22].
Our research data about VLPO-PnO circuits and melanocortinergic signaling
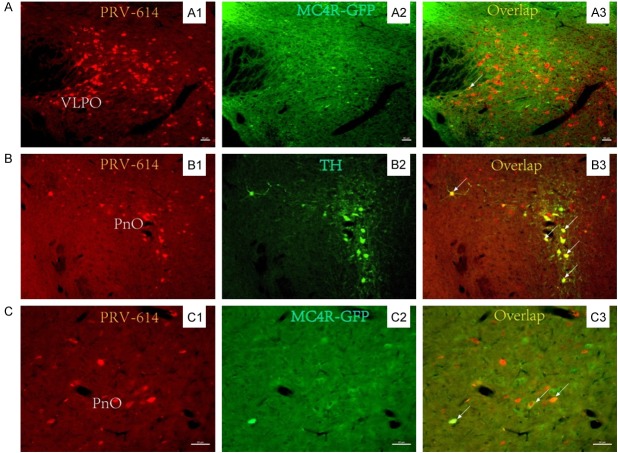
The ventrolateral preoptic area (VLPO) and the oral part of the pontine reticular formation (PnO) selectively express various receptors or peptides, the property that can define their phenotypes. Recent studies have indicated that the PnO may be involved in rapid eye movement (REM) sleep dysfunction in epilepsy by melanocortinergic signaling [28,29]. We examined the molecular basis of this selectivity in the VLPO and PnO by microinjections of pseudorabies virus vector PRV-614 expressing red fluorescent protein (RFP) into the kidney of transgenic mouse containing MC4R gene promoter using expressing green fluorescent protein (GFP) as the reporter [28-49]. 5 days following injection of the PRV-614, immunohistochemical assays of GFP expression from these constructs were done to determine whether the GFP reporter co-localizes with PRV-614-immunoreactivity in the VLPO and PnO. The results show that PRV-614/MC4R-GFP double-labeled neurons were detected in the VLPO and PnO (Figure 1), suggesting that there may exist the VLPO-PnO circuits and the key elements in the MC4R gene promoter may be involved in regulating the cell-type specific expression the VLPO and PnO (Figure 2).
Figure 1.
MC4R-GFP-positive neurons in the VLPO-PnO circuits. Injection of PRV-614 into the left kidney resulted in retrograde infection of neurons in the VLPO (A), NPO (B and C) by the sympathetic pathway. PRV-614/MC4R-GFP dual-labeled neurons were detected in the VLPO (A3) and NPO (C3). PRV-614/TH dual-labeled neurons were detected in the PPTg (B3). (A1, B1 and C1) show PRV-614-labeled cells; (A2 and C2) show MC4R-GFP-positive cells; (B2) shows TH-positive cells; and (A3, B3 and C3) show overlaid images of (A1, B1, and C1) plus (A2, B2, and C2), respectively. MC4R, melanocortin-4 receptor; PnO, pontine reticular formation; TH, tyrosine hydroxylase; VLPO, ventrolateral preoptic area. Arrows indicate double-labeled neurons. Scale bars, 50 μm. Some photomicrographs were taken from [28,29,42,43].
Figure 2.

Schematic representation showing the VLPO-PnO circuits. This figure has been updated according to that reported in previous studies. Greee line: The Cre-dependent adeno-associated virus (AAV) encoding humanized Renilla green fluorescent protein was injected into the the nucleus accumbens in A2AR-Cre mice. Immunohistochemistry was then used to visualize hrGFP, highlighting the perikarya of the A2AR neurons in the injection sites, and their axons in projection regions, suggesting that VLPO neurons that project to the PnO were revealed by retrograde labeling. Red line: injection of PRV-614 into the kidney resulted in retrograde infection of neurons in the IML, PnO, and VLPO by the sympathetic pathway. PRV-614/MC4R-GFP duallabeled neurons were detected in the IML, PnO, and VLPO. IML, intermediolateral cell column. PnO, pontine reticular formation; VLPO, ventrolateral preoptic area. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article). Some drawings were taken from [14,28,29].
The occurrence of seizures, sleep disturbances and loss of consciousness
The intimate relationship between the occurrence of seizures and loss of consciousness has long been recognized [31,50-53]. Wyllie et al. reported a 9-year-old girl case with loss of consciousness and seizures [54]. It is known that seizures are a devastating neurological disorder associated with sleep disturbance [55,56]. Our understanding of the mechanisms for sleep disturbances and loss of consciousness during the occurrence of seizures is changing rapidly.
The roles of GABA transporter subtype 1 (GAT1) inhibitors for the treatment of epileptic seizure activity have been extensively reported [57,58]. Xu et al observed sleep behaviors of mice treated with a selective GAT1 inhibitor NO-711, and found that NO-711 caused a marked enhancement of EEG activity during rapid eye movement (REM) sleep and wakefulness, indicating that NO-711 may be useful in sleep-wake regulation for the treatment of epilepsy [15]. Magnolol exerting anti-epileptic effects via the GABA receptor has been studied [18,19]. A study of Chen et al. showed that magnolol increased the number of state transitions from wakefulness to NREM sleep and increased c-Fos expression in the neurons of VLPO, a sleep center in the anterior hypothalamus [18]. These results suggest that the occurrence of seizures is tightly linked to sleep disturbances.
It is generally accepted that generalized seizures are commonly associated with ictal alterations in consciousness [59-62], whereas simple-partial seizures do not disrupt consciousness. Generalized seizures produce distinct, active and highly organized patterns on the electroencephalogram (EEG), the most common of which is synchronous or irregular slow spike-wave discharges [1,53]. Yang et al. used the prospective responsiveness in epilepsy scale (RES) to investigate impaired consciousness in 52 patients with epilepsy during continuous video-EEG monitoring, and found that RES impairment was greatest during and after tonic-clonic seizures whereas less in partial seizures, suggesting that RES impairment was related to EEG changes during seizures [62]. Blumenfeld and Taylor have indicated that generalized seizures result in abnormal increased activity in brain networks (e.g., fronto-parietal association cortex and related subcortical structures) to impedes the normal communications of arousal and cognition [1]. A study of Farzampour et al. has pointed that transient loss of consciousness accompanied by focal temporal lobe seizures is a complex life-threatening phenomenon [63].
Conclusion
Though the precise structures involved in loss of consciousness in epileptic seizures are far from complete, remarkable progress has been made in elucidating the neurobiological, neurophysiologic, and neuropharmacological mechanisms underlying the unconsciousness due to seizures [64-66]. Understanding these neural mechanisms could lead to a better comprehension of cerebral circuit function in the control of loss of consciousness in intractable epilepsy. We propose that VLPO-PnO circuits may serve a major role in the loss of consciousness in drug-refractory epilepsy. Future behavioural and neuroimaging studies are clearly needed to understand the functional connectivity between the VLPO and PnO during loss of consciousness in drug-refractory epilepsy, to greatly prevent unconsciousness in this disorder and improve the quality of life in patients with intractable epilepsy.
Acknowledgements
We gratefully acknowledge Dr. Lynn Enquist for kindly providing us with PRV-614. This work was supported by grants from China National Natural Science Foundation of PR China (81072152, 81670240), National Natural Science Foundation of Hubei Province (No. 2016CFB625 and No. 2016CFB 428), Research Foundation of Health and Family Planning Commission of Hubei Province (WJ2015M) and Key Programs of Science & Technology in Hainan Province (Applied Research and Industrialization Foundation, ZDXM 20130074).
Disclosure of conflict of interest
None.
References
- 1.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–310. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 2.Friedman MJ, Sharieff GQ. Seizures in children. Pediatr Clin North Am. 2006;53:257–277. doi: 10.1016/j.pcl.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Blumenfeld H. Epilepsy and the consciousness system: transient vegetative state? Neurol Clin. 2011;29:801–823. doi: 10.1016/j.ncl.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Perri C, Stender J, Laureys S, Gosseries O. Functional neuroanatomy of disorders of consciousness. Epilepsy Behav. 2014;30:28–32. doi: 10.1016/j.yebeh.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Di Perri C, Stender J, Laureys S, Gosseries O. Corrigendum to “functional neuroanatomy of disorders of consciousness” [Epilepsy Behav 30 (2014) 28-32] . Epilepsy Behav. 2014;30:28–32. doi: 10.1016/j.yebeh.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Holler Y, Trinka E. Is there a relation between eeg-slow waves and memory dysfunction in epilepsy? A critical appraisal. Front Hum Neurosci. 2015;9:341. doi: 10.3389/fnhum.2015.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luders H, Amina S, Bailey C, Baumgartner C, Benbadis S, Bermeo A, Carreno M, Devereaux M, Diehl B, Eccher M, Edwards J, Fastenau P, Fernandez Baca-Vaca G, Godoy J, Hamer H, Hong SB, Ikeda A, Kahane P, Kaiboriboon K, Kalamangalam G, Lardizabal D, Lhatoo S, Luders J, Mani J, Mayor C, Mesa Latorre T, Miller J, Morris HH, Noachtar S, O’Donovan C, Park J, Perez-Jimenez MA, Rona S, Rosenow F, Shahid A, Schuele S, Skidmore C, Steinhoff B, Szabo CA, Sweet J, Tandon N, Tanner A, Tsuji S. Proposal: different types of alteration and loss of consciousness in epilepsy. Epilepsia. 2014;55:1140–1144. doi: 10.1111/epi.12595. [DOI] [PubMed] [Google Scholar]
- 8.Blumenfeld H, Meador K, Jackson GD. Commentary: the return of consciousness to epilepsy seizure classification. Epilepsia. 2015;56:345–347. doi: 10.1111/epi.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blumenfeld H, Meador KJ. Consciousness as a useful concept in epilepsy classification. Epilepsia. 2014;55:1145–1150. doi: 10.1111/epi.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boly M, Massimini M, Tononi G. Theoretical approaches to the diagnosis of altered states of consciousness. Prog Brain Res. 2009;177:383–398. doi: 10.1016/S0079-6123(09)17727-0. [DOI] [PubMed] [Google Scholar]
- 11.Luo C, Li Q, Lai Y, Xia Y, Qin Y, Liao W, Li S, Zhou D, Yao D, Gong Q. Altered functional connectivity in default mode network in absence epilepsy: a resting-state fMRI study. Hum Brain Mapp. 2011;32:438–449. doi: 10.1002/hbm.21034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reuber M, Kurthen M. Consciousness in non-epileptic attack disorder. Behav Neurol. 2011;24:95–106. doi: 10.3233/BEN-2011-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Worrell GA, Nelson C, Brinkmann B, He B. Spectral and spatial shifts of post-ictal slow waves in temporal lobe seizures. Brain. 2012;135:3134–3143. doi: 10.1093/brain/aws221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang JP, Xu Q, Yuan XS, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A, Qu WM, Urade Y, Lazarus M, Huang ZL, Li RX. Projections of nucleus accumbens adenosine A2A receptor neurons in the mouse brain and their implications in mediating sleep-wake regulation. Front Neuroanat. 2013;7:43. doi: 10.3389/fnana.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu XH, Qiu MH, Dong H, Qu WM, Urade Y, Huang ZL. GABA transporter-1 inhibitor NO-711 alters the EEG power spectra and enhances non-rapid eye movement sleep during the active phase in mice. Eur Neuropsychopharmacol. 2014;24:585–594. doi: 10.1016/j.euroneuro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Alam MA, Kumar S, McGinty D, Alam MN, Szymusiak R. Neuronal activity in the preoptic hypothalamus during sleep deprivation and recovery sleep. J Neurophysiol. 2014;111:287–299. doi: 10.1152/jn.00504.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Yue XF, Qu WM, Tan R, Zheng P, Urade Y, Huang ZL. Morphine inhibits sleep-promoting neurons in the ventrolateral preoptic area via mu receptors and induces wakefulness in rats. Neuropsychopharmacology. 2013;38:791–801. doi: 10.1038/npp.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CR, Zhou XZ, Luo YJ, Huang ZL, Urade Y, Qu WM. Magnolol, a major bioactive constituent of the bark of magnolia officinalis, induces sleep via the benzodiazepine site of GABA(A) receptor in mice. Neuropharmacology. 2012;63:1191–1199. doi: 10.1016/j.neuropharm.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 19.Chen CR, Tan R, Qu WM, Wu Z, Wang Y, Urade Y, Huang ZL. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, exerts antiepileptic effects via the GABA/benzodiazepine receptor complex in mice. Br J Pharmacol. 2011;164:1534–1546. doi: 10.1111/j.1476-5381.2011.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiyashchenko LI, Mileykovskiy BY, Maidment N, Lam HA, Wu MF, John J, Peever J, Siegel JM. Release of hypocretin (orexin) during waking and sleep states. J Neurosci. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Induction of active (REM) sleep and motor inhibition by hypocretin in the nucleus pontis oralis of the cat. J Neurophysiol. 2002;87:2880–2888. doi: 10.1152/jn.2002.87.6.2880. [DOI] [PubMed] [Google Scholar]
- 24.Xi MC, Fung SJ, Yamuy J, Morales FR, Chase MH. Hypocretinergic facilitation of synaptic activity of neurons in the nucleus pontis oralis of the cat. Brain Res. 2003;976:253–258. doi: 10.1016/s0006-8993(03)02566-6. [DOI] [PubMed] [Google Scholar]
- 25.Xi M, Fung SJ, Yamuy J, Chase MH. Interactions between hypocretinergic and GABAergic systems in the control of activity of neurons in the cat pontine reticular formation. Neuroscience. 2015;298:190–199. doi: 10.1016/j.neuroscience.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Xi M, Chase MH. The injection of hypocretin-1 into the nucleus pontis oralis induces either active sleep or wakefulness depending on the behavioral state when it is administered. Sleep. 2010;33:1236–1243. doi: 10.1093/sleep/33.9.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu AJ, Liu TT, He ZG, Hong QX, Xiang HB. STN-PPTg circuits and REM sleep dysfunction in drug-refractory epilepsy. Epilepsy Behav. 2015;51:277–280. doi: 10.1016/j.yebeh.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 29.Xu AJ, Liu TT, He ZG, Wu W, Xiang HB. CeA-NPO circuits and REM sleep dysfunction in drug-refractory epilepsy. Epilepsy Behav. 2015;51:273–276. doi: 10.1016/j.yebeh.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 30.He ZG, Liu BW, Xiang HB. Cross interaction of melanocortinergic and dopaminergic systems in neural modulation. Int J Physiol Pathophysiol Pharmacol. 2015;7:152–157. [PMC free article] [PubMed] [Google Scholar]
- 31.Xu LJ, Liu TT, He ZG, Hong QX, Xiang HB. Hypothesis: CeM-RVLM circuits may be implicated in sudden unexpected death in epilepsy by melanocortinergic-sympathetic signaling. Epilepsy Behav. 2015;45:124–127. doi: 10.1016/j.yebeh.2015.02.034. [DOI] [PubMed] [Google Scholar]
- 32.Liu TT, Liu BW, He ZG, Feng L, Liu SG, Xiang HB. Delineation of the central melanocortin circuitry controlling the kidneys by a virally mediated transsynaptic tracing study in transgenic mouse model. Oncotarget. 2016;7:69256–69266. doi: 10.18632/oncotarget.11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He ZG, Zhang DY, Liu SG, Feng L, Feng MH, Xiang HB. Neural circuits of pain and itch processing involved in anterior cingulate cortex. Int J Clin Exp Med. 2016;9:22976–22984. [Google Scholar]
- 34.Liu BW, He ZG, Shen SE, Xiang HB. CeA-RVMM serotonergic circuits and sudden unexpected death in epilepsy. Int J Clin Exp Med. 2016;9:9752–9758. [Google Scholar]
- 35.Liu BW, Liu QQ, Liu SG, Xiang HB. Renal disease and neural circuits: brain-kidney crosstalk. Int J Clin Exp Med. 2016;9:5326–5333. [Google Scholar]
- 36.Hao Y, Tian XB, Liu TT, Liu C, Xiang HB, Zhang JG. MC4R expression in pedunculopontine nucleus involved in the modulation of midbrain dopamine system. Int J Clin Exp Pathol. 2015;8:2039–2043. [PMC free article] [PubMed] [Google Scholar]
- 37.He ZG, Wu ZF, Xia XH, Xu AJ, Zhang T, Xiang HB. Recurrent cervicodorsal spinal intradural enterogenous cyst: case report and literature review. Int J Clin Exp Med. 2015;8:16117–16121. [PMC free article] [PubMed] [Google Scholar]
- 38.Liu TT, Hong QX, Xiang HB. The change in cerebral glucose metabolism after electroacupuncture: a possible marker to predict the therapeutic effect of deep brain stimulation for refractory anorexia nervosa. Int J Clin Exp Med. 2015;8:19481–19485. [PMC free article] [PubMed] [Google Scholar]
- 39.Xu AJ, He ZG, Xia XH, Xiang HB. Anesthetic management for craniotomy in a patient with massive cerebellar infarction and severe aortic stenosis: a case report. Int J Clin Exp Med. 2015;8:11534–11538. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu TT, He ZG, Tian XB, Xiang HB. Neural mechanisms and potential treatment of epilepsy and its complications. Am J Transl Res. 2014;6:625–630. [PMC free article] [PubMed] [Google Scholar]
- 41.Xiang HB, Liu C, Liu TT, Xiong J. Central circuits regulating the sympathetic outflow to lumbar muscles in spinally transected mice by retrograde transsynaptic transport. Int J Clin Exp Pathol. 2014;7:2987–2997. [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang HB, Liu TT, Tian XB, Zhu WZ. Therapeutic mechanism of subthalamic nucleus stimulation for refractory epilepsy involved in melanocortin-4 receptor signaling. Molecular & Cellular Epilepsy. 2014;1:13–18. [Google Scholar]
- 43.Hong Q, Ke B, Yang H, Liu TT, Mei W, Xiang HB, Fang GG. Cuneiform nucleus stimulation as adjunct treatment for intractable epilepsy: a virally mediated transsynaptic tracing study in spinally transected transgenic mice. Epilepsy Behav. 2014;33:135–137. doi: 10.1016/j.yebeh.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 44.Hong Q, Fang G, Liu TT, Guan XH, Xiang HB, Liu Z. Posterior pedunculopontine tegmental nucleus may be involved in visual complaints with intractable epilepsy. Epilepsy Behav. 2014;34:55–7. doi: 10.1016/j.yebeh.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Xiang HB, Zhu WZ, Bu HL, Liu TT, Liu C. Possible mechanism of subthalamic nucleus stimulation-induced acute renal failure: a virally mediated transsynaptic tracing study in transgenic mouse model. Mov Disord. 2013;28:2037–2038. doi: 10.1002/mds.25632. [DOI] [PubMed] [Google Scholar]
- 46.Xiang HB, Zhu WZ, Guan XH, Ye DW. Possible mechanism of deep brain stimulation for pedunculopontine nucleus-induced urinary incontinence: a virally mediated transsynaptic tracing study in a transgenic mouse model. Acta Neurochir (Wien) 2013;155:1667–1669. doi: 10.1007/s00701-013-1743-8. [DOI] [PubMed] [Google Scholar]
- 47.Xiang HB, Zhu WZ, Guan XH, Ye DW. The cuneiform nucleus may be involved in the regulation of skeletal muscle tone by motor pathway: a virally mediated trans-synaptic tracing study in surgically sympathectomized mice. Brain. 2013;136:e251. doi: 10.1093/brain/awt123. [DOI] [PubMed] [Google Scholar]
- 48.Ke B, Liu TT, Liu C, Xiang HB, Xiong J. Dorsal subthalamic nucleus electrical stimulation for drug/treatment-refractory epilepsy may modulate melanocortinergic signaling in astrocytes. Epilepsy Behav. 2014;36:6–8. doi: 10.1016/j.yebeh.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 49.Qiu Q, Li RC, Ding DF, Liu C, Liu TT, Tian XB, Xiang HB, Cheung CW. Possible mechanism of regulating glucose metabolism with subthalamic nucleus stimulation in parkinson’s disease: a virally mediated trans-synaptic tracing study in transgenic mice. Parkinsonism Relat Disord. 2014;20:468–470. doi: 10.1016/j.parkreldis.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 50.Liu TT, He ZG, Tian XB, Liu C, Xiang HB, Zhang JG. Hypothesis: astrocytes in the central medial amygdala may be implicated in sudden unexpected death in epilepsy by melanocortinergic signaling. Epilepsy Behav. 2015;42:41–43. doi: 10.1016/j.yebeh.2014.10.028. [DOI] [PubMed] [Google Scholar]
- 51.Hao Y, Liu TT, He ZG, Wu W, Xiang HB. Hypothesis: CeM-PAG GABAergic circuits may be implicated in sudden unexpected death in epilepsy by melanocortinergic signaling. Epilepsy Behav. 2015;50:25–28. doi: 10.1016/j.yebeh.2015.04.070. [DOI] [PubMed] [Google Scholar]
- 52.Hao Y, Guan XH, Liu TT, He ZG, Xiang HB. Hypothesis: the central medial amygdala may be implicated in sudden unexpected death in epilepsy by melanocortinergic-sympathetic signaling. Epilepsy Behav. 2014;41:30–2. doi: 10.1016/j.yebeh.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wyllie E, Rincon SP, Pierce VM. Case records of the Massachusetts General Hospital. Case 16-2015. A 9-year-old girl with loss of consciousness and seizures. N Engl J Med. 2015;372:2050–2058. doi: 10.1056/NEJMcpc1501149. [DOI] [PubMed] [Google Scholar]
- 55.Mello CF, Oliveira MS. Commentary on Kaushik et al. : prostaglandin D2 is crucial for seizure suppression and postictal sleep. Novel evidence supporting a role for prostanoid receptors in seizure control. Exp Neurol. 2014;257:157–161. doi: 10.1016/j.expneurol.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Kaushik MK, Aritake K, Kamauchi S, Hayaishi O, Huang ZL, Lazarus M, Urade Y. Prostaglandin D(2) is crucial for seizure suppression and postictal sleep. Exp Neurol. 2014;253:82–90. doi: 10.1016/j.expneurol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Schousboe A, Madsen KK, Barker-Haliski ML, White HS. The GABA synapse as a target for antiepileptic drugs: a historical overview focused on GABA transporters. Neurochem Res. 2014;39:1980–1987. doi: 10.1007/s11064-014-1263-9. [DOI] [PubMed] [Google Scholar]
- 58.Madsen KK, White HS, Schousboe A. Neuronal and non-neuronal GABA transporters as targets for antiepileptic drugs. Pharmacol Ther. 2010;125:394–401. doi: 10.1016/j.pharmthera.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Cavanna AE, Ali F. Epilepsy: the quintessential pathology of consciousness. Behav Neurol. 2011;24:3–10. doi: 10.3233/BEN-2011-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavanna AE. Epilepsy as a pathology of consciousness. Epilepsy Behav. 2014;30:1. doi: 10.1016/j.yebeh.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 61.Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Shklyar I, Lee HW, Ezeani CC, Anaya J, Balakirsky S, Han X, Enamandram S, Men C, Cheng JY, Nunn A, Mayer T, Francois C, Albrecht M, Hutchison AL, Yap EL, Ing K, Didebulidze G, Xiao B, Hamid H, Farooque P, Detyniecki K, Giacino JT, Blumenfeld H. Impaired consciousness in epilepsy investigated by a prospective responsiveness in epilepsy scale (RES) Epilepsia. 2012;53:437–447. doi: 10.1111/j.1528-1167.2011.03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farzampour Z, Huguenard J. Seizing upon mechanisms for impaired consciousness. Neuron. 2015;85:453–455. doi: 10.1016/j.neuron.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol. 2013;23:239–244. doi: 10.1016/j.conb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Usami K, Matsumoto R, Kobayashi K, Hitomi T, Shimotake A, Kikuchi T, Matsuhashi M, Kunieda T, Mikuni N, Miyamoto S, Fukuyama H, Takahashi R, Ikeda A. Sleep modulates cortical connectivity and excitability in humans: direct evidence from neural activity induced by single-pulse electrical stimulation. Hum Brain Mapp. 2015;36:4714–29. doi: 10.1002/hbm.22948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koubeissi MZ, Bartolomei F, Beltagy A, Picard F. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav. 2014;37:32–35. doi: 10.1016/j.yebeh.2014.05.027. [DOI] [PubMed] [Google Scholar]