Abstract
Cold shock proteins (CSPs) enhance acclimatization of bacteria to adverse environmental circumstances. The Escherichia coli CSP genes CspA and CspB were modified to plant-preferred codon sequences and named as SeCspA and SeCspB. Overexpression of exogenous SeCspA and SeCspB in transgenic Arabidopsis lines increased germination rates, survival rates, and increased primary root length compared to control plants under drought and salt stress. Investigation of several stress-related parameters in SeCspA and SeCspB transgenic wheat lines indicated that these lines possessed stress tolerance characteristics, including lower malondialdehyde (MDA) content, lower water loss rates, lower relative Na+ content, and higher chlorophyll content and proline content than the control wheat plants under drought and salt stresses. RNA-seq and qRT-PCR expression analysis showed that overexpression of SeCsp could enhance the expression of stress-responsive genes. The field experiments showed that the SeCspA transgenic wheat lines had great increases in the 1000-grain weight and grain yield compared to the control genotype under drought stress conditions. Significant differences in the stress indices revealed that the SeCspA transgenic wheat lines possessed significant and stable improvements in drought tolerance over the control plants. No such improvement was observed for the SeCspB transgenic lines under field conditions. Our results indicated that SeCspA conferred drought tolerance and improved physiological traits in wheat plants.
Abiotic stresses such as drought, salt, heat, and cold are major environmental factors that affect plant growth and development1,2. Increasing numbers of genetically modified plants that have the ability to resist abiotic stresses have been reported recently3,4,5. Extensive field evaluations have demonstrated that the down-regulation of the ethylene biosynthetic pathway can improve grain yield in maize under drought conditions6. Overexpression of a C4 photosynthesis enzyme enhanced transgenic rice tolerance to drought stress7. Coexpression of ABA-Insensitive3 (ABI3)/Viviparous1 and an AtABI5 transcription factor in cotton enhanced drought stress adaptation under limited irrigation conditions8. Some studies showed that fungal and bacterial genes can be used to confer resistance to abiotic stresses in plants. For example, tobacco plants exhibited dehydration and necrosis under severe water-deficit conditions; but these symptoms were less severe in transgenic tobacco plants with the yeast trehalose-6-phosphate synthase gene9. Maize plants are sensitive to water-deficit stress throughout the growing season, but water deficit during the vegetative growth phase is of particular concern, as it typically leads to reduced grain yields. Bacterial cold shock protein (CSP) genes enhanced resistance to drought stress and improved grain yield in maize under drought conditions10.
CSPs are essential for assisting bacterial growth under low temperature conditions and in enhancing bacterial acclimatization to low temperatures11. The CspA and CspB genes play major roles in bacterial responses to low temperature12. The effect of temperature fluctuations on CspA and CspB has been experimentally defined13. During temperature cycling, the ratios of CspA or CspB to total protein change as temperatures vary14; transcription of both genes increases at low temperatures and decreases with increasing temperatures. CspA and CspB are thought to enhance protein translation at low temperatures through the elimination of stabilized RNA secondary structures15,16.
Water-deficit stress has become a serious problem in global agriculture and severely affects the growth of crops; it is of particular concern in northern and northwestern China, where the main winter wheat production areas are located17. In these areas, more than 70% of annual precipitation falls from June to September, whereas precipitation meets only 20 to 30% of the water requirement for winter wheat during the winter wheat growing season from February to the middle of June18. Therefore, the discovery of genetic sources of drought tolerance has become an urgent priority in wheat improvement efforts. The study reported here was conducted with field experiments and showed that the 1000-grain weight and the grain yield of wheat was significantly increased in transgenic wheat expressing SeCspA under drought conditions, resulting in improved wheat drought tolerance.
Results
Overexpression of SeCspA and SeCspB increased germination rates in Arabidopsis under abiotic stresses
We transformed Arabidopsis with SeCsp (SeCspA and SeCspB) and obtained four new 35 S:SeCsp transgenic lines. The expression levels of SeCsp in 4 lines are shown in Fig. S2. One hundred homozygous T3 seeds of the 35 S::SeCspA and 35 S::SeCspB transgenic lines, and control plants, were used for germination. The result showed, under normal growth conditions, the seed germination rates of the 35 S::SeCspA and 35 S::SeCspB transgenic lines and control plants showed no obvious differences, and exceeded 95%. With increasing NaCl concentrations, the germination rates of the 35 S::SeCspA and 35 S::SeCspB transgenic Arabidopsis lines were significantly higher than that of the control plants, although the germination of both the transgenic lines and the control plants showed a declining trend as the NaCl concentrations increased (Fig. S3). In media supplemented with 150 mM NaCl, the 35 S::SeCspA and 35 S::SeCspB transgenic Arabidopsis lines germinated at 70% and 56%, respectively, as compared with about 33% germination for the control plants.
To analyze the effect of polyethylene glycol (PEG 6000)-simulated drought stress on the germination of the 35 S::SeCspA and 35 S::SeCspB lines, seeds were placed on half-strength solid MS medium supplemented with various concentrations of PEG. After one week, the germination rates of the 35 S::SeCspA and 35 S::SeCspB transgenic lines were significantly higher than those of control plants under PEG stress at all PEG concentrations (Fig. S4). For example, the germination rates of the 35 S::SeCspA and 35 S::SeCspB transgenic lines were more than 62.3% and 56.3% under 8% PEG, respectively, as compared to the 37.5% rate of the control seeds (Fig. S4B,C). These results indicated that overexpression of SeCspA and SeCspB enhanced salt and PEG tolerance of the transgenic plants at the germination stage.
Tolerance of transgenic Arabidopsis against salt, cold and drought stresses
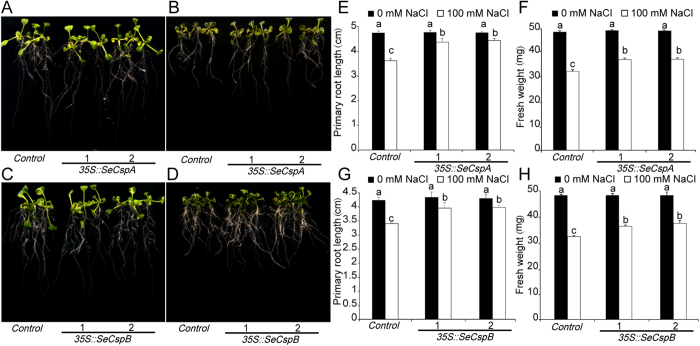
To investigate whether or not transformation of plants with the 35 S::SeCspA and 35 S::SeCspB constructs affected salt tolerance during plant growth, 9-day-old Arabidopsis seedlings were transferred to MS plates supplemented with 100 mM NaCl. In the absence of NaCl, there were no significant differences in growth characteristics between the transgenic lines and control plants (Fig. 1A,C). When grown in the presence of 100 mM NaCl for one week, some leaves of the control plants began to yellow, whereas those of the two 35 S::SeCspA transgenic lines remained green (Fig. 1B), and the primary root lengths of control plants were 23.5% shorter than the untreated controls, whereas the two 35 S::SeCspA transgenic lines showed reductions of 8.1 and 6.5%. Salt stress reduced the fresh weights of control plants by 33.0%, compared to 23.7% to 23.5% reductions for the two 35 S::SeCspA transgenic lines (Fig. 1E,F). Similar phenotypic results were observed for the 35 S::SeCspB transgenic plants. After salt treatment for one week the two 35 S::SeCspB transgenic lines had significantly longer primary root lengths and significantly higher fresh weights than did control plants (Fig. 1D,G and H). For example, under salt treatment, the two 35 S::SeCspB transgenic lines showed fresh weight reductions of 2.9 and 3.8% in comparison with normal conditions, compared to a corresponding reduction of 10.1% for control plants (Fig. 1H).
Figure 1. Responses of transgenic Arabidopsis lines and control plants under salt stress.
(A) and (C) Phenotypes of the SeCspA and SeCspB transgenic Arabidopsis lines grown on normal MS agar medium. (B) and (D) Phenotypes of the SeCspA and SeCspB transgenic Arabidopsis lines grown on high-salt medium (100 mM NaCl). (E) and (G) Primary root lengths of the Arabidopsis plants. (F) and (H) Fresh weights of the aerial parts of Arabidopsis plants. Three biological replicates were averaged and statistically analyzed using Duncan’s test. Vertical bars bearing different letters in (E), (F), (G) and (H) indicate significant differences at P < 0.05. Bars indicate the standard error of the mean (SE).
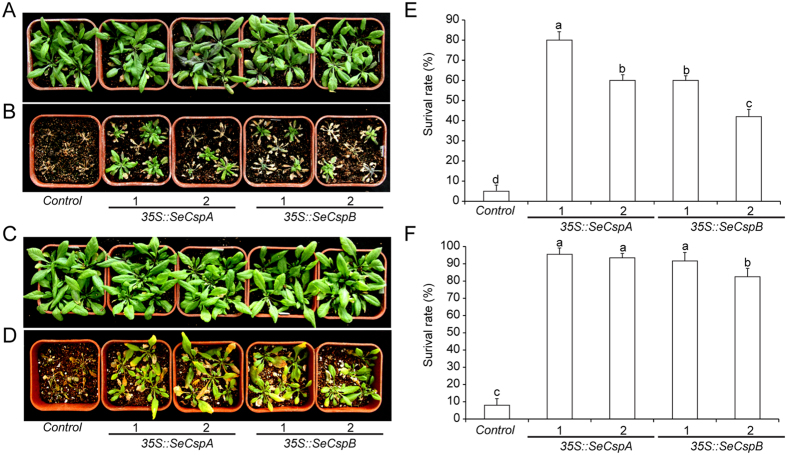
To investigate the tolerance of the transgenic Arabidopsis lines to cold stress, three-week-old Arabidopsis seedlings grown under normal conditions were transferred to −5 °C for 12 h for cold treatment and then returned to normal growth conditions for recovery. During the recovery phase, the transgenic Arabidopsis lines grew well and only a few leaves underwent yellowing, whereas the leaves of the control plants showed severe wilting and curling. After one week of recovery week, more than 40% of the 35 S::SeCspA and 35 S::SeCspB transgenic plants survived compared to only 5% survival for the control plants (Fig. 2B,E).
Figure 2. Phenotypes of transgenic Arabidopsis lines and control plants subjected to stress treatments.
(A) and (C) Phenotypes of transgenic Arabidopsis lines under normal condition. (B) Phenotypes after cold stress treatment. Plants were photographed after one week of recovery under normal conditions. (D) Phenotypes of transgenic Arabidopsis lines grown under dehydration conditions for one week. Photographs were taken after one week of recovery under normal conditions. (E) and (F) Survival rates of Arabidopsis lines. Vertical bars bearing different letters in (E) and (F) indicate significant differences at P < 0.05 and error bars represent standard errors.
To explore the drought tolerance of the transgenic Arabidopsis lines, three-week-old seedlings were exposed to water deficit stress for one week, and then rewatered in a recovery period of one week (Fig. 2D). As shown in Fig. 2F, after one week of recovery, more than 92% and 80%, respectively, of the 35 S::SeCspA and 35 S::SeCspB transgenic Arabidopsis plants had survived, compared to only 8% of the control plants. These results suggested that overexpression of SeCspA and SeCspB appeared to increase salt, cold, and drought tolerance in transgenic Arabidopsis plants.
The effect of salt stress on the transgenic wheat lines
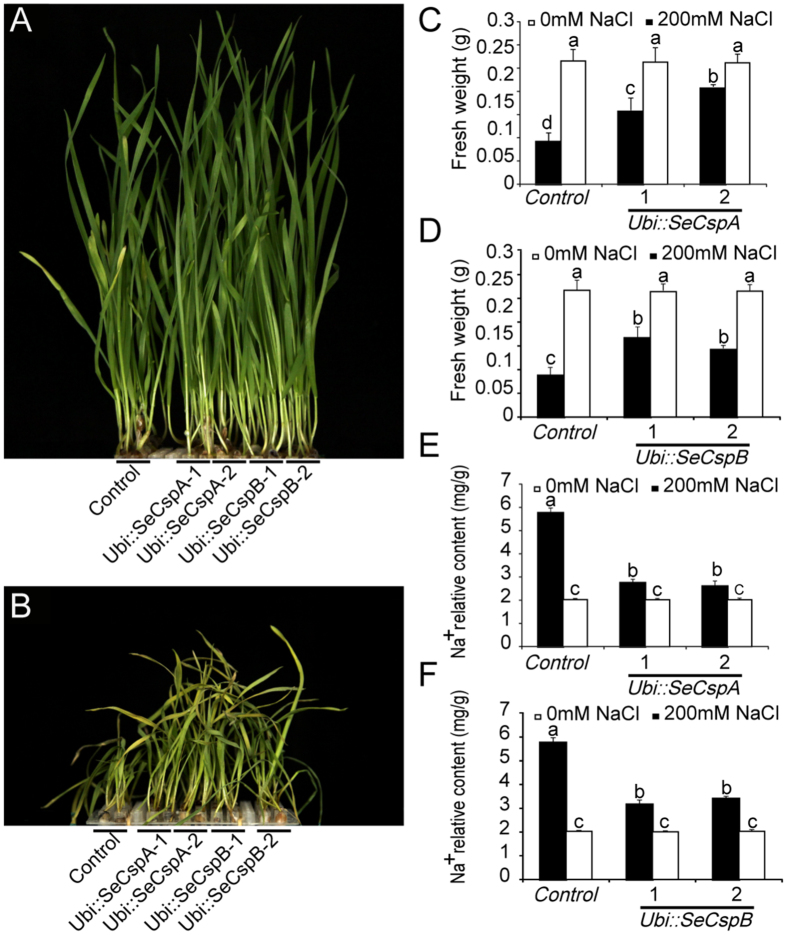
Ubi::SeCspA and Ubi::SeCspB transgenic wheat lines were subjected to high-salt stress (200 mM NaCl) (Fig. 3). As expected, control plants began to wilt after one week of salt stress, whereas the Ubi::SeCspA and Ubi::SeCspB transgenic lines continued to thrive (data not shown). After two weeks, the control plants had completely wilting, but the transgenic lines, especially the two Ubi::SeCspA lines, remained upright (Fig. 3B). The fresh weight measurement data showed that in the absence of salt treatment, there were no significant differences among the transgenic and control wheat plants. Salt stress reduced the fresh weights of control plants by 64.3%, compared to 19.4% to 39.8% reductions for the two Ubi::SeCspA transgenic lines and 31.9% to 41.2% reductions for the two Ubi::SeCspB transgenic lines (Fig. 3C,D).
Figure 3. Responses of the transgenic lines and parental wheat line (control) under salt stress.
(A) Phenotypes of transgenic wheat lines grown under normal conditions for two weeks. (B) Phenotypes of transgenic wheat lines grown under high-salt (200 mM NaCl) stress. (C) and (D) Fresh weights of the transgenic wheat lines and control wheat lines. Measurements were made after 7-day-old plants were treated with NaCl for one week. (E) and (F) Relative Na+ content. Measurements were made after 7-day-old plants were treated with NaCl for 6 h. Vertical bars bearing different letters in (C,D,E), and (F) indicate significant differences at P < 0.05 and error bars represent standard errors.
To further analyze the mechanism underlying the observed salt-tolerance phenotype, the relative Na+ content of the transgenic and control wheat plants was measured using an atomic absorption spectrometer. The results showed that the Na+ content of the control wheat plant and all of the Ubi::SeCspA and Ubi::SeCspB transgenic wheat lines was significantly increased after the plants were subjected to salt stress (200 mmol/L NaCl) for 6 h. However, the Na+ content of the transgenic wheat plants increased less dramatically than that of the control wheat plants (Fig. 3E,F). This result suggests that the transgenic wheat plants exhibited a decreased Na+ content into the cells under salt stress, but the control wheat plant presented a higher Na+ content into the cells due to their high membrane permeability.
The phenotypes of transgenic wheat lines were not affected by low temperature
To investigate potential plant responses to low temperature, 7-day-old transgenic and control wheat seedlings were transferred to −4 °C conditions for one week. As expected, the low temperature inhibited plant growth. There were no significant differences in the above-ground growth or root length among the transgenic lines and the control plants (Fig. S7E,F).
To further analyze whether transgenic wheat lines were affected by low temperature, the relative electric conductivity experiment was carried out. The result showed, under normal conditions (25 °C), there were no significant differences in the relative electric conductivity among the transgenic lines and the control plants. After −8 °C treatment for 2 h, although the relative electric conductivity of transgenic and control wheat seedlings leaves had a great increase, there were still no differences among the transgenic lines and the control plants (Fig. S7G,H).
Survival rates and effects on physiological indices of transgenic wheat lines under drought stress
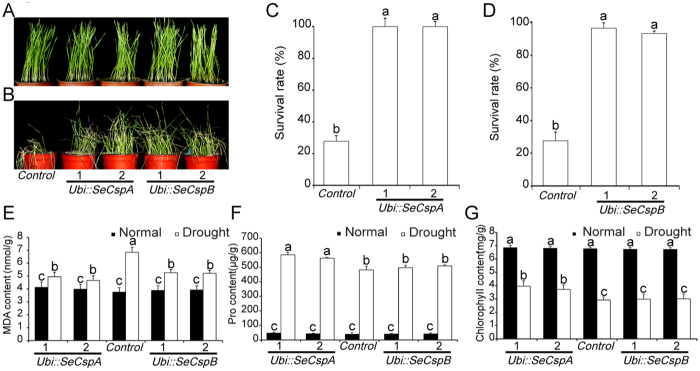
To test whether or not the Ubi::SeCspA and Ubi::SeCspB constructs conferred improved drought resistance to wheat plants, 7-day-old seedlings were exposed to severe water deficit. After one week, the control plants exhibited serious wilting and death; the symptoms of the Ubi::SeCspA and Ubi::SeCspB transgenic lines were considerably less severe (Fig. 4B). After re-watering for one week, 97.6% and 93.3%, respectively, of the Ubi::SeCspA and Ubi::SeCspB transgenic plants recovered and grew normally, while only 29.2% of the control plants survived and grew normally (Fig. 4C,D).
Figure 4. Phenotypes of transgenic wheat lines and parental wheat plants (control) under drought stress.
(A) Phenotypes of transgenic wheat lines grown under normal conditions for three weeks. (B) Phenotypes of the transgenic wheat lines after rehydration for one week. (C) and (D) Survival rates of the transgenic wheat lines. (E) MDA content. (F) Free proline content. (G) Chlorophyll content. Measurements were made after 7-day-old plants were treated with drought conditions for one week. Vertical bars bearing different letters in (C,D,E,F), and (G) indicate significant differences between transgenic and control wheat plants at P < 0.05 and error bars represent standard errors.
After 7 days of drought treatment, the malondialdehyde (MDA) content of the control plants increased rapidly and reached 6.85 nmol/g, an increase of 82.7% compared with growth under the normal irrigation condition. Drought treatment increased the MDA content of the Ubi::SeCspA and Ubi::SeCspB transgenic wheat lines by only 16.6%, 20.1%, 33.3%, and 35.7%, respectively, relative to their counterparts grown under normal irrigation conditions (Fig. 4E). Thus, the MDA content remained relatively stable in the transgenic wheat lines compared to the control plants.
Proline, an osmoprotectant that is known to promote drought resistance, accumulates in plant cells during drought stress19. When subjected to severe drought stress, significant increases in proline content were detected in both the Ubi::SeCspA and Ubi::SeCspB transgenic lines and in the control wheat plants (Fig. 4F). The two Ubi::SeCspA transgenic lines showed the highest proline content, reaching 563 and 586 μg/g under drought stress conditions, whereas the control wheat reached 482 μg/g. Although there were increases, relative to the control plants, in the proline content (497 and 510 μg/g) of the two Ubi::SeCspB transgenic lines, the differences were not statistically significant (Fig. 4F).
It is known that chlorophyll content directly affects photosynthetic efficiency20. Under normal conditions, there were no significant differences in chlorophyll content among the transgenic wheat lines and control plants (Fig. 4G). Drought stress decreased the chlorophyll content in the transgenic lines and in the control plants, relative to normal irrigation conditions. Under drought stress, the transgenic lines had less severe reductions in chlorophyll content (43.8 to 45.7%) than did the control plants (57.1%), but only the differences for the Ubi::SeCspA transgenic lines were statistically significant (Fig. 4G). The results of MDA content, free proline content and chlorophyll content revealed that the two Ubi::SeCspA transgenic lines had relatively stronger drought resistance than the control plants under water-deficit conditions.
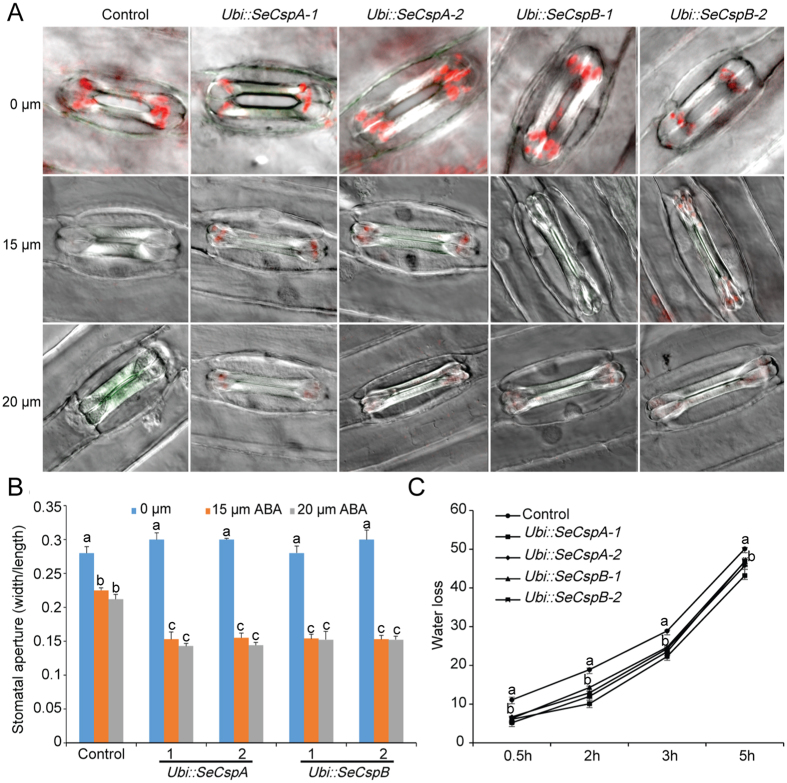
Effect of ABA on stomatal movement
ABA-mediated stomatal movement was monitored in the transgenic lines and the control plants. Seven-day-old wheat leaves were treated with ABA for 2 h, and stomatal aperture was calculated as the ratio of width to length. The mean stomatal apertures of the control and transgenic wheat leaves decreased concurrently as the concentrations of ABA increased (Fig. 5A). However, the guard cells of the transgenic lines exhibited greater sensitivity to ABA-induced stomatal closure than did those of the control leaves (Fig. 5B). The stomata of the transgenic wheat leaves closed relatively more rapidly than did the control stomata following treatment with exogenous ABA.
Figure 5. Measurements of stomatal closure and water loss in transgenic lines and control plants.
(A) and (B) Effects of ABA-induced stomatal closure. (C) Water loss from transgenic lines and control plants. Vertical bars bearing different letters in (B) indicate significant differences between transgenic and control wheat plants at P < 0.05. Different letters in (C) indicate in one treatment significant differences between transgenic and control wheat plants at P < 0.05. Error bars represent standard errors.
Further, we also compared the rate of water loss of detached leaves during dehydration between control and transgenic lines plants. The transgenic wheat line leaves showed lower rates of water loss than the control wheat plant leaves during dehydration (Fig. 5C). For example, at the 0.5 h time point of the dehydration treatment, the control wheat plant leaves revealed a water loss of 10.1% compared to 6.1–6.7% for the two Ubi::SeCspA transgenic lines, and 5.2–6.3% for the two Ubi::SeCspB transgenic lines. At the 2 h time point of the dehydration experiment, the water loss rates of the two Ubi::SeCspA transgenic line leaves were less than 10.1% and 12.9% and the two Ubi::SeCspB transgenic line leaves were less than 12% and 14.3%, respectively, compared to 19.2% for the control plant leaves. This result was consistent with the stomatal movement from transgenic wheat plants after drought stress, suggesting that detached leaves from the transgenic lines showed higher ability to withstand tissue dehydration.
Analysis of SeCSP enhanced the expression of stress-responsive genes
To better understand the differential expression of stress-related genes between the controls and transgenic wheat, an RNA sequencing (RNA-seq) approach was used. The raw sequence reads were deposited into the Ensembl Plants and the National Center for Biotechnology Information SRA database. The assembled transcripts that were differentially expressed in transgenic wheat plants are listed in Table S1.
In the context of the influence of abiotic stress by SeCspA and SeCspB, we focused on the transcripts that were differentially expressed between the controls and transgenic wheat. Based on their annotation, we selected transcripts that were upregulated 2-fold in SeCspA and SeCspB transgenic wheat plants compared with the control plants (Table S1). According to their functions, the corresponding genes are divided into three categories which are stress response-related genes (e.g., WD40, WRKY, LEA and GST), signal transduction-related genes (e.g., receptor kinase) and energy metabolism-related genes (e.g., Plasma membrane ATPase and Beta-glucosidase) (Table S1). These proteins have previously been reported to act either directly or indirectly in abiotic stress responses.
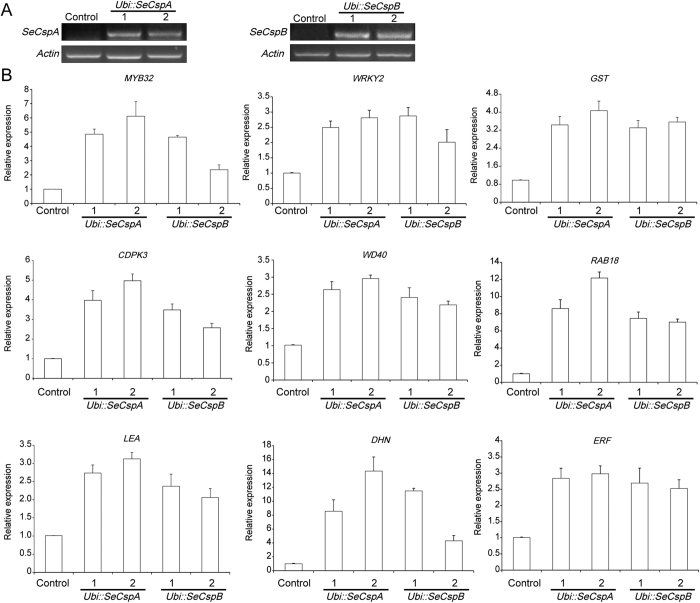
To validate the data from the RNA-seq digital expression analysis, we performed qRT-PCR assays of nine abiotic response-related genes, which investigated encode Calcium-dependent protein kinase (TaCDPK3), MYB transcriptional factor protein (TaMYB32), WD40 transcriptional factor protein (TaWD40), WRKY transcriptional factor protein (TaWRKY2), AP2-like ethylene-responsive transcription factor protein (TaERF3), ABA-responsive protein (TaRAB18), Late-embryogenesis abundant proteins (TaLEA), Glutathione S-transferase protein (GST) and a dehydrin protein (TaDHN) (Fig. 6). These genes are known to be involved in drought, salt, and ABA responses, and each of them had more than 2-fold higher expression in transgenic plants than in the control wheat plants (Fig. 6B). Although there were some anomalous quantitative differences, the trends of gene expression changes detected by the two different approaches were generally consistent (Fig. 6), thereby confirming the validity of the RNA-seq data. These results indicated that the introduction of exogenous Ubi::SeCspA and Ubi::SeCspB resulted in increased expression of some stress response genes.
Figure 6. Variation in the transcript levels of stress-related genes in transgenic and control wheat plants.
(A) Transcript levels of SeCspA and SeCspB in transgenic wheat lines. (B) The transcript levels of stress-related genes in transgenic wheat lines.
Drought resistance of transgenic wheat lines in the field
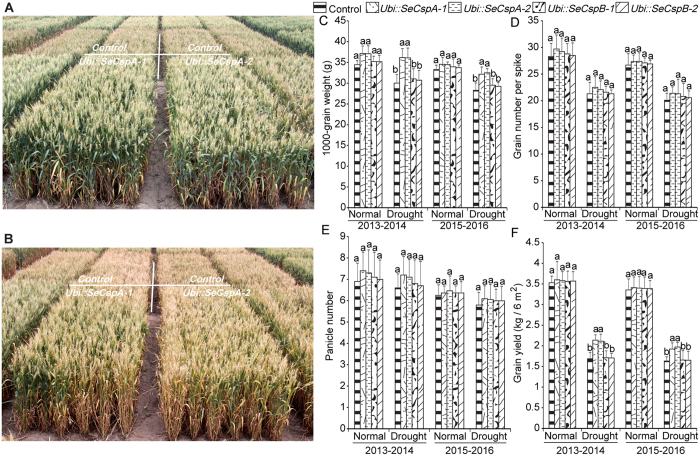
When grown under normal field conditions, the transgenic wheat lines and the control plants similar growth characteristics (Fig. 7A). At harvest, some agronomic traits of the transgenic and control wheat plants were analyzed. Panicle number, 1000-grain weight, and grain number per spike are important components of yield. Under normal irrigation conditions, despite small increases in the 1000-grain weight and the grain yield of the transgenic lines, The Duncan’s test showed no significant differences in agronomic traits among the transgenic lines and the control plants (Fig. 7C,F). Under drought stress, the 1000-grain weight of the two Ubi::SeCspA transgenic lines increased by 19.2 and 20.2% (average of two years), compared to the control plants (Fig. 7C). The grain yield of the two Ubi::SeCspA transgenic lines exceeded that of the control plants by 24.1 and 24.5% (average of two years) (Fig. 7F). Although there were increases, relative to the control plants, in the 1000-grain weight (1.5 and 2.0%) (average of two years) and the grain yield (1.3 and 2.1%) (average of two years) in the two Ubi::SeCspB transgenic lines, the differences were not statistically significant (Fig. 7C,F). We also investigated plant height and heading time, but no differences were found between the transgenic lines and control plants in Tables S2 and S3.
Figure 7. Phenotypes of transgenic lines and the control plants in May 2013 and 2016.
(A) Transgenic wheat lines grown under normal conditions. (B) Transgenic wheat lines grown under drought conditions. (C) 1000-grain weight. (D) Grain number per spike. (E) Panicle number. (F) Grain yield. Vertical bars bearing different letters in (C,D,E) and (F) indicate in one treatment significant differences between transgenic and control wheat plants at P < 0.05 and error bars represent standard errors.
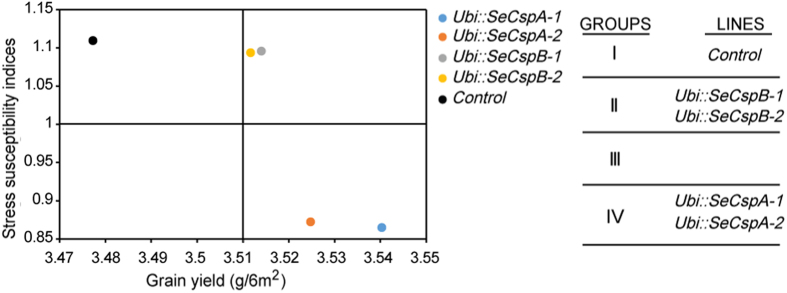
The stress tolerance index (STI) and stress tolerance (TOL) are two measures used to identify cultivars that produce high yields under both normal and stress conditions. When drought stress is severe, the stress susceptibility index (SSI) is thought to be a more useful index for evaluating drought tolerant cultivars21. Correlation analysis of the present data in Table S4 suggested that the SSI and TOL of each wheat variety were negatively correlated with the different traits under both normal and drought stress conditions, therefore, as shown in Table 1, the greater the TOL and SSI values, the more severe the reduction in yield in drought stress conditions, and the higher drought sensitivity. In order to better understand drought resistance of each wheat variety, we analyzed SSI and grain yield (under normal conditions) by cluster analysis, and found that the transgenic wheat lines and the control plants clustered into four groups: group I, low yield with the low drought resistance (control wheat plants); group II, high yield with the low drought resistance (Ubi::SeCspB-1 and Ubi::SeCspB-2); group IV, high yield with the high drought resistance (Ubi::SeCspA-1 and Ubi::SeCspA-2) (Fig. 8). The STI data in Table S4 shows the positive correlations between the different traits, and indicates that the higher STI values, and the stronger drought tolerance. These results revealed that transgenic Ubi::SeCspA lines had stronger drought tolerance than the control plants.
Table 1. Stress susceptibility indices (SSI), Stress tolerance (TOL), and Stress tolerance index (STI) for yield traits of the transgenic lines (average of two years).
| Indices | SSI |
TOL |
STI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Agronomic trait | 1000-grain weight | Panicle number | Grain yield | 1000-grain weight | Panicle number | Grain yield | 1000-grain weight | Panicle number | Grain yield |
| SeCspA-1 | 0.272a | 0.746a | 0.864a | 0.815a | 0.2 | 1.441a | 1.057a | 1.031a | 0.610a |
| SeCspA-2 | 0.317a | 0.756a | 0.872a | 0.940a | 0.2 | 1.448a | 1.048a | 1.005a | 0.601a |
| SeCspB-1 | 1.468 | 1.159 | 1.094 | 4.130 | 0.3 | 1.809 | 0.852 | 0.948 | 0.491 |
| SeCspB-2 | 1.535 | 1.167 | 1.097 | 4.310 | 0.3 | 1.810 | 0.841 | 0.935 | 0.487 |
| control | 1.514 | 1.193 | 1.109 | 4.180 | 0.3 | 1.817 | 0.813 | 0.893 | 0.474 |
aIndicates significant (P < 0.05) improvement relative to nontransgenic control.
Figure 8. Cluster analysis of grain yield (plants grown under normal conditions) and drought susceptibility index (average of two years).
Discussion
SeCspA and SeCspB enhanced cold tolerance in Arabidopsis, but not in wheat
Cold shock domain (CSD) proteins in plants differ from the CSPs identified in prokaryotes. Plant CSD proteins typically contain an N-terminal CSD and a C-terminal glycine-rich region, and these regions are often interspersed with varying numbers of retroviral-like CCHC zinc finger domains22. Four CSD protein genes (AtCSP1 to AtCSP4) were identified in Arabidopsis23. Expression of AtCSP1, AtCSP2, and AtCSP3 found to be induced by cold shock treatment24,25,26. Overexpression of AtCSP3 enhanced freezing tolerance in Arabidopsis27. In E. coli, CSPs are responsive to cold and function as RNA chaperones that are essential for bacterial growth under low temperature conditions15,16. E. coli CSPs share a domain with Arabidopsis AtCSP3 and play important roles in cold tolerance27. Our results show that modified E. coli CspA and CspB genes enhanced resistance to cold stress when they were overexpressed in Arabidopsis (Fig. 2A,B), suggesting that these synthetic genes had similar functions to Arabidopsis AtCSP3 in conferring cold tolerance in Arabidopsis.
At low temperatures, deletion of CspA is known to affect the growth of bacteria, whereas deletion of CspB did not significantly effect on growth13. Induction of E. coli cold shock protein CspA does not enhance transcription, but increases mRNA stability during low-temperature incubation28. Thus, CspA may be more important to cold adaptation than CspB. However, SeCspA, like SeCspB, did not significantly increase plant resistance to cold in transgenic wheat lines as compared to control plants (Fig. S7). It is possible that overexpression of SeCspA and SeCspB were insufficient to increase cold tolerance beyond the level already possessed by the particular winter wheat parent, which was already adapted to local conditions.
SeCspA and SeCspB changed physiological indices and affected the expression of stress-responsive genes in transgenic plants
We measured drought-related physiological indices of transgenic Ubi::SeCspA and Ubi::SeCspB wheat lines in an attempt to determine the effects of these genes when they were overexpressed in wheat. The MDA content and the free proline content, and the rates of water loss, can be used experimentally as physiological indices of plant drought tolerance. Chlorophyll content directly affects plant photosynthesis. Under drought stress, the free proline content and the chlorophyll content of the transgenic wheat lines were higher than those of the control plants, while the MDA content and the water loss rates of the transgenic wheat lines were lower than those of the control plants (Figs 4E–G and 5C).
E. coli CSPs share a domain with Arabidopsis AtCSP3. Overexpression of AtCSP3 in Arabidopsis enhanced salt and drought tolerance through up-regulated expression of stress-related proteins29. SeCspA and SeCspB share a domain with Arabidopsis AtCSP3 and influenced expression of stress-related genes in the transgenic Arabidopsis and wheat plants (Fig. 6). Our study showed that overexpression of SeCspA and SeCspB in wheat resulted in up-regulation of TaCDPK3. CDPKs play important roles in stress signal transduction and can regulate the expression of salt and drought tolerance-related genes, which can be activated by ABA30. RAB plays a crucial role in plant responses to ABA31. However, overexpression of SeCspA and SeCspB in wheat affected the expression of ABA signaling-related genes under stress conditions, TaRAB. TaMYB32, a salt-inducible gene, enhanced salt stress tolerance in transgenic Arabidopsis overexpressing TaMYB3232. TaWRKY2, a WRKY-type transcription factor, is involved in multiple aspects of plant growth, development and stress response. Transgenic Arabidopsis plants overexpressing TaWRKY2 exhibited improved salt and drought tolerance compared with controls33. Wheat ERF transcription factor members play crucial roles in regulating stress responses. Recent investigations indicated that overexpression of TaERF3 promotes tolerance to salt and drought stresses in wheat34. TaWD40, repeat-containing WD40 proteins, acting as scaffolding molecules and promoting protein activity in protein–protein or protein–DNA interactions35. TaWD40D overexpression wheat lines enhanced tolerance to salt and drought stresses in wheat36. GST, plays an important role in resisting various stresses, is ROS-scavenging enzymes are known to be involved in redox homeostasis of cells37,38. DHN and LEA positively contributed to plant tolerance to cold, drought, and salt stresses in other plant species39. In this study, the transcript levels of these stress-related genes were up-regulated in the SeCspA and SeCspB overexpressing wheat lines relative to the control wheat plants. These results show that overexpression of SeCspA and SeCspB in wheat affected the expression of these stress-related genes.
Drought stress induces the accumulation of the plant hormone abscisic acid (ABA), and this compound is known to contribute to stomatal closure and thereby prevent water loss40. We observed that the stomata of Ubi::SeCspA and Ubi::SeCspB transgenic wheat plants closed rapidly following ABA treatment (Fig. 5A,B), which implied that the Ubi::SeCspA and Ubi::SeCspB transgenic wheat plants were more sensitive to ABA than were the control wheat plants, which maybe contribute the stomata of Ubi::SeCspA and Ubi::SeCspB transgenic wheat plants close rapidly under drought stress conditions and resulted in reduced transpiration and water loss, improving drought resistance.
SeCspA improves wheat stress tolerance
Field experiments are essential to verify experimental differences that are observed in greenhouse experiments10. We performed field tests on our transgenic Ubi::SeCspA and Ubi::SeCspB wheat plants. Wheat was sown in early October and harvested in mid-June of the following year. Rainfall in Shijiazhuang is mainly concentrated in mid-June to August (Fig. S8). During the whole growing season of wheat, the Shijiazhuang area is in a period of low rainfall. Rainfall in Shijiazhuang at this time does not meet the water requirement for wheat growth; this site is therefore suitable for drought response trials with transgenic wheat (Fig. S8). The soil water content of the experimental plots was monitored during the whole wheat growing season. Under the well-irrigated conditions, the soil water content of the experimental plot area was about 18.5% to 26.9% (average of two years) during the entire growth cycle (Table S5), which suggested there should have enough water for wheat to grow. Under the water-limited conditions, the soil water content of the experimental plot area was about 8.7% to 12.1% (average of two years) during the late growth stage (Table S5), which suggested that wheat grown in this area would experience drought conditions.
Different parameters have been used to evaluate the drought resistance of crops in the field. For example, growth and senescence of the maize canopy was evaluated using normalized differences in vegetation indices under drought stress41. Seedling leaf senescence scores were used as an index for evaluating drought resistance in rice42. We used SSI, STI, and TOL to evaluate drought resistance of our transgenic wheat plants. SSI, in particular, can represent the drought resistance of the transgenic wheat. Cluster analysis between SSI and grain yield showed that group I (Control) performed poorly in all environments and could not acclimatize to the changing environment, the Ubi::SeCspB transgenic wheat lines in group II performed well only under normal irrigated conditions, indicating that Ubi::SeCspB transgenic wheat lines would not be suitable for cultivation in arid regions (Fig. 8). Group IV members performed best in all environments (Fig. 8), suggesting that Ubi::SeCspA transgenic wheat lines have relatively less drought sensitivity than the control genotype under drought conditions.
Materials and Methods
Modification of the E. coli CspA and CspB genes
In order to improve the expression of exogenous E. coli CspA and CspB in plant cells, nucleotide changes were made to the DNA sequence in order to increase the overall G+C content; the purpose behind these changes was to increase the number plant-preferred codons without changing the amino acid sequence of the CspA and CspB proteins43. The modified sequences were named SeCspA and SeCspB (Fig. S1).
Generation of transgenic Arabidopsis and wheat plants
SeCspA and SeCspB, with SmaI and SpeI enzyme sites, were recombined into the pBI121 vector (kanamycin) under control of the cauliflower mosaic virus (CaMV) 35 S promoter, resulting in 35 S::SeCspA and 35 S::SeCspB constructs. The recombinant constructs were introduced into Agrobacterium tumefaciens strain EHA105, which was then used for the transformation of plant by the floral dip method. Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) were grown until flowering under normal growth conditions. The recombinant strains carrying recombinant vector SeCSP-pBI121 were then used to transform Arabidopsis. Homozygous T3 lines were obtained and used for further experiments. The methods were described by Clough and Bent, 199844. The leaves of three-week-old T3 transgenic Arabidopsis lines were taken and used for extracting total RNA (RNAprep plant kit, TIANGEN), and then reverse transcribed into cDNA using a PrimeScript First-Strand cDNA Synthesis kit (Takara). Transcript levels of SeCspA and SeCspB in transgenic Arabidopsis lines were detected by semi-quantitative PCR.
For generation of the transgenic wheat expression vectors, SeCspA and SeCspB were recombined into the pAHC25 vector45 under control of the ubiquitin (Ubi) promoter, and bar was used as the plant selection marker gene (Fig. S5A). Calli of immature embryos of common wheat cultivar KN199 (winter wheat) were cultured for one week at 26 °C in darkness and used in the transformation experiments. Bombardment was performed as described by Xu et al.46. After bombardment, calli were cultured and incubated on 1/2 MS medium containing 3 mg/L Bialaphos for 4 weeks (Fig. S5B). Regenerated plantlets were transferred to plantlet strengthening medium to allow vigorous root development following the methods described in Pellegrineschi et al.47, (Fig. S5C). Rooted plants were potted into soil and grown to maturity in a greenhouse. The transgenic wheat lines were generated by particle bombardment and propagated for two generations in the greenhouse.
Genomic DNA was extracted from the transgenic wheat lines and the control wheat plant tissue samples for PCR analysis using the CTAB method48. All T1 seeds from the T0 parents were planted in the field and allowed to set T2 seeds. When T1 wheat seedlings had grown into three-leaf plantlets, the genomic DNA from the T1 seedlings was extracted and analyzed by PCR analysis using the specific primers detailed in Table S6 (UBI-F from the vector, SeCspA-R, SeCspB-R). All PCR-positive T2 wheat seeds from unique (single) T1 wheat plants were harvested together and then planted in a field to allow plants to set T3 seeds. One of the homozygous T3 wheat plants from each PCR analysis-positive T2 wheat line was randomly examined via for Southern blot analysis; Southern blot-positive transgenic wheat plants were allowed to produce T4 seeds (Fig. S6). Four homozygous T4 transgenic wheat line progenies from each Southern blot-positive T3 transgenic wheat lines were harvested together and used for subsequent biochemical analyses.
Performance of transgenic Arabidopsis under stress treatment
One hundred homozygous T3 seeds of the 35 S::SeCspA and 35 S::SeCspB transgenic lines, and control plants, were sown on half-strength MS medium [1/2 strength Murashige and Skoog medium with salt and vitamins (PhytoTechnology Laboratories) further supplemented with 0.8% (w/v) agar, 30 mg L–1 hygromycin B (Sigma-Aldrich), and 2% (w/v) sucrose, pH 5.7] containing different concentrations of NaCl (0, 50, 100, and 150 mM) or PEG 6000 (0, 4% and 8%) and stored in a refrigerator (4 °C) and vernalized for 3 days before transfer to a controlled environment chamber (16 h light/8 h darkness, 23 °C, 70% relative humidity) for growth. After 4 days, germination rates were scored prescribed days, essentially as described by Kim et al.49,50.
For seedling phenotype analysis of the control and the 35 S::SeCspA and 35 S::SeCspB lines under salt treatment, 9-day-old seedlings of transgenic lines and control plants (including three-day vernalization treatment) were transferred to half-strength MS (Murashige and Skoog) agar plates supplemented with 100 mM NaCl and grown for one week under long-day conditions (16 h light/8 h dark) at 23 °C with 70% relative humidity, and the fresh weights of the aerial parts and the primary root lengths of the plants were measured after one week of treatment51.
For the drought treatment, three-week-old plants grown in soil (including three-day vernalization treatment) under normal conditions (16 h light/8 h dark, 23 °C, 70% relative humidity) were exposed to drought conditions for one week (plants were not watered), and the plants were then re-watered for growth recovery for one week. Similarly, for cold tests, three-week-old seedlings were transferred to −5 °C for 12 h and then returned to normal growing conditions for recovery for one week. Survival rates were recorded after recovery for one week. The experiments were repeated for several times and the results were consistent. One set of the experiments was shown.
Effect of salt, drought and cold stress on the growth of wheat
Wheat seeds were sown in a 96-well plates that were level with the surface of a hydroponic solution and grown in a controlled environment chamber under normal conditions (23 °C, 16 h light/8 h darkness) for one week. The seedlings were then treated with salt stress (200 mM NaCl in the hydroponic solution). After one week of salt treatment, the phenotypes of transgenic and control wheat plants were observed and the relative Na+ content in the leaves were measured using an atomic absorption spectrometer. The fresh weights of the transgenic and control plants were measured with MS Analytical Balances (Mettler Toledo).
The effects of drought on physiological indices were evaluated for two-week-old seedlings. Seven-day-old plants were treated with drought conditions for one week before measuring physiological indices. All of the measurements were repeated three times. MDA content was assayed according to the method reported by Lv et al.52. Wheat leaf samples (0.1 g) were used, and absorbance values at 450, 532, and 600 nm were measured with a spectrophotometer (Perkin-Elmer Lambda 25, Boston, MA, USA). The MDA content was calculated using the following formula: C (1 mol/L) = 6.45 (OD532–OD600)–0.56 OD450. About 0.1 g of wheat leaf was used for the measuring the proline content; the samples were treated with 3% (w/v) sulphosalicylic acid and were boiled for 1 h. The proline content was measured with ninhydrin, which was measured at 520 nm, and a proline standard liquid was used as a reference for generation of a standard curve53. Chlorophyll content was measured with a traditional spectrophotometer method; pigments were extracted overnight in 90% ethanol54.
Seven-day-old transgenic and control wheat seedlings were exposed to low temperature (−4 °C) for one week in a controlled environment chamber (16 h light/8 h darkness). The phenotypes were then observed and the root lengths were measured.
Relative electric conductivity
The sample leaves (0.1 g, wheat seedlings in the two leaf stage) of transgenic wheat lines and control plants seedlings (with natural growth condition and with 2 h of −8 °C cold treatment) were put into 20 ml of distilled water and a vacuum (test tube) was applied for 30 min, and then surged for 2 h to measure the initial electric conductance (S1) (25 °C). A test tube was filled with leaf discs and distilled water, the mixture was cooked (100 °C) for 30 min and then reduced to room temperature (25 °C) to determine the final electric conductance (S2). The relative electric conductivity was evaluated as: REC = S1 × 100/S2.
Stomatal aperture and water loss analysis
Leaves of 7-day-old wheat plants were collected and incubated for 4 h in stomatal-opening solution (0.05 M KNO3/10 mM, MES/50 μM CaCl2, pH = 6.15) under high light conditions (810 μmol•m−2•s−1, 25 °C). The leaves were then transferred into an ABA-containing solution (0, 15 μM, and 20 μM) for 2 h. The adaxial sides of the leaf epidermis was peeled off using a cutter blade and leaves were then mounted on slides and observed with a confocal laser scanning microscope (CLSM).
To measure water loss, leaves of 7-day wheat plants were excised and placed on a bench (25 °C, 30% relative humidity), and the initial fresh weights of plants was measured at 0 h and recorded W0, and then after 0.5 h, 2 h, 3 h and 5 h, the fresh weights of plants was measured and recorded Wn (n = 0.5, 2, 3, 5), respectively. The water loss rate was evaluated as: (W0 – Wn)*100/W0.
De novo sequencing of the control and transgenic wheat plants
Triplicate samples (one-week-old wheat seedlings, the two-leaf stage wheat seedlings), comprising three independent plants of each line, were used for RNA sequencing experiment. Total RNA was extracted from control wheat plants and the transgenic wheat lines using TRIzol reagent (Invitrogen) and then treated with RNase-free DNaseI to remove contaminating DNA. The quality and quantity of RNA were examined using an Agilent 2100 Bioanalyzer (Agilent Technologies) using the procedures described in Duan et al.55. RNA sequencing was performed on a HiSeq 2000 instrument (Illumina).
We used pair-end reads to assemble the hexaploid wheat transcriptome. Three state-of-the-art assemblers were used: Trinity was employed with the ALLPATHSLG error correction56; ABySS version 1.2.5 with the scaffolding option off and contig end erosion off was carried out for individual k-mer assemblies57; and trans-ABySS version 1.2.0 was used to merge the individual k-mer assemblies with default parameters58. The de novo assembly process was divided into the following four steps: pre-assembly, merging different samples, removal of redundancy, and scaffolding. Every detail of these steps and how these steps influenced assembly performance are reported in Duan et al.55. After optimization, the assembled transcripts were compared to the Sangerderived ESTs in terms of both continuity and accuracy. The differentially expressed genes were estimated by using DEseq package and considered to be significant at p-value < 0.05 and absolute fold-change ≥2- fold. Gene ontology analysis was performed by Blast2go software59.
QRT-PCR
Wheat seedlings at the two leaf stage that were grown under normal condition were sampled and frozen in liquid nitrogen for 10 min, and then transferred into −80 °C refrigerator for saving. Total RNA was extracted from wheat leaves using an RNAprep plant kit (TIANGEN) and reverse transcribed into cDNA using a PrimeScript First-Strand cDNA Synthesis kit (Takara). SYBR master mix (TIANGEN) was used for qRT-PCR. qRT-PCR was performed following the methods described in Liu et al.60 using an ABI Prism 7500 real-time PCR system (Lifetech). Wheat actin, as an internal reference, was used to normalize all data. Three biological replications for each line were performed in each test. The relative transcript levels of stress-responsive genes (TaMYB32, TaWRKY2, TaWD40, TaCDPK3, TaGST, TaRAB18, TaLEA, TaDHN and TaERF3) were calculated using the 2−ΔΔCT method61. All primers for the stress-responsive genes are listed in Table S6.
Response of transgenic wheat to drought stress in the field
Field trials for the drought response of the transgenic wheat lines were conducted at Shijiazhuang in Hebei province, China. The soil type was a loam. Soil properties at the 0–40 cm depth were pH 7.5–8.5, 1–2.2% organic matter, 30–99 ppm of inorganic N, 3–20 ppm of available Olsen P, and 100–200 ppm of K. The trace elements iron, copper, and manganese were abundant (4.5–10 ppm Fe, 1–2 ppm Cu, 10–15 ppm Mn), the soil bulk density was 1.301 g cm3, the saturation point was 43%, and the wilting coefficient was 6–7%. During the 2013 and 2016 growing season, the mean monthly temperatures in Shijiazhuang varied from 3 °C (January) to 28 °C (June), and Shijiazhuang received 159.4 and 120.1 mm of rainfall from January to June (Fig. S8).
Drought response tests were conducted from 2013 to 2014 and 2015 to 2016 with T5 and T7 generation plants. T5 and T7 generation control wheat plants and the transgenic wheat lines were grown during the 2013 to 2014 and 2015 to 2016 growing season on an experimental farm near Shijiazhuang in Hebei province, China. The experimental design was a two factor split-plot design with three replications (separate plots at the same site), water treatments were applied to entire main plots; genotypes were included as subplots. Each genotype was planted in 6 m2 plots with 4 m length, 1.5 m wide and seeds were sown by a bedder planter with an interval of 5 cm.
The field was irrigated before sowing in order to saturate the soil profile and to facilitate germination. T5 and T7 generation control wheat plants and the transgenic wheat lines were planted on October 2, 2013 and October 5, 2015 (average soil water content of 0–40 cm was 20.01%). The well-watered treatment plots were given a total of 240 mm irrigation (across four applications) at the over-wintering stage (September 19–20, 2013 and 2015), the stem elongation stage (March 3–5, 2014 and 2016), the flowering stage (April 25–30, 2014 and 2016), and the grain filling stage (May 21–25, 2014 and 2016). While the water-stress treatment was given a total of 90 mm irrigation (two applications) at the wintering stage (September 19–20, 2013 and 2015) and the stem elongation stage (March 3–5, 2014 and 2016). The average soil water content of 0–40 cm at the flowering stage (April 25–30, 2014 and 2016) was 10.3% (Water Stress) and 24.5% (Well-Watering), respectively. The average soil water content of 0–40 cm at the grain filling stage (May 21–25, 2014 and 2016) was 9.2% (Water Stress) and 24% (Well-Watering), respectively. The major agronomic traits of the transgenic and control wheat lines were observed for 20 plants from each of five sampling points for each plot. Drought resistance indices of the transgenic and control wheat lines were calculated using the following relationships: (1) SSI = [1 − (Ys)/(Yp)]/[1 − (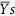 )/(
)/(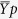 )]62, where Ys and Yp are the indices of the transgenic and control wheat lines evaluated under stress and non-stress conditions, and
)]62, where Ys and Yp are the indices of the transgenic and control wheat lines evaluated under stress and non-stress conditions, and 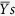 and
and 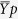 are the mean yields over all of the transgenic and control wheat lines evaluated under stress and non-stress conditions; (2) TOL = Yp − Ys63; (3) STI = (Yp − Ys)/
are the mean yields over all of the transgenic and control wheat lines evaluated under stress and non-stress conditions; (2) TOL = Yp − Ys63; (3) STI = (Yp − Ys)/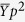 64. The soil samples of each plot were weighed as fresh weights (FW) and then dried in an electric drying oven at 80 °C for 48 h and weighed as dry weights (DW). The soil water content (%) was calculated using the following formula: [(FW − DW)/DW] * 100.
64. The soil samples of each plot were weighed as fresh weights (FW) and then dried in an electric drying oven at 80 °C for 48 h and weighed as dry weights (DW). The soil water content (%) was calculated using the following formula: [(FW − DW)/DW] * 100.
Additional Information
How to cite this article: Yu, T.-F. et al. Improved drought tolerance in wheat plants overexpressing a synthetic bacterial cold shock protein gene SeCspA. Sci. Rep. 7, 44050; doi: 10.1038/srep44050 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This research was financially supported by the National Transgenic Key Project of the Chinese Ministry of Agriculture (2014ZX08009-016B and 2016ZX08002-002). We are grateful to Dr Robert A McIntosh (University of Sydney, Australia) and Dr John Hugh Snyder (Cornell University, USA) for suggestions on the manuscript.
Footnotes
The authors declare no competing financial interests.
Author Contributions Z.S.X. coordinated the project, conceived and designed experiments, and edited the manuscript; T.F.Y. performed experiments and wrote the first draft; J.K.G., Y.X.W., X.C. and Y.B.Z. conducted performed the field experiments; J.D.F. and T.F.Y. generated and analyzed data; M.C. and X.G.Y. contributed with valuable discussions. B.A. reviewed and revised this paper; Y.Z.M. coordinated the project and edited the manuscript. All authors have read and approved the final manuscript.
References
- Xu Z. S., Chen M., Li L. C. & Ma Y. Z. Functions of the ERF transcription factor family in plants. Botany 86, 969–977 (2008). [Google Scholar]
- Xu Z. S., Chen M., Li L. C. & Ma Y. Z. Functions and application of the AP2/ERF transcription factor family in crop improvement. J Integr Plant Biol 53, 570–585 (2011). [DOI] [PubMed] [Google Scholar]
- Amara I., Capellades M., Ludevid M. D., Pagès M. & Goday A. Enhanced water stress tolerance of transgenic maize plants over-expressing LEA Rab28 gene. J Plant Physiol 170, 864–873 (2013). [DOI] [PubMed] [Google Scholar]
- Saad A. S. et al. A rice stress-responsive NAC gene enhances tolerance of transgenic wheat to drought and salt stresses. Plant Sci 204, 33–40 (2013). [DOI] [PubMed] [Google Scholar]
- Jiang X. et al. RhNAC3, a stress-associated NAC transcription factor, has a role in dehydration tolerance through regulating osmotic stress-related genes in rose petals. Plant Biotechnol J 12, 38–48 (2014). [DOI] [PubMed] [Google Scholar]
- Habben J. E. et al. Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol J 12, 685–693 (2014). [DOI] [PubMed] [Google Scholar]
- Gu J. F., Qiu M. & Yang J. C. Enhanced tolerance to drought in transgenic rice plants over-expressing C4 photosynthesis enzymes. Crop J 2, 105–114 (2013). [Google Scholar]
- Mittal A. et al. Related to ABA-Insensitive3 (ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol J 12, 578–589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C., Bellés J. M., Vayá J. L., Serrano R. & Culiáñez-Macià F. A. Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201, 293–297 (1997). [DOI] [PubMed] [Google Scholar]
- Castiglioni P. et al. Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 147, 446–455 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R. et al. The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 (1997). [DOI] [PubMed] [Google Scholar]
- Wang N., Yamanaka K. & Inouye M. CspI, the ninth member of the CspA family of Escherichia coli, is induced upon cold shock. J Bacteriol 181, 1603–1609 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon R. R., Lang E. A., Silva C. A. & Marques M. V. Cold shock genes CspA and CspB from Caulobacter crescentus are post transcriptionally regulated and important for cold adaptation. J Bacteriol 194, 6507–6517 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivancic T., Jamnik P. & Stopar D. Cold shock CspA and CspB protein production during periodic temperature cycling in Escherichia coli. BMC Res Notes 6, 248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W., Hou Y. & Inouye M. CspA, the major cold-shock protein of Escherichia coli, is an RNA chaperone. J Biol Chem 272, 196–202 (1997). [DOI] [PubMed] [Google Scholar]
- Graumann P. L. & Marahiel M. A. A superfamily of proteins that contain the cold-shock domain. Trends Biochem Sci 23, 286–290 (1998). [DOI] [PubMed] [Google Scholar]
- Deng X. P., Shan L., Zhang H. P. & Turner N. C. Improving agricultural water use efficiency in arid and semiarid areas of China. Agr Water Manage 80, 23–40 (2006). [Google Scholar]
- Mei X. R., Zhong X. L., Vadez V. & Liu X. Y. Improving Water Use Efficiency of Wheat Crop Varieties in the North China Plain: Review and Analysis. J Integr Agr 12, 1243–1250 (2013). [Google Scholar]
- Kesari R. et al. Intron-mediated alternative splicing of Arabidopsis P5CS1 and its association with natural variation in proline and climate adaptation. Proc Natl Acad Sci USA 109, 9197–9202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y. J. et al. Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of tetrastigma hemsleyanum diels et gilg. Environ Exp Bot 65, 177–182 (2009). [Google Scholar]
- Sio-Se Mardeh A., Ahmadi A., Poustini K. & Mohammadi V. Evaluation of Drought Resistance Indices under Various Environmental Conditions, Field Crops Res 98, 222–229 (2006). [Google Scholar]
- Sasaki K. & Imai R. Pleiotropic roles of cold shock domain proteins in plants. Front Plant Sci 2, 116–119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson D., Nakaminami K., Toyomasu T. & Imai R. A cold-regulated nucleic acid-binding protein of winter wheat shares a domain with bacterial cold shock proteins. J Biol Chem 277, 35248–35256 (2002). [DOI] [PubMed] [Google Scholar]
- Sasaki K., Kim M. H. & Imai R. Arabidopsis COLD SHOCK DOMAIN PROTEIN2 is a RNA chaperone that is regulated by cold and developmental signals. Biochem Bioph Res Co 364, 633–638 (2007). [DOI] [PubMed] [Google Scholar]
- Fusaro A. F. et al. AtGRP2, a cold-induced nucleo-cytoplasmic RNA-binding protein, has a role in flower and seed development. Planta 225, 1339–1351 (2007). [DOI] [PubMed] [Google Scholar]
- Kim J. S. et al. Cold shock domain proteins and glycine-rich RNA-binding proteins from Arabidopsis thaliana can promote the cold adaptation process in Escherichia coli. Nucleic Acids Res 35, 506–516 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. H., Sasaki K. & Imai R. Cold shock domain protein 3 regulates freezing tolerance in Arabidopsis thaliana. J Biol Chem 284, 23454–23460 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Jiang W., Bae W. & Inouye M. Promoter-independent cold-shock induction of cspA and its derepression at 37 degrees by mRNA stabilization. Mol Microbiol 23, 355–364 (1997). [DOI] [PubMed] [Google Scholar]
- Kim M. H. et al. COLD SHOCK DOMAIN PROTEIN 3 is involved in salt and drought stress tolerance in Arabidopsis. FEBS 3, 438–442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D., Pelloux J. & Brownlee C. Calcium at the crossroads of signaling. Plant Cell 14, 401–417 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. L. et al. A Glycine soja ABA-responsive receptor-like cytoplasmic kinase, GsRLCK, positively controls plant tolerance to salt and drought stresses. Planta 237, 1527–1545 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang L. C., Zhao G. Y., Jia J. Z., Liu X. & Kong X. Y. Molecular characterization of isolated wheat MYB genes and analysis of their expression during abiotic stress. J Exp Bot 63, 203–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu C. F. et al. Wheat WRKY genes TaWRKY2 and TaWRKY19 regulate abiotic stress tolerance in transgenic Arabidopsis plants. Plant Cell Environ 35, 1156–1170 (2012). [DOI] [PubMed] [Google Scholar]
- Rong W. et al. The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12, 468–479 (2014). [DOI] [PubMed] [Google Scholar]
- Xu C. & Min J. Structure and function of WD40 domain proteins. Protein Cell 2, 202–214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong D. J., Li M. J., Dong Z. H., Ji H. T. & Li X. Identification of TaWD40D, a wheat WD40 repeat-containing protein that is associated with plant tolerance to abiotic stresses. Plant Cell Rep 34, 395–410 (2015). [DOI] [PubMed] [Google Scholar]
- Bhavanath J., Anubha S. & Avinash M. Expression of SbGSTU (tau class glutathione S-transferase) gene isolated from Salicornia brachiata in tobacco for salt tolerance. Mol. Biol. Rep. 38, 4823–4832 (2011). [DOI] [PubMed] [Google Scholar]
- Huang X. S., Wang W., Zhang Q. & Liu J. H. A basic helix-loop-helix transcription factor, PtrbHLH, of poncirus trifoliata confers cold tolerance and modulates peroxidase-mediated scavenging of hydrogen peroxide. Plant Physiol 162, 1178–1194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhawat U. K., Srinivas L. & Ganapathi T. R. MusaDHN-1, a novel multiple stress-inducible SK (3)-type dehydrin gene, contributes affirmatively to drought- and salt-stress tolerance in banana. Planta 234, 915–932 (2011). [DOI] [PubMed] [Google Scholar]
- Chandler P. M. & Robertson M. Gene expression regulated by abscisic acid and its relation to stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 45, 113–141 (1994). [Google Scholar]
- Liu Y. L. et al. Large-scale screening for maize drought resistance using multiple selection criteria evaluated under water-stressed and well-watered environments. Field Crops Res 124, 37–45 (2011). [Google Scholar]
- Mitchell J. H. et al. The use of seedling leaf death score for evaluation of drought resistance of rice. Field Crops Res 55, 129–139 (1998). [Google Scholar]
- Perlak F. J., Fuchs R. L., Dean D. A., McPherson S. L. & Fischhoff D. A. Modification of the coding sequence enhances plant expression of insect control protein genes. Proc Natl Acad Sci USA 88, 3324–3328 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium- mediated transformation of Arabidopsis thaliana. Plant J 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Christensen A. H. & Quailm P. H. Ubiquitin promoter-based vectors for high level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res 5, 213–218 (1996). [DOI] [PubMed] [Google Scholar]
- Xu Z. S. et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress resistance. Plant Mol Biol 65, 719–732 (2007). [DOI] [PubMed] [Google Scholar]
- Pellegrineschi A. et al. Warburton, M., Hoisington, D. Identification of highly transformable wheat genotypes for mass production of fertile transgenic plants. Genome 45, 421–430 (2002). [DOI] [PubMed] [Google Scholar]
- Saghai-Maroof M. A., Soliman K. M., Soliman K. M., Jorgensen R. A.& Allard R. W. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81, 8014–8019 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. O., Kim J. S. & Kang H. Cold-inducible zinc finger-containing glycine-rich RNA binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J 42, 890–900 (2005). [DOI] [PubMed] [Google Scholar]
- Kim J. S. et al. Glycine-rich RNA-binding protein7 affects abiotic stress responses by regulating stomata opening and closing in Arabidopsis thaliana. Plant J 55, 455–466 (2008). [DOI] [PubMed] [Google Scholar]
- Joshi R., Ramanarao M. V. & Baisakh N. Arabidopsis plants constitutively overexpressing a myo-inositol 1-phosphate synthase gene (SaINO1) from the halophyte smooth cordgrass exhibits enhanced level of tolerance to salt stress. Plant Physiol Bioch 65, 61–66 (2013). [DOI] [PubMed] [Google Scholar]
- Lv S., Yang A., Zhang K., Wang L. & Zhang J. Increase of glycine betaine synthesis improves drought tolerance in cotton. Mol Breeding 20, 233–248 (2007). [Google Scholar]
- Bates L. S., Waldren R. P. & Teare I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973). [Google Scholar]
- Sartory D. P. & Grobbelaar J. U. Extraction of chlorophylla from fresh water phytoplankton for spectrophotometric analysis, Hydrobiologia 114, 177–187 (1984). [Google Scholar]
- Duan J. l., Xia C., Zhao G. Y., Jia J. Z. & Kong X. Y. Optimizing de novo common wheat transcriptome assembly using short-read RNA-Seq data. BMC Genomics 13, 392–403 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr M. G. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29, 644–652 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birol I. et al. De novo transcriptome assembly with ABySS. Bioinformatics 25, 2872–2877 (2009). [DOI] [PubMed] [Google Scholar]
- Robertson G. et al. De novo assembly and analysis of RNAseq data. Nat Methods 7, 909–912 (2010). [DOI] [PubMed] [Google Scholar]
- Cones A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 (2005). [DOI] [PubMed] [Google Scholar]
- Liu P., Xu Z. S., Lu P. P., Hu D., Chen M., Li L. C. & Ma Y. Z. A wheat PI4K gene whose product possesses threonine autophophorylation activity confers tolerance to drought and salt in Arabidopsis. J Exp Bot 10, 2915–2927 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le D. T. et al. Genome-wide expression profiling of soybean two-component system genes in soybean root and shoot tissues under dehydration stress. DNA Res 18, 17–29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer R. A. & Maurer R. Drought resistance in spring wheat cultivars. Part 1: grain yield response. Aust J Agr Res 29, 897–912 (1978). [Google Scholar]
- Hossain A. B., Sears A. G., Cox T. S. & Paulsen G. M. Desiccation tolerance and its relationship to assimilate partitioning in winter wheat. Crop Sci 30, 622–627 (1990). [Google Scholar]
- Fernandez G. C. Effective selection criteria for assessing stress tolerance. In Kuo C. G. (Ed.), Proceedings of the International Symposium on Adaptation of Vegetables and Other Food Crops in Temperature and Water Stress, Publication, Tainan, Taiwan (1992).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.