Abstract
Dendritic cells (DCs) are known to induce the growth and function of natural killer (NK) cells. Here, we address the capacity of DCs to interact with NK cells in human lymphoid organs and identify the role of specific DC-derived cytokines. We demonstrate that DCs colocalize with NK cells in the T cell areas of lymph nodes. In culture, DCs from either blood or spleen primarily stimulate the CD56brightCD16– NK cell subset, which is enriched in secondary lymphoid tissues. Blocking of IL-12 abolished DC-induced IFN-γ secretion by NK cells, whereas membrane-bound IL-15 on DCs was essential for NK cell proliferation and survival. Maturation by CD40 ligation promoted the highest IL-15 surface presentation on DCs and led to the strongest NK cell proliferation induced by DCs. These results identify secondary lymphoid organs as a potential DC/NK cell interaction site and identify the distinct roles for DC-derived IL-12 and IL-15 in NK cell activation.
Keywords: lymph node, spleen, IFN-γ secretion, proliferation, survival
Natural killer (NK) cells are an important component of innate immunity, able to limit viremia and tumor burden even before the adaptive immune system can be activated. Whereas human peripheral blood NK cells have been characterized in considerable detail (1), the NK cell subset in secondary lymphoid organs has just recently been identified (2, 3). Secondary lymphoid tissues harbor NK cells that are CD56bright CD16–. This subset of NK cells secretes large amounts of IFN-γ, tumor necrosis factor (TNF), and granulocyte–macrophage colony-stimulating factor (GM-CSF) upon activation but requires longer stimulation to become cytolytic (3). In contrast, the majority of NK cells in peripheral blood are CD56dimCD16+ and cytolytic but poor in cytokine secretion (4–6). Therefore, there are substantial functional differences and distinct anatomical sites for NK cells, and these need to be considered to understand NK cell biology.
Dendritic cells (DCs) are now understood to be potent stimulators of NK cell activation (7–10). DCs reside in peripheral tissues, and their homing to secondary lymphoid organs is enhanced upon inflammation or encounter of pathogenic products (11). Early NK cell activation is essential for the immune control of viral infections, for example, by herpesviruses (12). In mice, during infection with murine cytomegalovirus, a β-herpesvirus, CD8α+ myeloid DCs are essential for the expansion of the protective Ly49H+ NK cell subset (13, 14). However, in humans, the sites of DC/NK cell interaction, the NK cell subsets activated by DCs and the stimuli involved in NK cell activation by DCs are poorly defined.
Several cytokines have been implicated in NK cell activation by DCs. Human DCs were most consistently found to stimulate cytotoxicity (15, 16) and IFN-γ secretion (17, 18) of NK cells from peripheral blood through IL-12. Mouse DCs, on the contrary, stimulate NK cell cytotoxicity through type I IFNs and IFN-γ secretion of NK cells in part through IL-2 (19) and/or IL-15 together with IL-12 (20). In addition, IL-15 has been implicated in NK cell accumulation/survival after murine cytomegalovirus infection (21). However, the degree to which these mechanisms contribute to human NK cell activation in secondary lymphoid organs and the requirements for DC-induced human NK cell proliferation have not been elucidated so far.
Here, we show that DCs and NK cells are colocalized in the T cell areas of lymph nodes and that, upon maturation, both monocyte-derived DCs as well as DCs directly isolated from the human spleen activate the CD56brightCD16– NK cell subset of secondary lymphoid tissues. Furthermore, we demonstrate that DCs induce IFN-γ secretion of CD56brightCD16– NK cells through IL-12, whereas IL-15 mediates NK cell proliferation upon DC encounter. Because DC-induced NK cell proliferation is sensitive to transwell separation, and because the active DCs present IL-15 on their surface, we suggest that membrane-bound IL-15 is the relevant cytokine form for NK cell expansion mediated by DCs.
Materials and Methods
Human Secondary Lymphoid Organs and Blood. The regional organ procurement organization, the New York Organ Donor Network, procured human lymph nodes, autologous spleens, and blood from individual brain-dead donors after obtaining informed consent from appropriate individuals. All tissues were obtained as part of the Institutional Review Board-approved protocols of Weill Medical College, Cornell University, and The Rockefeller University. Soon after their removal, tissues were dissociated to obtain single-cell suspensions and cryopreserved as described in ref. 3.
Preparation of DCs. DCs were generated from CD14+ cells or isolated directly from human spleens. Positive selection for CD14+ blood or spleen cells was performed by using magnetic cell separation (Miltenyi Biotec, Bergisch Gladbach, Germany). DCs were differentiated from monocytes as described in ref. 8. DCs were matured by the addition of 10 ng/ml IL-1β, 1,000 units/ml IL-6, and 10 ng/ml TNF-α, all from R&D Systems, and 1 μg/ml prostaglandin E2 from Sigma for 2 days. Alternatively, DCs were matured by adding 100 ng/ml lipopolysaccharide (LPS) from Sigma, bacillus Calmette–Guérin [multiplicity of infection of 1 (one DC per one bacterial body)] from Pasteur Merieux (Lyon, France) (22), by CD40 ligand (CD40L)-expressing D1.1 cells [one DC per five D1.1 cells (D1.1 cell line, ATCC catalog no. CRL-10915)], or by CD40L-expressing murine fibroblasts (five DCs per one CD40L L cell) (23). DCs from human spleen were obtained by flow cytometric cell sorting (FACSVantage SE, Becton Dickinson) after staining of splenic mononuclear cells with phycoerythrin (PE)-conjugated anti-CD11c mAb and then gating on large/granular/CD11cbright cells. Sorted cells were then cultured in RPMI medium 1640 in the presence of 10% FCS and 100 ng/ml LPS for 24 h.
Preparation of NK Cells. Whole blood from leucocyte concentrates served as the source of peripheral blood mononuclear cells (PBMC), isolated by density-gradient centrifugation on Ficoll/Paque (Amersham Pharmacia). Alternatively, splenic mononuclear cells were used. NK cells were isolated with the NK Cell Isolation Kit (Miltenyi Biotec). The percentage of NK cells after isolation was evaluated by using FITC-conjugated anti-CD3 and PE-conjugated anti-CD56 mAbs (Becton Dickinson) in flow cytometry and was routinely >98.5%. NK cell/DC cocultures for functional experiments were performed in RPMI medium 1640 plus 10% FCS at a 10:1 NK cell:DC ratio.
mAbs and Flow Cytometry. Analysis of cell surface markers was performed by using the following mAbs in direct immunofluorescence assays: anti-CD3, anti-CD56, anti-CD11c, anti-HLA-DR, and anti-CD14 (Becton Dickinson); anti-CD83 (Immunotech, Marseille, France); anti-DC-SIGN (CD209); anti-IL-15 (clone 34593.1, purified, and clone 34559, FITC- or PE-conjugated from R & D Systems); and anti-DEC-205 (CD205) (eBioscience, San Diego). Immunof luorescence staining was performed by diluting fluorochrome-labeled mAb with 1 mg/ml human γ-globulin (human therapy grade; Biotest, Milan), to block nonspecific Fc-receptor binding. For indirect immunofluorescence staining, nonspecific binding sites were saturated with human γ-globulin, and then the relevant mAbs were added, followed by FITC- or PE-conjugated isotype-specific goat anti-mouse antibodies (Southern Biotechnology Associates). Negative controls included directly labeled or unlabeled isotype-matched irrelevant mAbs. IL-15Rα was stained indirectly with the goat anti-IL-15Rα polyclonal antibody (R & D Systems) and donkey anti-goat IgG-PE secondary antibody (Jackson ImmunoResearch). The control for IL-15Rα polyclonal goat antibody staining was polyclonal goat anti-mouse IgG antibody (Jackson ImmunoResearch) with the same secondary reagent. Cells were analyzed with a FACScalibur flow cytometer (Becton Dickinson).
Deconvolution Microscopy. Human lymph node and spleen samples were embedded in Tissue-Tek OTC (Sakura, Torrance, CA) and stored at –80° until use. Cryosections at 8 μm of thickness were fixed with acetone for 10 min and then stained with antibodies against CD20, CD3, and CD56 (clone NCAM16.2; Becton Dickinson) and against DEC-205 (CD205; eBioscience), followed by isotype-specific Alexa Fluor 488 or Alexa Fluor 546 (Molecular Probes). After washing, the specimens were mounted with Aqua Poly/Mount (Polysciences). An AX70 deconvolution microscope (Olympus, Melville, NY) was used to analyze the specimens.
IFN-γ Production Assay. To evaluate DC-mediated IFN-γ production by NK cells, purified NK cells or mononuclear cells were cultured in the presence of 2 μM monensin (Sigma) with 10% autologous monocyte-derived DCs or DCs sorted from spleen. In some experiments, blocking mAbs against IL-12, IL-15, and IL-2 (R & D Systems) were added to the cultures 2 h before the addition of NK cells. Where indicated, NK cells and DCs were separated by 0.4-mm polycarbonate membranes (Nalge). After 6 h of coculture, cells were harvested and stained with PE-conjugated anti-CD56 and FITC-conjugated anti-CD3 (Becton Dickinson). Cells were then fixed in 1% paraformaldehyde, permeabilized, and stained with allophycocyanin-conjugated anti-IFN-γ (Becton Dickinson).
Proliferation Assay by 5,6-Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Dilution. Mononuclear cells or purified NK cells were labeled with 5 μM CFSE in PBS plus 0.1% BSA for 10 min at 37°C. After washing twice with PBS plus 0.1% BSA, cells were incubated with autologous DCs (NK cell or mononuclear cell:DC ratio 10:1). NK cells and DCs were then cultured for 6 days at 37°C. CFSE fluorescence (FL-1) was evaluated on CD3–CD56+ cells by flow cytometry. In select experiments, blocking mAbs against IL-12, IL-15, and IL-2 (R & D Systems) were added to the cultures at the beginning and on day 3 of culture. Where indicated, NK cells and DCs were separated by 0.4-mm polycarbonate membranes (Nalge).
Preparation of Cell Lysates, SDS/PAGE, and Western Blot Analysis. Cells were washed twice in PBS and boiled in reducing SDS sample buffer. Lysates of 3 × 105 cells per lane were separated on a 12% SDS/polyacrylamide gel. Proteins were transferred to a poly(vinylidene difluoride) (PVDF) membrane (Hybond-P, Amersham Pharmacia), and IL-15 was detected with the 34593.11 mAb (R & D Systems). The mouse anti-β-actin (clone AC-40) mAb was purchased from Sigma. Proteins were visualized by using horseradish peroxidase-coupled goat anti-mouse IgG secondary antibody (Bio-Rad) and the ECLplus detection system (Amersham Pharmacia).
Results
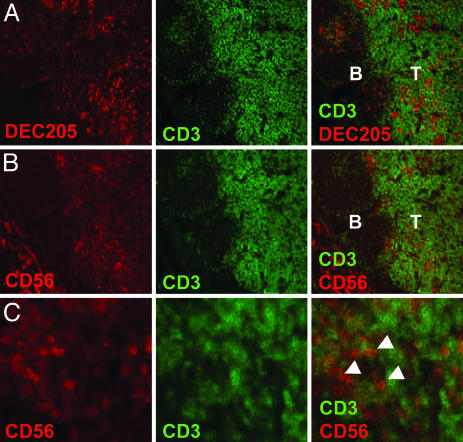
NK Cells and DCs Colocalize in T Cell Areas of Human Lymph Nodes. We recently demonstrated that human lymph nodes harbor an abundant subset of NK cells characterized by a CD16–CD56bright phenotype (3). A recent report concluded by using immunohistochemistry and in situ PCR that CD56+CD3– cells are located in the T cell area of inflamed human lymph nodes (2). We investigated whether DCs and CD3–CD56bright NK cells localize to the same regions of uninflamed lymph nodes from different donors. We could confirm the presence of CD3–CD56+ NK cells in T cell areas, including clusters in the parafollicular regions of the T cell zone (Fig. 1 B and C). No CD56 signal was detected in the B cell follicles. CD3+CD56+ double-positive cells were observed rarely (data not shown). This finding probably reflects the fact that the levels of CD56 on activated T cells is 10-fold less by fluorescence-activated cell sorter (FACS) analysis than CD56 on the CD56bright NK subset (3). In addition, DEC-205+ DCs also were found in the T cell areas of human lymph nodes (Fig. 1 A). These results indicate that DCs and NK cells meet in the T cell areas of human secondary lymphoid tissues.
Fig. 1.
NK cells and DCs colocalize in T cell areas of normal human lymph node. Sequential cryosections of human lymph node show that DEC-205-positive DCs (A) and CD3–CD56+ NK cells (B) are in close proximity in the T cell area (T) of the tissue, but not in B cell follicles (B). (C) Arrowheads indicate NK cells expressing CD56 but not CD3 in the T cell area of lymph nodes. (Magnification: ×10 in A and B and ×40 in C.)
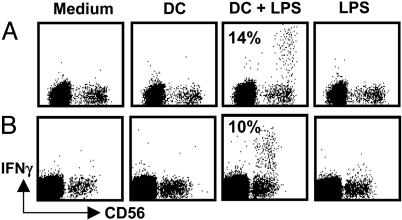
DCs Induce Prompt IFN-γ Production of Autologous Lymph Node NK Cells. We recently demonstrated that NK cells of human lymph nodes and tonsils produce high levels of IFN-γ upon stimulation with IL-2 and IL-12 (3). Because DCs are known to migrate to secondary lymphoid organs upon maturation and because we find DEC-205+ DCs in close proximity to NK cells in lymph nodes, we investigated whether lymph node NK cells might secrete IFN-γ upon stimulation with mature DCs. To this end, we cocultured resting NK cells of lymph nodes with autologous DCs for 6 h. The DCs were either derived from autologous splenic monocytes or directly isolated from autologous spleen as large CD11chigh cells (24). Only DCs that had been matured with LPS were able to induce IFN-γ production by NK cells (Fig. 2). We found that 14% of NK cells produced IFN-γ after coculture with monocyte-derived DCs (median value range, 10–35% in five independent experiments) and 15% of NK cells produced IFN-γ after coculture with spleen DCs (median value range, 10–19% in three experiments). Neither immature DCs nor LPS alone were able to induce the cytokine production by lymph node NK cells during the same time interval. These data indicate that, in analogy to DC-mediated activation of human peripheral blood NK cells (8–10), DCs can activate NK cells of secondary lymphoid organs to produce IFN-γ.
Fig. 2.
Human lymph node NK cells produce IFN-γ upon interaction with autologous DCs. (A) Lymph node mononuclear cells were cultured for 6 h alone (Medium), in the presence of 100 ng/ml LPS (LPS), or in the presence of immature (DC) or LPS-matured (DC + LPS) autologous monocyte-derived DCs. One of five experiments is shown. (B) Lymph node mononuclear cells were cultured for 6 h alone, with LPS and in the presence of spleen DCs, which were matured (DC + LPS) or not (DC) for 24 h in the presence of 100 ng/ml LPS. IFN-γ production was analyzed on CD3– cells; numbers inside the dot plots indicate the percentage of NK cells producing IFN-γ in these representative experiments. One of three experiments is shown.
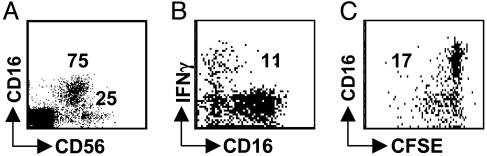
DCs Primarily Stimulate CD16–CD56bright NK Cells to Secrete IFN-γ and Proliferate. We initially concentrated our studies on normal human spleen, because this lymphoid organ contains large numbers of cells from which CD3–CD56+ NK cells can be sorted and cryopreserved and which contain both the CD56brightCD16– and the CD56dimCD16+ NK cell subsets in substantial amounts (25% and 75% of the CD3– mononuclear cells, respectively; Fig. 3A) (3). We analyzed whether DCs selectively stimulate one or the other NK cell subset. We found that IFN-γ secretion was restricted to CD16–CD56bright NK cells after short-term (6 h) stimulation of purified CD3–CD56+ NK cells with autologous monocyte-derived mature DCs (Fig. 3B). Approximately 10% of total CD3–CD56+ NK cells and 40% of the CD16–CD56+ NK cell subset responded to DC stimulation by IFN-γ secretion, whereas no IFN-γ secretion of the CD56dimCD16+ subset was detectable after this short-term stimulation (Fig. 3B). Furthermore, only CD16–CD56bright NK cells proliferated during 6 days of stimulation with autologous monocyte-derived DCs (Figs. 3C and 5C). CD56dimCD16+ NK cells showed very little proliferation under the same conditions. These findings are consistent with our previous report (3), in which we demonstrated that even lymph node NK cells, after maturation to cytolytic NK cells expressing killer-Ig-like receptors (KIRs), stop proliferating, whereas KIR– NK cells proliferate vigorously upon activation. Similar to the percentage of DC-activated IFN-γ-producing CD16–CD56bright NK cells, about half of the CD16–CD56bright NK cells entered proliferation upon DC stimulation. Therefore, DCs selectively stimulate the CD16–CD56bright NK subset to secrete IFN-γ as well as proliferate, and this subset is enriched in secondary lymphoid tissues.
Fig. 3.
DCs stimulate IFN-γ secretion and proliferation mainly by CD56bright NK cells. (A) Splenic CD56+CD3– NK cells were analyzed for their CD56brightCD16– and CD56dimCD16+ subsets. (B) IFN-γ secretion by purified splenic CD56+CD3– NK cells upon monocyte-derived DC stimulation was analyzed. (C) Proliferation of purified splenic CD56+CD3– NK cells upon monocyte-derived DC stimulation. The numbers in the blots indicate the percentages of the responding NK populations. One of two experiments is shown.
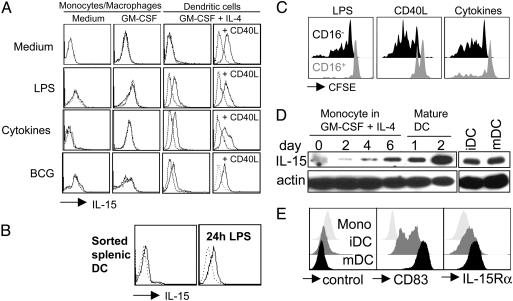
Fig. 5.
DCs present IL-15 on their surface upon maturation. DCs derived from monocytes by culture in the presence of GM-CSF and IL-4 (GM-CSF + IL-4) (A) and DCs directly isolated from spleen (B) present IL-15 on their surface upon maturation with LPS, proinflammatory cytokines (IL-1β, IL-6, TNF-α, and prostaglandin E2) or live bacillus Calmette–Guérin. Monocyte/macrophages cultured without cytokines (Medium) or in the presence of GM-CSF alone (GM-CSF) and then activated by the same stimuli used for DC maturation failed to express IL-15 on their surfaces. Addition of CD40L-transfected cells to DC cultures (CD40L) in the last 48 h was sufficient, even in the absence of inflammatory stimuli, to induce surface IL-15 expression in DCs but not in monocyte/macrophages (data not shown). Dotted lines represent isotype controls. One of three experiments is shown. (C) NK cells purified from peripheral blood were labeled with CFSE and cultured in the presence of autologous DCs matured by different stimuli. At day 6 of the cultures, cells were harvested, and CFSE dilution was evaluated (LPS, DCs matured by 100 ng/ml LPS; CD40L, DCs matured by CD40L-transfected cells; Cytokines, DCs matured by proinflammatory cytokines). One of three experiments is shown. (D) Western blot analysis of IL-15 at the indicated time points of DC differentiation from peripheral blood monocytes and with (mDC) and without (iDC) maturation by proinflammatory cytokines for 2 days. One of three experiments is shown. (E) Flow cytometric analysis of CD83 (Center) and IL-15Rα (Right) on monocytes (Mono), immature DCs (iDC; day 8) and mature DCs (mDC; day 8 and last 2 days incubated with proinflammatory cytokines). As a control for the polyclonal anti-IL-15Rα goat antibody a goat anti-mouse polyclonal antibody was used (control). One of three experiments is shown.
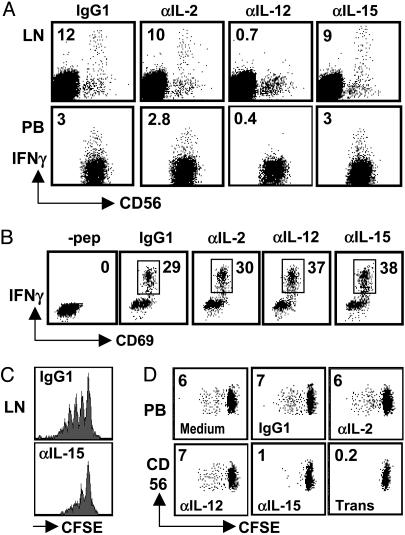
DCs Induce NK Cells to Produce IFN-γ Through IL-12 and to Proliferate Through IL-15. We next analyzed the contribution of select cytokines to NK cell activation by DCs. In previous studies, we found that DC-mediated NK cell activation correlated well with the capacity of IL-12 secretion by different DC subtypes (25). In addition, murine DCs produce the NK cell-activating cytokine IL-2 (26), and human DCs secrete the NK cell-activating cytokine IL-15 (27, 28). Therefore, we compared the contributions of IL-12, IL-15, and IL-2 for DC-induced IFN-γ secretion by NK cells. For these experiments, we either purified NK cells (spleen and peripheral blood) or gated on CD3–CD56+ cells during the analysis (lymph node). For the stimulation of lymph node NK cells, we used autologous DCs isolated directly from human spleen (Fig. 4A Upper) or DCs derived from autologous splenic monocytes (data not shown). And for the stimulation of purified peripheral blood or splenic NK cells, we used autologous monocyte-derived DCs (Fig. 4A Lower and data not shown). IFN-γ secretion of NK cells was blocked completely by antibodies against IL-12 but not by antibodies against IL-2 and IL-15 (Fig. 4A). Consistent with secreted IL-12 being the main factor for DC-induced IFN-γ secretion by NK cells, IFN-γ secretion was blocked only partially by transwell separation of DCs and NK cells (data not shown).
Fig. 4.
IL-12 and IL-15 are crucial for DC-mediated IFN-γ secretion and proliferation by NK cells, respectively. (A Upper) Lymph node mononuclear cells were cultured for 6 h in the presence of mature DCs isolated from autologous spleen in the presence of the indicated blocking antibodies. Analyses were performed on CD3– cells. Similar results were obtained when autologous monocyte-derived DCs were used to activate lymph node NK cells (data not shown). (A Lower) NK cells purified from peripheral blood were cultured in the presence of autologous monocyte-derived DCs. One of three experiments is shown. (B) IFN-γ production by the EBNA1-specific CD4+ T cell clone A4.E116 in response to autologous monocyte-derived DCs, which had been pulsed with the cognate EBNA1514-527 peptide, in the presence of blocking antibodies. One of three experiments is shown. NK cells purified from human lymph nodes (C) or from peripheral blood (D) were labeled with CFSE and cultured for 6 days in the presence of autologous DCs alone (Medium) and, where indicated, with blocking antibodies (IgG1, isotype control; αIL-2, anti-IL-2; αIL-12, anti-IL-12; αIL-15, anti-IL-15) or separated by transwell (Trans). Numbers inside dot plots indicate the percentage of cycling NK cells. One of two experiments is shown for C, and one of three experiments is shown for D.
In contrast, IL-15 and cell-to-cell contact mediated DC-induced CD56+ NK cell proliferation (Fig. 4 C and D). For the proliferation assays, NK cells also were purified from lymph nodes. Because relatively few cells were available from uninflamed lymph nodes, we could test only isotype control and IL-15 blocking (Fig. 4C). Interestingly, blocking of IL-15 as well as transwell separation also decreased the recovery of NK cells (data not shown). Therefore, DC-induced NK cell proliferation in spleen, lymph node, and peripheral blood depends on IL-15.
To exclude nonspecific effects of our antibody treatments, we analyzed IFN-γ production by the Epstein–Barr virus-encoded nuclear antigen 1 (EBNA1)-specific CD4+ T cell clone A4.E116 (29) in response to autologous monocyte-derived DCs, which had been pulsed with the cognate EBNA1514–527 peptide, in the presence or absence of IL-2, IL-12, or IL-15 blocking antibodies (Fig. 4B). IFN-γ production by the A4.E116 T cell clone was not dependent on any of the three cytokines, and none of the blocking antibodies inhibited unspecifically activation of the T cell clone. We conclude from these data that DCs mainly expand and activate the CD56brightCD16– NK cells and that this activation proceeds through IL-12 and IL-15. Therefore, DCs are especially suited to activate NK cells in secondary lymphoid tissues, where CD56brightCD16– NK cells are enriched.
Mature, but Not Immature, DCs Present IL-15 on Their Surface. Maturing DCs have been reported to increase IL-15 messenger RNA but secrete only small amounts of IL-15 protein (25, 27, 28). Indeed, we have found recently that the concentrations of DC-secreted IL-15 are too low to activate NK cells directly when administered as recombinant cytokines (25). Recent evidence that IL-15 might be presented to NK cells in trans through the IL-15Rα (30, 31) prompted us to analyze the expression of this cytokine on the surface of human DCs. As shown in Fig. 5, both monocyte-derived DCs and DCs isolated directly from the spleen expressed IL-15 on their surfaces upon maturation with LPS, proinflammatory cytokines (TNF-α, IL-1β, IL-6, and prostaglandin E2), live bacteria (bacillus Calmette–Guérin), or CD40 ligation. Membrane-bound IL-15 was never detectable on the surface of immature DCs. Monocyte/macrophages activated by the same stimuli used for DC maturation failed to express IL-15 on their surfaces. Because both blocking of IL-15 and separation of NK cells and DCs by a transwell device virtually abrogated DC-mediated NK cell expansion, these results suggest that membrane-bound IL-15 might be responsible for DC-mediated effects on NK cell proliferation. Accordingly, CD40L-matured DCs, which express the highest IL-15 levels on their surface of all conditions tested, stimulated the proliferation of CD56brightCD16– NK cells most efficiently (Fig. 5 A and C).
We next analyzed the mechanism by which mature DCs express IL-15 on their surface. Therefore, we analyzed whether differences in IL-15 protein levels could account for the difference in IL-15 surface presentation between immature and mature DCs. For this purpose, we performed Western blot analysis for IL-15 during DC differentiation from CD14+ blood monocytes and for comparison of IL-15 levels between cultures matured with proinflammatory cytokines and untreated cultures (Fig. 5D). Whereas accumulation of IL-15 was clearly evident during DC differentiation, mature and immature DCs had similar levels of total cellular IL-15. Blocking of IL-15 secretion with brefeldin A did not increase the IL-15 signal in mature or immature DCs (data not shown). Because differences in IL-15 production seemed not to explain the different IL-15 surface expression by mature and immature DCs, we analyzed the levels of the IL-15-presenting molecule, IL-15Rα (Fig. 5E). We found that IL-15Rα was nearly absent on monocytes but expressed on immature DCs and further up-regulated on mature DCs. We assessed the maturation status of these DC populations by CD83 staining. At day 1 after maturation, IL-15Rα levels differed only slightly between mature and immature DCs (data not shown), but 2 days after maturation, mature DCs expressed significantly more IL-15Rα than did immature DCs (Fig. 5E). Interestingly, even within the immature DC population, IL-15Rα expression was found mainly on CD83+CD25+CCR7+ partially matured DCs (data not shown). Therefore, the high level of IL-15Rα on DCs matured for 2 days seems to be required for the activation of NK proliferation and survival by monocyte-derived DCs in trans.
Discussion
In this study, we have investigated the interaction of DCs with NK cells from human secondary lymphoid organs. We demonstrate that DCs selectively activate the CD56brightCD16– NK cell subset, which is enriched in secondary lymphoid organs, and characterize the distinct roles of two crucial cytokines that mediate NK cell activation by DCs. Whereas IL-12 is required for DCs to stimulate rapid IFN-γ production of NK cells, IL-15 seems essential for the induction of NK cell proliferation. Transwell separation of DCs and NK cells abolished IL-15-dependent NK cell proliferation but not IL-12-dependent IFN-γ secretion. Thus, IL-15 presented to NK cells on the surface of mature monocyte-derived and splenic DCs may be mainly responsible to cause NK cell proliferation, whereas IFN-γ secretion is probably induced by secreted IL-12.
We show that DCs mainly stimulate IFN-γ secretion and proliferation of the NK cell subset that is enriched in secondary lymphoid organs (2, 3). Most human CD56brightCD16– NK cells are localized in the T cell area of lymph nodes (Fig. 1 and refs. 2 and 32), to which mature DCs home at increased numbers after activation in the periphery. In addition, mature DCs seem to be the most efficient stimulators of cytokine secretion and proliferation of NK cells. This finding suggests that NK cell activation might be favored in secondary lymphoid tissues by virtue of the enrichment and colocalization of mature DCs and CD56brightCD16– NK cells, cellular subsets ideally suited for productive DC/NK cell interaction. We therefore suggest that secondary lymphoid tissues are the main site for DC-mediated NK cell activation.
The interaction between DCs and NK cells in T cell areas of secondary lymphoid organs might facilitate the initiation of T cell immunity and polarize emerging immune responses toward a T helper cell 1 (Th1) phenotype. Immunoregulatory CD56brightCD16– NK cells could directly polarize T cells during priming or edit DCs to become more potent inducers of Th1 immunity. There is evidence for both mechanisms. IFN-γ has been reported to facilitate Th1 polarization directly in mouse and human (33, 34), and IFN-γ secreted by NK cells upon DC-mediated stimulation would be optimally localized to the T cell area to support Th1-type T cell priming. In addition, NK cells support human DC1 development (35). DC1 cells are DCs that optimally drive Th1 responses by virtue of their elevated IL-12 secretion. However, DC1 induction by NK cells required specific in vitro conditions and the presence of type I IFNs as well as IL-18. Therefore, it remains to be established whether NK cells can influence DCs for better Th1 induction in general or just under these specific stimulation conditions. Interestingly, in a murine model of skin-graft rejection, DC/NK cell interaction led to a modulation of Th1/Th2 polarization (36). NK cell depletion in the graft recipient polarized developing antigraft alloresponses to Th2 but failed to prolong graft survival. Therefore, NK cells might play an important role during DC-mediated T cell priming and polarization in T cell areas of secondary lymphoid organs.
Both secreted and cell-contact-dependent components of NK activation by DCs have been postulated (7, 15, 37). We propose that IL-12 and surface IL-15 respectively mediate secretion and cell-contact-dependent components of human NK activation by DCs. IL-15Rα is able to present IL-15 on the surface of cells (31), and we demonstrate that IL-15Rα up-regulation correlates with IL-15 surface presentation on mature DCs. Previously, it has been shown that both IL-15 and IL-15Rα are required for NK cell survival (38, 39). However, IL-15Rα did not need to be present on NK cells, and IL-15Rα expression on bone marrow-derived cells could support NK cell survival in the periphery (30). We suggest, therefore, that the complex of IL-15 and IL-15Rα on the surface of mature DCs stimulates NK cell proliferation. Two mechanisms for NK cell activation by the IL-15/IL-15Rα complex on the surface of antigen-presenting cells have been suggested. Autocrine signaling to the antigen-presenting cell could lead to the up-regulation of NK stimulatory molecules (37), and paracrine signaling could engage the common IL-2R/IL-15Rβ and γc chains on NK cells for direct activation (31). Only DC maturation in the presence of type I IFNs up-regulates the MHC class I chain-related gene A/B (MICA/B) molecules, which stimulate NK cells through NKG2D (15), and autocrine IL-15 mediates this effect (37). In contrast, the majority of DC maturation stimuli, like LPS, polyI:C, CD40L, and TNF-α, including the maturation stimuli used in this study, do not up-regulate MICA/B and do not use IL-15 in this autocrine fashion (15, 37). Therefore, the mature DCs in our study activate NK cells probably in trans by the paracrine mechanism of the IL-15/IL-15Rα complex.
This study suggests that DCs activate CD56brightCD16– NK cells upon homing to secondary lymphoid organs and that the capacities of DCs to secrete IL-12 and present IL-15 are crucial. To harness DCs for NK cell activation during immunotherapy of tumors and persistent viral infections, DC preparation should be optimized for IL-12 and IL-15 production as well as efficient homing to secondary lymphoid organs.
Acknowledgments
We thank Ralph M. Steinman and James W. Young for critically reading the manuscript. This work was supported by the Leukemia and Lymphoma Society, the New York Academy of Medicine, National Cancer Institute Grant R01CA108609 (to C.M.), a fellowship from the American Society of Transplantation, the Juvenile Diabetes Foundation (D.T.), and grants from the Associazione Italiana per la Ricerca sul Cancro and the Italian Ministero della Salute (to G.F.).
Author contributions: G.F. and C.M. designed research; G.F., M.P., C.P., D.S., T.S., G.B., and C.M. performed research; D.T., W.A.M., and L.M. contributed new reagents/analytic tools; G.F., M.P., C.P., D.S., T.S., G.B., and C.M. analyzed data; and C.M. wrote the paper.
Abbreviations: CD40L, CD40 ligand; DC, dendritic cell; NK, natural killer; GM-CSF, granulocyte–macrophage colony-stimulating factor; TNF, tumor necrosis factor; LPS, lipopolysaccharide; PE, phycoerythrin; CFSE, 5,6-carboxyfluorescein diacetate succinimidyl ester; EBNA1, Epstein–Barr virus-encoded nuclear antigen 1; Th, T helper cell.
References
- 1.Trinchieri, G. (1989) Adv. Immunol. 47, 187–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehniger, T. A., Cooper, M. A., Nuovo, G. J., Cella, M., Facchetti, F., Colonna, M. & Caligiuri, M. A. (2003) Blood 101, 3052–3057. [DOI] [PubMed] [Google Scholar]
- 3.Ferlazzo, G., Thomas, D., Lin, S. L., Goodman, K., Morandi, B., Muller, W. A., Moretta, A. & Münz, C. (2004) J. Immunol. 172, 1455–1462. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs, R., Hintzen, G., Kemper, A., Beul, K., Kempf, S., Behrens, G., Sykora, K. W. & Schmidt, R. E. (2001) Eur. J. Immunol. 31, 3121–3127. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, M. A., Fehniger, T. A., Turner, S. C., Chen, K. S., Ghaheri, B. A., Ghayur, T., Carson, W. E. & Caligiuri, M. A. (2001) Blood 97, 3146–3151. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, J. J., Qin, S., Unutmaz, D., Soler, D., Murphy, K. E., Hodge, M. R., Wu, L. & Butcher, E. C. (2001) J. Immunol. 166, 6477–6482. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez, N. C., Lozier, A., Flament, C., Ricciardi-Castagnoli, P., Bellet, D., Suter, M., Perricaudet, M., Tursz, T., Maraskovsky, E. & Zitvogel, L. (1999) Nat. Med. 5, 405–411. [DOI] [PubMed] [Google Scholar]
- 8.Ferlazzo, G., Tsang, M. L., Moretta, L., Melioli, G., Steinman, R. M. & Münz, C. (2002) J. Exp. Med. 195, 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerosa, F., Baldani-Guerra, B., Nisii, C., Marchesini, V., Carra, G. & Trinchieri, G. (2002) J. Exp. Med. 195, 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N. M. (2002) J. Exp. Med. 195, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin-Fontecha, A., Sebastiani, S., Hopken, U. E., Uguccioni, M., Lipp, M., Lanzavecchia, A. & Sallusto, F. (2003) J. Exp. Med. 198, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biron, C. A., Byron, K. S. & Sullivan, J. L. (1989) N. Engl. J. Med. 320, 1731–1735. [DOI] [PubMed] [Google Scholar]
- 13.Andrews, D. M., Scalzo, A. A., Yokoyama, W. M., Smyth, M. J. & Degli-Esposti, M. A. (2003) Nat. Immunol. 4, 175–181. [DOI] [PubMed] [Google Scholar]
- 14.Brown, M. G., Dokun, A. O., Heusel, J. W., Smith, H. R., Beckman, D. L., Blattenberger, E. A., Dubbelde, C. E., Stone, L. R., Scalzo, A. A. & Yokoyama, W. M. (2001) Science 292, 934–937. [DOI] [PubMed] [Google Scholar]
- 15.Jinushi, M., Takehara, T., Kanto, T., Tatsumi, T., Groh, V., Spies, T., Miyagi, T., Suzuki, T., Sasaki, Y. & Hayashi, N. (2003) J. Immunol. 170, 1249–1256. [DOI] [PubMed] [Google Scholar]
- 16.Yu, Y., Hagihara, M., Ando, K., Gansuvd, B., Matsuzawa, H., Tsuchiya, T., Ueda, Y., Inoue, H., Hotta, T. & Kato, S. (2001) J. Immunol. 166, 1590–1600. [DOI] [PubMed] [Google Scholar]
- 17.Vitale, M., Della Chiesa, M., Carlomagno, S., Romagnani, C., Thiel, A., Moretta, L. & Moretta, A. (2004) Eur. J. Immunol. 34, 1715–1722. [DOI] [PubMed] [Google Scholar]
- 18.Borg, C., Abdelali, J., Laderach, D., Maruyama, K., Wakasugi, H., Charrier, S., Ryffel, B., Vainchenker, W., Galy, A., Caignard, A., et al. (July 8, 2004) Blood, 10.1182/blood-2004-01-0380. [DOI] [PubMed]
- 19.Granucci, F., Zanoni, I., Pavelka, N., Van Dommelen, S. L., Andoniou, C. E., Belardelli, F., Degli Esposti, M. A. & Ricciardi-Castagnoli, P. (2004) J. Exp. Med. 200, 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koka, R., Burkett, P., Chien, M., Chai, S., Boone, D. L. & Ma, A. (2004) J. Immunol. 173, 3594–3598. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen, K. B., Salazar-Mather, T. P., Dalod, M. Y., Van Deusen, J. B., Wei, X. Q., Liew, F. Y., Caligiuri, M. A., Durbin, J. E. & Biron, C. A. (2002) J. Immunol. 169, 4279–4287. [DOI] [PubMed] [Google Scholar]
- 22.Ferlazzo, G., Morandi, B., D'Agostino, A., Meazza, R., Melioli, G., Moretta, A. & Moretta, L. (2003) Eur. J. Immunol. 33, 306–313. [DOI] [PubMed] [Google Scholar]
- 23.de Saint-Vis, B., Vincent, J., Vandenabeele, S., Vanbervliet, B., Pin, J. J., Ait-Yahia, S., Patel, S., Mattei, M. G., Banchereau, J., Zurawski, S., et al. (1998) Immunity 9, 325–336. [DOI] [PubMed] [Google Scholar]
- 24.McIlroy, D., Troadec, C., Grassi, F., Samri, A., Barrou, B., Autran, B., Debre, P., Feuillard, J. & Hosmalin, A. (2001) Blood 97, 3470–3477. [DOI] [PubMed] [Google Scholar]
- 25.Münz, C., Dao, T., Ferlazzo, G., de Cos, M. A., Goodman, K. & Young, J. W. (August 26, 2004) Blood, 10.1182/blood-2004-06-2482.
- 26.Granucci, F., Vizzardelli, C., Pavelka, N., Feau, S., Persico, M., Virzi, E., Rescigno, M., Moro, G. & Ricciardi-Castagnoli, P. (2001) Nat. Immunol. 2, 882–888. [DOI] [PubMed] [Google Scholar]
- 27.Jonuleit, H., Wiedemann, K., Muller, G., Degwert, J., Hoppe, U., Knop, J. & Enk, A. H. (1997) J. Immunol. 158, 2610–2615. [PubMed] [Google Scholar]
- 28.Mattei, F., Schiavoni, G., Belardelli, F. & Tough, D. F. (2001) J. Immunol. 167, 1179–1187. [DOI] [PubMed] [Google Scholar]
- 29.Paludan, C., Bickham, K., Nikiforow, S., Tsang, M. L., Goodman, K., Hanekom, W. A., Fonteneau, J. F., Stevanovic, S. & Münz, C. (2002) J. Immunol. 169, 1593–1603. [DOI] [PubMed] [Google Scholar]
- 30.Koka, R., Burkett, P. R., Chien, M., Chai, S., Chan, F., Lodolce, J. P., Boone, D. L. & Ma, A. (2003) J. Exp. Med. 197, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. (2002) Immunity 17, 537–547. [DOI] [PubMed] [Google Scholar]
- 32.Ferlazzo, G. & Münz, C. (2004) J. Immunol. 172, 1333–1339. [DOI] [PubMed] [Google Scholar]
- 33.Parronchi, P., De Carli, M., Manetti, R., Simonelli, C., Sampognaro, S., Piccinni, M. P., Macchia, D., Maggi, E., Del Prete, G. & Romagnani, S. (1992) J. Immunol. 149, 2977–2983. [PubMed] [Google Scholar]
- 34.Bradley, L. M., Dalton, D. K. & Croft, M. (1996) J. Immunol. 157, 1350–1358. [PubMed] [Google Scholar]
- 35.Mailliard, R. B., Son, Y. I., Redlinger, R., Coates, P. T., Giermasz, A., Morel, P. A., Storkus, W. J. & Kalinski, P. (2003) J. Immunol. 171, 2366–23673. [DOI] [PubMed] [Google Scholar]
- 36.Coudert, J. D., Coureau, C. & Guery, J. C. (2002) J. Immunol. 169, 2979–2987. [DOI] [PubMed] [Google Scholar]
- 37.Jinushi, M., Takehara, T., Tatsumi, T., Kanto, T., Groh, V., Spies, T., Suzuki, T., Miyagi, T. & Hayashi, N. (2003) J. Immunol. 171, 5423–5429. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy, M. K., Glaccum, M., Brown, S. N., Butz, E. A., Viney, J. L., Embers, M., Matsuki, N., Charrier, K., Sedger, L., Willis, C. R., et al. (2000) J. Exp. Med. 191, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lodolce, J. P., Boone, D. L., Chai, S., Swain, R. E., Dassopoulos, T., Trettin, S. & Ma, A. (1998) Immunity 9, 669–676. [DOI] [PubMed] [Google Scholar]