Abstract
Cardiac resynchronization therapy (CRT) threw lights on heart failure treatment, however, parts of patients showed nonresponse to CRT. Unfortunately, it lacks effective parameters to predict CRT non-response. In present study, we try to seek effective electro-echocardiographic predictors on CRT non-response. This is a retrospective study to review a total of 227 patients of dyssynchronous heart failure underwent CRT implantation. Logistic analysis was performed between CRT responders and CRT non-responders. The primary outcome was the occurrence of improved left ventricular ejection fraction 1 year after CRT implantation. We concluded that LVEDV > 255 mL (OR = 2.236; 95% CI, 1.016–4.923) rather than LVESV > 160 mL (OR = 1.18; 95% CI, 0.544–2.56) and TpTe/QTc > 0.203 (OR = 5.206; 95% CI, 1.89–14.34) significantly predicted CRT non-response. Oppositely, S wave > 5.7 cm/s (OR = 0.242; 95% CI, 0.089–0.657), E/A > 1 (OR = 0.211; 95% CI, 0.079–0.566), E’/A’ > 1 (OR = 0.054; 95% CI, 0.017–0.172), CLBBB (OR = 0.141; 95% CI, 0.048–0.409), and QRS duration >160 ms (OR = 0.52; 95% CI, 0.305–0.922) surprisingly predicted low-probability of CRT non-response.
Heart failure (HF), as a common endpoint of different heart disease, brings great burden to our society1. To date, there is a lack of effective treatment to alleviate this disease burden. However, recent 20 years have witnessed a great progress in the area of dyssynchronous heart failure (DHF) treated with cardiac resynchronization therapy (CRT)2. A phenomenon that not all failing hearts assume synchronized movements makes CRT a promising way for patients with DHF. And CRT has been proved to be effective in treating DHF3. But, some patients whose clinical characteristics met the guidelines of CRT implantation didn’t respond to CRT4. Moreover, a bit of patients suffered from ventricular arrhythmia, abnormal hemodynamics, even aggravation of cardiac function after CRT implantation. Many factors are associated with CRT non-response, including aetiology of heart disease, sex, age, and cardiac function at baseline, etc5. Unfortunately, it still lacks of credible and convenient parameters. Electrocardiography parameters, especially QRS duration is very important to patient selection for CRT implantation6. Besides, other parameters reflecting the abnormal ventricular repolarization potentially predict CRT non-response7, e.g. QT interval, TpTe, and J wave, etc. Moreover, echocardiography dynamically presenting cardiac mechanical function makes itself conductive to predict CRT non-response8. To date, however, it still lacked relatively large sample size study to integratively summarize and analyze these factors mentioned above, especially for Chinese. In this study, electrocardiography, echocardiography, and blood bio-marker indices were combined to comprehensively find out CRT non-response predictors in a relatively large Chinese cohort.
Methods
Study population
This was a retrospective clinical study to review the patients of DHF underwent CRT implantation (n = 362) in our hospital from February 2013 to February 2016. Total 227 patients were finally included in this study, with informed consent being obtained from all subjects, while other 135 patients were excluded based on following exclusion criteria. The inclusion criteria were: (1) patients ranging from 18 to 85 years old; (2) diagnosed as decompensated heart failure with non-ischemic aetiology; (3) with LVEF less than 35%, with complete left bundle branch block (CLBBB), or with QRS more than 130 ms9,10; (4) having guideline based optimal medical treatment for at least 3 months (medical therapy was conducted by related guideline11, and each kind of medication was prescribed with its maximal dose that the patient could tolerate); (5) with New York Heart Association (NYHA) class II or higher levels (NYHA IV is acceptable after careful assessment of patient’s condition). The exclusion criteria were as follows: (1) incomplete clinical history record (n = 20); (2) upgraded to dual-chamber pacing with a prior right ventricular pacemaker (n = 7); (3) without follow-up clinical data especially echocardiogram because of any reason (n = 25); (4) confirmed cardiac ion channel diseases (n = 3); (5) active chronic inflammation (n = 6); (6) continuous renal replacement therapy because of severe renal dysfunction with stage 5 of Chronic Kidney Disease (CKD-5) defined by KDOQI guideline12,13 (n = 4); (7) underlying in ischemic cardiomyopathy (n = 66); (8) or disorder in hematological and immunological system (n = 4). Design of this study and informed consent were approved by our local ethics committee (Ethics Committee of Zhongshan Hospital affiliated to Fudan University), and were carried out in accordance with the principles of the Declaration of Helsinki. Consent for publication of these data was obtained from each patient.
CRT implantation
Patient met the indication for CRT implantation was firstly receiving selective coronary angiography to verify any lesion on coronary artery and optimal medical treatment for at least 3 months, then receiving CRT implantation if there was no improvement. The position of pacing lead was recorded in every patient. Left ventricular lead was targeted in coronary vein including posterio-lateral vein, posterior vein, lateral vein, major vein, and middle vein. Right ventricular lead was placed in apex or out flow tract of right chamber. These lead positions were chosen based on program control during operation. Besides, Patients were performed CRT optimization by UCG 3 months after implantation. AV delay optimization was performed by assessing the pattern of pulsed wave Doppler recorded through the mitral valve using the iterative method14. The optimal VV delay by the UCG was defined as the delay associated with the largest average the aortic velocity time integral (VTI). Aortic VTI measurements were obtained in accordance with the American Society of Echocardiography guidelines15.
Electrocardiography
12-lead electrocardiograms (ECG) were performed in admission and within 48 hours after CRT implantation respectively. QRS duration, QT interval, corrected QT interval (QTc by Bazzet formula), T wave from peak to end interval (TpTe), ratio between TpTe and QTc (TpTe/QTc), and QRS morphology were measured through an electronic ECG analysis system by 2 independent cardiologists who didn’t know the study design and patients’ condition. When their opinions went against each other, another physician should be brought in to give an ultimate decision. For paroxysmal atrial fibrillation, the ECG without atrial fibrillation (AF) presentation were adopted. For persistent AF, parameter was calculated as mean value from measurement of 3 continuous complexes.
Echocardiography
Echocardiograms (UCG) were performed within 1 month before and 1 year after CRT implantation in all patients using a Philips IE33 instrument (Philips, Netherlands) with a 2–3.5 MHz transducer (X3-1) to detect left ventricular ejection fraction (LVEF). Left atrial diameter (LAD), left ventricular end systolic diameter (LVESD), and left ventricular end diastolic diameter (LVEDD) were measure by M-mode. Left atrial volume (LAV), left ventricular end systolic volume (LVESV), and left ventricular end diastolic volume (LVEDV) were calculated by corrected Teichholz formula: V = 7.0/(2.4 + D) × D3 (V = LV volume, D = LV diameter) which was suitable for non-ischemic aetiology16. Pulmonary artery systolic pressure (PASP) was estimated from the tricuspid regurgitant jet velocity using the modified Bernoulli equation and adding the estimated right atrial pressure (RAP) on the basis of inferior vena cava (IVC) diameter and collapsibility according to the ASE guidelines for echocardiographic assessment of the right heart in adults: IVC diameter ≤2.1 cm that collapses >50% with a sniff suggests a normal RAP of 3 mm Hg (range, 0–5 mm Hg), whereas an IVC diameter >2.1 cm that collapses <50% with a sniff suggests a high RAP of 15 mm Hg (range, 10–20 mm Hg). In indeterminate cases in which the IVC diameter and collapse do not fit this paradigm, an intermediate value of 8 mm Hg (range, 5–10 mm Hg) was used17. Besides, E/A and E’/A were introduced as parameters reflecting cardiac diastolic function. E/A was the ratio of early and late peak values of mitral transvalvular blood flow speed through Doppler imaging. E’/A’ referred to the ratio of early and late peak values of mitral annulus tissue speed through Doppler imaging. S wave indicating the maximal velocity of mitral annulus motion revealed cardiac systolic function18,19. CRT response was defined as relative increase (≥15%) or absolute increase (≥10%) of LVEF after 1 year20,21. And if it didn’t meet the CRT response criteria, CRT non-response was defined. For paroxysmal AF, the UCG without atrial fibrillation presentation were adopted. For persistent AF, measurement was not different except for unavailability of E/A.
Serology
Routine blood test items of HF patients in admission were collected from our medical record system, including serum creatinine (Scr), N-terminal Prohormone of Brain Natriuretic Peptide (NT-proBNP), troponine-T (cTnT), creatine kinase-MB (CK-MB) and C-reactive protein (CRP), etc. Furthermore, estimated glomerular filtration rate (eGFR) was calculated by MDRD formula and CKD-EPI formula based on the value of Scr and adjusted by patient’s age and sex22,23. MDRD formula was described as follows: eGFR for male = 175 × (Scr × 0.0113)−1.154 × age−0.203 and eGFR for female = 175 × (Scr × 0.0113)−1.154 × age−0.203 × 0.742, respectively. Well, CKD-EPI formula was more complicated because it was dependent on not only sex but also the range of Scr level, and the specific equations were depicted as below: for male with Scr ≤ 80: eGFR = 141 × (Scr × 0.0113/0.9)−0.411 × 0.993age, for male with Scr > 80: eGRF = 141 × (Scr × 0.0113/0.9)−1.209 × 0.993age, for female with Scr ≤ 62: eGFR = 144 × (Scr × 0.0113/0.7)−0.329 × 0.993age, and for female with Scr > 62: eGFR = 144 × (Scr × 0.0113/0.7)−1.209 × 0.993age. As these formulae mentioned above, the unit of eGFR was ml/min/1.73 m2, the unit of Scr was μmol/L, and the unit of age was year.
Statistics
All statistical analyses were performed with SPSS software 19.0. Data were presented as the percentage, mean ± standard deviation (SD), or median values with their 25–75th percentiles. Chi-square analysis was used to compare the frequency for categorical variables, Mann-Whitney test was used for ordinal categorical variables, and Student’s t tests were used to compare means for continuous variables. Multivariable logistic analysis was performed to identify the independent predictors for CRT non-response. All statistical analyses and graphs were performed using SPSS 19.0 software or Stata 12.0 software. All P-values were two-sided, and P < 0.05 was considered to indicate statistical significance.
Ethics approval
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University.
Results
Demographics and Baseline Characteristics
All 227 DHF patients enrolled with average 60.4 ± 12.3 years, including 163 men (71.8%). The prevalence of hypertension, diabetes, and atrial fibrillation (AF) were 40.1%, 18.1%, and 16.3% (30 paroxysmal AF and 7 persistent AF) respectively. Cardiac function (LVEF), renal function (Scr and eGFR), comorbidity (hypertension, diabetes, and AF), and medication prescriptions had no difference at baseline between CRT responders and CRT non-responders. Besides, markers of cardiac injury (cTnT, CK-MB, and NT-proBNP) showed equal levels in each group before CRT implantation. Baseline clinical characteristics of patients were shown in Table 1.
Table 1. Comparison of clinical characteristics between CRT response and CRT non-response HF patients in baseline.
| Factors | Total | Response (n = 138) | non-Response (n = 89) | p value |
|---|---|---|---|---|
| (1) Demography | ||||
| Age (years) | 60.4 ± 12.3 | 61.2 ± 12.6 | 59.3 ± 11.5 | 0.266 |
| Male n, (%) | 163 (71.8) | 94 (68.1) | 69 (77.5) | 0.124 |
| NYHA | 2.9 ± 0.5 | 2.9 ± 0.6 | 2.9 ± 0.5 | 0.334 |
| II n, (%) | 47 (20.7) | 32 (23.2) | 15 (16.9) | |
| III n, (%) | 158 (69.6) | 93 (67.4) | 65 (73) | |
| IV n, (%) | 22 (9.7) | 13 (9.4) | 9 (10.1) | |
| DM n, (%) | 41 (18.1) | 28 (20.3) | 13 (14.6) | 0.277 |
| HTN n, (%) | 91 (40.1) | 58 (42) | 33 (37.1) | 0.457 |
| VT/VF history n, (%) | 26 (11.5) | 12 (8.7) | 14 (15.7) | 0.104 |
| HF history (years) | 4.8 ± 5.2 | 4.8 ± 5.3 | 4.6 ± 5 | 0.827 |
| AF n, (%) | 37 (16.3) | 23 (16.7) | 14 (15.7) | 0.852 |
| Hemoglobin (g/L) | 132.1 ± 19.5 | 131.9 ± 17.6 | 132.4 ± 22.1 | 0.863 |
| Albumin (g/L) | 38.9 ± 3.6 | 38.8 ± 3.6 | 39 ± 3.7 | 0.386 |
| (2) Echocardiography | ||||
| LVEF (%) | 30 ± 7 | 30.3 ± 6.2 | 32.5 ± 8.7 | 0.084 |
| PASP (mmHg) | 44.4 ± 13.9 | 43.5 ± 13 | 45.8 ± 15.1 | 0.23 |
| LAD (mm) | 48.9 ± 7.1 | 47.9 ± 7 | 50.6 ± 7 | 0.005 |
| LVESD (mm) | 58.6 ± 9.4 | 57.3 ± 9.6 | 60.6 ± 8.7 | 0.01 |
| LVEDD (mm) | 70.3 ± 8.4 | 68.9 ± 8.4 | 72.6 ± 7.9 | 0.001 |
| LAV (mL) | 116 ± 42 | 110.3 ± 39.9 | 124.8 ± 44 | 0.011 |
| LVESV (mL) | 176.5 ± 64 | 168.4 ± 64.5 | 189.2 ± 61.5 | 0.015 |
| LVEDV (mL) | 263.1 ± 70.9 | 251.3 ± 69.6 | 281.3 ± 69.2 | 0.002 |
| S wave (cm/s) | 5.6 ± 1.4 | 5.8 ± 1.3 | 5.3 ± 1.3 | 0.003 |
| E/A > 1 n, (%) | 122 (53.7) | 107 (77.5) | 15 (16.9) | <0.001 |
| E’/A’ > 1 n, (%) | 120 (52.9) | 108 (78.3) | 12 (13.5) | <0.001 |
| (3) Electrocardiography | ||||
| CLBBB n, (%) | 136 (59.9) | 100 (72.5) | 36 (40.4) | <0.001 |
| QRS duration (ms) | 160.8 ± 35.8 | 163.7 ± 25.9 | 156.3 ± 47.1 | 0.178 |
| QTc (ms) | 481.9 ± 52.4 | 491 ± 41.1 | 472.8 ± 40 | 0.001 |
| TpTe (ms) | 97.7 ± 19.5 | 96.4 ± 17.5 | 99.7 ± 22.1 | 0.211 |
| TpTe/QTc | 0.202 ± 0.037 | 0.196 ± 0.034 | 0.211 ± 0.039 | 0.005 |
| (4) Serology | ||||
| Serum potassium (mmol/L) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.1 ± 0.5 | 0.943 |
| CRP (mg/L) | 12.3 ± 19 | 13.4 ± 23.3 | 10.7 ± 9 | 0.31 |
| Serum creatinine (μmol/L) | 94.4 ± 33.4 | 92.5 ± 31.5 | 97.3 ± 36.2 | 0.291 |
| eGFR (ml/min/1.73 m2, calculated by 2 different equations) | ||||
| by MDRD formula | 72.4 ± 23.1 | 73.4 ± 23.6 | 71 ± 22.4 | 0.439 |
| by CKD-EPI formula | 74.1 ± 21.2 | 74.6 ± 21.3 | 73.4 ± 21.2 | 0.663 |
| NT-proBNP (pg/mL) | 4505.5 ± 4738.9 | 4196.5 ± 4343.7 | 4984.6 ± 5283.7 | 0.222 |
| Troponin T (ng/mL) | 0.059 ± 0.167 | 0.056 ± 0.174 | 0.064 ± 0.157 | 0.723 |
| CK-MB (U/L) | 12.4 ± 4.7 | 12.7 ± 5.4 | 12.1 ± 3.2 | 0.299 |
| (5) Medication | ||||
| ACEI/ARB n, (%) | 189 (83.3) | 115 (83.3) | 74 (83.1) | 0.971 |
| β- blocker (%) | 183 (80.6) | 108 (78.3) | 75 (84.3) | 0.264 |
| Spironolactone n, (%) | 188 (82.8) | 116 (84.1) | 72 (80.9) | 0.538 |
| Digoxin n, (%) | 104 (45.8) | 65 (47.1) | 39 (43.8) | 0.628 |
| Amiodorane n, (%) | 39 (17.2) | 21 (15.2) | 18 (20.2) | 0.329 |
| Loop-diuretics n, (%) | 179 (78.9) | 108 (78.3) | 71 (79.8) | 0.785 |
Indicated clinical characteristics between CRT response and CRT non-response HF patients before CRT implantation. From this table, age, sex, concomitant disease, HF history, baseline EF, electrolyte and other bio-marker, and medication were not different between two groups. However, indice of UCG and ECG were significantly different.
Follow-up of the echocardiograph after 1 year
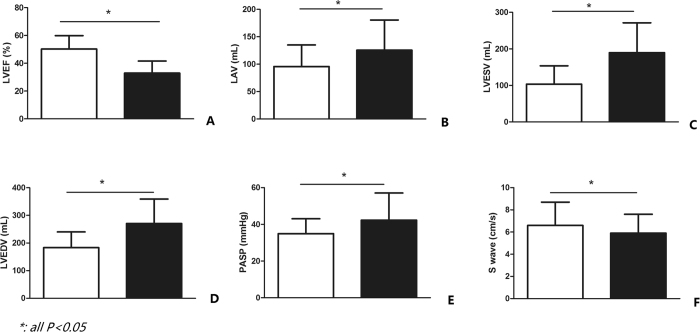
UCG data after 1 year of CRT implantation were collected properly, and patients according with inclusion criteria were then separated into two groups, CRT responders and CRT non-responders respectively, based on the above mentioned definition of CRT response. LVEF was significantly increased in CRT responders (50.2 ± 9.7% vs 32.9 ± 8.7%, p < 0.001, Fig. 1A). Compared to CRT responders whose mean value of LAD was 44.7 ± 7 mm, the mean value of CRT non-responders was 50.2 ± 8.9 mm (p < 0.001). Additionally, the mean value of LVESD was higher in CRT non-responders than CRT responders (44.7 ± 9.8 mm vs 61.3 ± 19.9 mm, p < 0.001). Moreover, in comparison with CRT responders, the value of LVEDD was higher in CRT non-responders (59.2 ± 8.2 mm vs 70.9 ± 9.9 mm, p < 0.001). The value of LAV, LVESV, and LVEDV were higher in CRT non-responders (95.8 ± 39.3 vs 125.5 ± 55 mL, 103.3 ± 50.3 vs 189.6 ± 81.7 mL, and 183.2 ± 57.1 vs 270.4 ± 88.7 mL respectively, p < 0.001, Fig. 1B,C,D). Besides, the mean value of PASP in CRT responders was significantly lower than CRT non-responders’ (34.9 ± 8.1 mmHg vs 42.3 ± 14.8 mmHg, p < 0.001, Fig. 1E). In addition for S wave, the summit velocity of mitral annulus’ motion in CRT non-responders was distinctly slower than the one in CRT responders (6.6 ± 2.1 cm/s vs 5.9 ± 1.7 cm/s, p = 0.012, Fig. 1F). Scr level was lower in CRT responders (75.2 ± 23.4 μmol/L vs 92.5 ± 30.1 μmol/L, Fig. 1).
Figure 1. Indicated the difference of related UCG parameters between CRT responders and CRT non-responders.
All bars in white color referred to CRT responders, while bars in black color represented for CRT non-responders. Besides, all of the p values here were less than 0.001 (CRT responders vs CRT non-responders).
Analysis of CRT non-response predictors
Univariate analysis
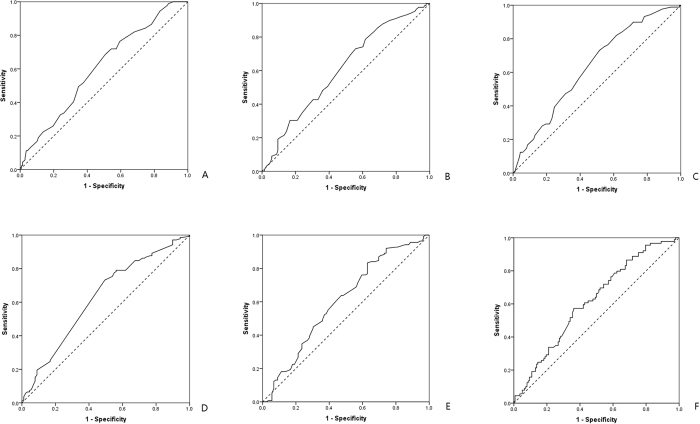
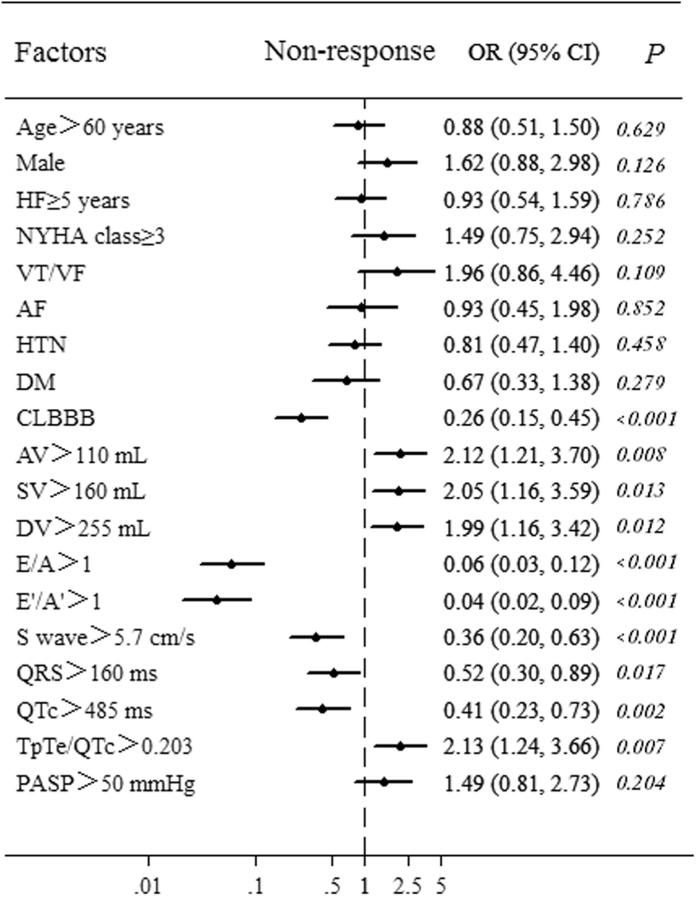
Univariate analysis was performed to preliminarily filter risk factors of CRT non-response. The optimal cut-off points of risk predictors were selected by receiver operating characteristic (ROC) curve based on the maximal Youden index (sensitivity + specificity − 1) or median. ROC analysis was applied to electro-echocardiographic indices (Fig. 2). Area under the curve (AUC) and the optimal cut-off point’s sensitivity and specificity were shown in Table 2. LAV > 110 mL [Odds Ratio (OR) = 2.116, p = 0.008], LVESV > 160 mL (OR = 2.045, p = 0.013), and LVEDV > 255 mL (OR = 1.994, p = 0.012) showed great potential to predict CRT non-response. Besides, S wave > 5.7 cm/s (OR = 0.358, p < 0.001), E/A > 1 (OR = 0.059, p < 0.001), E’/A’ > 1 (OR = 0.043, p < 0.001), QRS > 160 ms (OR = 0.517, p = 0.017), QTc > 485 ms (OR = 0.413, p = 0.002), and TpTe/QTc > 0.203 (OR = 0.216, p = 0.007), as well as CLBBB (OR = 0.258, p < 0.001) a widely known CRT response predictor, indicated low-probability of CRT non-response. However, HF history, NYHA class, comorbidity of AF, hypertension, and diabetes, and PASP failed to predict CRT non-response (Fig. 3).
Figure 2. Showed ROC curve to predict the optimal cut off of CRT non-response predictors, and all p values were less than 0.01.
The optimal cut-off point was in the upper-left area and was calculated based on the maximal Youden index (sensitivity + specificity − 1). (A) Was ROC curve of LAV; (B) was ROC curve of LVESV; (C) was ROC curve of LVEDV; (D) was ROC curve of S wave; (E) was ROC curve of QRS duration; (F) was ROC curve of TpTe/QTc.
Table 2. Optimal cut-off points and related diagnostic value by ROC analysis.
| Factors | Cut-off points | Sensitivity | Specificity | AUC | P value |
|---|---|---|---|---|---|
| LAV | 110 mL | 0.685 | 0.493 | 0.606 | 0.007 |
| LVESV | 160 mL | 0.73 | 0.442 | 0.606 | 0.007 |
| LVEDV | 255 mL | 0.73 | 0.486 | 0.632 | 0.001 |
| S wave | 5.7 cm/s | 0.732 | 0.506 | 0.626 | 0.001 |
| QRSduration | 160 ms | 0.833 | 0.371 | 0.603 | 0.009 |
| QTcinterval | 485 ms | 0.522 | 0.697 | 0.631 | 0.001 |
| TpTe/QTc | 0.203 | 0.573 | 0.638 | 0.616 | 0.003 |
Showed optimal cut-off points and related diagnostic value by ROC analysis with related sensitivity, specificity, AUC, and p value (LAV = left atrial volume; LVESV = left ventricular end systolic volume; LVEDV = left ventricular end diastolic volume; AUC = area under the curve). Larger AUC indicated better diagnostic value.
Figure 3. Showed univariate logistic regression analysis of electro-echocardiographic indices and other clinical factors for predicting CRT non-response.
Odds ratio (OR) and its 95% credential interval (CI) of each factor was presented as point and line respectively. Risk of CTR non-response increased when OR > 1, while it decreased when OR < 1. HF = heart failure; NYHA = New York Heart Association; AF = atrial fibrillation; HTN = hypertension; DM = diabetes mellitus; AV = left atrial volume; SV = left ventricular end systolic volume; DV = left ventricular end diastolic volume; CLBBB = complete left bundle branch block.
Multivariate analysis
Multivariate logistic analysis was performed to demonstrate the independent effect of these predictors (confirmed statistic difference in univariate analysis) on the occurrence of CRT non-response. Moreover, since previous studies reported age, sex, and NYHA class5 were related to CRT response, these factors were included in multivariate analysis to correct the latent bias. In this analysis, CRT non-response was employed as a dependent variable, while CLBBB, LAV > 110 mL, LVESV > 160 mL, LVEDV > 255 mL, S wave > 5.7 cm/s, E/A > 1, E’/A’ > 1, QRS > 160 ms, QTc > 485 ms, TpTe/QTc > 0.203, age > 60 years, male, and NYHA class > III were set as independent variables. LVEDV > 255 mL (OR = 2.236; 95% CI, 1.016–4.923) rather than LVESV > 160 mL (OR = 1.18; 95% CI, 0.544–2.56) and TpTe/QTc > 0.203 (OR = 5.206; 95% CI, 1.89–14.34) significantly predicted CRT non-response. Oppositely, S wave > 5.7 cm/s (OR = 0.242; 95% CI, 0.089–0.657), E/A > 1 (OR = 0.211; 95% CI, 0.079–0.566), E’/A’ > 1 (OR = 0.054; 95% CI, 0.017–0.172), CLBBB (OR = 0.141; 95% CI, 0.048–0.409), and QRS duration >160 ms (OR = 0.52; 95% CI, 0.305–0.922) surprisingly predicted low-probability of CRT non-response (Table 3).
Table 3. Odds ratios of independent predictors for CRT non-response in HF patients (multivariate logistic analysis).
| Predictors | Odds Ratio | 95% confidence intervals | p value |
|---|---|---|---|
| Age >60 years | 0.917 | 0.527–1.596 | 0.759 |
| Male | 1.643 | 0.885–3.05 | 0.116 |
| HF ≥ 5 years | 0.872 | 0.506–1.506 | 0.624 |
| NYHA class ≥ 3 | 1.563 | 0.782–3.126 | 0.206 |
| AF | 0.892 | 0.427–1.864 | 0.761 |
| HTN | 0.794 | 0.451–1.506 | 0.425 |
| DM | 0.543 | 0.167–1.769 | 0.311 |
| LAV > 110 mL | 1.001 | 0.987–1.015 | 0.889 |
| LVESV > 160 mL | 1.18 | 0.544–2.56 | 0.676 |
| LVEDV > 255 mL | 2.236 | 1.016–4.923 | 0.046 |
| E/A > 1 | 0.211 | 0.079–0.566 | 0.002 |
| E’/A’ > 1 | 0.054 | 0.017–0.172 | <0.001 |
| S > 5.7 cm/s | 0.242 | 0.089–0.657 | 0.005 |
| CLBBB | 0.141 | 0.048–0.409 | <0.001 |
| QRS > 160 ms | 0.53 | 0.305–0.922 | 0.025 |
| QTc > 485 ms | 0.539 | 0.189–1.535 | 0.247 |
| TpTe/QTc > 0.203 | 5.206 | 1.89–14.34 | 0.001 |
Presented multivariate logistic regression analysis of risk factors with odds ratio and its 95% confidence interval. LVEDV but not LVESV, TpTe/QTc, CLBBB, QRS, E/A, E’/A’ and S wave were valuable to predict CRT non-response.
Discussion
Existed researches showed ischemic aetiology, female, and non-CLBBB, etc. are related to CRT non-response5,24, while it still lacks of credible and efficient indices to predict CRT non-response since there exist amount of non-responders to CRT (almost 30%) with unclear reasons2. The present study indicated higher values of TpTe/QTc and LVEDV but not LAV or LVESV were promising to independently predict CRT non-response. While, higher values of S wave and QRS duration, E/A > 1, E’/A’ > 1 at baseline predicted lower possibility of CRT non-response except for the traditional predictor CLBBB. Oppositely, QTc failed to independently predict CRT non-response though it showed significant difference in single-variate analysis. We thought QTc interval should be dependent on QRS duration. Results in this study are partly consistent with prior studies25,26, for example LVEDD, LVEDV and non-CLBBB, however, TpTe/QTc, E/A, E’/A’, and S wave as risk factors predicting CRT non-response were seldom reported before. PASP can reflect the function of right ventricle25,27 and the pumping function is normally maintained by synchronous movement between left and right ventricular walls. Besides, increased PASP brought problems to pulmonary leading to hypoxemia which aggravating oxygen deficit of cardiomyocyte. Thus, abnormal PASP related to devastating cardiac function at baseline predicted CRT non-response However, in present study, PASP estimated by empirical equation failed to be filtered through single-variate analysis. That may be related to the limited sample size and the way of ultrasonic Doppler to measure PASP, because Doppler could underestimate PASP if there was no tricuspid regurgitation being detected. In addition, parameters reflecting left ventricular diastolic function such as LVEDV, E/A, and E’/A’ showed close relationship with CRT non-response18,19. As is known to us, heart failure with impaired ejection fraction is indicated for CRT. However, it is thought-provoking that LVEDV rather than LVESV predicted CRT non-response in this study. To some extent, LVEDV reflected diastolic function which was crucial for cardiac filling volume, while LVESV determined pumping volume. Studies showed that instead of systolic function, clinical prognosis post-CRT implantation was predicted by diastolic function28,29,30. Badly impaired diastolic function was hardly reversed by CRT, so that LVEDV played important roles to predict CRT non-response. On the one hand, LVESV were important to evaluate the effect of CRT; while on the other hand, LVEDD and LVEDV were valuable for predicting CRT non-response30. What’s more, E/A indicating the condition of blood flow over mitral valve was valuable for CRT non-response prediction, but E’/A’ referring to the motion of mitral annulus was insignificant. As mentioned above, CRT was especially effective in dyssynchronous HF, and it was reported that E/A can predict CRT response within 6 months31, however, little was known about the effect of E’/A’ and S wave. Present study showed the value of S wave and E’/A’ to predict CRT non-response.
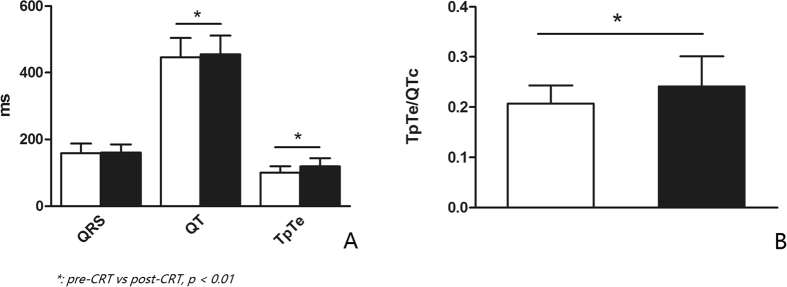
It was reported that both QTc and TpTe/QTc reflect the degree of cardiac transmural dispersion of repolarization (TDR)32,33. Shorter QTc predicted CRT non-response increment. On the contrary, smaller TpTe/QTc value predicted CRT-nonresponse reduction. Unfortunately, TpTe was not statistically significant in predicting CRT non-response, so the effect of TpTe/QTc in this study may be largely determined by QTc. Interestingly, QTc interval is related to the degree of TDR, and the increase of TDR is underlying arrhythmogenesis34. Thus, shorter QTc interval indicating less TDR should do good. However, present study showed shorter QTc as a harmful factor, having high risk in CRT non-response. It seemed QTc as a double-edged sword increased arrhythmia when lengthening, but decreased CRT non-response when shortening. Moreover, CRT changes the direction of cardiac repolarization, and it could contribute to the increase of TDR. Under physiological condition, hearts depolarize from endocardium to epicardium, and repolarize in an opposite direction35. However, CRT totally changes left ventriclular direction of depolarization and repolarization since its epicardial pacing, leading to TDR increasing36. It was reported that occurrence of tachycardia was higher in early stage of CRT implantation37. In this study, compared to baseline, TpTe and QT which reflect the degree of TDR were significantly increased within 72 h after CRT implantation (100 ± 20 ms vs. 119 ± 24 ms, and 446 ± 58 ms vs. 455 ± 56 ms respectively, p < 0.01, indicating that CRT increases the dispersion of repolarization and has potential to cause arrhythmia in the early stage (Fig. 4). It indicates that cardiac dispersion of repolarization may have dual effect on patients with CRT.
Figure 4. Was the comparison of electrocardiographic indice about dispersion of repolarization between pre-CRT placement and post-CRT placement.
(A) and (B) showed QT interval, TpTe and TpTe/QTc significantly increased within 3 days after CRT implantation indicating CRT prompting TDR increasing in early stage (white bars represented for indices of pre-CRT, and black bars referred to indices of post-CRT).
Since severe renal dysfunction could be a confounding factor, and end stage renal disease is always complicated with multiple-organ disorder including heart problems. Thus, we excluded patients in CKD-5 with continuous renal replacement therapy. Scr can somewhat reflect cardiac function since cardiac dysfunction resulting in low pumping volume could directly affect the perfusion of kidney, thus Scr could predict prognosis of HF38. What’s more, chronic kidney disease could influence CRT non-response as concomitant disease39. However, oppositely, lower level of Scr, reflecting muscle mass decreasing, was related to poor prognosis in HF40. It seemed that on the one hand, increased Scr indicating renal function worsening was in relationship with poor prognosis after CRT implantation; while on the other hand, decreased Scr indicating over self-consumption prognosed bad outcome. Considering renal function might be related to CRT non-response, we analyzed both the value of Scr and eGFR. However, at baseline, there was no difference between CRT responders and CRT non-responders. While, after one year of CRT implantation, Scr level of CRT responders was significantly lower than CRT non-responders’ (Fig. 5). It hinted that Scr or eGFR might be more suitable for evaluation the effect of CRT.
Figure 5. Showed difference of Scr level between CRT responders and CRT non-responders after 1 year.
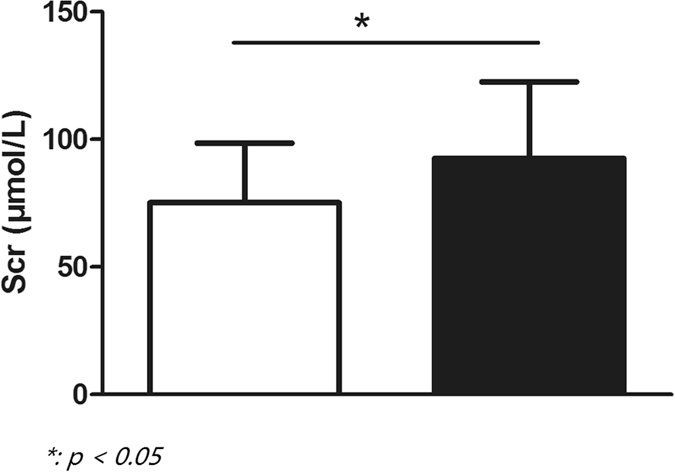
Bar in white color referred to CRT responders, while bar in black color represented for CRT non-responders.
Limitations of our study included a retrospective design, single centre participation and short study duration. Thus, a prospective, large scale, single blind to patients, randomized, controlled, and multi-center collaborative clinical trial is still in high need in future. Besides, other valuable parameters such as left ventricular volume, E’, and E/E’ were not included because they were not available with a retrospective design.
Conclusion
The present study revealed that both electrocardiographic and echocardiographic indice, especially TpTe/QTc > 0.203, and LVEDV > 255 mL are valuable to predict CRT non-response. CLBBB, QRS duration >160 ms, S wave >5.7 cm, E/A > 1, and E’/A’ > 1 showed lower probability of CRT non-response. The findings of this study provide some useful parameters on predicting CRT non-response. However the sample size in this study is not large enough, though 227 objects included for single-center in this area is less common. Consequently, large scale, multi-center, and prospective research is highly wanted in future.
Additional Information
How to cite this article: Yu, Z. et al. Electro-echocardiographic Indices to Predict Cardiac Resynchronization Therapy Non-response on Non-ischemic Cardiomyopathy. Sci. Rep. 7, 44009; doi: 10.1038/srep44009 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We appreciate SQ Hou, ZW Chen and GJ Liu on giving modification of this article to improve its language. Besides, Q wan and NW Zhou helped us a lot on understanding the UCG data. This study was supported by National Natural Science Foundation of China (Grant No: 81671934) and Zhongshan hospital Science Funding (Grant No: fund-044). Shanghai Municipal Planning Commission of Science and Research Fund (No.20164Y006).
Footnotes
The authors declare no competing financial interests.
Author Contributions Zq Yu participated in conception and design, acquisition of data and drafting and revising the manuscript; Xy Chen took part in acquisition of data and analysis of data as well as giving revised edition of the manuscript; F Han carried out acquisition of data and statistical analysis; Sm Qin took part in acquisition of data; Yg Su participated in design, writing the response letter to reviewers, and having given final approval of the version to be published; Mh Li and Y Wu took part in applying for examination of ethics and interpretation of data; Jb Ge carried out design, having given final approval of the version to be published. All authors read and approved the final manuscript.
References
- Braunwald E. Heart Failure. JACC Heart Fail. 1, 1–20 (2013). [DOI] [PubMed] [Google Scholar]
- Leyva F., Nisam S. & Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 64, 1047–1058 (2014). [DOI] [PubMed] [Google Scholar]
- Aiba T. et al. Electrophysiological consequences of dyssynchronous heart failure and its restoration by resynchronization therapy. Circulation. 119, 1220–1230 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auricchio A. & Prinzen F. W. Non-responders to cardiac resynchronization therapy: the magnitude of the problem and the issues. Circ J. 75, 521–527 (2011). [DOI] [PubMed] [Google Scholar]
- Daubert C. et al. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. (2016). [DOI] [PubMed] [Google Scholar]
- Braunschweig F. et al. New York Heart Association functional class, QRS duration, and survival in heart failure with reduced ejection fraction: implications for cardiac resychronization therapy. Eur J Heart Fail. (2016). [DOI] [PubMed] [Google Scholar]
- Timineri S. et al. Selection of patient for cardiac resynchronization therapy: role of QT corrected dispersion. Pacing Clin Electrophysiol. 35, 850–5 (2012). [DOI] [PubMed] [Google Scholar]
- Doltra A. et al. Mechanical abnormalities detected with conventional echocardiography are associated with response and midterm survival in CRT. JACC Cardiovasc Imaging. 7, 969–979 (2014). [DOI] [PubMed] [Google Scholar]
- Brenyo A. et al. Predictors of spontaneous reverse remodeling in mild heart failure patients with left ventricular dysfunction. Circ Heart Fail 7, 565–572 (2014). [DOI] [PubMed] [Google Scholar]
- Veazie P. J. et al. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol. 60, 1940–1944 (2012). [DOI] [PubMed] [Google Scholar]
- Priori S. G. et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 17, 1601–1687 (2015). [DOI] [PubMed] [Google Scholar]
- KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. National Kidney Foundation. et al. Am J Kidney Dis. 66, 884–930 (2015). [DOI] [PubMed] [Google Scholar]
- Huang W. H. et al. Negative Relationship between Erythropoietin Dose and Blood Lead Level in Patients Undergoing Maintenance Hemodialysis. Sci Rep. 6, 34313 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland J. G. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 352, 1539–1549 (2005). [DOI] [PubMed] [Google Scholar]
- Gorcsan J. R. et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting–a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr 21, 191–213 (2008). [DOI] [PubMed] [Google Scholar]
- Dele-Michael A. O. et al. Left Ventricular Stroke Volume Quantification by Contrast Echocardiography - Comparison of Linear and Flow-Based Methods to Cardiac Magnetic Resonance. Echocardiography 30, 880–888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudski L. G., Bra. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23, 685–713 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y. et al. Diastolic Left Ve tricular Function in Relation to Circulating Metabolic Biomarkers in a General Population. J Am Heart Assoc. 5, e2681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. A. et al. Determinants of preserved diastolic function at the lateral annulus in patients with isolated diastolic dysfunction. Eur Heart J Cardiovasc Imaging 14, 443–448 (2013). [DOI] [PubMed] [Google Scholar]
- Wang N. C. et al. New-onset left bundle branch block-associated idiopathic nonischemic cardiomyopathy and left ventricular ejection fraction response to guideline-directed therapies: The NEOLITH study. Heart Rhythm 13, 933–942 (2016). [DOI] [PubMed] [Google Scholar]
- Melman Y. F. et al. Circulating MicroRNA-30 d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 131, 2202–2216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell T., Hadlow N. & Chakera A. Impact of routine reporting of estimated glomerular filtration rate using the CKD-EPI formula in a community population: A cross-sectional cohort study. Nephrology (Carlton) 19, 581–586 (2014). [DOI] [PubMed] [Google Scholar]
- Boriani G. et al. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes Sci Rep. 6, 30271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N. A. & Singh J. P. Cardiac resynchronization therapy: past, present, and future. Heart Fail Clin. 11, 287–303 (2015). [DOI] [PubMed] [Google Scholar]
- Wang J. et al. Elevated pulmonary artery pressure predicts poor outcome after cardiac resynchronization therapy. J Interv Card Electrophysiol 40, 171–178 (2014). [DOI] [PubMed] [Google Scholar]
- Rossi L. et al. Indexed maximal left atrial volume predicts response to cardiac resynchronization therapy. Int J Cardiol. 168, 3629–3633 (2013). [DOI] [PubMed] [Google Scholar]
- Chatterjee N. A. et al. Pre-capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail 2, 230–237 (2014). [DOI] [PubMed] [Google Scholar]
- Ciampi Q. et al. Additive value of severe diastolic dysfunction and contractile reserve in the identification of responders to cardiac resynchronization therapy. Eur J Heart Fail 13, 1323–1330 (2011). [DOI] [PubMed] [Google Scholar]
- Egnaczyk G. F. & Chung E. S. The relationship between cardiac resynchronization therapy and diastolic function. Curr Heart Fail Rep. 11, 64–69 (2014). [DOI] [PubMed] [Google Scholar]
- Facchini E. et al. Systolic heart failure and cardiac resynchronization therapy: a focus on diastole. Int J Cardiovasc Imaging 30, 897–905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R. M. et al. Do baseline diastolic echocardiographic parameters predict outcome after resynchronization therapy? Results from the PROSPECT trial. Pacing Clin Electrophysiol 36, 214–220 (2013). [DOI] [PubMed] [Google Scholar]
- Karaman K. et al. New markers for ventricular repolarization in coronary slow flow: Tp-e interval, Tp-e/QT ratio, and Tp-e/QTc ratio. Ann Noninvasive Electrocardiol 20, 338–344 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner S. B. et al. Repolarization Heterogeneity: Beyond the QT Interval. J Am Heart Assoc. 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antzelevitch C. Cellular basis and mechanism underlying normal and abnormal myocardial repolarization and arrhythmogenesis. Ann Med. 36, 5–14 (2004). [DOI] [PubMed] [Google Scholar]
- Yan G. X. et al. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol. 42, 401–409 (2003). [DOI] [PubMed] [Google Scholar]
- Ozcan E. E. et al. Comparison of the Effects of Epicardial and Endocardial Cardiac Resynchronization Therapy on Transmural Dispersion of Repolarization. Pacing Clin Electrophysiol 38, 1099–1105 (2015). [DOI] [PubMed] [Google Scholar]
- Itoh M. et al. Time-dependent effect of cardiac resynchronization therapy on ventricular repolarization and ventricular arrhythmias. Europace. 15, 1798–1804 (2013). [DOI] [PubMed] [Google Scholar]
- Pimentel R. et al. Prognostic value of worsening renal function in outpatients with chronic heart failure. Eur J Intern Med. 25, 662–668 (2014). [DOI] [PubMed] [Google Scholar]
- Daly D. D. Jr. et al. The Effect of Chronic Kidney Disease on Mortality with Cardiac Resynchronization Therapy . Pacing Clin Electrophysiol (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Maaten J. M. et al. Creatinine excretion rate, a marker of muscle mass, is related to clinical outcome in patients with chronic systolic heart failure. Clin Res Cardiol 103, 976–983 (2014). [DOI] [PubMed] [Google Scholar]