Abstract
Successful species develop strategies to optimize their reproductive performance. This optimization likely includes the evolution of genes that specifically permit reproduction in physiologically challenging conditions. The prolactin (PRL) family gene cluster is one of 25 mouse-specific gene clusters, the majority of which are associated with reproduction. A prevailing theme characterizing the PRL family is its connection with pregnancy and mechanisms controlling viviparity. PRL-like protein A (PLP-A) is one of 26 genes located within the PRL family locus. It is a nonclassical member of the PRL family (e.g., PLP-A does not use the PRL receptor) produced by trophoblast cells of the chorioallantoic placenta and acts on uterine natural killer cells. In this report, the biology of PLP-A has been investigated by generating mice with a PLP-A null mutation. Under standardized animal husbandry conditions, PLP-A possesses modest effects on reproductive performance. However, this same gene is critical for reproduction when mice are exposed to a physiological stressor. Wild-type mice exposed to hypobaric hypoxia during gestation readily adapt and maintain their pregnancies, whereas PLP-A null mutant mice fail to adapt, resulting in pregnancy failure. PLP-A contributes to hypoxia-induced adaptations critical to hemochorial placentation and thus nutrient flow to extraembryonic and embryonic tissues. The findings provide insights into species-specific reproductive adaptations.
Keywords: natural killer cell, placenta, prolactin
Reproductive adaptations to environmental challenges are a key to the success of a species (1, 2). Among mammals, few species have achieved the reproductive efficiency of mice and rats. The biological mechanisms underlying these increases in reproductive efficiency are not well understood. Sequencing of the mouse and rat genomes has provided insights that might help explain the heightened reproductive efficiencies of rodents (3, 4). The mouse and rat genomes were impacted by gene duplication and selection creating gene families not evident in other species, including the human genome. Many of these expanded gene families are associated with reproduction, as exemplified by the prolactin (PRL) gene family (3, 5).
In the mouse, the PRL family consists of at least 26 genes clustered within a 1-Mb region on chromosome 13, whereas in the human only a single member of the PRL family exists (5–7). Members of the mouse PRL gene family are expressed in tissues pivotal to the regulation of reproduction, including the anterior pituitary, uterus, and placenta (6, 7). The PRL gene family encodes for structurally related hormones/cytokines implicated in the biology of pregnancy. The best-studied member of the PRL gene family is PRL. Orthologous PRL genes are expressed in the anterior pituitary of vertebrates (8, 9). In the mouse, PRL is a key hormone regulating the development and functioning of the corpus luteum and mammary gland, actions critical to successful pregnancy and lactation (10, 11). Null mutations of either the PRL gene or the gene that encodes the PRL receptor result in female infertility (12, 13). A subset of four additional members of the mouse PRL family, referred to as placental lactogen I (α, β, and γ) and placental lactogen II and produced by the placenta during early and late pregnancy, respectively, also activate the PRL receptor signaling pathway (5–7). The remaining 21 members of the PRL family do not appear to use the PRL receptor and are referred to as nonclassical members. Among the nonclassical members, intriguing targets and biological actions have been elucidated, including actions on cells associated with hematopoietic, vasculature, and immune systems (14–21). However, the biological significance of these nonclassical and species-specific hormones/cytokines is not known. PRL-like protein A (PLP-A) is a nonclassical member produced by trophoblast cells of the chorioallantoic placenta with actions on uterine natural killer (NK) cells (20–28). We have gained insights into the biology of PLP-A and the PRL family by generating mice with a PLP-A null mutation.
Materials and Methods
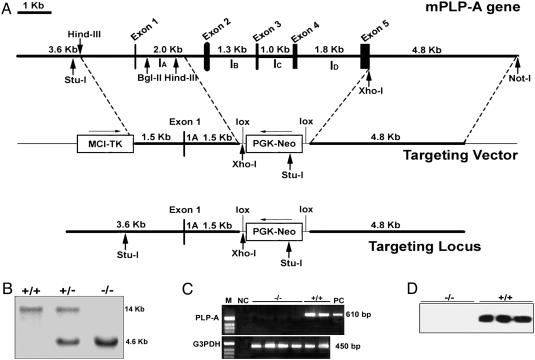
Gene Targeting. A replacement mutation was generated through the substitution of a neomycin (neo) resistance gene cassette for exons 2–5 of the mouse PLP-A gene (29). A 3.0-kb DNA fragment containing 1.5 kb of 5′ flanking DNA and 1.5 kb of exon 1 and intron A of the mouse PLP-A genomic construct was subcloned upstream of the neo cassette. A 4.8-kb DNA fragment containing 3′ flanking DNA located immediately downstream of exon 5 was subcloned downstream of the neo cassette and upstream of a thymidine kinase gene cassette. The accuracy of the vector was verified by restriction enzyme and DNA sequence analyses (Fig. 1). The targeting vector was introduced into R1 embryonic stem cells (ref. 30; a generous gift from Janet Rossant, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto) by electroporation. Cells were selected by exposure to G418 and gangciclovir. Southern blot analysis was used to identify clones that appropriately underwent homologous recombination with the targeting vector. Genomic DNA was isolated, digested with StuI, and fractionated in 0.8% agarose gels. Southern blots were performed with a probe derived from intron A. Wild-type samples are characterized by a 14.0-kb hybridization signal; homozygous mutant samples are characterized by a 4.6-kb hybridization signal. Chimeras were generated by injection into C57BL/6 blastocysts and transferred into pseudopregnant (C57BL/6 × CBA) F1 females. Mice with the PLP-A mutation were backcrossed for 10 generations to the C57BL/6, 129SvJ, or CD-1 genetic background.
Fig. 1.
Targeted disruption of the PLP-A gene in mice. (A) Schematic maps of the mouse PLP-A gene (Top), the targeting vector (Middle), and the homologous recombinant mutant allele (Bottom). (B) Southern blot analysis of tail DNA from littermates generated by heterozygous crossing. (C) RT-PCR analysis of day-11.5 placental RNA from PLP-A null mutant and wild-type mice. (D) Western blot analyses of conditioned media generated by day-11.5 placental explants cultured for 24 h in serum-free culture medium.
Western Blot Analysis. Perforin and PLP-A proteins were detected in tissue extracts by immunoblotting as described in refs. 21 and 26. Protein concentrations were determined for each sample by using the DC protein assay (Bio-Rad).
Immunocytochemistry. Immunocytochemical analyses were used for the purpose of determining the distribution of NK, trophoblast, and endothelial cells (31). NK cells were detected with a rabbit polyclonal anti-perforin antibody (Torrey Pines Biolabs, Houston). Trophoblast cells were monitored by using a rat monoclonal anti-mouse cytokeratin antibody (TROMA-1, Developmental Studies Hybridoma Bank, Iowa City, IA). Mesometrial endothelial cells were localized by using a rat monoclonal anti-mouse endoglin antibody (Developmental Studies Hybridoma Bank).
In Situ Hybridization. In situ hybridization was performed as described in ref. 31. Ten-micrometer cryosections of tissues were prepared and stored at –80°C until use. Plasmids containing cDNAs for mouse PLP-A and mouse PL-II (28, 32) were used as templates to synthesize sense and antisense digoxigenin-labeled riboprobes according to the manufacturer's instructions (Roche Molecular Biochemicals).
In Situ PLP-A Binding. PLP-A interactions with targets were evaluated by using an alkaline-phosphatase–PLP-A fusion protein (20). In situ alkaline-phosphatase–PLP-A binding to tissues and cells were conducted as described in ref. 20.
PRL Family Miniarray Assay. The PRL family miniarray assay is a hybridization-based tool for simultaneously monitoring expression of each member of the PRL family (32). It has been effectively used to monitor the phenotypes of the placenta and trophoblast cells. The PRL family miniarray assay was performed as described in ref. 32.
Hypobaric Hypoxia. On day 7.5 of pregnancy, female mice were placed in hypobaric chambers. Under these conditions, air is circulated at a barometric pressure of ≈420 torr (1 torr = 133 Pa), which results in an inspired PO2 of ≈78 torr, equivalent to breathing 11% O2 at sea level. The chambers were opened daily to clean cages and replenish food and water (15–20 min).
Results
Generation of a PLP-A Null Mouse. PLP-A is encoded by a fiveexon–four-intron gene that possesses homology to the PRL gene structure (5, 29). PLP-A null mutant mice were generated by gene targeting strategies culminating in the replacement of a region of the PLP-A gene located between exons 2 and 5 with a neomycin resistance gene (Fig. 1 A and B). The portion of the coding sequence remaining in the mutated gene (exon 1) encodes for only the first 10 aa of the PLP-A signal peptide. Breeding of mice heterozygous for the PLP-A null mutation resulted in offspring exhibiting a typical Mendelian ratio without an apparent reproductive phenotype (Table 1). The mutation was moved to two inbred strains (C57BL/6 and 129SvJ) and the CD-1 outbred strain, after 10 generations of backcrosses.
Table 1. Reproductive performance and Mendelian ratios of mice heterozygous for the PLP-A null mutation.
| Parental genetic background
|
No. of litters
|
No. of newborns
|
Litter size (mean ± SEM)
|
Genotype of offspring
|
||
|---|---|---|---|---|---|---|
| +/+ | +/- | -/- | ||||
| 129SvJ × C57BL/6 | 14 | 111 | 7.9 ± 1.3 | 28 | 57 | 26 |
| CD-1 | 12 | 153 | 12.7 ± 2.9 | 38 | 76 | 39 |
| C57BL/6 | 15 | 132 | 8.8 ± 1.9 | 32 | 65 | 35 |
| 129SvJ | 11 | 60 | 5.4 ± 1.7 | 16 | 31 | 13 |
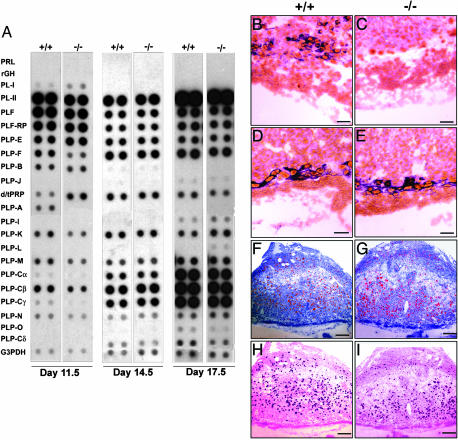
Characterization of the PLP-A Null Mouse. Placentas from homozygous null mutant mice were devoid of PLP-A mRNA and protein, verifying the accuracy of the gene targeting and production of a null mutant (Figs. 1 C and D and 2 A–C). The absence of PLP-A and the presence of the neo resistance gene did not significantly affect expression patterns of other members of the PRL gene family (as has been seen in targeted alleles of other tandemly repeated multigene families), except for PRL-like protein F (PLP-F) (Fig. 2 A, D, and E). The impact on PLP-F was limited to day 12 of gestation and disappeared as gestation progressed. Thus, the PLP-A mutation did not appear to have a disruptive influence over other genes within the PRL gene family cluster. PLP-F has been implicated in the regulation of hematopoiesis (33), and the transient change in its expression may reflect a physiological compensation to the PLP-A deficiency. Breeding of mice homozygous for the PLP-A null mutation from a mixed genetic background (129SvJ × C57BL/6), from two inbred strains, and from the CD-1 outbred strain under standard animal husbandry practices was unremarkable. Litter sizes were within normal ranges (Table 2). Furthermore, PLP-A deficiency did not markedly affect NK cell distributions (Fig. 2 F and G) or PLP-A binding to uterine mesometrial NK cells (Fig. 2 H and I).
Fig. 2.
Characterization of the uteroplacental compartment in PLP-A null mutant mice. (A) PRL family miniarray analysis of placental RNA from days 11.5, 14.5, and 17.5 of pregnancy in wild-type and PLP-A null mutant mice. Note that the differences in PLP-F and PLP-A expression between wild-type and PLP-A null day-11.5 placentas are significant; however, the apparent difference in dPRP (decidual PRL-related protein) expression is not reproducible. (B–E) In situ hybridization of day-9.5 uteroplacental compartment with antisense PLP-A RNA (B and C) and PL-II RNA (D and E) probes. (F and G) Perforin immunohistochemistry on the day-9.5 uteroplacental compartment showing uterine NK cell distribution. (H and I) Binding of alkaline-phosphatase–PLP-A fusion protein to tissue sections from the day-9.5 uteroplacental compartment. (Scale bars: B–E, 75 μm; F–I, 250 μm.)
Table 2. Reproductive performance in PLP-A null mutant mice.
| Parental genetic background | Genotype | No. of litters | No. of newborns | Litter size (mean ± SEM) |
|---|---|---|---|---|
| 129SvJ × C57BL/6 | +/+ | 6 | 49 | 8.1 ± 0.9 |
| -/- | 7 | 54 | 7.7 ± 1.2 | |
| CD-1 | +/+ | 11 | 145 | 13 ± 2.7 |
| -/- | 19 | 237 | 12.5 ± 3.1 | |
| C57BL/6 | +/+ | 12 | 106 | 8.8 ± 1.1 |
| -/- | 11 | 96 | 8.7 ± 2.1 | |
| 129SvJ | +/+ | 10 | 59 | 5.9 ± 1.7 |
| -/- | 14 | 77 | 5.5 ± 1.1 |
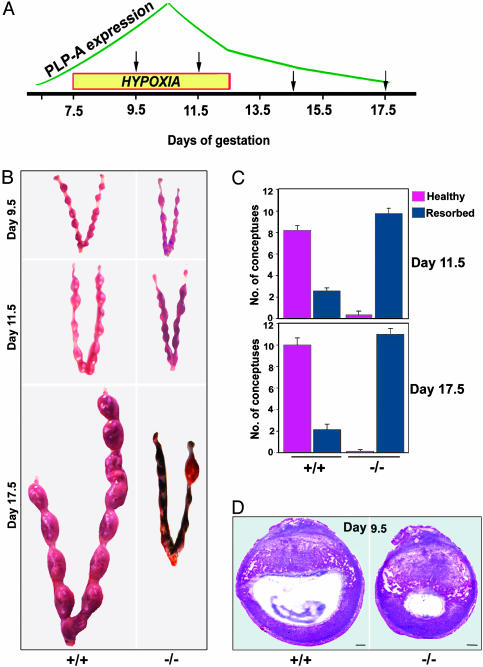
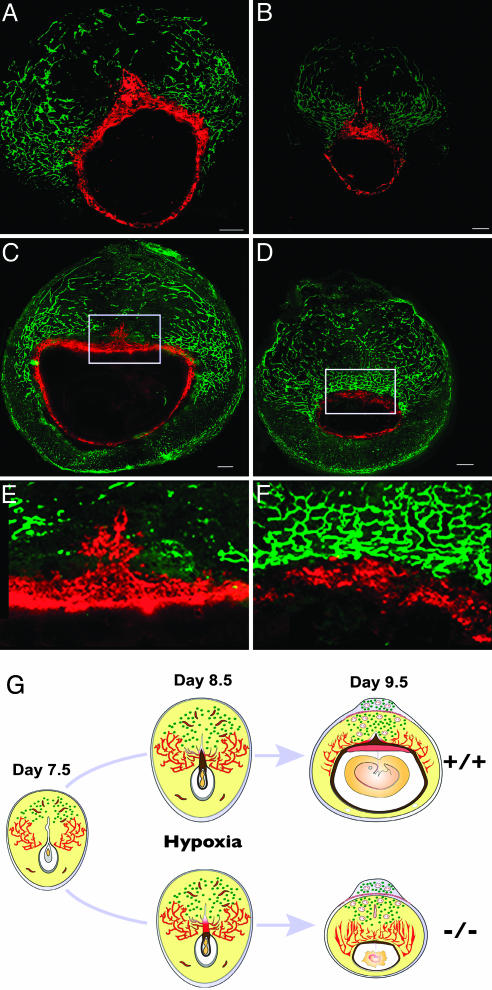
Effects of a Physiological Stressor on the Mouse PLP-A Null Phenotype. Retention of PLP-A in the mouse and rat genomes suggests that it likely contributes to the success of at least these two murid species. Because we did not observe a prominent phenotype when PLP-A null mice were maintained under standard housing conditions, we hypothesized that PLP-A may participate in adaptive responses to physiological stressors. The linkages between PLP-A and uterine NK cells and between uterine NK cells and the uteroplacental vasculature (34, 35) prompted us to investigate environmental challenges that would impact the uteroplacental vasculature. We selected hypoxia as a physiological stressor. Low oxygen tension is a well established stimulus promoting vascular remodeling (36–38), including blood vessels associated with the uterus and placenta (39). We challenged the uteroplacental vasculature by exposing pregnant CD-1 mice (wild-type and PLP-A null mutant mice) to hypobaric hypoxia during the gestational interval of maximal PLP-A expression and maximal density of uterine NK cells (days 7.5 to 12.5 of gestation; Fig. 3A). Pregnancies were monitored at days 9.5, 11.5, 14.5, and 17.5 of gestation. Wild-type mice were able to successfully adapt to the hypoxia challenge and maintained viable and healthy conceptuses, whereas PLP-A null mutants could not sustain their pregnancies (Fig. 3 B and C). Almost all embryo–placental structures (conceptuses) of the PLP-A null mutants were undergoing resorption by day 11.5 of gestation (Fig. 3 B and C). Histological analyses of the uteroplacental structures 2 days after initiation of hypobaric hypoxia showed that the absence of PLP-A resulted in profound growth retardation of the conceptuses (Fig. 3D). Upon closer examination of the distribution of trophoblast (stained with TROMA-1) and endothelial (stained with endoglin antibodies) cells within the implantation sites, it became evident that trophoblast cell–vascular interactions were impaired in the PLP-A-deficient mice exposed to hypoxia (Fig. 4 A–F). Between days 7.5 and 9.5 of pregnancy, there is a progression of events within the mesometrial chamber that is essential for the establishment of the hemochorial placenta (40, 41). They include degeneration of the associated uterine luminal epithelium, mesometrial-associated angiogenesis and opening of the maternal vessels into the mesometrial chamber, and expansion of the mesometrial chamber, followed by growth of the ectoplacental cone into the mesometrial chamber (40, 41). These events occur in wild-type mice and in PLP-A null mice exposed to ambient conditions. Hemochorial placentation fails in PLP-A null mice exposed to hypobaric hypoxia. The uterine epithelium lining the mesometrial chamber does degenerate, and there is a marked increase in angiogenesis within the mesometrial uterine compartment; however, trophoblast cells associated with the ectoplacental cone do not grow into the mesometrial chamber (compare E and F in Fig. 4; summarized in G). The net result is a failure of adequate nutrient flow to support extraembryonic and embryonic development.
Fig. 3.
Pregnancies in PLP-A null mice are vulnerable to maternal hypoxia. (A) Schematic representation of placental PLP-A expression patterns (18–20), maternal hypoxia exposure, and gestation days for tissue harvesting. (B) Pregnant uteri of wild-type and PLP-A null mutant mice exposed to hypoxia. (C) Quantitation of pregnancy outcomes in wild-type and PLP-A null mutant mice exposed to hypoxia. (D) Hematoxylin/eosin stained sections of implantation sites in wild-type and PLP-A null mutant mice after 48 h of hypoxia exposure. (Scale bars: 250 μm.)
Fig. 4.
Defect in hemochorial placentation and trophoblast–vascular interactions in PLP-A null mutant mice exposed to hypoxia. (A–F) Double immunohistochemical staining for trophoblast cells by using a cytokeratin-8-specific antibody (TROMA-1) and for endothelial cells by using an endoglin antibody within implantation sites of wild-type and PLP-A null mutant mice after 24 h (A and B) and 48 h (C and D) of hypoxia starting on gestation day 7.5. (Scale bars: 250 μm.) E and F are high-magnification images of the boxed sections in C and D, respectively. (G) Schematic representation of cellular dynamics at the implantation sites of wild-type and PLP-A null mutant mice exposed to hypoxia for 48 h starting on gestation day 7.5. Note the lack of ectoplacental cone expansion into the mesometrial chamber on days 8.5 and 9.5 of pregnancy in PLP-A null mutant mice after exposure to hypoxia. Also note the aberrant vasculature and underdeveloped placentas at the implantation sites on day 9.5 of pregnancy in PLP-A null mutant mice exposed to hypoxia.
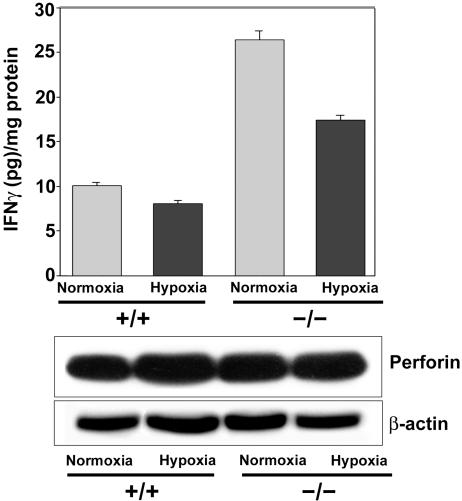
PLP-A Influences Mesometrial IFN-γ Expression. The mechanisms underlying the increased sensitivity to hypoxia during pregnancy in PLP-A null mice are not known. Uterine NK cells may be involved. They associate with the uterine mesometrial vasculature, and their function is affected by PLP-A (20, 21). Uterine mesometrial NK cells contribute to the uteroplacental cytokine milieu through their production of IFN-γ (42). PLP-A is a negative modulator of uterine mesometrial NK cell production of IFN-γ. PLP-A inhibits NK cell production of IFN-γ in vitro (21), and uterine mesometrial IFN-γ concentrations are significantly increased in PLP-A null mutant conceptuses (Fig. 5). IFN-γ is known to inhibit trophoblast growth (31) and may be a contributing factor to the trophoblast growth restriction seen in the pregnant PLP-A null mice exposed to hypoxia; however, dysregulated IFN-γ is not the complete explanation. Uterine mesometrial IFN-γ levels are also elevated in the pregnant PLP-A null mouse housed under ambient conditions, yet their conceptuses do not exhibit any evidence of growth arrest. Any involvement of NK cells or IFN-γ in the adaptations to maternal hypoxia will need to be directly evaluated.
Fig. 5.
IFN-γ content in the mesometrial decidua of wild-type and PLP-A null mutant implantation sites without (normoxia) and with hypoxia challenge. IFN-γ concentrations were measured by ELISA and normalized to protein content. Data presented are mean ± SEM of triplicate experiments. Shown below the bar graph are Western blot analyses for perforin (uterine NK cell differentiation marker) and β-actin.
Discussion
Investigation of the phenotype of the PLP-A null mouse has generated unexpected insights into mechanisms underlying species-specific reproductive adaptations. PLP-A, a placental hormone and member of the mouse PRL family, is dispensable when mice are maintained under standard animal husbandry practices. However, it is evident that the selective pressures driving the evolution of the mouse PRL family or, more specifically, the PLP-A gene did not occur in the laboratory with the availability of a reliable food supply and modern veterinary hygienic practices. Accordingly, we reasoned that the biological importance of PLP-A might become apparent when the mice were presented with an environmental challenge. Support for this notion comes from ideas presented by Kaplan and Grumbach (43). They proposed that members of the primate placental PRL/growth hormone family serve as regulatory molecules ensuring adaptations to physiological stressors, especially those associated with increased metabolic demands. PRL has similarly been advanced as a key regulatory molecule involved in immune-system adaptations to stress (44–46). In our study, the PLP-A gene was found to be critically involved in pregnancy-associated adaptive responses to hypoxia.
Selection of hypoxia as a physiological stressor was based on current knowledge of the physiology of PLP-A and the function of its targets. PLP-A interacts with uterine NK cells (20, 21), and uterine NK cells are well established modulators of the uteroplacental vasculature (34, 35). We manipulated oxygen tension because of the known action of hypoxia as a stimulus for vascular remodeling (36–38). At this juncture, we do not know whether the phenotype we elicited in PLP-A null mice is specific and/or restricted to hypoxia. PLP-A may also participate in adaptive responses to other stressors (e.g., metabolic, immunologic, etc.) during pregnancy.
Exposure of pregnant PLP-A null mice to hypoxia resulted in the unmasking of a homeostatic mechanism controlling hemochorial placentation. In this mutant model, trophoblast cells associated with the developing chorioallantoic placenta do not succeed in establishing connectivity with the uterine mesometrial vasculature (Fig. 4). The consequence is impaired nutrient delivery and, ultimately, growth arrest. We predict that PLP-A, arising from trophoblast cells, participates in the regulation of NK cells that accumulate in the uterine mesometrial compartment. The nature of this interaction becomes critical when the pregnant female is exposed to hypoxia during the establishment of the chorioallantoic placenta. PLP-A may modulate the production of NK cell-derived regulatory molecules that directly or indirectly regulate trophoblast expansion into the mesometrial chamber and its interaction with the uterine mesometrial vasculature. One such NK cell product may be IFN-γ. Evidence is accumulating that PLP-A is a negative modulator of uterine NK cell IFN-γ production (ref. 21 and this study), and IFN-γ has been shown to negatively impact trophoblast development (31). Additional experimentation will be required to determine the precise involvement of uterine NK cells and IFN-γ in the adaptive responses mediated by PLP-A.
Species-specific gene-family expansions appear to represent a conserved mechanism contributing to the regulation of reproductive adaptations. The mouse and rat have used the ancestral PRL gene as a template to create an assortment of pregnancy-specific modulators. Other species-specific gene families linked to pregnancy exist in the mouse and rat (3, 4), as well as in other mammals (47, 48). The choice of template for the species-specific gene-family expansions is sometimes conserved across divergent species and other times restricted to only more closely related species. Each pregnancy-associated gene-family expansion likely represents a strategy for improving reproductive success. Although PRL-family ligands are not well conserved between mice and humans (6, 7), the selection of targets for the murine PRL family ligands is intriguing. It is logical to assume that modulating the functioning of hematopoietic, vascular, and immune systems during pregnancy represents an effective strategy for ensuring the safe flow of nutrients from mother to fetus (14–21).
A hierarchy of genes critical to reproduction can be constructed. Key conserved genes regulating the hypothalamic–anterior pituitary–gonadal axis and the uterus (e.g., gonadotropin-releasing hormone, luteinizing hormone, progesterone receptor, etc.) are fundamental regulators of reproduction across a broad spectrum of vertebrates. PLP-A and, likely, other nonorthologous members of the PRL family improve reproductive efficiency and promote adaptive responses to physiological stressors, but they are not intrinsic regulators of reproduction. Such genes have been retained in the mouse and rat genomes because they provide selective advantages. They allow species to reproduce in a range of habitats during an assortment of environmental challenges.
Acknowledgments
We thank Drs. Norberto C. Gonzalez, John G. Wood, and William E. Truog for helpful discussions on the biology of hypoxia; Dustin O. Wiemers, Lu Lu, and My-Linh Trinh for technical assistance; and Kathryn Vasicek for animal care. This work was supported by grants from the National Institutes of Health, the Hall Family Foundation, the Philip Astrowe Trust, and the American Heart Association.
Author contributions: R.A., G.D., A.R.G., and M.J.S. designed research; R.A., G.D., and J.H.D. performed research; A.R.G. contributed new reagents/analytic tools; R.A. and M.J.S. analyzed data; and R.A. and M.J.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PRL, prolactin; PLP-A, PRL-like protein A; NK, natural killer.
See Commentary on page 16397.
References
- 1.Bock, W. J. (2003) Zool. Sci. 20, 279–289. [DOI] [PubMed] [Google Scholar]
- 2.Feder, M. E. & Mitchell-Olds, T. (2003) Nat. Rev. Genet. 4, 649–655. [DOI] [PubMed] [Google Scholar]
- 3.Mouse Genome Sequencing Consortium (2002) Nature 420, 520–562. [DOI] [PubMed] [Google Scholar]
- 4.Rat Genome Sequencing Project Consortium (2004) Nature 428, 493–521.15057822 [Google Scholar]
- 5.Wiemers, D. O., Shao, L.-J., Ain, R., Dai, G. & Soares, M. J. (2003) Endocrinology 144, 313–325. [DOI] [PubMed] [Google Scholar]
- 6.Soares, M. J. & Linzer, D. I. H. (2001) in Prolactin, ed. Horseman, N. D. (Kluwer, Boston), pp. 139–167.
- 7.Soares, M. J. (2004) Reprod. Biol. Endocrinol. 2, 51, www.rbej.com/content/2/1/51.
- 8.Bern, H. A. & Nicoll, C. S. (1968) Recent Prog. Horm. Res. 24, 681–720. [DOI] [PubMed] [Google Scholar]
- 9.Forsyth, I. A. & Wallis, M. (2002) J. Mammary Gland Biol. Neoplasia 7, 291–312. [DOI] [PubMed] [Google Scholar]
- 10.Goffin, V., Binart, N., Touraine, P. & Kelly, P. A. (2002) Annu. Rev. Physiol. 64, 47–67. [DOI] [PubMed] [Google Scholar]
- 11.Bole-Feysot, C., Goffin, V., Edery, M., Binart, N. & Kelly, P. A. (1998) Endocrine Rev. 19, 225–268. [DOI] [PubMed] [Google Scholar]
- 12.Horseman, N. D., Zhao, W., Montecino-Rodriguez, E., Tanaka, M., Nakashima, K., Engle, S. J., Smith, F., Markoff, E. & Dorshkind, K. (1997) EMBO J. 16, 6926–6935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ormandy, C. J., Camus, A., Barra, J., Damotte, D., Lucas, B., Buteau, H., Edery, M., Brousse, N., Babinet, C., Binart, N. & Kelly, P. A. (1997) Genes Dev. 11, 167–178. [DOI] [PubMed] [Google Scholar]
- 14.Jackson, D., Volpert, O. V., Bouck, N. & Linzer, D. I. H. (1994) Science 266, 1581–1584. [DOI] [PubMed] [Google Scholar]
- 15.Bengtson, N. W. & Linzer, D. I. H. (2000) Mol. Endocrinol. 14, 1934–1943. [DOI] [PubMed] [Google Scholar]
- 16.Lin, J. & Linzer, D. I. H. (1999) J. Biol. Chem. 274, 21485–21489. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharyya, S., Lin, J. & Linzer, D. I. (2002) Mol. Endocrinol. 16, 1386–1393. [DOI] [PubMed] [Google Scholar]
- 18.Bittorf, T., Jaster, R., Soares, M. J., Seiler, J., Brock, J., Friese, K. & Müller, H. (2000) J. Mol. Endocrinol. 25, 253–262. [DOI] [PubMed] [Google Scholar]
- 19.Wang, D., Ishimura, R., Walia, D. S., Müller, H., Dai, G., Hunt, J. S., Lee, N. A., Lee, J. J. & Soares, M. J. (2000) J. Endocrinol. 167, 15–29. [DOI] [PubMed] [Google Scholar]
- 20.Müller, H., Liu, B., Croy, B. A., Head, J. R., Hunt, J. S., Dai, G. & Soares, M. J. (1999) Endocrinology 140, 2711–2720. [DOI] [PubMed] [Google Scholar]
- 21.Ain, R., Tash, J. S. & Soares, M. J. (2003) Mol. Cell. Endocrinol. 204, 65–74. [DOI] [PubMed] [Google Scholar]
- 22.Duckworth, M. L., Peden, L. M. & Friesen, H. G. (1986) J. Biol. Chem. 261, 10879–10884. [PubMed] [Google Scholar]
- 23.Deb, S., Youngblood, T., Rawitch, A. & Soares, M. J. (1989) J. Biol. Chem. 264, 14348–14353. [PubMed] [Google Scholar]
- 24.Campbell, W. J., Deb, S., Kwok, S. C., Joslin, J. A. & Soares, M. J. (1989) Endocrinology 125, 1565–1574. [DOI] [PubMed] [Google Scholar]
- 25.Deb, S. & Soares, M. J. (1990) Mol. Cell. Endocrinol. 74, 163–172. [DOI] [PubMed] [Google Scholar]
- 26.Deb, S., Hamlin, G. P., Kwok, S. C. M. & Soares, M. J. (1993) J. Biol. Chem. 268, 3298–3305. [PubMed] [Google Scholar]
- 27.Lin, J., Poole, J. & Linzer, D. I. H. (1997) Endocrinology 138, 5541–5549. [DOI] [PubMed] [Google Scholar]
- 28.Müller, H., Ishimura, R., Orwig, K. E., Liu, B. & Soares, M. J. (1998) Biol. Reprod. 58, 45–51. [DOI] [PubMed] [Google Scholar]
- 29.Dai, G., Chapman, B. M., Wang, D., White, R. A., Preuett, B. & Soares, M. J. (1999) Mammal. Genome 10, 78–80. [DOI] [PubMed] [Google Scholar]
- 30.Nagy, A., Rossant, J., Nagy, R., Abramow-Newerly, W. & Roder, J. C. (1993) Proc. Natl. Acad. Sci. USA 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ain, R., Canham, L. N. & Soares, M. J. (2003) Dev. Biol. 260, 176–190. [DOI] [PubMed] [Google Scholar]
- 32.Dai, G., Lu, L., Tang, S., Peal, M. J. & Soares, M. J. (2002) Reproduction 124, 755–765. [DOI] [PubMed] [Google Scholar]
- 33.Zhou, B., Lum, H. E., Lin, J. & Linzer, D. I. H. (2002) Endocrinology 143, 4281–4286. [DOI] [PubMed] [Google Scholar]
- 34.Guimond, M. J., Luross, J. A., Wang, B., Terhorst, C., Danial, S. & Croy, B. A. (1997) Biol. Reprod. 56, 169–179. [DOI] [PubMed] [Google Scholar]
- 35.Ashkar, A. A. & Croy, B. A. (2001) Semin. Immunol. 13, 235–241. [DOI] [PubMed] [Google Scholar]
- 36.Czyzyk-Krzeska, M. F. (1997) Respir. Physiol. 110, 99–111. [DOI] [PubMed] [Google Scholar]
- 37.Bruick, R. K. & McKnight, S. L. (2001) Genes Dev. 15, 2497–2502. [DOI] [PubMed] [Google Scholar]
- 38.Semenza, G. L. (2003) Annu. Rev. Med. 54, 17–28. [DOI] [PubMed] [Google Scholar]
- 39.Zamudio, S. (2003) High Alt. Med. Biol. 4, 171–191. [DOI] [PubMed] [Google Scholar]
- 40.Welsh, A. O. & Enders, A. C. (1991) Am. J. Anat. 192, 347–365. [DOI] [PubMed] [Google Scholar]
- 41.Enders, A. C. & Welsh, A. O. (1993) J. Exp. Zool. 266, 578–587. [DOI] [PubMed] [Google Scholar]
- 42.Ashkar, A. A. & Croy, B. A. (1999) Biol. Reprod. 61, 493–502. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan, S. L. & Grumbach, M. M. (1981) in Fetal Endocrinology, eds. Novy, M. J. & Resko, J. A. (Academic, New York), pp. 127–139.
- 44.Dorshkind, K. & Horseman, N. D. (2001) BioEssays 23, 288–294. [DOI] [PubMed] [Google Scholar]
- 45.Dugan, A. L., Thellin, O., Buckley, D. J., Buckley, A. R., Ogle, C. K. & Horseman, N. D. (2002) Endocrinology 143, 4147–4151. [DOI] [PubMed] [Google Scholar]
- 46.Dugan, A. L., Schwemberger, S., Babcock, G. F., Buckley, D., Buckley, A. R., Ogle, C. K. & Horseman, N. D. (2004) Shock 21, 151–159. [DOI] [PubMed] [Google Scholar]
- 47.Xie, S., Green, J., Bixby, J. B., Szafranska, B., DeMartini, J. C., Hecht, S. & Roberts, R. M. (1997) Proc. Natl. Acad. Sci. USA 94, 12809–12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts, R. M., Ezashi, T., Rosenfeld, C. S., Ealy, A. D. & Kubisch, H. M. (2003) Reprod. Suppl. 61, 239–251. [PubMed] [Google Scholar]