Abstract
The role of groundwater as a resource in sustaining terrestrial vegetation is widely recognized. But the global prevalence and magnitude of groundwater use by vegetation is unknown. Here we perform a meta-analysis of plant xylem water stable isotope (δ2H and δ18O, n = 7367) information from 138 published papers – representing 251 genera, and 414 species of angiosperms (n = 376) and gymnosperms (n = 38). We show that the prevalence of groundwater use by vegetation (defined as the number of samples out of a universe of plant samples reported to have groundwater contribution to xylem water) is 37% (95% confidence interval, 28–46%). This is across 162 sites and 12 terrestrial biomes (89% of heterogeneity explained; Q-value = 1235; P < 0.0001). However, the magnitude of groundwater source contribution to the xylem water mixture (defined as the proportion of groundwater contribution in xylem water) is limited to 23% (95% CI, 20–26%; 95% prediction interval, 3–77%). Spatial analysis shows that the magnitude of groundwater source contribution increases with aridity. Our results suggest that while groundwater influence is globally prevalent, its proportional contribution to the total terrestrial transpiration is limited.
Water uptake by vegetation and transpiration return about half of terrestrial precipitation to the global water cycle1. Partitioning of terrestrial precipitation inputs among transpiration, evaporation, soil water storage and groundwater recharge in different environments, however, remains poorly understood2. Quantifying the influence of groundwater on transpiration is important in part because of recent evidence suggesting widespread separation between groundwater and transpiration3,4, and because of the recognized relevance of groundwater to large-scale land surface processes5,6,7. While the percent uptake of groundwater by plants has been estimated at large scales using Geographic Information System-based methods8,9 and land surface model approaches10,11, only stable isotope techniques12 can provide direct means for tracking the water sources of plants13. Since root water uptake is generally a non-fractionating process14,15,16, the isotopic composition (δ2H and δ18O) of xylem (i.e. plant stem) water should reflect its sources within the rooting zone. This fundamental understanding of water uptake and transport from roots to shoots underlies the utility of stable isotopes in plant water uptake investigations17. While many site-based studies have now been completed18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152, a global synthesis of these data has not yet been made.
Here we employ a meta-analytic review of 138 published papers (hereafter ‘source papers’), representing 7,367 xylem water measurements from 251 genera and 414 species of angiosperms (n = 376) and gymnosperms (n = 38) in 162 sites across 12 terrestrial biomes worldwide. To make appropriate quantification of groundwater influence and address publication bias, our systematic review encompasses all physiographic settings. We define “groundwater” following the operational definitions in the respective source papers, which includes ‘water below permanent groundwater tables’ or ‘aquifers to springs’ and ‘groundwater reservoirs in bedrock cracks and fissures’. We quantify this broad groundwater influence on xylem water by calculating the prevalence and magnitude of groundwater use. We define prevalence as the number of samples out of a universe of plant samples reported to have groundwater contribution to xylem water. We define magnitude as the proportion of groundwater contribution reported in the xylem water mixture (see Methods).
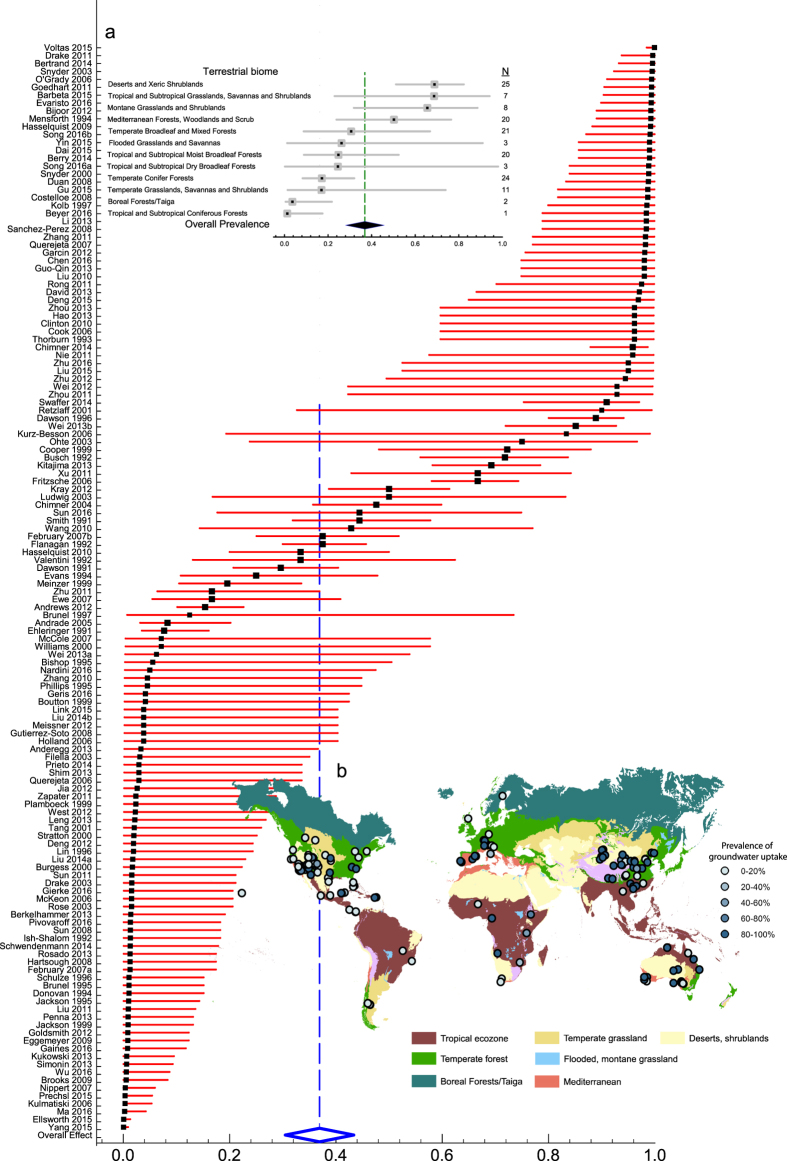
To quantify prevalence of groundwater influence, we compute prevalence point estimates and 95% confidence intervals (CIs) using a random-effects model with the source paper as the unit of analysis (see Methods). Figure 1 shows that the global mean prevalence of groundwater influence is 37% (95% CI, 28–46%). There is substantial variability between studies from a low of 0.05% to a high of 99%, reflecting site- and species-level differences across studies. Almost 90% (I2 = 88.91; Q-value = 1235; df = 137; P < 0.0001) of the observed variance in prevalence estimates is real. That is, on a global scale the true prevalence estimates for each study would be only a little smaller (~11%) than the wide site-to-site variability shown in Fig. 1. The high value of I2 further suggests that on a global scale, the true mean prevalence of groundwater influence on plant transpiration is in the CI range of 28–48% for 95% of all possible meta-analysis cases. On a terrestrial biome basis (Fig. 1a), groundwater influence is most prevalent in deserts 69% (95% CI, 51–82%), tropical grasslands 69% (95% CI, 23–94%), and montane grasslands 66% (95% CI, 32–88%). Groundwater influence is least prevalent in tropical coniferous forests 1% (95% CI, 0–17%), boreal forests 4% (95% CI, 0–22%), and temperate coniferous forests 17% (95% CI, 8–32%). These biome-level estimates of mean prevalence reflect the variability between major habitat types (Fig. 1b; Q-value = 39.7; df = 11; P < 0.0001), underlining differences across study sites and species.
Figure 1. Prevalence of groundwater influence (x-axis: 0 corresponds to no groundwater influence, greater than 0 to groundwater influence).
Main plot, prevalence estimates grouped by source paper (first author-year format). Filled black squares are prevalence point estimates, error bars are 95% confidence intervals (CI, red horizontal lines). Open diamond represents overall prevalence value and its 95% CI is represented by the width of diamond. (a), prevalence estimates grouped by terrestrial biome with N representing corresponding number of sites; (b), Map of prevalence estimates in 162 sites in the global meta-analysis database. Terrestrial biomes delineated by The Nature Conservancy http://www.nature.org. Map was generated using ArcMap 10.2 (http://services.arcgisonline.com/arcgis/rest/services/World_Street_Map/MapServer).
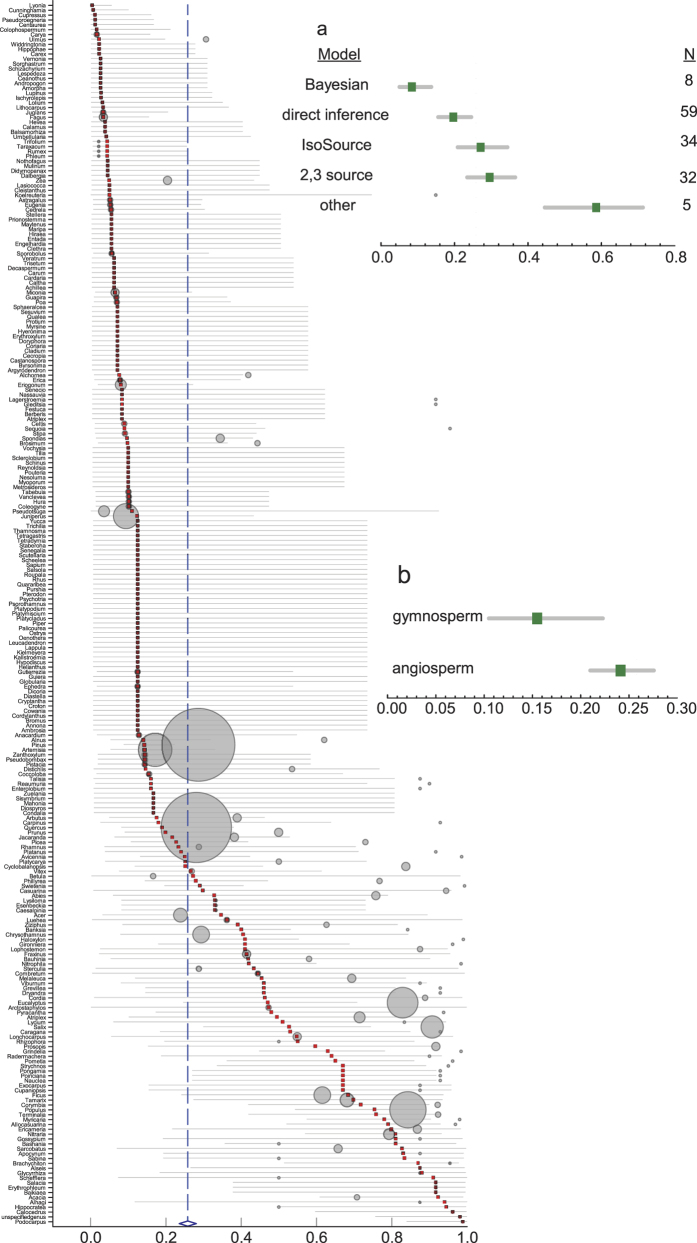
To quantify magnitude of groundwater influence, we compute magnitude point estimates and 95% CIs using a random-effects model with genus as the unit of analysis (see Methods). Figure 2 shows that the global mean magnitude of groundwater influence is 23% (95% CI, 20–26%). As with the meta-analysis on prevalence, there is substantial variability in magnitude of groundwater use between genera, from exclusively soil water (e.g. Cupressus sp. and Vernonia sp.) to almost exclusively groundwater (phreatophytes Sarcobatus sp. and Ericameria sp.). About 67% (I2 = 66.62; Q-value = 1375; df = 250; P < 0.0001) of the observed variance in magnitude estimates is real. The high value of I2 further suggests that on a global scale, the true mean magnitude of groundwater influence on plant transpiration is in the CI range of 20–26% for 95% of all possible meta-analysis cases. Calculating the 95% prediction interval (PI) results in a true magnitude for any single study in the range of 3–77%, reflecting variability due to site-, species-, and genus-level differences. We note that a meta-analysis using species as the unit of analysis yields similar overall mean magnitude and 95% CI estimates compared to genus as the unit of analysis. This suggests that taxonomic control on the magnitude of groundwater influence is as important at the genus level as it is at the species level.
Figure 2. Magnitude of groundwater influence.
Forest plot, magnitude estimates grouped by genus. Filled squares are magnitude point estimates, error bars are 95% confidence intervals (CI). Open blue diamond represents overall magnitude value and its 95% CI is represented by the width of diamond. Bubble plot, mean prevalence point estimates grouped by genus. Bubble size represents the corresponding number of studies (i.e. source papers) about one particular plant genus (e.g. Pinus and Quercus with N = 26 and 25, respectively). (a), magnitude estimates grouped by source identification/apportionment approach with N representing corresponding number of source papers; (b), magnitude estimates grouped by angiosperm and gymnosperm.
Figure 2 shows a superposition of prevalence (bubble plot) and magnitude (error bar plot), illustrating that both measures of groundwater influence are similar in 155 of 251 genera. This reflects studies where prevalence is of the same degree as magnitude, which results from exclusive use of either soil water (i.e. vadose zone water) or groundwater. In 96 of 251 genera, prevalence is greater than magnitude. This reflects studies where plant water use is a mixture of various sources. We explore these effects by performing an analysis across different source identification approaches. Bayesian mixing model results (using SIAR153 and MixSIR154; n = 8) tend to fall outside the lower limit of overall 95% CI (20–26%), while IsoSource155 model results (n = 34) and 2,3-source mixing model results (n = 32) tend to approximate the overall CI (Fig. 2a). A direct inference approach (n = 59), defined as direct comparison of hydrogen or oxygen isotopic composition between xylem water and subsurface water sources, lies between the Bayesian source-mixture approaches, while the customized (i.e. unique to papers) mixing model approaches (n = 5) tend to fall outside the upper limit of overall CI. While these show the differences between mixing model approaches in quantifying the magnitude of groundwater influence (Q-value = 54.25; df = 4; P < 0.0001), the variability resulting from these differences are reflected in the overall prevalence and magnitude estimates and corresponding 95% CI. This suggests that mixing model differences are important, but at the aggregated scale of this meta-analysis, these differences converge to yield the overall magnitude estimate of 23% (95% CI, 20–26%).
We next grouped the meta-analysis data into angiosperms and gymnosperms. Figure 2b shows that angiosperms exhibit greater magnitude of groundwater influence at 24% (95% CI, 21–28%) than gymnosperms at 16% (95% CI, 10–22%) (Q-value = 5.72; df = 1; P = 0.01). The main conducting elements in angiosperms (called xylem vessels) allow for wider variability in element size and wall thicknesses156 than their conducting element counterparts in gymnosperms (called tracheids). Moreover, angiosperms have a greater number of parenchyma cells157 that are linked to improved hydraulic system efficiency after stressful conditions such as drought158. These xylem anatomical differences may explain our finding that angiosperms tend to have greater groundwater influence than gymnosperms.
Finally, we explore the physical controls on our magnitude findings. Several factors are known to affect groundwater influence on vegetation, from physiological159, water table and rooting depths5,6,160, and species161,162, to rainfall patterns9, groundwater extractions8 and changes in stream flow regimes163. We supplement the primary information available from the source papers with site-specific datasets (see Methods) on rooting depth, rooting zone water storage amount, water table depth, potential evapotranspiration and aridity index, precipitation amount, and streamflow amount. We then use a batch algorithm and a locally weighted linear smoother (self-organizing maps clustering technique) to quantify the multivariate controls on the magnitude of groundwater influence on xylem water and the spatial structure of the meta-analysis database (see Methods). Our analysis shows that the database falls into seven optimal clusters. Figure 3 shows how these clusters plot along a theoretical curve (the Budyko curve, ref. 164) of evaporative index as a function of dryness index. The Budyko curve predicts actual evapotranspiration (AET) by potential evapotranspiration (PET) based on a simple water balance (streamflow = precipitation – evapotranspiration).
Figure 3. Cluster analysis (self-organizing maps) plot in evaporative index (actual evapotranspiration/precipitation166) as a function of dryness index (potential evapotranspiration.
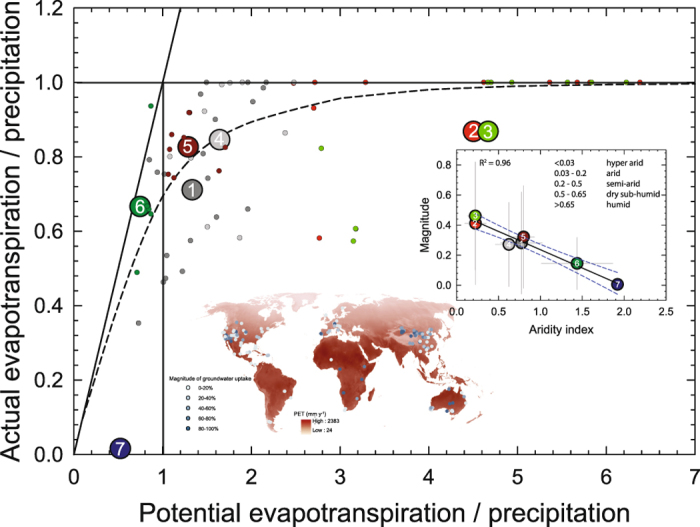
180/precipitation). Actual evapotranspiration (AET) was calculated as the difference between precipitation and streamflow181. Small dots are sites in cluster analysis with colors representing corresponding cluster assignments. Inset, magnitude estimates of cluster assignments as a function of aridity index. Error bars are 1 SD. Also shown is the generalized climate classification scheme for global aridity index180 and map of magnitude of groundwater influence over potential evapotranspiration (PET) layer (mm y−1) annual average 1950–2000180. Map was generated using ArcMap 10.2 (http://services.arcgisonline.com/arcgis/rest/services/World_Street_Map/MapServer).
The computed magnitude of groundwater influence is strongly negatively correlated with aridity index of the modeled clusters (Fig. 3 inset, R2 = 0.96). This suggests that the interactions between variables (e.g. rooting depth, moisture conditions, water table depth, etc.) within a cluster are strong enough to predict the magnitude of groundwater influence (Fig. 3 map inset). Exceptions are clusters 2, 3, and 7, as well as four sites in highly urbanized settings, that fall below the theoretical prediction. That these deviations are along the y-axis (AET/P in Fig. 3, see figure caption for definition of terms) suggest that in those locations vegetation dynamics could not be predicted from a simple water balance.
Overall, our results demonstrate that while groundwater influence on vegetation is prevalent (~37% globally), its magnitude may not be as significant as is increasingly being argued in the literature5. Transpiration has been estimated to comprise between 39 ± 10%1 and 48 ± 10%4 of total terrestrial precipitation. Without making any assumptions about plant-level (e.g. stomatal regulation160) controls on plant water use strategies, our finding that the magnitude of groundwater contribution to total xylem water mixture is limited to ~23% globally translates to an estimate of groundwater contribution to xylem as a fraction of total terrestrial precipitation between 9 and 11%. This is consistent with the 11 ± 8% estimate of ‘connected water’ as a fraction of total terrestrial precipitation by ref. 4 based on remotely-sensed global deuterium mass balance. We note that these estimates (including our own) do not necessarily equal the magnitude of groundwater contribution to transpiration fluxes, due to the existence of strong vegetation ecophysiological controls on transpiration fluxes (e.g. stomatal regulation of transpiration rates). Like ref. 132 we note that field-based sap flow measurements of total tree water use would be needed to make such estimates.
Our finding that most plants have limited groundwater use may have several implications for land surface models (LSMs) designed for explicit representation of saturated zone water in climate-vegetation feedbacks167. Regional- to global-scale climate projections are informed in part by LSMs that describe root water uptake as a function of changes in soil moisture168. Furthermore, our findings may also have implications for the assessment of plant vulnerability to, and growth impairment after, drought stress at plant and ecosystem scales18,169.
Methods
Data compilation and treatment
We searched the published literature for water stable isotope papers in ecology and hydrology. The search initially returned 615 and 533 papers from ISI Web of Science and Scopus, respectively. Papers without xylem (i.e. plant stem) isotope measurements were excluded. After title/abstract screening and removal of duplicates, a total of 138 records remained and were included in the systematic review and meta-analysis. We used Comprehensive Meta-analysis (version 3, BioStat Software, Engelwood, NJ, USA) as the statistical platform for all meta-analysis and associated statistical tests. We defined “groundwater” following the operational definitions in the respective source papers, which included ‘water below permanent groundwater tables’ or ‘aquifers to springs’ and ‘groundwater reservoirs in bedrock cracks and fissures’. We described groundwater influence on vegetation by quantifying prevalence and magnitude. We defined prevalence as the number of samples out of a universe of plant samples reported to have groundwater contribution to xylem water. We defined magnitude as the proportion of groundwater contribution in xylem water mixture. We acknowledge that future work involving newly collected data should go beyond the necessarily loose definition of “groundwater” used here (as mandated by the original paper synthesis) and consider how bedrock groundwater132 and other shallower groundwater108,109 might impact plant water sourcing. To summarize prevalence and magnitude findings, we computed prevalence and magnitude point estimates using the following:
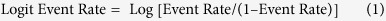 |
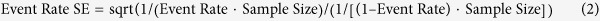 |
Confidence interval (CI) indicates the range within which the true mean (prevalence or magnitude) estimates will fall in 95% of all possible meta-analyses. 95% CI was computed using the following:
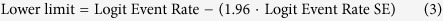 |
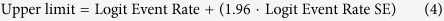 |
Prediction interval (PI) indicates the range within which 95% of all (prevalence or magnitude) estimates will fall in 95% of all meta-analyses. 95% PI was computed using the following:
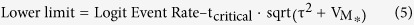 |
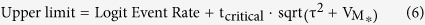 |
where tcritical is the two-tailed inverse of t-distribution at 95% interval, τ2 is the variance of true effects, and VM* is variance of the summary effect estimated as the reciprocal of the sum of the weights.
Associated statistics, particularly Q-value and I2, were then calculated to supplement the description of the point and 95% CI estimates of prevalence and magnitude of groundwater influence. Q-value is a measure of weighted squared deviations, which quantifies true variation from observed variation170. That is, it provides a test of the null hypothesis that all studies in a particular analysis share a common prevalence (or as applicable, magnitude) estimate. This means that if the Q-value is equal to the degrees of freedom (df; n − 1) then all studies share the same prevalence or magnitude estimate. I2 is computed as ((Q − df)/Q) ∙ 100, and provides an estimate of the ratio of true heterogeneity to total variance across the observed prevalence (or magnitude) estimates170. That is, it indicates what proportion of the observed variance reflects differences in true effect sizes (i.e. prevalence or magnitude) rather than sampling error.
The ultimate goal of any meta-analysis is to estimate an overall effect size (in this case, prevalence or magnitude of groundwater influence) and associated CI. If the precision across all 138 published papers in our database were equal, we could readily compute for the simple mean of all prevalence (or magnitude) estimates. Since this is obviously not the case, we needed to compute a weighted mean by assigning weights to the studies. There are two computational model approaches in meta-analysis to achieve this: the fixed effect model and the random effects model. A fixed effect model would be appropriate if there was plausible reason to believe that all studies in the synthesis were functionally identical. This is generally not a valid assumption in any meta-analysis that synthesizes data from primary literature170. Thus, here we used the random effects model. In so doing, we weighted each study by the inverse of its original (within-studies) variance plus between-studies variance.
In the above equations, event rate with respect to prevalence of groundwater influence calculations refers to the number of samples out of a universe of xylem samples reported to have groundwater contribution in xylem. Two input variables were required: (1) number of samples reported to have groundwater contribution in xylem, and (2) total number of xylem samples. Event rate with respect to magnitude of groundwater influence calculations refers to the mean proportion of groundwater contribution in xylem. Two input variables were required: (1) mean proportion of groundwater contribution in xylem, and (2) total number of xylem samples. Our database consists of source papers (n = 138) that represent five main source identification or source apportionment approaches: (1) direct inference (n = 59); (2) IsoSource155, (n = 34); 2, 3-source mass balance (n = 32); Bayesian153,154 (n = 8); and custom author (“other”, n = 5). All data syntheses on prevalence and magnitude of groundwater influence were performed at a species level. Genus and other categorical variables (e.g. terrestrial biome, angiosperm/gymnosperm, source apportionment approach, etc.) were assigned accordingly to allow for associated statistical tests. To be conservative and avoid underestimation from a meta-analysis of IsoSource155 papers, we calculated the mean value of the reported maximum proportions. We also performed a meta-analysis using the mean value of reported minimum proportions and grand mean of reported mean values in IsoSource papers. Performing these resulted in estimates biased towards the lower end of the range reported in Fig. 2a. Where a source paper used any of the other four source identification/apportionment approaches, we calculated the mean value of all reported proportions. For studies with reported measurements at multiple time points (e.g. multiple dates through the year), we calculated the mean values of reported proportions at multiple time points and used the mean score in the calculation of prevalence and magnitude estimates172,173.
Ref. 9 showed that groundwater dependent ecosystems span a wide range, from wettest to driest environments. This implies that controls on groundwater use by vegetation are manifold, possibly a combination of a host of factors that vary from one site and/or species to the next160,174,175,176. For example, ref. 160 showed that in an extremely arid setting (annual precipitation ~35 mm, potential evaporation ~2600 mm), plant physiological parameters (e.g. leaf-specific hydraulic conductance and leaf water relations of phreatophytes) were related to groundwater depth. By extension, rooting depth and rooting zone water storage were also related to groundwater depth. To achieve a multivariate characterization of the magnitude of groundwater influence, we supplemented the primary information available from source papers with site-specific datasets: rooting depth177, rooting zone water storage amount178, water table depth179, potential evapotranspiration and aridity index180, precipitation amount166, and streamflow amount165. We also derived actual evapotranspiration (AET) as the difference between precipitation and streamflow180. This resulted in a table with the aforementioned variables as data columns in addition to the mean magnitude estimates of groundwater contribution from the random effects model in meta-analysis. We then used a batch algorithm and a locally weighted linear smoother (self-organizing maps clustering technique171) to explore the structure of the meta-analysis database. Like classical k-means clustering, self-organizing maps (SOMs) technique performs an iterative alternating fitting process that results in the formation of a number of clusters.
Each cluster is a set of points that share similar values across a number of variables. Unlike the classical k-means clustering, SOMs form clusters in a particular layout on a grid. This results in the formation of clusters with points that are near each other in the SOM grid as well as in multivariate space. The SOM technique was an appropriate treatment of our data because of the wide range of variability in the magnitude estimates of groundwater influence. The SOM was implemented first by using principal components analysis thereby determining the two directions that captured the most variation in the data. It then laid out a grid in the principal component space thereby forming clusters back in the original space of the variables. The same clustering principle and estimation of means as in k-means approach was then used in assigning clusters. This analysis resulted in the identification of seven clusters shown in Fig. 3. The inset in Fig. 3 shows an intuitive outcome of the SOM approach used here wherein the magnitude of groundwater influence increases with aridity (i.e. inversely proportional to aridity index).
Publication bias
Any meta-analysis is prone to publication bias (i.e. the file-drawer problem182). This is a function, by and large, of the inherent propensity of many journals to not publish negative results. A meta-analysis such as this contribution, therefore, may not be immune to such publication bias in the most fundamental sense. Nevertheless, here we addressed this issue in two phases: (Phase 1) structural intervention to mitigate against bias (before data synthesis and meta-analysis); (Phase 2) statistical assessment of bias (after data synthesis and meta-analysis).
Phase 1 was achieved by not discriminating against studies performed in any particular physiographic setting. For example, rather than synthesizing information exclusively from source papers in settings reported to have groundwater influence, we synthesized information from source papers across all habitat types. Phase 2 was achieved by performing graphical and statistical analysis to assess bias. Extended Data Fig. 1 shows a funnel plot of standard error by logit event rate. Large studies tend to cluster near the mean effect size and toward the top of the graph. Smaller studies tend to be more dispersed (i.e. greater sampling variation in prevalence estimates) and toward the bottom of the graph. In the absence of publication bias studies would be distributed symmetrically about the combined effect size. The absence of publication bias in our study is confirmed as shown in Extended Data Fig. 1. We also employed three statistical approaches to assess bias: Classic Fail-safe N183, Begg and Mazumdar Rank Correlation Test170,184, and Duval and Tweedie’s Trim and Fill185. Classic Fail-safe N yielded a z-value of −5.37594, a corresponding 2-tailed P < 0.0001, and a Fail-safe N of 901. This means that we would need to locate and include 901 ‘null’ studies in order for the combined 2-tailed P-value to exceed 0.050, and therefore nullify our results. Given that the initial number of returned studies from our database search was between 533 and 615, here we rule out bias based on this approach.
The Begg and Mazumdar Rank Correlation Test170,184 computes the rank order correlation between the prevalence and magnitude estimates and the standard error (driven primarily by sample size). They suggest that a significant correlation points to the existence of bias. Based on this approach the 2-tailed P-value was 0.35739, which means that we could rule out the existence of bias. Finally, the Duval and Tweedie Trim and Fill185 suggests a way to determine where the missing studies were likely to fall if at all bias existed, add them to the database and then reanalyze the overall prevalence estimates. Using this approach, the prevalence estimate and 95% CI is 0.36915 (0.28443, 0.46277), which remained unchanged from the original (Fig. 1). The unchanged values suggest, and confirm earlier indications of, the absence of publication bias. We note the approaches that we employed in Phase 1 (literature search process) and Phase 2 (assessment of publication bias) are in compliance with “state of the art” and “state of the practice” in meta-analyses, particularly the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines186.
Sensitivity analysis
To be able to test the robustness of the overall estimate of groundwater influence [23 (95% CI, 20–26%)], we performed a sensitivity analysis by removing one study at a time and recalculating the effect of this “removal” on the overall estimate. This was performed for all 138 source papers and at each corresponding genus (n = 251) and species (n = 414). Results of the sensitivity analysis showed markedly minimal effect on the overall estimate, with mean point estimates ranging between 22.68% and 23.36%; 95% lower limit ranging between 19.83% and 20.59%; 95% upper limit ranging between 25.81% and 26.38%.
Isotope systems
When the analysis is grouped by isotope system used, we note that source papers that used δ2H (n = 35), δ18O (n = 31), or δ2H-δ18O (n = 72) isotope systems yield prevalence and magnitude estimates that may be different from each other. With respect to prevalence estimates, δ2H and δ2H- δ18O source papers tended to be similar (not statistically different) to each other: 32% (95% CI, 20–46%) and 31% (95% CI, 17–50%), respectively. δ18O tended to overestimate prevalence at 55% (95% CI, 38–72%). With respect to magnitude estimates, δ2H and δ18O source papers tended to be comparable (not statistically different) with each other: 32% (95% CI, 23–41%) and 44% (95% CI, 33–55%), respectively. δ2H-δ18O tended to underestimate magnitude at 15% (95% CI, 11–20%).
Taxonomic and biome effects on groundwater use
Approximately 93% of taxonomic information in our database were referenced from National Center for Biotechnology Information (NCBI http://www.ncbi.nlm.nih.gov/taxonomy).
Additional Information
How to cite this article: Evaristo, J. and McDonnell, J. J. Prevalence and magnitude of groundwater use by vegetation: a global stable isotope meta-analysis. Sci. Rep. 7, 44110; doi: 10.1038/srep44110 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Footnotes
The authors declare no competing financial interests.
Author Contributions J.E. designed the study, compiled the data set, and conducted the statistical analyses. J.E. wrote the first paper draft. J.J.M. helped conceive the study, edited and commented on the manuscript and contributed to the text in later iterations.
References
- Schlesinger W. H. & Jasechko S. Transpiration in the global water cycle. Agric. For. Meteorol. 189, 115–117 (2014). [Google Scholar]
- Soulsby C., Birkel C. & Tetzlaff D. Characterizing the age distribution of catchment evaporative losses. Hydrol. Process. 30, 1308–1312 (2016). [Google Scholar]
- Evaristo J., Jasechko S. & McDonnell J. J. Global separation of plant transpiration from groundwater and streamflow. Nature 525, 91–94 (2015). [DOI] [PubMed] [Google Scholar]
- Good S. P., Noone D. & Bowen G. Hydrologic connectivity constrains partitioning of global terrestrial water fluxes. Science 349, 175–177 (2015). [DOI] [PubMed] [Google Scholar]
- Fan Y. Groundwater in the Earth’s critical zone: Relevance to large-scale patterns and processes. Water Resour. Res. 51, 3052–3069 (2015). [Google Scholar]
- Goedhart C. M. & Pataki D. E. Ecosystem effects of groundwater depth in Owens Valley, California. Ecohydrology 4, 458–468 (2011). [Google Scholar]
- Eamus D. & Froend R. Groundwater-dependent ecosystems: the where, what and why of GDEs. Aust. J. Bot. 54, 91–96 (2006). [Google Scholar]
- Elmore A., Manning S., Mustard J. & Craine J. Decline in alkali meadow vegetation cover in California: the effects of groundwater extraction and drought. J. Appl. Ecol. 43, 770–779 (2006). [Google Scholar]
- Howard J. & Merrifield M. Mapping groundwater dependent ecosystems in California. Plos One 5, e11249 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutanto S. J. A perspective on isotope versus non-isotope approaches to determine the contribution of transpiration to total evaporation. Hydrol. Earth Syst. Sci. 18, 2815–2827 (2014). [Google Scholar]
- Clark M. P. et al. Improving the representation of hydrologic processes in Earth System Models. Water Resour. Res. 51, 5929–5956 (2015). [Google Scholar]
- Good S. P., Noone D., Kurita N., Benetti M. & Bowen G. J. D/H isotope ratios in the global hydrologic cycle. Geophys. Res. Lett. 42, 5042–5050 (2015). [Google Scholar]
- Ehleringer J. R. & Dawson T. E. Water uptake by plants: perspectives from stable isotope composition. Plant, Cell Environ. 15, 1073–1082 (1992). [Google Scholar]
- Zimmermann U. et al. Tracers determine movement of soil moisture and evapotranspiration. Science 152, 346–347 (1966). [DOI] [PubMed] [Google Scholar]
- Dawson T. E. & Ehleringer J. R. Streamside trees that do not use stream water. Nature 350, 335–337 (1991). [Google Scholar]
- White J. W. C. In Stable Isotopes in Ecological Research (eds Rundel P. W., Ehleringer J. R. & Nagy K. A.) 142–162 (Springer New York, New York, NY, 1989). [Google Scholar]
- Berkelhammer M. et al. Convergent approaches to determine an ecosystem’s transpiration fraction. Global Biogeochem. Cycles 30, 933–951 (2016). [Google Scholar]
- Fritzsche F. et al. Soil-plant hydrology of indigenous and exotic trees in an Ethiopian montane forest. Tree Physiol. 26, 1043–1054 (2006). [DOI] [PubMed] [Google Scholar]
- Andrade J., Meinzer F., Goldstein G. & Schnitzer S. Water uptake and transport in lianas and co-occurring trees of a seasonally dry tropical forest. Trees-Structure and Function 19, 282–289 (2005). [Google Scholar]
- Andrews S. F., Flanagan L. B., Sharp E. J. & Cai T. Variation in water potential, hydraulic characteristics and water source use in montane Douglas-fir and lodgepole pine trees in southwestern Alberta and consequences for seasonal changes in photosynthetic capacity. Tree Physiol. 32, 146–160 (2012). [DOI] [PubMed] [Google Scholar]
- Barbeta A. et al. The combined effects of a long-term experimental drought and an extreme drought on the use of plant-water sources in a Mediterranean forest. Global Change Biol. 21, 1213–1225 (2015). [DOI] [PubMed] [Google Scholar]
- Berkelhammer M. et al. The nocturnal water cycle in an open-canopy forest. Journal of Geophysical Research-Atmospheres 118, 10225–10242 (2013). [Google Scholar]
- Berry Z. C., Hughes N. M. & Smith W. K. Cloud immersion: an important water source for spruce and fir saplings in the southern Appalachian Mountains. Oecologia 174, 319–326 (2014). [DOI] [PubMed] [Google Scholar]
- Bertrand G. et al. Determination of spatiotemporal variability of tree water uptake using stable isotopes (d 18O, d 2H) in an alluvial system supplied by a high- altitude watershed, Pfyn forest, Switzerland. Ecohydrology 7, 319–333 (2014). [Google Scholar]
- Beyer M. et al. A deuterium-based labeling technique for the investigation of rooting depths, water uptake dynamics and unsaturated zone water transport in semiarid environments. Journal of Hydrology 533, 627–643 (2016). [Google Scholar]
- Bijoor N. S., McCarthy H. R., Zhang D. & Pataki D. E. Water sources of urban trees in the Los Angeles metropolitan area. Urban Ecosystems 15, 195–214 (2012). [Google Scholar]
- Bishop K. & Dambrine E. Localization of Tree Water-Uptake in Scots Pine and Norway Spruce with Hydrological Tracers. Canadian Journal of Forest Research-Revue Canadienne De Recherche Forestiere 25, 286–297 (1995). [Google Scholar]
- Boutton T. W., Archer S. R. & Midwood A. J. Stable isotopes in ecosystem science: Structure, function and dynamics of a subtropical savanna. Rapid Commun. Mass Spectrom. 13, 1263–1277 (1999). [DOI] [PubMed] [Google Scholar]
- Brooks J. R., Barnard H. R., Coulombe R. & McDonnell J. J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nature Geoscience 3, 100–104 (2010). [Google Scholar]
- Brunel J. P., Walker G. R., Dighton J. C. & Monteny B. Use of stable isotopes of water to determine the origin of water used by the vegetation and to partition evapotranspiration. A case study from HAPEX-Sahel. Journal of Hydrology 189, 466–481 (1997). [Google Scholar]
- Brunel J. P., Walker G. R. & Kennettsmith A. K. Field validation of isotopic procedures for determining sources of water used by plants in a semiarid environment. Journal of Hydrology 167, 351–368 (1995). [Google Scholar]
- Burgess S., Adams M., Turner N. & Ward B. Characterisation of hydrogen isotope profiles in an agroforestry system: implications for tracing water sources of trees. Agric. Water Manage. 45, 229–241 (2000). [Google Scholar]
- Busch D., Ingraham N. & Smith S. Water-uptake in woody riparian phreatophytes of the Southwestern United-States - A stable isotope study. Ecol. Appl. 2, 450–459 (1992). [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen Y., Xu C. & Li W. The effects of groundwater depth on water uptake of Populus euphratica and Tamarix ramosissima in the hyperarid region of Northwestern China. Environmental Science and Pollution Research 23, 17404–17412 (2016). [DOI] [PubMed] [Google Scholar]
- Chimner R. & Cooper D. Using stable oxygen isotopes to quantify the water source used for transpiration by native shrubs in the San Luis Valley, Colorado USA. Plant Soil 260, 225–236 (2004). [Google Scholar]
- Chimner R. A. & Resh S. C. Are Riparian Bur Oak Phreatophytic? A stable water isotope study in Homestead National Monument, Nebraska. Nat. Areas J. 34, 56–64 (2014). [Google Scholar]
- Clinton B. D., Vose J. M., Vroblesky D. A. & Harvey G. J. Determination of the relative uptake of ground vs. surface water by Populus deltoides during phytoremediation. Int. J. Phytoremediation 6, 239–252 (2004). [DOI] [PubMed] [Google Scholar]
- Cook P. & O’Grady A. Determining soil and ground water use of vegetation from heat pulse, water potential and stable isotope data. Oecologia 148, 97–107 (2006). [DOI] [PubMed] [Google Scholar]
- Cooper D., Merritt D., Andersen D. & Chimner R. Factors controlling the establishment of Fremont cottonwood seedlings on the upper Green River, USA. Regulated Rivers-Research & Management 15, 419–440 (1999). [Google Scholar]
- Costelloe J. F. et al. Water sources accessed by arid zone riparian trees in highly saline environments, Australia. Oecologia 156, 43–52 (2008). [DOI] [PubMed] [Google Scholar]
- Dai Y., Zheng X., Tang L. & Li Y. Stable oxygen isotopes reveal distinct water use patterns of two Haloxylon species in the Gurbantonggut Desert. Plant Soil 389, 73–87 (2015). [Google Scholar]
- David T. S. et al. Root functioning, tree water use and hydraulic redistribution in Quercus suber trees: A modeling approach based on root sap flow. For. Ecol. Manage. 307, 136–146 (2013). [Google Scholar]
- Dawson T. & Pate J. Seasonal water uptake and movement in root systems of Australian phraeatophytic plants of dimorphic root morphology: A stable isotope investigation. Oecologia 107, 13–20 (1996). [DOI] [PubMed] [Google Scholar]
- Deng Y., Jiang Z. & Qin X. Water source partitioning among trees growing on carbonate rock in a subtropical region of Guangxi, China. Environmental Earth Sciences 66, 635–640 (2012). [Google Scholar]
- Deng Y., Kuo Y., Jiang Z., Qin X. & Jin Z. Using stable isotopes to quantify water uptake by Cyclobalanopsis glauca in typical clusters of karst peaks in China. Environmental Earth Sciences 74, 1039–1046 (2015). [Google Scholar]
- Donovan L. & Ehleringer J. Water-stress and use of summer precipitation in a Great-Basin shrub community. Funct. Ecol. 8, 289–297 (1994). [Google Scholar]
- Drake P. L., Froend R. H. & Franks P. J. Linking hydraulic conductivity and photosynthesis to water-source partitioning in trees versus seedlings. Tree Physiol. 31, 763–773 (2011). [DOI] [PubMed] [Google Scholar]
- Drake P. & Franks P. Water resource partitioning, stem xylem hydraulic properties, and plant water use strategies in a seasonally dry riparian tropical rainforest. Oecologia 137, 321–329 (2003). [DOI] [PubMed] [Google Scholar]
- Duan D., Ouyang H., Song M. & Hu Q. Water sources of dominant species in three alpine ecosystems on the Tibetan Plateau, China. Journal of Integrative Plant Biology 50, 257–264 (2008). [DOI] [PubMed] [Google Scholar]
- Eggemeyer K. D. et al. Seasonal changes in depth of water uptake for encroaching trees Juniperus virginiana and Pinus ponderosa and two dominant C(4) grasses in a semiarid grassland. Tree Physiol. 29, 157–169 (2009). [DOI] [PubMed] [Google Scholar]
- Ehleringer J., Phillips S., Schuster W. & Sandquist D. Differential utilization of summer rains by desert plants. Oecologia 88, 430–434 (1991). [DOI] [PubMed] [Google Scholar]
- Ellsworth P. Z. & Sternberg L. S. L. Seasonal water use by deciduous and evergreen woody species in a scrub community is based on water availability and root distribution. Ecohydrology 8, 538–551 (2015). [Google Scholar]
- Evans R. & Ehleringer J. Water and nitrogen dynamics in an arid Woodland. Oecologia 99, 233–242 (1994). [DOI] [PubMed] [Google Scholar]
- Evaristo J., McDonnell J. J., Scholl M. A., Bruijnzeel L. A. & Chun K. P. Insights into plant water uptake from xylem-water isotope measurements in two tropical catchments with contrasting moisture conditions. Hydrol. Process. 30, 3210–3227 (2016). [Google Scholar]
- Ewe S. M. L., Sternberg L. d. S. L. & Childers D. L. Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia 152, 607–616 (2007). [DOI] [PubMed] [Google Scholar]
- February E. C., Higgins S. I., Newton R. & West A. G. Tree distribution on a steep environmental gradient in an arid savanna. J. Biogeogr. 34, 270–278 (2007). [Google Scholar]
- February E. C., West A. G. & Newton R. J. The relationship between rainfall, water source and growth for an endangered tree. Austral Ecol. 32, 397–402 (2007). [Google Scholar]
- Filella I. & Penuelas J. Partitioning of water and nitrogen in co-occurring Mediterranean woody shrub species of different evolutionary history. Oecologia 137, 51–61 (2003). [DOI] [PubMed] [Google Scholar]
- Flanagan L., Ehleringer J. & Marshall J. Differential Uptake of Summer Precipitation among Cooccurring Trees and Shrubs in a Pinyon-Juniper Woodland. Plant Cell and Environment 15, 831–836 (1992). [Google Scholar]
- Gaines K. P. et al. Reliance on shallow soil water in a mixed-hardwood forest in central Pennsylvania. Tree Physiol. 36, 444–458 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcin Y. et al. Hydrogen isotope ratios of lacustrine sedimentary n-alkanes as proxies of tropical African hydrology: Insights from a calibration transect across Cameroon. Geochim. Cosmochim. Acta 79, 106–126 (2012). [Google Scholar]
- Geris J. et al. Ecohydrological separation in wet, low energy northern environments? A preliminary assessment using different soil water extraction techniques. Hydrol. Process. 29, 5139–5152 (2015). [Google Scholar]
- Gierke C., Newton B. T. & Phillips F. M. Soil-water dynamics and tree water uptake in the Sacramento Mountains of New Mexico (USA): a stable isotope study. Hydrogeol. J. 24, 805–818 (2016). [Google Scholar]
- Goldsmith G. R. et al. Stable isotopes reveal linkages among ecohydrological processes in a seasonally dry tropical montane cloud forest. Ecohydrology 5, 779–790 (2012). [Google Scholar]
- Gu D. et al. Seasonal water use strategy of Cyclobalanopsis glauca in a karst area of southern China. Environmental Earth Sciences 74, 1007–1014 (2015). [Google Scholar]
- Gutierrez-Soto M. V. & Ewel J. J. Water use in four model tropical plant associations established in the lowlands of Costa Rica. Rev. Biol. Trop. 56, 1947–1957 (2008). [DOI] [PubMed] [Google Scholar]
- Hao X., Chen Y., Guo B. & Ma J. Hydraulic redistribution of soil water in Populus euphratica Oliv. in a central Asian desert riparian forest. Ecohydrology 6, 974–983 (2013). [Google Scholar]
- Hartsough P., Poulson S. R., Biondi F. & Estrada I. G. Stable isotope characterization of the ecohydrological cycle at a tropical treeline site. Arctic Antarctic and Alpine Research 40, 343–354 (2008). [Google Scholar]
- Hasselquist N. J. & Allen M. F. Increasing demands on limited water resources: Consequences for two endangered plants in Amargosa Valley, USA. Am. J. Bot. 96, 620–626 (2009). [DOI] [PubMed] [Google Scholar]
- Hasselquist N. J., Allen M. F. & Santiago L. S. Water relations of evergreen and drought-deciduous trees along a seasonally dry tropical forest chronosequence. Oecologia 164, 881–890 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland K. L., Tyerman S. D., Mensforth L. J. & Walker G. R. Tree water sources over shallow, saline groundwater in the lower River Murray, south-eastern Australia: implications for groundwater recharge mechanisms. Aust. J. Bot. 54, 193–205 (2006). [Google Scholar]
- Ishshalom N., Sternberg L., Ross M., Obrien J. & Flynn L. Water utilization of tropical hardwood hammocks of the Lower Florida Keys. Oecologia 92, 108–112 (1992). [DOI] [PubMed] [Google Scholar]
- Jackson P., Cavelier J., Goldstein G., Meinzer F. & Holbrook N. Partitioning of water-resources among plants of a lowland tropical forest. Oecologia 101, 197–203 (1995). [DOI] [PubMed] [Google Scholar]
- Jackson P. et al. Partitioning of soil water among tree species in a Brazilian Cerrado ecosystem. Tree Physiol. 19, 717–724 (1999). [DOI] [PubMed] [Google Scholar]
- Jia G., Yu X., Deng W., Liu Y. & Li Y. Determination of minimum extraction times for water of plants and soils used in isotopic analysis. J. Food Agric. Environ. 10, 1035–1040 (2012). [Google Scholar]
- Kitajima K., Allen M. F. & Goulden M. L. Contribution of hydraulically lifted deep moisture to the water budget in a Southern California mixed forest. Journal of Geophysical Research-Biogeosciences 118, 1561–1572 (2013). [Google Scholar]
- Kolb T., Hart S. & Amundson R. Boxelder water sources and physiology at perennial and ephemeral stream sites in Arizona. Tree Physiol. 17, 151–160 (1997). [DOI] [PubMed] [Google Scholar]
- Kray J. A., Cooper D. J. & Sanderson J. S. Groundwater use by native plants in response to changes in precipitation in an intermountain basin. J. Arid Environ. 83, 25–34 (2012). [Google Scholar]
- Kukowski K. R., Schwinning S. & Schwartz B. F. Hydraulic responses to extreme drought conditions in three co-dominant tree species in shallow soil over bedrock. Oecologia 171, 819–830 (2013). [DOI] [PubMed] [Google Scholar]
- Kulmatiski A., Beard K. H. & Stark J. M. Exotic plant communities shift water-use timing in a shrub-steppe ecosystem. Plant Soil 288, 271–284 (2006). [Google Scholar]
- Kurz-Besson C. et al. Hydraulic lift in cork oak trees in a savannah-type Mediterranean ecosystem and its contribution to the local water balance. Plant Soil 282, 361–378 (2006). [Google Scholar]
- Leng X. et al. Differential water uptake among plant species in humid alpine meadows. Journal of Vegetation Science 24, 138–147 (2013). [Google Scholar]
- Lin G., Phillips S. & Ehleringer J. Monosoonal precipitation responses of shrubs in a cold desert community on the Colorado Plateau. Oecologia 106, 8–17 (1996). [DOI] [PubMed] [Google Scholar]
- Link C. M., Thevathasan N. V., Gordon A. M. & Isaac M. E. Determining tree water acquisition zones with stable isotopes in a temperate tree-based intercropping system. Agrofor. Syst. 89, 611–620 (2015). [Google Scholar]
- Liu S. et al. Use of H-2 and O-18 stable isotopes to investigate water sources for different ages of Populus euphratica along the lower Heihe River. Ecol. Res. 30, 581–587 (2015). [Google Scholar]
- Liu W. et al. Vertical patterns of soil water acquisition by non-native rubber trees (Hevea brasiliensis) in Xishuangbanna, southwest China. Ecohydrology 7, 1234–1244 (2014). [Google Scholar]
- Liu W., Li P., Duan W. & Liu W. Dry-season water utilization by trees growing on thin karst soils in a seasonal tropical rainforest of Xishuangbanna, Southwest China. Ecohydrology 7, 927–935 (2014). [Google Scholar]
- Liu W., Liu W., Li P., Duan W. & Li H. Dry season water uptake by two dominant canopy tree species in a tropical seasonal rainforest of Xishuangbanna, SW China. Agric. For. Meteorol. 150, 380–388 (2010). [Google Scholar]
- Liu Y. et al. Analyzing relationships among water uptake patterns, rootlet biomass distribution and soil water content profile in a subalpine shrubland using water isotopes. Eur. J. Soil Biol. 47, 380–386 (2011). [Google Scholar]
- Ludwig F., Dawson T., de Kroon H., Berendse F. & Prins H. Hydraulic lift in Acacia tortilis trees on an East African savanna. Oecologia 134, 293–300 (2003). [DOI] [PubMed] [Google Scholar]
- Ma Y. & Song X. Using stable isotopes to determine seasonal variations in water uptake of summer maize under different fertilization treatments. Sci. Total Environ. 550, 471–483 (2016). [DOI] [PubMed] [Google Scholar]
- McCole A. A. & Stern L. A. Seasonal water use patterns of Juniperus ashei on the Edwards Plateau, Texas, based on stable isotopes in water. Journal of Hydrology 342, 238–248 (2007). [Google Scholar]
- McKeon C. et al. Growth and water and nitrate uptake patterns of grazed and ungrazed desert shrubs growing over a nitrate contamination plume. J. Arid Environ. 64, 1–21 (2006). [Google Scholar]
- Meinzer F. et al. Partitioning of soil water among canopy trees in a seasonally dry tropical forest. Oecologia 121, 293–301 (1999). [DOI] [PubMed] [Google Scholar]
- Meissner M., Koehler M., Schwendenmann L. & Hoelscher D. Partitioning of soil water among canopy trees during a soil desiccation period in a temperate mixed forest. Biogeosciences 9, 3465–3474 (2012). [Google Scholar]
- Mensforth L. J., Thorburn P. J., Tyerman S. D. & Walker G. R. Sources of water used by riparian Eucalyptus-Camaldulensis overlying highly saline groundwater. Oecologia 100, 21–28 (1994). [DOI] [PubMed] [Google Scholar]
- Nardini A. et al. Rooting depth, water relations and non-structural carbohydrate dynamics in three woody angiosperms differentially affected by an extreme summer drought. Plant Cell and Environment 39, 618–627 (2016). [DOI] [PubMed] [Google Scholar]
- Nie Y. et al. Seasonal water use patterns of woody species growing on the continuous dolostone outcrops and nearby thin soils in subtropical China. Plant Soil 341, 399–412 (2011). [Google Scholar]
- Nippert J. B. & Knapp A. K. Linking water uptake with rooting patterns in grassland species. Oecologia 153, 261–272 (2007). [DOI] [PubMed] [Google Scholar]
- O’Grady A., Eamus D., Cook P. & Lamontagne S. Groundwater use by riparian vegetation in the wet-dry tropics of northern Australia. Aust. J. Bot. 54, 145–154 (2006). [DOI] [PubMed] [Google Scholar]
- Ohte N. et al. Water utilization of natural and planted trees in the semiarid desert of Inner Mongolia, China. Ecol. Appl. 13, 337–351 (2003). [Google Scholar]
- Penna D. et al. Tracing the water sources of trees and streams: isotopic analysis in a small pre-alpine catchment. Four Decades of Progress in Monitoring and Modeling of Processes in the Soil-Plant-Atmosphere System: Applications and Challenges 19, 106–112 (2013). [Google Scholar]
- Phillips S. L. & Ehleringer J. R. Limited uptake of summer precipitation by Bigtooth Maple (Acer-Grandidentatum Nutt) and Gambels Oak (Quercus-Gambelii Nutt). Trees Structure and Function 9, 214–219 (1995). [Google Scholar]
- Pivovaroff A. L. et al. Multiple strategies for drought survival among woody plant species. Funct. Ecol. 30, 517–526 (2016). [Google Scholar]
- Plamboeck A., Grip H. & Nygren U. A hydrological tracer study of water uptake depth in a Scots pine forest under two different water regimes. Oecologia 119, 452–460 (1999). [DOI] [PubMed] [Google Scholar]
- Prechsl U. E., Burri S., Gilgen A. K., Kahmen A. & Buchmann N. No shift to a deeper water uptake depth in response to summer drought of two lowland and sub-alpine C 3-grasslands in Switzerland. Oecologia 177, 97–111 (2015). [DOI] [PubMed] [Google Scholar]
- Prieto I. & Ryel R. J. Internal hydraulic redistribution prevents the loss of root conductivity during drought. Tree Physiol. 34, 39–48 (2014). [DOI] [PubMed] [Google Scholar]
- Querejeta J. I., Estrada-Medina H., Allen M. F. & Jimenez-Osornio J. J. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia 152, 26–36 (2007). [DOI] [PubMed] [Google Scholar]
- Querejeta J. I., Estrada-Medina H., Allen M. F., Jimenez-Osornio J. J. & Ruenes R. Utilization of bedrock water by Brosimum alicastrum trees growing on shallow soil atop limestone in a dry tropical climate. Plant Soil 287, 187–197 (2006). [Google Scholar]
- Retzlaff W., Blaisdell G. & Topa M. Seasonal charges in water source of four families of loblolly pine (Pinus taeda L.). Trees- Structure and Function 15, 154–162 (2001). [Google Scholar]
- Rong L., Chen X., Chen X., Wang S. & Du X. Isotopic analysis of water sources of mountainous plant uptake in a karst plateau of southwest China. Hydrol. Process. 25, 3666–3675 (2011). [Google Scholar]
- Rosado B. H. P., De Mattos E. A. & Sternberg L. D. S. L. Are leaf physiological traits related to leaf water isotopic enrichment in restinga woody species? An. Acad. Bras. Cienc. 85, 1035–1045 (2013). [DOI] [PubMed] [Google Scholar]
- Rose K. L., Graham R. C. & Parker D. R. Water source utilization by Pinus jeffreyi and Arctostaphylos patula on thin soils over bedrock. Oecologia 134, 46–54 (2003). [DOI] [PubMed] [Google Scholar]
- Sanchez-Perez J. M., Lucot E., Bariac T. & Tremolieres M. Water uptake by trees in a riparian hardwood forest (Rhine floodplain, France). Hydrol. Process. 22, 366–375 (2008). [Google Scholar]
- Schulze E. et al. Rooting depth, water availability, and vegetation cover along an aridity gradient in Patagonia. Oecologia 108, 503–511 (1996). [DOI] [PubMed] [Google Scholar]
- Schwendenmann L., Pendall E., Sanchex-Bragado R., Kunert N. & Holsher D. Tree water uptake in a tropical plantation varying in tree diversity: interspecific differences, seasonal shifts and complementarity. Ecohydrol. 8, 1–12 (2014). [Google Scholar]
- Shim J. H. et al. Hydrologic control of the oxygen isotope ratio of ecosystem respiration in a semi-arid woodland. Biogeosciences 10, 4937–4956 (2013). [Google Scholar]
- Simonin K. A. et al. Vegetation induced changes in the stable isotope composition of near surface humidity. Ecohydrology 7, 936–949 (2014). [Google Scholar]
- Smith S., Wellington A., Nachlinger J. & Fox C. Functional-responses of riparian vegetation to streamflow diversion in the Eastern Sierra-Nevada. Ecol. Appl. 1, 89–97 (1991). [DOI] [PubMed] [Google Scholar]
- Snyder K. A. & Williams D. G. Water sources used by riparian trees varies among stream types on the San Pedro River, Arizona. Agric. For. Meterol. 105, 227–240 (2000). [Google Scholar]
- Snyder K. & Williams D. Defoliation alters water uptake by deep and shallow roots of Prosopis velutina (Velvet Mesquite). Funct. Ecol. 17, 363–374 (2003). [Google Scholar]
- Song L., Zhu J., Li M. & Zhang J. Water use patterns of Pinus sylvestris var. mongolica trees of different ages in a semiarid sandy lands of Northeast China. Environ. Exp. Bot. 129, 94–107 (2016). [Google Scholar]
- Song L., Zhu J., Li M., Zhang J. & Lv L. Sources of water used by Pinus sylvestris var. mongolica trees based on stable isotope measurements in a semiarid sandy region of Northeast China. Agric. Water Manage. 164, 281–290 (2016). [Google Scholar]
- Stratton L., Goldstein G. & Meinzer F. Temporal and spatial partitioning of water resources among eight woody species in a Hawaiian dry forest. Oecologia 124, 309–317 (2000). [DOI] [PubMed] [Google Scholar]
- Sun S., Meng P., Zhang J. & Wan X. Variation in soil water uptake and its effect on plant water status in Juglans regia L. during dry and wet seasons. Tree Physiol. 31, 1378–1389 (2011). [DOI] [PubMed] [Google Scholar]
- Sun S., Huang J., Han X. & Lin G. Comparisons in water relations of plants between newly formed riparian and non-riparian habitats along the bank of Three Gorges Reservoir, China. Trees-Structure and Function 22, 717–728 (2008). [Google Scholar]
- Sun Z., Long X. & Ma R. Water uptake by saltcedar (Tamarix ramosissima) in a desert riparian forest: responses to intra-annual water table fluctuation. Hydrol. Process. 30, 1388–1402 (2016). [Google Scholar]
- Swaffer B. A., Holland K. L., Doody T. M., Li C. & Hutson J. Water use strategies of two co-occurring tree species in a semi-arid karst environment. Hydrol. Process. 28, 2003–2017 (2014). [Google Scholar]
- Tang K. L. & Feng X. H. The effect of soil hydrology on the oxygen and hydrogen isotopic compositions of plants’ source water. Earth Planet. Sci. Lett. 185, 355–367 (2001). [Google Scholar]
- Thorburn P., Walker G. & Brunel J. Extraction of water from Eucalyptus trees for analysis of deuterium and O-18 – Laboratory and field techniques. Plant Cell and Environment 16, 269–277 (1993). [Google Scholar]
- Valentini R., Mugnozza G. & Ehleringer J. Hydrogen and carbon isotope ratios of selected species of a Mediterranean Macchia ecosystem. Funct. Ecol. 6, 627–631 (1992). [Google Scholar]
- Voltas J., Lucabaugh D., Regina Chambel M. & Pedro Ferrio J. Intraspecific variation in the use of water sources by the circum-Mediterranean conifer Pinus halepensis. New Phytol. 208, 1031–1041 (2015). [DOI] [PubMed] [Google Scholar]
- Wang L., Mu Y., Zhang Q. & Zhang X. Groundwater use by plants in a semi-arid coal-mining area at the Mu Us Desert frontier. Environmental Earth Sciences 69, 1015–1024 (2013). [Google Scholar]
- Wang P., Song X., Han D., Zhang Y. & Liu X. A study of root water uptake of crops indicated by hydrogen and oxygen stable isotopes: A case in Shanxi Province, China. Agric. Water Manage. 97, 475–482 (2010). [Google Scholar]
- Wei Y., Fang J., Zhao X., Yi M. Zhang R. & Li S. Isotopic model estimate of relative contribution of potential water pools to water uptake of Pinus sylvestris var. mongolica in Horqin Sandy Land. Journal of Resources and Ecology 3, 308–315 (2012). [Google Scholar]
- Wei L., Lockington D. A., Poh S., Gasparon M. & Lovelock C. E. Water use patterns of estuarine vegetation in a tidal creek system. Oecologia 172, 485–494 (2013). [DOI] [PubMed] [Google Scholar]
- Wei Y. F., Fang J., Liu S., Zhao X. Y. & Li S. G. Stable isotopic observation of water use sources of Pinus sylvestris var. mongolica in Horqin Sandy Land, China. Trees-Structure and Function 27, 1249–1260 (2013). [Google Scholar]
- West A. G. et al. Diverse functional responses to drought in a Mediterranean-type shrubland in South Africa. New Phytol. 195, 396–407 (2012). [DOI] [PubMed] [Google Scholar]
- Williams D. G. & Ehleringer J. R. Intra- and interspecific variation for summer precipitation use in pinyon-juniper woodlands. Ecol. Monogr. 70, 517–537 (2000). [Google Scholar]
- Wu H. et al. Contrasting water use pattern of introduced and native plants in an alpine desert ecosystem, Northeast Qinghai-Tibet Plateau, China. Sci. Total Environ. 542, 182–191 (2016). [DOI] [PubMed] [Google Scholar]
- Xu Q. et al. Water use patterns of three species in subalpine forest, Southwest China: the deuterium isotope approach. Ecohydrology 4, 236–244 (2011). [Google Scholar]
- Yang B., Wen X. & Sun X. Seasonal variations in depth of water uptake for a subtropical coniferous plantation subjected to drought in an East Asian monsoon region. Agric. For. Meteorol. 201, 218–228 (2015). [Google Scholar]
- Yin L. et al. Interaction between groundwater and trees in an arid site: Potential impacts of climate variation and groundwater abstraction on trees. Journal of Hydrology 528, 435–448 (2015). [Google Scholar]
- Zapater M. et al. Evidence of hydraulic lift in a young beech and oak mixed forest using O-18 soil water labelling. Trees-Structure and Function 25, 885–894 (2011). [Google Scholar]
- Zhang C. et al. Coupling a two-tip linear mixing model with a delta D-delta O-18 plot to determine water sources consumed by maize during different growth stages. Field Crops Res. 123, 196–205 (2011). [Google Scholar]
- Zhang W. et al. Using stable isotopes to determine the water sources in alpine ecosystems on the east Qinghai-Tibet plateau, China. Hydrol. Process. 24, 3270–3280 (2010). [Google Scholar]
- Zhao G., Li X., Wu H., Zhang S. & Li G. Study on plant water use in Myricaria squamosa with stable hydrogen isotope tracer in Qinghai Lake basin. Chinese Journal of Plant Ecology 37, 1091–1100 (2013). [Google Scholar]
- Zhou H., Zheng X., Tang L. & Li Y. Differences and similarities between water sources of Tamarix ramosissima, Nitraria sibirica and Reaumuria soongorica in the southeastern Junggar Basin. Chinese Journal of Plant Ecology 37, 665–673 (2013). [Google Scholar]
- Zhou Y., Chen S., Song W., Lu Q. & Lin G. Water-use strategies of two desert plants along a precipitation gradient in northwestern China. Chinese Journal of Plant Ecology 35, 789–800 (2011). [Google Scholar]
- Zhu L., Xu X. & Mao G. Water sources of shrubs grown in the northern Ningxia Plain of China characterized by shallow groundwater table. Chinese Journal of Plant Ecology 36, 618–628 (2012). [Google Scholar]
- Zhu J. et al. Soil-water interacting use patterns driven by Ziziphus jujuba on the Chenier Island in the Yellow River Delta, China. Archives of Agronomy and Soil Science 62, 1614–1624 (2016). [Google Scholar]
- Zhu Y., Jia Z. & Yang X. Resource-dependent water use strategy of two desert shrubs on interdune, Northwest China. Journal of Food Agriculture & Environment 9, 832–835 (2011). [Google Scholar]
- Parnell A. C., Inger R., Bearhop S. & Jackson A. L. Source partitioning using stable isotopes: Coping with too much Variation. PLoS One 5, e9672 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W. & Semmens B. X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480 (2008). [DOI] [PubMed] [Google Scholar]
- Phillips D. L. & Gregg J. W. Source partitioning using stable isotopes: coping with too many sources. Oecologia 136, 261–269 (2003). [DOI] [PubMed] [Google Scholar]
- Anderegg W. R. L. Spatial and temporal variation in plant hydraulic traits and their relevance for climate change impacts on vegetation. New Phytol. 205, 1008–1014 (2015). [DOI] [PubMed] [Google Scholar]
- Olano J. M., Arzac A., Garcia-Cervigon A. I., von Arx G. & Rozas V. New star on the stage: amount of ray parenchyma in tree rings shows a link to climate. New Phytol. 198, 486–495 (2013). [DOI] [PubMed] [Google Scholar]
- McDowell N. G. Mechanisms linking drought, hydraulics, carbon metabolism, and vegetation mortality. Plant Physiol. 155, 1051–1059 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. L. & White D. A. Plasticity in the Huber value contributes to homeostasis in leaf water relations of a mallee Eucalypt with variation to groundwater depth. Tree Physiol. 29, 1407–1418 (2009). [DOI] [PubMed] [Google Scholar]
- Gries D. et al. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant Cell and Environment 26, 725–736 (2003). [Google Scholar]
- Horton J., Kolb T. & Hart S. Physiological response to groundwater depth varies among species and with river flow regulation. Ecol. Appl. 11, 1046–1059 (2001). [Google Scholar]
- Turnbull L. et al. Understanding the role of ecohydrological feedbacks in ecosystem state change in drylands. Ecohydrology 5, 174–183 (2012). [Google Scholar]
- Pringle C. Hydrologic connectivity and the management of biological reserves: A global perspective. Ecol. Appl. 11, 981–998 (2001). [Google Scholar]
- Budyko M. I. Climate and Life (Academic Press: New York, USA 1974). [Google Scholar]
- Fekete B., Vörösmarty C. & Grabs W. Global composite runoff fields on observed river discharge and simulated water balances. Water System Analysis Group, University of New Hampshire, and Global Runoff Data Centre. Koblenz, Germany: Federal Institute of Hydrology (BfG) (2002).
- GPCC. ISLSCP II Global Precipitation Climatology Centre (GPCC) Monthly Precipitation. In Hall, Forrest G. G., Collatz B., Meeson S., Los E., Brown de Colstoun & Landis D. (eds). ISLSCP Initiative II Collection. Data set. Available on-line http://daac.ornl.gov/ from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, USA (2011). [Google Scholar]
- Reichstein M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295 (2013). [DOI] [PubMed] [Google Scholar]
- Feddes R. et al. Modeling root water uptake in hydrological and climate models. Bull. Am. Meteorol. Soc. 82, 2797–2809 (2001). [Google Scholar]
- Anderegg L. D. et al. Drought characteristics’ role in widespread aspen forest mortality across Colorado, USA. Global Change Biol. 19, 1526–1537 (2013). [DOI] [PubMed] [Google Scholar]
- Borenstein M., Hedges L. V., Higgins J. P. T. & Rothstein H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods 1, 97–111 (2010). [DOI] [PubMed] [Google Scholar]
- Kohonen T. Self-Organization and Associative Memory. Springer-Verlag, Berlin (1984). [Google Scholar]
- Auge R. M., Toler H. D. & Saxton A. M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: a meta-analysis. Mycorrhiza 25, 13–24 (2015). [DOI] [PubMed] [Google Scholar]
- Mayerhofer M. S., Kernaghan G. & Harper K. A. The effects of fungal root endophytes on plant growth: a meta-analysis. Mycorrhiza 23, 119–128 (2013). [DOI] [PubMed] [Google Scholar]
- Soylu M. E., Istanbulluoglu E., Lenters J. D. & Wang T. Quantifying the impact of groundwater depth on evapotranspiration in a semi-arid grassland region. Hydrology and Earth System Sciences 15, 787–806 (2011). [Google Scholar]
- Stromberg J., Tiller R. & Richter B. Effects of groundwater decline on riparian vegetation of semiarid regions: The San Pedro, Arizona. Ecol. Appl. 6, 113–131 (1996). [Google Scholar]
- Zencich S., Froend R., Turner J. & Gailitis V. Influence of groundwater depth on the seasonal sources of water accessed by Banksia tree species on a shallow, sandy coastal aquifer. Oecologia 131, 8–19 (2002). [DOI] [PubMed] [Google Scholar]
- Schenk H. & Jackson R. Rooting depths, lateral root spreads and below-ground/above-ground allometries of plants in waterlimited ecosystems. J. Ecol. 90, 480–494 (2002). [Google Scholar]
- Kleidon A. ISLSCP II Total plant-available soil water storage capacity of the rooting zone. In Hall, Forrest G., G. Collatz, B. Meeson, S. Los, E. Brown de Colstoun, and D. Landis (eds). ISLSCP Initiative II Collection. Data set. Available on-line [http://daac.ornl.gov/] from Oak Ridge National Laboratory Distributed Active Archive Center, Oak Ridge, Tennessee, USA (2011). [Google Scholar]
- Fan Y., Li H. & Miguez-Macho G. Global patterns of groundwater table depth. Science 339, 940–943 (2013). [DOI] [PubMed] [Google Scholar]
- Trabucco A. & Zomer R. J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR Consortium for Spatial Information. (2009). [Google Scholar]
- Jones J. A. et al. Ecosystem processes and human influences regulate streamflow response to climate change at Long-Term Ecological Research sites. Bioscience 62, 390–404 (2012). [Google Scholar]
- Mueck L. Report the awful truth! Nature Nanotechnology 8, 693–695 (2013). [DOI] [PubMed] [Google Scholar]
- Rosenthal R. The file drawer problem and tolerance for null results. Psychol. Bull. 86, 638–641 (1979). [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
- Duval S. & Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in metaanalysis. Biometrics 56, 455–463 (2000). [DOI] [PubMed] [Google Scholar]
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 151 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.