Abstract
We have used total chemical synthesis to perform high-resolution dissection of the pharmacophore of a potent anti-HIV protein, the aminooxypentane oxime of [glyoxylyl1]RANTES(2-68), known as AOP–RANTES, of which we designed and made 37 analogs. All involved incorporation of one or more rationally chosen nonnatural noncoded structures, for which we found a clear comparative advantage over coded ones. We investigated structure–activity relationships in the pharmacophore by screening the analogs for their ability to block the HIV entry process and produced a derivative, PSC-RANTES {N-nonanoyl, des-Ser1[l-thioproline2, l-cyclohexylglycine3]-RANTES(2–68)}, which is 50 times more potent than AOP–RANTES. This promising group of compounds might be optimized yet further as potential prophylactic and therapeutic anti-HIV agents. The remarkable potency of our RANTES analogs probably involves the unusual mechanism of intracellular sequestration of CC-chemokine receptor 5 (CCR5), and it has been suggested that this arises from enhanced affinity for the receptor. We found that inhibitory potency and capacity to induce CCR5 down-modulation do appear to be correlated, but that unexpectedly, inhibitory potency and affinity for CCR5 do not. We believe this study represents the proof of principle for the use of a medicinal chemistry approach, above all one showing the advantage of noncoded structures, to the optimization of the pharmacological properties of a protein. Medicinal chemistry of small molecules is the foundation of modern pharmaceutical practice, and we believe we have shown that techniques have now reached the point at which the approach could also be applied to the many macromolecular drugs now in common use.
Keywords: AIDS, CCR5, chemokines
HIV/AIDS was responsible for an estimated 3 million deaths in 2003, with an estimated 5 million people newly infected with HIV, mostly in the developing world (1). Agents capable of preventing HIV transmission during sexual contact could potentially save many lives, particularly in the absence of an effective HIV vaccine. Among the substances currently being evaluated for this purpose are molecules that block the entry of HIV into target cells (2). HIV entry inhibitors can also be used therapeutically (3) and are of particular interest in light of the emergence of HIV strains that are resistant to highly active antiretroviral therapy (4). HIV entry requires CD4 plus a chemokine receptor, generally either CXC-chemokine receptor 4 (CXCR4) or CC-chemokine receptor 5 (CCR5), with CCR5 almost exclusively used in transmission and in early stages of disease (5). Studies involving individuals homozygous for a null CCR5 allele (Δ32) underline the importance of CCR5 in HIV transmission and strongly suggest that its inactivation would not generate adverse side effects (6–8). The natural ligands of CCR5, which include RANTES (CCL5), all inhibit entry of R5-tropic HIV strains, i.e., those strains requiring CCR5 to enter the cell (9, 10). These ligands belong to the chemokine family, which numbers ≈40 small proteins principally involved in control of leukocyte trafficking, notably in the modulation of inflammatory processes and in the maintenance of the adaptive immune system (11). Current models (12–14) suggest that structures on the surface of the core domain of chemokines are responsible for “address” functions, i.e., docking to receptors with high affinity and specificity, whereas the flexible N-terminal region is responsible for receptor activation.
Several engineered analogs of chemokine ligands of CCR5 with enhanced anti-HIV activity have been described. The first was AOP-RANTES (15), the aminooxypentane oxime of [glyoxylyl1]RANTES(2–68). We designed this molecule to incorporate a more hydrophobic and nearly isosteric replacement for the side chain of the N-terminal methionine of Met0-RANTES, a known CCR5 antagonist (16). All of the analogs have hydrophobic extensions to the N-terminal region (15, 17–21), and most do not act as simple receptor antagonists. Instead, their inhibitory mechanism seems to be the induction of intracellular sequestration of CCR5 (20, 22–24).
Studies of the structure–activity relationships of proteins mainly use recombinant DNA technology to delete and/or substitute residues suspected to be important for activity. Although molecular evolution techniques permit parallel evaluation of vast combinatorial libraries of protein variants (25), exploration of shape space using recombinant DNA is restricted by the limited number of coded amino acids. A wide range of amino acid analogs that are directly compatible with peptide synthesis approaches are now available. Total chemical synthesis of proteins of the size of chemokines is now relatively simple and can rapidly yield several milligrams of material in a high state of purity (26). Hence, it is now possible to extend to proteins what has often been fruitfully applied to peptides, the rational incorporation of unnatural amino acid analogs during synthesis, to explore shape space for a given region of the molecule with higher resolution than is possible with only the natural amino acids. Here we present such a high-resolution protein study, carried out on the N-terminal region of AOP-RANTES, and show how it led to the identification of even better HIV inhibitors (17, 20). We also discuss structure–activity relationships with respect to the probable anti-HIV inhibitory mechanism of this promising series of analogs.
Materials and Methods
Total Chemical Synthesis. RANTES analogs were prepared by polymer-supported organic synthesis of two fragments equivalent to the whole proteins after cleavage between residues 33 and 34. t-Butoxycarbonyl (Boc) chemistry was used, as described in ref. 27. The fragments were coupled in a native chemical ligation (28). More complete details, including the particular methods used to introduce the hydrophobic substituents at the N terminus, are provided as Supporting Text, which is published as supporting information on the PNAS web site. Folding of each synthetic protein with concomitant disulfide formation was carried out in the presence of a Cys-SH/(Cys-S)2 redox couple. Purity and integrity of RANTES analogs were routinely verified by HPLC and mass spectrometry.
Semisynthesis. We prepared AOP-RANTES and CAP-RANTES, the 1-carboxy,5-aminooxypentane oxime of [glyoxylyl1] RANTES, by the general method described in ref. 15. AOP-RANTES was also made by total chemical synthesis (27), with no detectable difference in properties.
Cell Fusion Assay. CCR5-tropic viral envelope-mediated cell fusion assays were carried out in triplicate essentially as described in ref. 21. Results were expressed as the ratio of the IC50 of the analog to that of n-nonanoyl-RANTES(2-68) (NNY-RANTES).
Competition Binding Assay. Experiments were done in quadruplicate as described in ref. 21 by using a clonal Chinese hamster ovary–CCR5 cell line that had been obtained by transduction with lentiviral vectors (29).
CCR5 Down-Modulation on CD4+ T Cells. CCR5 expression at the cell surface of CD4+ T cells was measured in culture as a function of time after addition and then removal of chemokine (see Supporting Text for further details). Immunofluorescent labeling was used with the PA12 antibody, which is directed against the N terminus of CCR5. Staining with PA12 is not affected by ligand binding (30). Relative expression of CCR5 was determined by quantitative flow cytometry, as described (23).
Anti-HIV Activity of Selected Analogs in Vivo. Human peripheral blood mononuclear cell-reconstituted severe combined immunodeficient (SCID) (hu-PBL-SCID) mice (n = 3–5) were injected i.p. with various amounts of PSC-RANTES or NNY-RANTES in a volume of 0.5 ml of Dulbecco's PBS (DPBS) or with 0.5 ml of DPBS. Thirty minutes later, the mice were infected by i.p. injection of 103 tissue culture infectious doses of the 242 R5 molecular clone of HIV-1 (31). Infection of hu-PBL-SCID mice was monitored by weekly plasma viral RNA determinations (Amplicor HIV Monitor; Roche Molecular Systems, Somerville, NJ), as described (17). Uninfected mice had undetectable (<200 copies per ml) HIV viral RNA for 4 consecutive weeks. All infected mice had >10,000 HIV viral RNA copies per ml by week 2 after infection.
Results
We set out to enhance the anti-HIV potency of AOP-RANTES, using cycles of design, synthesis, and activity assay in an R5-tropic envelope-dependent cell fusion assay.
A Hydrophobic N-Terminal Extension Is Crucial for Potent Anti-HIV Activity. We first wished to test the hypothesis that the engineered N-terminal extension must be hydrophobic for a RANTES analog to show strong anti-HIV activity. Hence we designed CAP-RANTES, which is structurally identical to AOP-RANTES save for the addition of a carboxy group at the distal end of the pentane chain (Fig. 1). In support of the hypothesis, CAP-RANTES is indeed orders of magnitude less active than AOP-RANTES as an HIV entry inhibitor (Fig. 1; see also Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 1.
First round of optimization; structure and anti-HIV activity of AOP-RANTES analogs. Potencies (IC50), which were determined in cell fusion assay, are shown to the left of each structure, with 95% confidence intervals shown in parentheses.
First Cycle of Optimization. Increasing the hydrophobicity of the N-terminal substituent is beneficial up to a point. In the first cycle of optimization (Fig. 1), we increased the hydrophobicity of the N-terminal substituent beyond that of the aminooxypentane oxime moiety by systematically eliminating its heteroatoms. Through evaluation of this series, we identified NNY-RANTES, a significantly improved analog of AOP-RANTES (7-fold increase in potency in the cell fusion assay, Fig. 1; see also Fig. 5), whose improved activity has subsequently been verified in vitro and in vivo (17, 23). However, beyond a certain point, further elimination of heteroatoms led to a reversal of the improvements gained [NNA-RANTES and DDY-RANTES; see Fig. 1 for structures]. In NNA-RANTES, unlike NNY-RANTES, the imino nitrogen of proline-2 is alkylated and can therefore ionize. It may be that the loss of improvement occurs because the increase in hydrophobicity on elimination of the carbonyl oxygen in NNY-RANTES is more than offset by the acquisition of a charge by the proline nitrogen. Moving to DDY-RANTES, in which the proline nitrogen has been eliminated, does not restore any lost advantage, but we note that this change involves the removal of the proline side chain as well.
Second Cycle of Optimization. In the second cycle, we fixed the N-terminal substitution as that present in the best derivative from the second cycle, the n-nonanoyl group of NNY-RANTES, and we designed, synthesized, and screened a set of 28 proteins, into which we had introduced rationally chosen substituents at positions 2 and 3. The structures of these variants, and their activity indices, are shown in Fig. 2. For every substituent mentioned, where the α-carbon is asymmetric, the configuration was that of a natural l-amino acid.
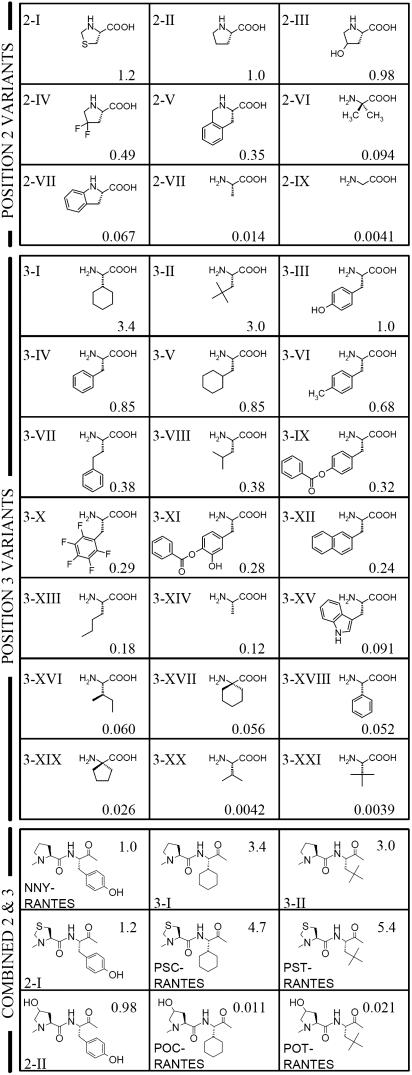
Fig. 2.
Second and third rounds of optimization; structure and anti-HIV activity of NNY-RANTES analogs. Activity indices (two significant figures) are expressed as the potency (IC50) of a given molecule relative to that of NNY-RANTES measured in the same experiment and are shown to the right of each structure. For the second cycle of optimization, Roman numerals are used to denote structures of amino acid substituents used in NNY-RANTES analogs, which are displayed in order of decreasing potency (see below). For the third cycle of optimization (combined substitution at positions 2 and 3), structures corresponding to positions 2 and 3 of NNY-RANTES are shown. Position 2 variants: 2-I; thiazolidine-4-carboxylic acid, 2-II; proline, 2-III; 4-hydroxyproline, 2-IV; 4,4-difluoroproline, 2-V; 1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid, 2-VI; aminoisobutyric acid, 2-VII; indoline-2-carboxylic acid, 2-VIII; alanine, 2-IX; glycine. Position 3 variants: 3-I; cyclohexylglycine, 3-II; t-butylalanine, 3-III; tyrosine, 3-IV; phenylalanine, 3-V; cyclohexylalanine, 3-VI; 4-methylphenylalanine, 3-VII; homophenylalanine, 3-VIII; leucine, 3-IX; p-benzoyl-tyrosine, 3-X; pentafluorophenylalanine, 3-XI; 3-hydroxy, 4-benzoyl-tyrosine, 3-XII; 2-naphthylalanine, 3-XIII; norleucine, 3-XIV; alanine, 3-XV; tryptophan, 3-XVI; isoleucine, 3-XVII; 1-amino-1-cyclohexanecarboxylic acid, 3-XVIII; phenylglycine, 3-XIX; and 1-amino-1-cyclopentanecarboxylic acid, 3-XX; and valine, 3-XXI; t-leucine.
Position 2. Eight of the variant forms of NNY-RANTES represented single substitutions at position 2 (proline). Proline's particular conformational constraints and size of substituent seem important for activity. Five variants incorporated analogs of proline that conserve proline's conformational constraints. Those analogs, if the ring substitutions had not increased the residue size too dramatically [thiazolidine-4-carboxylic acid (2-I), 4-hydroxyproline (2-III), and 4,4-difluoroproline (2-IV)], had potency similar to or slightly higher than that of NNY-RANTES. Those with bulkier structures [1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid (2-V) and indoline-2-carboxylic acid (2-VII)] were somewhat less potent than NNY-RANTES. Substitutions with freer rotation about the C—N bond [alanine (2-VII) and glycine (2-IX)] gave quite weak inhibitors compared with NNY-RANTES. One nonimino-acid analog [aminoisobutyric acid (2-VI)], which would nonetheless be expected on steric grounds to share some of proline's constraints, seems to occupy an intermediate place in terms of activity.
Position 3. A group of 20 variants of NNY-RANTES each had a single substitution, at position 3 (tyrosine).
The hydroxyl group is not essential. The –OH appears unimportant for activity, because it can be either removed [phenylalanine (3-IV)] or replaced by a methyl group [4-methylphenylalanine (3-VI)] without affecting activity.
Bulk must be kept within limits. A moderate increase in bulk somewhat decreased activity [pentafluorophenylalanine (3-X)]. A significant increase in the bulkiness of the substituent [p-benzoyl-tyrosine (3-IX), 3-hydroxy, 4-benzoyl-tyrosine (3-XI), 2-naphthylalanine (3-XII), and tryptophan (3-XV)] was clearly detrimental to activity.
Within limits, the distance of the aromatic ring from the peptide chain can vary. In tyrosine and phenylalanine, the phenyl moiety is separated from the peptide backbone by a single methylene group. Increasing the separation to two such groups [homophenylalanine (3-VII)] was well tolerated, whereas removal of the methylene [phenylglycine (3-XVIII)] caused a 100-fold loss in potency.
Aromaticity is not essential. Substitution of the phenyl moiety by cyclohexane [cyclohexylalanine (3-V)] was well tolerated. The nonaromatic substituent need not be cyclic for there to be an improvement [t-butylalanine (3-II)].
The effects of distance of nonaromatic substituents from the peptide chain differ from those seen with aromatic ones. Remarkably, when the substituent was a nonaromatic ring, eliminating the methylene spacer could actually increase potency, rather than decreasing it. Cyclohexylglycine (3-I) improved potency 3.4-fold, compared with the 100-fold drop shown with its aromatic counterpart [phenylglycine (3-XVIII)]. Reducing the separation still further, however, was strongly detrimental to activity [1-amino-1-cyclohexanecarboxylic acid (3-XVII)].
Small changes to branched aliphatic chains give major activity shifts. Substitution by t-leucine (3-XXI), which is simply equivalent to removal of the methylene from (3-II), the t-butylalanine variant, all but abolished activity. Removal of a methyl group from (3-II) gave leucine (3-VIII) with a reduction in potency of only 10-fold, whereas moving it from a tertiary to a primary position [isoleucine (3-XVI)] gave a 500-fold decrease. Variants featuring smaller or less-branched aliphatic substituents at this position [norleucine (3-XIII), valine (3-XX), and alanine (3-XIV)] were significantly less active than NNY-RANTES.
Third Cycle of Optimization (PSC-RANTES). Improvements can be additive or better. A preliminary scan of changes in positions further down the chain from tyrosine-3 gave no strong leads (data not shown), and so in the final cycle, we combined the two most promising structures identified at position 2 [4-hydroxyproline (2-III) and thiazolidine-4-carboxylic acid (2-I)] with the two most promising structures identified at position 3 [cyclohexylglycine (3-I) and t-butylalanine (3-II)]. The insertion of a sulfur atom in the proline ring at position 2 enhanced, probably more than additively, the activity gain that had resulted from placing cyclohexylglycine or t-butylalanine at position 3 (PSC-RANTES and PST-RANTES; Fig. 2).
Small differences can have large effects. The delicacy of the molecular interactions involved is well illustrated by the fact that the same substitutions at position 3 in the presence of an –OH group on the proline ring at position 2 led not to an improvement but to a 300-fold loss in potency (POC-RANTES and POT-RANTES, Fig. 2). We saw above that this same –OH substitution at position 2 led to no loss of activity whatever, so long as tyrosine was at position 3 (2-III).
Inhibitory Mechanism. The activity of key molecules identified by screening with the cell fusion assay was confirmed in tests for their ability to inhibit replication of the R5-tropic laboratory HIV strain BaL in human peripheral blood mononuclear cells (Fig. 5).
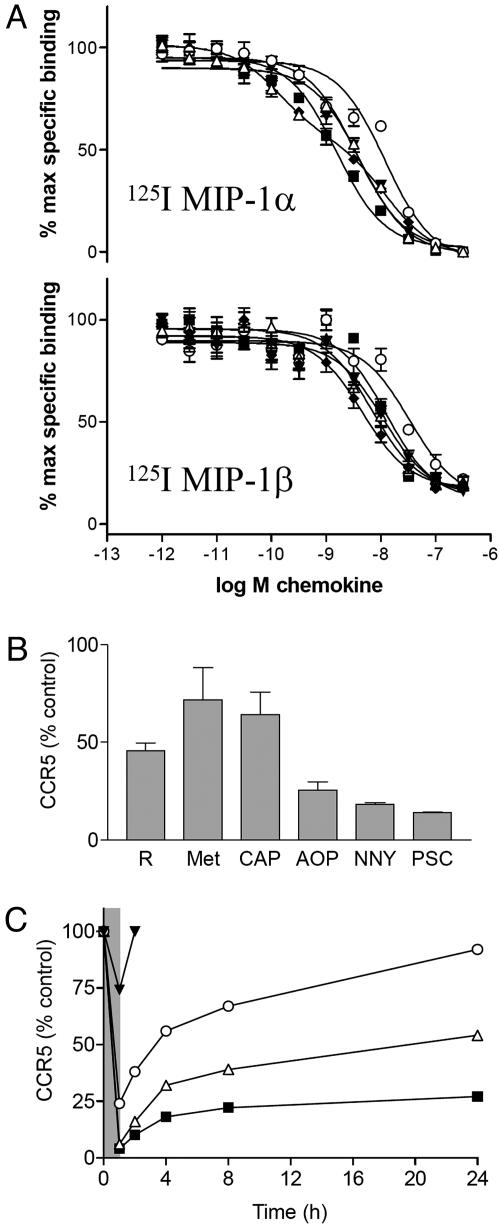
CCR5-binding affinity of RANTES analogs. Increased affinity for CCR5 has been proposed as an explanation for the increased potency of certain chemokine analogs, either because this enables them to compete more effectively with the HIV envelope for a common binding site on cell-surface receptors (15), or because it reduces ligand dissociation during endocytosis and hence contributes to receptor sequestration by interfering with the recycling/resensitization process (22, 24, 32). We therefore compared the CCR5-binding affinity of a representative group of RANTES analogs, whose inhibitory potencies vary from almost undetectable to the picomolar range (see also Fig. 5). The propensity of RANTES to aggregate on cell-surface proteoglycans complicates the interpretation of competition binding assays (33), so we worked with iodinated forms of other CCR5 ligands [macrophage inflammatory protein (MIP)-1α (CCL3) and MIP-1β (CCL4)] (Fig. 3A). In general, although the analogs appear to compete more effectively with MIP-1α than MIP-1β (IC50 values generally lower in competition with MIP-1α), both tracers give broadly similar results. The apparent affinities (IC50) of all five analogs lie within 1 order of magnitude of each other (in the range 1.5–15 nM when iodinated MIP-1α is used, and in the range 4–40 nM when iodinated MIP-1β is used) and, where relative affinities can be distinguished, in the same rank order (CAP-RANTES > PSC-RANTES ≈ NNY-RANTES ≈ RANTES > AOP-RANTES). This order bears no relationship to that established for antiviral potency. In contrast to data presented by Simmons et al. (15), we find that AOP-RANTES has a particularly poor apparent affinity relative to other analogs (12 nM against MIP-1α and 33 nM against MIP-1β). The methodology we used for this assay was matched as closely as possible to that used by Simmons et al. (15), and we produced the AOP-RANTES used in both studies. A potential explanation for this discrepancy would be our use of Chinese hamster ovary rather than human embryonic kidney cells for CCR5 expression, but we have carried out similar assays on other CCR5-expressing cell lines and obtained results broadly similar to those presented here (data not shown).
Fig. 3.
Data relating to mechanisms underlying the anti-HIV activity of RANTES analogs. (A) RANTES analogs exhibit similar affinity in competition binding assays on Chinese hamster ovary–CCR5 cells. (Upper) Assay using 125I MIP-1α. (Lower) Assay using 125I MIP-1β. Each data point represents the mean ± SEM of quadruplicate determinations, expressed as a percentage of maximum specific binding (binding in the absence of competitor). Curves for data sets were fitted with a one-site (monophasic) competition model except for CAP-RANTES against 125I MIP-1α, for which a two-site (biphasic) competition model appeared to be best. (B) RANTES analogs differ in their capacity to modulate cell surface expression of CCR5 in CD4+ T cells. Steady-state surface levels of CCR5 were determined after a 1-h incubation with RANTES analogs at 30 nM. R, RANTES; Met, Met-RANTES; CAP, CAP-RANTES; AOP, AOP-RANTES; NNY, NNY-RANTES; PSC, PSC-RANTES. (C) Measurements of steady-state surface CCR5 levels on CD4+ T cells were made in order to follow the reappearance of CCR5 at the cell surface after a 1-h pulse with RANTES analogs at 30 nM (shaded area on graph). Filled inverted triangle, RANTES; filled lozenge, CAP-RANTES; open triangle, NNY-RANTES; open circle, AOP-RANTES; and filled square, PSC-RANTES.
Receptor down-modulation. To ascertain whether increases in anti-HIV potency are accompanied by increases in capacity to sequester receptors, we tested a group of analogs in a steady-state CCR5 down-modulation assay using primary CD4-positive human lymphocytes. In contrast to the affinity determinations, the rank order of the compounds tested here, in terms of both magnitude of receptor down-modulation and its duration, is the same as their rank order for potency as HIV inhibitors (PSC-RANTES > NNY-RANTES > AOP-RANTES > Met-RANTES ≈ CAP-RANTES; Fig. 3 B and C). These results, in agreement with other studies (20–24), suggest that the capacity of chemokine variants to induce coreceptor sequestration is a key parameter for anti-HIV potency but show that affinity plays no role.
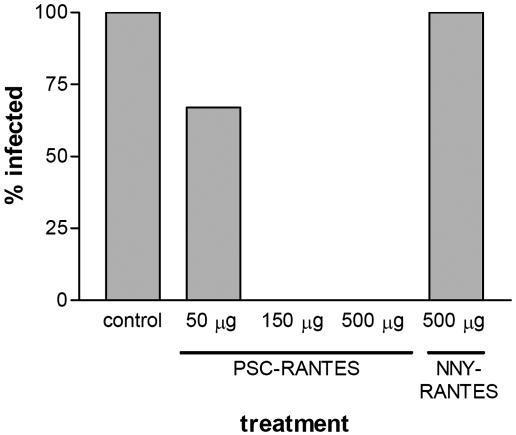
Protection from Infection by HIV-1 in Vivo. We have previously shown that NNY-RANTES was superior to AOP-RANTES in protecting SCID mice grafted with human peripheral blood lymphocytes (hu-PBL-SCID mice) from infection by HIV-1 (17). Protection was achieved by treating mice with a single bolus injection of 1 mg of NNY-RANTES just before HIV-1 challenge and providing continuous low-dose administration of NNY-RANTES by osmotic pumps. Here we report that a single injection of 500 μg of NNY-RANTES was unable to protect against infection, whereas a single injection of PSC-RANTES at either 500 μg or 150 μg protected all of the challenged mice against HIV-1 infection. Injection of 50 μg was partially protective (Fig. 4). The PSC-RANTES was in no way formulated for bioavailability, and we note that the continuous infusion experiments mentioned above suggested that an equilibrium concentration of NNY-RANTES between 75 and 96 pM was sufficient to sustain protection against infection (ref. 17; D.M., unpublished results).
Fig. 4.
Anti-HIV activity of selected analogs in vivo. Human peripheral blood mononuclear cell-reconstituted SCID (hu-PBL-SCID) mice received bolus injections of various quantities of chemokine analogs or vehicle alone (control) before challenge by HIV-1.
Thus, the third cycle of optimization produced a compound that gave complete protection from HIV-1 under circumstances in which the best compound from the second cycle had completely failed. These results suggest that the superior activity of PSC-RANTES observed in vitro may predict greater clinical efficacy in preventing HIV-1 infection, and that the optimization procedure reported here was worthwhile and probably necessary.
Discussion
A Medicinal Chemistry Approach Applied to a Protein. To our knowledge, a high-resolution structure–activity study carried out on a complete protein has not previously been described. We have confirmed the importance of the N-terminal region for anti-HIV activity, because our modifications to it brought an ≈50-fold improvement in the potency over our starting molecule, AOP-RANTES. Although incomplete, our panel of variants has begun to delineate a pharmacophore responsible for their potent anti-HIV activity. First, anti-HIV activity benefits from a hydrophobic N-terminal extension, in the form of either a straight aliphatic chain or a bulky hydrophobic amino acid (19, 21). A similar benefit was seen when a hydrophobic extension (an aminooxypentane oxime) was added to the N terminus of MIP-1α variant LD78β (18), and N-terminal extension by methionine improves the anti-HIV activity of stromal cell-derived factor 1 (SDF-1) against ×4-tropic strains (34). Second, although it appears that serine-1 of RANTES does not contribute to activity, because potency can be increased through removal of its side chain and backbone heteroatoms, substitution of proline-2 appears to be tolerated only when the substituent both is small and retains the conformational constraints characteristic of proline. The importance of proline-2 for interaction with CCR5 is underlined not only by the results shown here, but also by the results of a study (21) carried out using a library of phage-displayed RANTES mutants that permitted alanine, proline, serine, or threonine at this position. All variants that met the functional criteria for selection had proline at this position. Furthermore, in native RANTES itself, substitution of proline-2 by alanine has been shown to greatly reduce its capacity to interact with CCR5 (35). Third, position 3 is clearly a key element of the pharmacophore: in a relatively small group of substituents at this position, we found those that gave rise to both significant increases and significant decreases in potency. We have, for example, seen that addition, subtraction, or displacement of a single methyl group in a side chain at this position can lead to order-of-magnitude changes in potency. The utility of the medicinal chemistry approach, as opposed to conventional mutagenesis limited to natural amino acids, is underlined by the fact that substitution with natural amino acids did not lead to improved potency, whereas substitution with two unnatural ones gave significant improvements. The dramatically different effects seen when substitutions at adjacent positions are combined suggests that to fully optimize a region, it might be necessary to vary a group of adjacent positions in a combinatorial manner. We have done this with phage display (21), but the present study was able to exploit the greater breadth of possible substitutions, and thus higher degree of spatial resolution, afforded by total chemical synthesis.
Inhibitory Mechanism and Structure–Activity Relationships of RANTES Analogs. Chemokine analogs with N termini modified according to our methods are thought to have an unusual mechanism of action: the induction of long-term intracellular sequestration of the receptor (18, 20–23, 32). The explanation most frequently put forward (18, 22, 32) is that the increased capacity of such ligands to induce sequestration of CCR5 depends on their increased affinity for the receptor. Our data are not consistent with this explanation, because we find, unusually for pharmacologically active substances, no correlation between their affinity and their potency. On the other hand, our observation does not contradict the sequestration hypothesis itself, and indeed we report here an apparent correlation between inhibitory potency and capacity to induce CCR5 sequestration across a panel of compounds whose anti-HIV potencies span several orders of magnitude. Nonetheless, the exact mechanisms by which our modifications increase intracellular retention of CCR5 remain to be fully elucidated, and they obviously offer an important new target for drug development. Because G protein-coupled receptors share common pathways for the processes of desensitization and resensitization (36), the capacity to be modulated by inhibitors in this way may not be unique to CCR5, and it is possible that inhibitors that act like PSC-RANTES could be found for other therapeutically important receptors.
Potential of PSC-RANTES for Clinical Development. HIV entry inhibitors, including those that act via blockade of HIV coreceptors, could be of clinical interest. First, they might be used to prevent person-to-person transmission of infection during sexual contact (ref. 2; such agents are generally, although incorrectly, known as “microbicides”). Second, they could become therapeutic agents to treat infected individuals.
Preliminary evaluation of PSC-RANTES for use as a microbicide has shown that it potently blocks HIV replication in epithelial Langerhans cells, which are believed to be among the first target cells encountered by the virus during transmission across genital mucosa (37). In the same context, PSC-RANTES showed no detectable toxic effects in macaques when applied intravaginally at concentrations as high as 1 mM (R. Veazey, M. Lederman, O.H., D.M. & R.O, unpublished data) and then protected from infection all macaques in a group that had received a high-titer intravaginal challenge of R5-tropic immunodeficiency virus (38). This experiment was necessarily carried out not with HIV but with a hybrid simian/human immunodeficiency virus, but results presented in the present paper show that PSC-RANTES will prevent, in vivo, infection of human cells by a strain of HIV-1 itself.
A number of orally available low-molecular-weight CCR5 antagonists are currently in clinical development as systemically administered therapeutic anti-HIV agents. PSC-RANTES may hold certain advantages over these molecules because of its apparently long-acting and noncompetitive inhibitory mechanism and may be worthy of further optimization.
Conclusion
We have reported the application of a previously undescribed high-resolution “medicinal chemistry” approach to the improvement of activity of a synthetic protein. Significant gains in potency were achieved through the rational incorporation of nonnatural, i.e., noncoded structures at key sites to produce a molecule with potential for further clinical development. Techniques have now reached the point at which this would be a promising approach to the optimization of other small proteins of clinical interest.
Supplementary Material
Acknowledgments
We acknowledge the support of the AIDS section of the Swiss National Science Foundation (Project 3339-62032-00), the Stanley Thomas Johnson Foundation, the National Institutes of Health (Projects PO1 AI 51649-01, R01 AI52778, and M01 GM00833), and General Clinical Research Centers, National Institutes of Health Grant M01 RR00833 for costs of providing blood samples from normal donors.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: SCID, severe combined immunodeficient; CCR5, CC-chemokine receptor 5; MIP, macrophage inflammatory protein.
References
- 1.UNAIDS/World Health Organization (2003) AIDS Epidemic Update (UNAIDS/World Health Organization, Geneva).
- 2.Shattock, R. J. & Moore, J. P. (2003) Nat. Rev. Microbiol. 1, 25–34. [DOI] [PubMed] [Google Scholar]
- 3.Robertson, D. (2003) Nat. Biotechnol. 21, 470–471. [DOI] [PubMed] [Google Scholar]
- 4.Michael, N. L. & Moore, J. P. (1999) Nat. Med. 5, 740–742. [DOI] [PubMed] [Google Scholar]
- 5.Berger, E. A., Murphy, P. M. & Farber, J. M. (1999) Annu. Rev. Immunol. 17, 657–700. [DOI] [PubMed] [Google Scholar]
- 6.Dean, M., Carrington, M., Winkler, C., Huttley, G. A., Smith, M. W., Allikmets, R., Goedert, J. J., Buchbinder, S. P., Vittinghoff, E., Gomperts, E., et al. (1996) Science 273, 1856–1862. [DOI] [PubMed] [Google Scholar]
- 7.Liu, R., Paxton, W. A., Choe, S., Ceradini, D., Martin, S. R., Horuk, R., MacDonald, M. E., Stuhlmann, H., Koup, R. A. & Landau, N. R. (1996) Cell 86, 367–377. [DOI] [PubMed] [Google Scholar]
- 8.Samson, M., Libert, F., Doranz, B. J., Rucker, J., Liesnard, C., Farber, C. M., Saragosti, S., Lapoumeroulie, C., Cognaux, J., Forceille, C., et al. (1996) Nature 382, 722–725. [DOI] [PubMed] [Google Scholar]
- 9.Cocchi, F., DeVico, A. L., Garzino, D. A., Arya, S. K., Gallo, R. C. & Lusso, P. (1995) Science 270, 1811–1815. [DOI] [PubMed] [Google Scholar]
- 10.Blanpain, C., Migeotte, I., Lee, B., Vakili, J., Doranz, B. J., Govaerts, C., Vassart, G., Doms, R. W. & Parmentier, M. (1999) Blood 94, 1899–1905. [PubMed] [Google Scholar]
- 11.Zlotnik, A. & Yoshie, O. (2000) Immunity 12, 121–127. [DOI] [PubMed] [Google Scholar]
- 12.Siciliano, S. J., Rollins, T. E., DeMartino, J., Konteatis, Z., Malkowitz, L., Van, R. G., Bondy, S., Rosen, H. & Springer, M. S. (1994) Proc. Natl. Acad. Sci. USA 91, 1214–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells, T., Proudfoot, A., Power, C. A., Lusti-Narasimhan, M., Alouani, S., Hoogewerf, A. J. & Peitsch, M. C. (1996) Methods 10, 126–134. [DOI] [PubMed] [Google Scholar]
- 14.Blanpain, C., Doranz, B. J., Bondue, A., Govaerts, C., De Leener, A., Vassart, G., Doms, R. W., Proudfoot, A. & Parmentier, M. (2002) J. Biol. Chem. 278, 5179–5187. [DOI] [PubMed] [Google Scholar]
- 15.Simmons, G., Clapham, P. R., Picard, L., Offord, R. E., Rosenkilde, M. M., Schwartz, T. W., Buser, R., Wells, T. N. C. & Proudfoot, A. E. (1997) Science 276, 276–279. [DOI] [PubMed] [Google Scholar]
- 16.Proudfoot, A. E., Power, C. A., Hoogewerf, A. J., Montjovent, M. O., Borlat, F., Offord, R. E. & Wells, T. N. (1996) J. Biol. Chem. 271, 2599–2603. [DOI] [PubMed] [Google Scholar]
- 17.Mosier, D. E., Picchio, G. R., Gulizia, R. J., Sabbe, R., Poignard, P., Picard, L., Offord, R. E., Thompson, D. A. & Wilken, J. (1999) J. Virol. 73, 3544–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Townson, J. R., Graham, G. J., Landau, N. R., Rasala, B. & Nibbs, R. J. (2000) J. Biol. Chem. 275, 39254–39261. [DOI] [PubMed] [Google Scholar]
- 19.Polo, S., Nardese, V., Santis, C. D., Arcelloni, C., Paroni, R., Sironi, F., Verani, A., Rizzi, M., Bolognesi, M. & Lusso, P. (2000) Eur. J. Immunol. 30, 3190–3198. [DOI] [PubMed] [Google Scholar]
- 20.Pastore, C., Picchio, G. R., Galimi, F., Fish, R., Hartley, O., Offord, R. E. & Mosier, D. E. (2003) Antimicrob. Agents Chemother. 47, 509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartley, O., Dorgham, K., Perez-Bercoff, D., Cerini, F., Heimann, A., Gaertner, H., Offord, R. E., Pancino, G., Debre, P. & Gorochov, G. (2003) J. Virol. 77, 6637–6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack, M., Luckow, B., Nelson, P. J., Cihak, J., Simmons, G., Clapham, P. R., Signoret, N., Marsh, M., Stangassinger, M., Borlat, F., et al. (1998) J. Exp. Med. 187, 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabbe, R., Picchio, G. R., Pastore, C., Chaloin, O., Hartley, O., Offord, R. & Mosier, D. E. (2001) J. Virol. 75, 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt, S. M., Mariani, R., Holland, A. U., Hope, T. J. & Landau, N. R. (2002) J. Biol. Chem. 277, 17291–17299. [DOI] [PubMed] [Google Scholar]
- 25.Dower, W. J. & Mattheakis, L. C. (2002) Curr. Opin. Chem. Biol. 6, 390–398. [DOI] [PubMed] [Google Scholar]
- 26.Wilken, J. & Kent, S. B. (1998) Curr. Opin. Biotechnol. 9, 412–426. [DOI] [PubMed] [Google Scholar]
- 27.Wilken, J., Hoover, D., Thompson, D. A., Barlow, P. N., McSparron, H., Picard, L., Wlodawer, A., Lubkowski, J. & Kent, S. B. (1999) Chem. Biol. 6, 43–51. [DOI] [PubMed] [Google Scholar]
- 28.Dawson, P. E., Muir, T. W., Clark-Lewis, I. & Kent, S. B. (1994) Science 266, 776–779. [DOI] [PubMed] [Google Scholar]
- 29.Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M. & Trono, D. (1996) Science 272, 263–267. [DOI] [PubMed] [Google Scholar]
- 30.Olson, W. C., Rabut, G. E., Nagashima, K. A., Tran, D. N., Anselma, D. J., Monard, S. P., Segal, J. P., Thompson, D. A., Kajumo, F., Guo, Y., et al. (1999) J. Virol. 73, 4145–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesebro, B., Wehrly, K., Nishio, J. & Perryman, S. (1996) J. Virol. 70, 9055–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Signoret, N., Pelchen-Matthews, A., Mack, M., Proudfoot, A. E. & Marsh, M. (2000) J. Cell Biol. 151, 1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoogewerf, A. J., Kuschert, G. S., Proudfoot, A. E., Borlat, F., Clark-Lewis, I., Power, C. A. & Wells, T. N. (1997) Biochemistry 36, 13570–13578. [DOI] [PubMed] [Google Scholar]
- 34.Yang, O. O., Swanberg, S. L., Lu, Z., Dziejman, M., McCoy, J., Luster, A. D., Walker, B. D. & Herrmann, S. H. (1999) J. Virol. 73, 4582–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pakianathan, D. R., Kuta, E. G., Artis, D. R., Skelton, N. J. & Hebert, C. A. (1997) Biochemistry 36, 9642–9648. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson, S. S. (2001) Pharmacol. Rev. 53, 1–24. [PubMed] [Google Scholar]
- 37.Kawamura, T., Bruce, S. E., Abraha, A., Sugaya, M., Hartley, O., Offord, R. E., Arts, E. J., Zimmerman, P. A. & Blauvelt, A. (2004) J. Virol. 78, 7602–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lederman, M. M., Veazey, R. S., Offord, R. E., Mosier, D. E., Dufour, J., Mefford, M., Piatak, M., Lifson, J. D., Salkowitz, J. R., Rodriguez, B., et al. (2004) Science, 306, 485–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.