Abstract
Transgenic mice overexpressing in B lymphocytes either Bcl-2 or a TNF receptor-associated factor (TRAF)2 mutant lacking the N-terminal RING and zinc finger domains located at the N terminus of the molecule (TRAF2DN), which mimics TRAF1, developed lymphadenopathy and splenomegaly due to polyclonal B cell expansion. Remarkably, TRAF2DN/Bcl-2 double-transgenic mice contained B cell populations similar to those observed in TRAF2DN mice. However, over time, they developed severe splenomegaly and lymphadenopathy, and most animals also developed leukemia, pleural effusion, and, in some cases, ascites associated with monoclonal and oligoclonal B cell neoplasms. The life span of TRAF2DN/Bcl-2 mice was markedly reduced compared with Bcl-2 and TRAF2DN single-transgenics or wild-type littermates. The expanded B cell population of TRAF2DN/Bcl-2 double-transgenic mice was primarily comprised of small/medium-size noncycling B220M/IgMH/IgDL/CD21L/CD23NULL/CD11b+/CD5+ cells that were Bcl-6-negative, consistent with a B-1 phenotype. The cells also expressed high levels of CD54 and other adhesion molecules. In vitro, these B cells showed comparable proliferation rates to those of wild-type counterparts but exhibited markedly increased survival and were resistant to apoptosis induced by chemotherapeutic agents and glucocorticoids. Histopathologic features were consistent with mouse small B cell lymphoma progressing to leukemia with many similarities to human chronic lymphocytic leukemia. Given that many human chronic lymphocytic leukemias overexpress TRAF1 and Bcl-2, our findings suggest that cooperation between Bcl-2 and TRAF pathways contributes to the development of this type of leukemia.
B cell chronic lymphocytic leukemia (CLL) is the most common form of leukemia in adults in the western hemisphere and still is considered an incurable disease (1). CLL is characterized by a gradual accumulation of malignant B cells in patients as a result of deficient programmed cell death pathways rather than accelerated cell division (2). Although the underlying defect(s) in apoptosis in CLL B cells is undefined, a key feature of CLL cells is the expression of high levels of the antiapoptotic protein Bcl-2, which confers an apoptosis-resistant phenotype (3). Other lymphoid malignancies are also characterized by the expression of high levels of Bcl-2, as a consequence of various molecular mechanisms, including chromosome translocations, gene amplification, and transcriptional deregulation of the Bcl-2 gene.
TNF receptor-associated factors (TRAFs) are a family of adapter proteins that link TNF-family receptors (TNFRs) to intracellular signal transduction. Gene ablation studies in mice have demonstrated a critical role for specific TRAF-family members in signaling by many TNFRs, where they participate in signaling cascades involved in gene expression, cell proliferation, and control of apoptosis. Deregulation of these pathways is causative of several autoimmune and inflammatory diseases (4, 5). Elevated expression of some TRAF-family proteins, in particular TRAF1, is found in hematopoietic malignancies such as CLL and non-Hodgkin's lymphomas (NHL) (6–8). In this regard, roles for TRAF1 and TRAF2 in mediating apoptosis protection have been demonstrated (9–11), suggesting that these TRAF family members could participate in the apoptosis-resistant phenotype of CLL and NHL.
Transgenic mouse lines expressing Bcl-2 in B lymphocytes develop age-dependent lymphadenopathy and splenomegaly (12), associated with lymphoid cell expansions resembling certain human low-grade B cell malignancies (12, 13). Transgenic mice expressing in lymphocytes a TRAF2 mutant lacking the RING and zinc finger domains located at the N terminus of the molecule (TRAF2DN) also develop splenomegaly and lymphadenopathy, as a result of a polyclonal expansion of B lymphocytes (14). Interestingly, TRAF2DN is structurally similar to TRAF1, which is the only TRAF family member that lacks a RING finger domain (6). TRAF1 and TRAF2DN can heterodimerize with TRAF2, modulating various TRAF2 activities (6, 10, 14, 15).
In this report, we show that transgenic mice expressing both TRAF2DN and Bcl-2 in the B cell lineage develop an agedependent B cell leukemia and lymphoma having striking similarities to human CLL. These findings provide direct evidence that TRAFs can contribute to malignancy. Furthermore, our results provide functional evidence that the high levels of TRAF1 and Bcl-2 coexpression commonly found in human CLL cells contribute to the pathogenesis of this leukemia.
Materials and Methods
Transgenic Mice. Transgenic BALB/c mice expressing human Bcl-2 specifically in B lymphocytes have been described (12). The transgene mimics the (14, 18)(q32;21) translocation involving Bcl-2 and IgH found in human follicular lymphomas (FLs) (Fig. 1A). Transgenic FVB/N mice expressing a TRAF2 mutant lacking the N-terminal 240 amino acids encompassing the RING and zinc finger domains (TRAF2DN) have been described (14). Bcl-2 and TRAF2DN heterozygous mice were bred to produce litters with progeny of all four genotypes (wild-type, Bcl-2 single-positive, TRAF2DN single-positive, and TRAF2DN/Bcl-2 double-positive mice) expressed on mixed BALB/c × FVB/N. Analysis of the transgenic mouse genotypes was performed by PCR by using primers specific for human Bcl-2 and TRAF2DN, and verification of the transgene expression was accomplished by immunoblotting. Euthanasia was performed by following the rules of the American Veterinarian Medical Association.
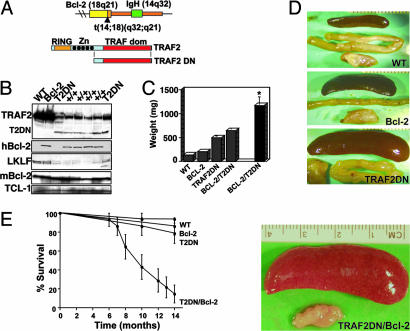
Fig. 1.
Characterization of transgenic mice. (A) Schematic representation of the Bcl-2 (Upper) and TRAF2DN (Lower) transgenic constructs. The Bcl-2 transgene is of human origin and mimics the t(14, 18)(q32-q21) chromosomal translocation found in most non-Hodgkin's B cell lymphomas by fusing a Bcl-2 minigene to a 14;18 breakpoint and adjacent IgH enhancer (12). TRAF2DN transgenic mice express a murine TRAF2 mutant lacking the RING finger domain and the four N-terminal zinc finger domains under the control of an H-2k promoter and an IgH enhancer that drives the transgene expression only in lymphocytes (14). (B) Immunoblot analysis was performed by using lysates from splenocytes isolated from age-matching mice having different genotypes, as indicated. Samples were normalized for protein content and blotted with Abs recognizing mouse TRAF2, human Bcl-2, mouse Bcl-2, lung Krüppel-like factor, and T cell lymphoma-1. +/+, TRAF2DN/Bcl-2 double-transgenic mice. (C) Spleen weights are compared from euthanized mice (gray columns) or from mice that died from disease or old age (black columns). The average weight ± SE of spleens from the different transgenic mice was calculated. WT, 138 ± 13.9 mg, n = 10; Bcl-2, 205 ± 6.3 mg, n = 9; TRAF2DN, 496 ± 37.7 mg, n = 10; TRAF2DN/Bcl-2, 647 ± 54 mg, n = 7; TRAF2DN/Bcl-2 dead from disease or old age, 1,247 ± 180 mg, n = 16. *, P < 0.05 when TRAF2DN/Bcl-2 (dead by natural causes) are compared with euthanized TRAF2DN/Bcl-2 littermates, and P < 0.0001 compared with age-matched wild-type mice by unpaired t test. (D) Representative examples of spleens and submaxilary lymph nodes from age-matched (12–13 months) transgenic mice. WT, wild-type. The spleen of a TRAF2DN/Bcl-2 double-transgenic mouse dead from disease is shown. (E) Kaplan–Meier analysis of survival of wild-type, Bcl-2 single-, TRAF2DN single-, and TRAF2DN/Bcl-2 double-transgenic mice (n = 14 per genotype). The proportion of animals remaining alive over time is plotted. Survival analysis was performed by using the nonparametric model of Kaplan–Meier, and statistical significance was determined by using the log-rank test.
Cell Isolation. Spleens and lungs were carefully crushed to release the lymphocytes. Ascitic fluid was obtained from the peritoneal cavity, and blood was collected in tubes coated with heparin from the heart of euthanized mice or from the cavernous sinus. Cell suspensions were depleted of red cells and neutrophils by density centrifugation (Lympholite-M, Cedarlane Laboratories) or hypotonic lysis in the case of blood.
Flow Cytometry. Lymphocytes were incubated with 25 μg/ml human γ-globulin to block Fc-receptors. Then 105 to 106 cells were incubated with a combination of allophycocyanin (APC)-, FITC-, or phycoerythrin (PE)-conjugated Abs recognizing different surface markers. Propidium iodide (PI) staining (1 μg/ml) was performed in some experiments to assess the viability of the cells, which was always >95%. Depending on the experiment, gating was performed either on the B220-positive population or on the lymphocyte populations, as determined by forward and right-angle scatter. The Abs used were APC-conjugated anti-B220 and CD3; FITC-conjugated anti-IgD, CD21, B220, CD29, CD11a, and CD4; and PE-conjugated anti-CD23, CD5, CD54, CD49d, CD11b, and CD8a (all from BD Biosciences); PE-conjugated anti-IgM (Southern Biotechnology Associates); and isotype controls (BD Biosciences). Flow cytometry analysis was accomplished by using a FACScalibur equipped with detectors for four colors (BD Biosciences).
For cell quantification, blood was collected in heparinized capillary tubes, diluted five times in PBS containing 10% FCS, 1% BSA, 0.05% sodium azide, 5 mM EDTA, and 50 μg/ml human γ-globulin, and incubated on ice for 15 min. Then the mixture was incubated with anti-CD45 PE-Cy5 and anti-B220 PE or the respective isotype controls and incubated for 20 min at room temperature. Erythrocytes were lysed by using 10 volumes of hypotonic lysis buffer (PharmLyse, BD Biosciences), and quantification was performed on a personal cell analysis and counter microfluorocytometer (Guava Technologies, Hayward, CA).
For DNA content analysis, cells were washed and resuspended in 0.4 ml of hypotonic buffer containing 0.1% sodium citrate, 0.37% Nonidet P-40, 20 μg/ml RNase A, and 50 μg/ml PI. Cells were incubated for 5 min on ice and analyzed by flow cytometry.
Immunohistochemistry. Tissues and organs from transgenic mice were fixed in Bouin's solution (Sigma) and embedded in paraffin, and tissue sections (5 μm) were stained with hematoxylin/eosin and for immunohistochemistry as described (7, 16). Bone marrow, blood smears, and ascites were stained with Wright–Giemsa staining.
Immunoblotting. Cell lysates from frozen mouse lymphoid tissues were prepared in modified Laemmli buffer, as described (17) Tissue lysates were normalized for total protein content (50 μg per lane) and subjected to SDS/PAGE followed by immunoblot analysis using anti-human Bcl-2 (16), anti-mouse Bcl-2 (18), anti-TRAF2 (Santa Cruz Biotechnology, C-20), antilung Krüppel-like factor (19), and anti-Tcl-1 (Santa Cruz Biotechnology, F-14) Abs. Detection was accomplished by using appropriate secondary horseradish peroxidase-conjugated Abs (Bio-Rad), followed by an enhanced chemiluminescence assay (Amersham Pharmacia).
Southern Blotting. Ig heavy chain (IgH) gene rearrangements were analyzed by Southern blotting by using a mouse IgH probe that spans JH2 and Eμ, essentially as described (20).
In Vitro Cell Survival Assays. Purified splenocytes (5 × 105 cells per well) were cultured in RPMI medium 1640 containing 10% FCS (HyClone), 50 μM 2-mercaptoethanol, oxalacetate, pyruvate and insulin supplement (Sigma), 1 mM l-glutamine, and antibiotics in the presence of different chemotherapeutic drugs. Cells were recovered by centrifugation at various times and stained with APC-anti-B220 mAb, and cell death of the B220+ lymphocytes was determined by double staining with FITC-labeled annexin-V and PI (BioVision, Mountain View, CA), followed by fluorescence-activated cell sorter analysis using the FL-1 and FL-3 channels. Annexin-V-positive cells were considered apoptotic.
Results
Mice Expressing TRAF2DN and Bcl-2 Develop Massive Splenomegaly and Die Prematurely. Many B cell malignancies, including CLL and FL, are characterized by excessive Bcl-2 expression, which is important to the etiology of these diseases (3). In addition, increased levels of TRAF1 expression have also been observed in many CLLs and FLs (6, 7). However, the relevance to B cell malignancy of combined up-regulation of Bcl-2 and TRAF1 has not been established. Interestingly, mice overexpressing either Bcl-2 or TRAF2DN developed polyclonal expansions of B cells, although they rarely progressed to lethal malignancies. To test the hypothesis that TRAF2DN and Bcl-2 might cooperate in the development of B cell malignancies, we took advantage of the availability of Bcl-2 and TRAF2DN transgenic mice and produced double-transgenic mice expressing both Bcl-2 and TRAF2DN in B lymphocytes.
Analysis of spleen size in transgenic mice expressing Bcl-2 or TRAF2DN alone or in combination revealed that splenomegaly was already detectable at birth in all three lines, although it became more evident over time. Thus, Bcl-2 mice from ages 10–16 months had spleens averaging 2 times the weight of wild-type littermates, whereas TRAF2DN mice had spleens 3 to 4 times larger and also developed lymphadenopathy, as described (14). Age-matched TRAF2DN/Bcl-2 double-transgenic mice had spleens similar in size and weight to TRAF2DN (647 ± 54 mg vs. 496 ± 38 mg, respectively). However, a majority of TRAF2DN/Bcl-2 double-transgenic mice died prematurely, unlike Bcl-2 and TRAF2DN single-transgenic mice. At death, TRAF2DN/Bcl-2 mice displayed severe splenomegaly (1,246 ± 180 mg, n = 16), with spleen weights that were at least twice those of age-matched TRAF2DN single- and asymptomatic TRAF2DN/Bcl-2 double-transgenic mice (P < 0.05) and 10 times higher than wild-type littermates (138 ± 13.9 mg, n = 10; P < 0.0001). Furthermore, these mice also had severe lymphadenopathy, and roughly half of them developed ascites. The presence of pleural effusion was a common event, and, thus, cardiorespiratory insufficiency might be the ultimate cause of death of these mice. Representative examples of the spleens and submaxillary lymph nodes from the various transgenic lines are shown in Fig. 1D.
Bcl-2 single-transgenic mice did not develop major health problems and had a normal life span, as indicated above. In contrast, TRAF2DN/Bcl-2 double-transgenic mice had a much-reduced survival. Some of these mice died as early as 6 months after birth. By 14 months, <20% of the TRAF2DN/Bcl-2 mice remained alive (Fig. 1E). In contrast, only 10% of TRAF2DN mice (n = 21) developed massive splenomegaly.
TRAF2DN and Bcl-2 transgene expression was examined by immunoblotting by using spleens from age-matched mice of the different genotypes (Fig. 1B). Interestingly, expression of TRAF2DN correlates with a striking reduction of endogenous TRAF2 protein. The mechanism of TRAF2DN-mediated downregulation of TRAF2 is not yet known, but it further implies an impairment of TRAF2 activities in the TRAF2DN transgenic mice. Accordingly, expression of the lung Krüppel-like factor, which depends on TRAF2 (11), is severely reduced in TRAF2DN and TRAF2DN/Bcl-2 splenocytes compared with wild-type and Bcl-2 transgenic littermates (Fig. 1B). In contrast, similar levels of T cell lymphoma (TCL)-1 protein, previously implicated in development of CLL and lymphoma (21, 22), were found in all mouse lines.
TRAF2DN/Bcl-2 Double-Transgenic Mice Develop Small B Cell Lymphoma and CLL-Like Leukemia. Histological analysis of TRAF2DN and TRAF2DN/Bcl-2 spleens consistently showed the expansion of the marginal zone with infiltrating small B lymphocytes (Fig. 2). In contrast, Bcl-2 mice also had an expanded population of small B cells consisting of centrocytes that typically expanded the splenic white pulp (not shown).
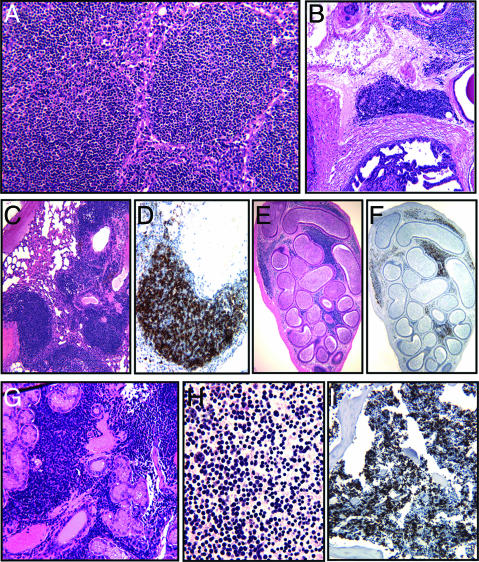
Fig. 2.
Histochemical analysis of tissues from the TRAF2DN/Bcl-2 double-transgenic mice. Immunohistochemical analysis of organs and tissues from the TRAF2DN/Bcl-2 double-transgenic mice was performed. Spleen (A), prostate (B), lung (C and D), epididymis (F and G), submaxillary gland lymph node (H), ascites (I), and bone marrow (J). Hematoxylin/eosin staining is shown in A–C, E, and G. Staining with anti-B220 mAb is shown in D, F, and I to illustrate that the infiltrating cells are predominately B lymphocytes. Wright–Giemsa staining of ascites cells is shown in H.
Immunohistochemical analysis of TRAF2DN/Bcl-2 mice with overt disease showed massive infiltration of B220-positive lymphocytes throughout major organs and tissues (Fig. 2). TRAF2DN single-transgenic mice also showed lymphocyte infiltration in lungs, but never to the extent of TRAF2DN/Bcl-2 double-transgenic mice (data not shown). The bone marrow of mice with overt disease was massively infiltrated with B220-positive small lymphocytes, which would be consistent with the development of leukemia (see below).
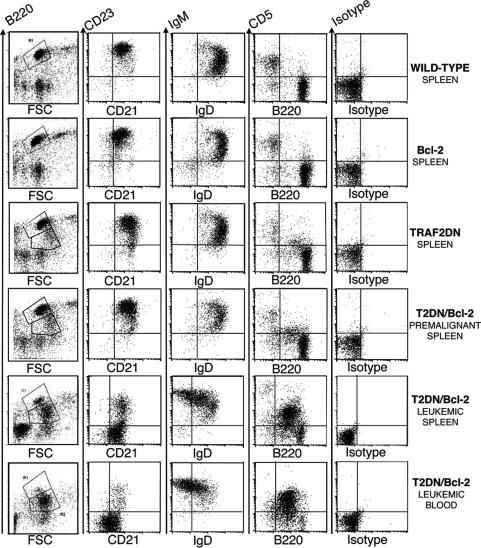
We characterized the lymphoid cell populations from these mice by fluorescence-activated cell sorter (FACS) analysis. The B cells found in Bcl-2 mice are similar to those found in wild-type mice, which are mainly mature B cells expressing B220H/IgMMtoL/IgDH/CD21L/CD23H/CD5null (Fig. 3). TRAF2DN mice have an expanded subpopulation of B220M/CD21M/CD23M B cells. (Fig. 3). Young or asymptomatic TRAF2DN/Bcl-2 mice contained expanded B cell populations similar to those found in the TRAF2DN single-transgenic mice (Fig. 3). In contrast, FACS analysis of splenocytes from TRAF2DN/Bcl-2 mice that died naturally or that had developed leukemia consistently showed a dramatic expansion of a population of B220M/IgMH/IgDL to null/CD21L to null/CD23null/CD11bL B cells. In the majority of the mice, these cells also expressed CD5, consistent with a B-1a phenotype. In one-quarter of the mice, this cell population did not express CD5, consistent with a B-1b phenotype (23) (Fig. 3 and Figs. 7 and 8, which are published as supporting information on the PNAS web site). These mice also developed leukemia, with B cell counts in blood as high as 167 × 106 cells per ml, compared with an average for wild-type mice of 4 × 106 B cells per ml. The accumulation of leukemic B cells in blood over a period of 3 weeks is shown in Fig. 9A, which is published as supporting information on the PNAS web site. Analysis of the blood B lymphocytes of leukemic mice consistently shows the expansion of the B-1-like B220M/IgMH/IgDLtonull/CD21Ltonull/CD23null B cells (Fig. 3 and Fig. 9B, which is published as supporting information on the PNAS web site).
Fig. 3.
Analysis of B lymphocyte populations in wild-type vs. transgenic mice. Four-color flow-cytometry analysis was performed to determine the phenotype of B lymphocytes. Gating was performed on the B220+ population (R1 and R2, Left) in the case of CD23/CD21 and IgM/IgD analyses or on the lymphocyte population for B220/CD5 and isotype control analyses. Splenocytes were analyzed from representative 11-month-old wild-type, Bcl-2, TRAF2DN, and TRAF2DN/Bcl-2 double-transgenic mice at a premalignant stage and splenocytes or blood lymphocytes of a representative TRAF2DN/Bcl-2 mouse (12 months old) with acute disease.
As indicated above, we also found this B-1-like population in both nodal and extranodal locations. However, some terminally ill mice also had expansions of phenotypically different B lymphocyte subpopulations, especially in ascitic fluid or lung compared with spleen or blood of the same mouse (Fig. 8). This population heterogeneity might be caused by oligoclonal expansion of malignant lymphocytes or could reflect phenotypic alterations caused by the microenvironment.
Bcl-6 expression was not detected in the expanded B cell populations of the TRAF2DN/Bcl-2 double-transgenic mice, thus confirming a pregerminal center origin (24) (data not shown). Total numbers of T cells (CD3 positive) as well as the CD4/CD8 ratio were similar in wild-type and transgenic mice (data not shown).
The combined histopathologic and phenotypic features of the B cell expansions observed in the TRAF2DN/Bcl-2 mice are thus fully consistent with the diagnosis of small B cell lymphoma (SBL) with a leukemic phase (25). This mouse SBL is immunophenotypically and morphologically similar in many respects to human CLL.
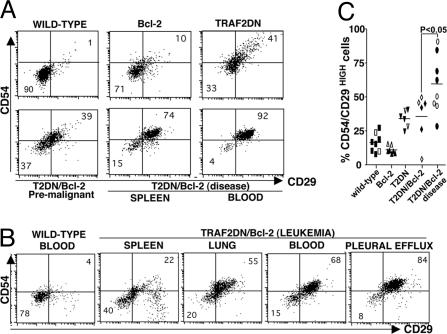
Increased Expression of Adhesion Molecules on the TRAF2DN/Bcl-2 B Lymphocytes. Increased expression of cell adhesion molecules has been implicated in invasiveness of malignant B cells (26). Because TRAF2DN/Bcl-2 double-transgenic mice developed massive lymphoid infiltration in nonlymphoid tissues (Fig. 2), we analyzed the expression of various cell adhesion molecules on the surface of B lymphocytes from transgenic mice, including CD54 (intercellular adhesion molecule-1) and CD29 (β1 integrin) (Fig. 4). B cells from wild-type and Bcl-2 transgenic mice had comparable CD54 and CD29 expression levels. However, B cells from TRAF2DN and asymptomatic TRAF2DN/Bcl-2 mice showed increased expression of CD54 and CD29 (Fig. 4A). This increase in CD54 and CD29 expression was even more evident in B cells from TRAF2DN/Bcl-2 mice in the acute phase of the disease (P < 0.05) (Fig. 4 B and C). The B cells with high CD54 and CD29 are those expressing B220M/IgMH/IgDLtonull/CD21Ltonull/CD23null, whereas normal mature B cells expressing B220H/IgMM/IgDH/CD21L/CD23H bear normal levels of CD54 and CD29 (Fig. 9B). Analysis of the expression of CD11a (LFA-1) and CD49d (α4 integrin) also revealed a moderate increase in the expression of these adhesion proteins on B cells in TRAF2DN and TRAF2DN/Bcl-2 mice (not shown).
Fig. 4.
Increased levels of adhesion molecules on the surface of B lymphocytes from TRAF2DN and TRAF2DN/Bcl-2 transgenic mice. (A) Surface expression of intercellular adhesion molecule-1 (CD54) and β1-integrin (CD29) was measured on the B220-positive B lymphocytes by flow cytometry. FITC anti-CD29 and PE-anti-CD54 mAbs were used to stain splenic B cells from age-matched wild-type, Bcl-2, TRAF2DN, premalignant TRAF2DN/Bcl-2 double-positive, and TRAF2DN/Bcl-2 double-positive in the acute phase of the disease and blood lymphocytes from this TRAF2DN/Bcl-2 double-positive mouse in the acute phase. (B) A similar analysis was performed by using blood from another representative wild-type mouse or lymphocytes from spleen, lungs, blood, or pleural effusion of a TRAF2DN/Bcl-2 mouse that had developed leukemia. (C) The percentage of B220-positive lymphocytes with high levels of surface CD54/CD29 expression (upper right quadrant) was compared. B cells from spleen (black dots) or lung (white dots) were analyzed. Each dot represents an individual mouse. Statistical significance was determined by unpaired t test.
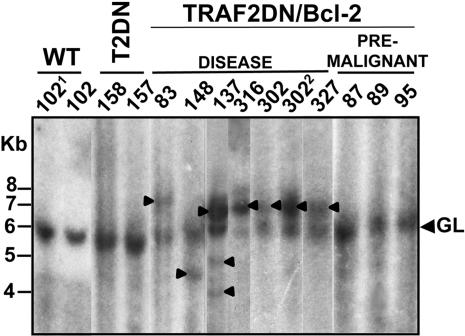
Analysis of Ig Gene Rearrangements in B Cells from the TRAF2DN/Bcl-2 Double-Transgenic Mice Reveals Clonal Neoplasms. The increase in B cell numbers seen in TRAF2DN/Bcl-2 mice upon progression to the symptomatic phase could result from either the outgrowth of neoplastic B cell clones or a gradual unrelenting accumulation of polyclonal B cells. To distinguish between these possibilities, we analyzed DNA from splenocytes and pleural effusion of transgenic mice for the presence of clonal Ig gene rearrangements. As shown in Fig. 5, the existence of a single JH rearrangement (mice 83, 148, 316, 302, and 327) is indicative of the expansion of a single B cell clone in the spleens of these TRAF2DN/Bcl-2 mice that progressed to overt disease, whereas multiple rearrangements were observed in mouse 137, indicative of the expansion of different clones. In contrast, no evidence of clonality was observed in Bcl-2 (data not shown), TRAF2DN, or asymptomatic (premalignant) TRAF2DN/Bcl-2 mice (Fig. 5).
Fig. 5.
Southern blot analysis of IgH rearrangement in B cells from TRAF2DN/Bcl-2 mice. DNA was isolated from spleen, liver (1), or pleural effusion (2), and digested with EcoR1 and analyzed by Southern blotting by using a IgH probe. The band of 6 kb corresponds to the germ line (GL) IgH locus. IgH rearrangements corresponding to clonal expansions are indicated with an arrowhead. Wild-type, TRAF2DN, and TRAF2DN/Bcl-2 mice either asymptomatic (premalignant) or with overt lymphoma (disease) were analyzed. The ages in months of the mice (identified by number) analyzed were 17 (102), 15 (137), 14 (158, 157, and 148), 13 (83 and 316), and 12 (302, 327, 87, 89, and 95).
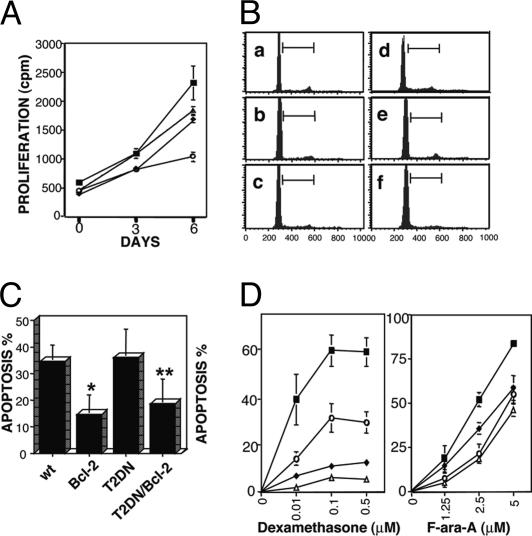
Apoptosis Resistance of TRAF2DN/Bcl-2 B Cells. The expansion of B cells in the double-transgenic mice could have occurred because of accelerated cell division, reduced cell death, or both. To explore the basis for the expansion, we performed cell proliferation assays of lymphocytes isolated from the various transgenic mice. As shown in Fig. 6A, cells from Bcl-2, TRAF2DN, and asymptomatic TRAF2DN/Bcl-2 double-transgenic mice had proliferation rates comparable to those of cells from wild-type littermates (Fig. 6A). Consistent with these results, no increase in the number of cells over a period of 2 weeks was observed in cultures of TRAF2DN/Bcl-2 splenocytes, ascites, or blood lymphocytes isolated from mice with leukemia and overt lymphoma (n = 4). DNA content analyses were then performed to assess the cell cycle distribution of lymphocytes isolated from TRAF2DN/Bcl-2 double-transgenic mice that developed overt lymphoma. The percentage of cycling cells as evidenced by DNA content >2n (i.e., cells in S, G2, or M phase) was only slightly higher in splenocytes of TRAF2DN/Bcl-2 double-transgenic mice with overt lymphoma (9.1 ± 2%), compared with age-matching wild-type mice (6.3 ± 0.9; P > 0.2), Bcl-2 single-(6.2 ± 0.2%), and TRAF2DN single-transgenic mice (5.6 ± 0.6%) (Fig. 6B a–d). However, these differences were not observed in lymphocytes isolated from ascitic fluid or extracted from lungs of symptomatic TRAF2DN/Bcl-2 double-transgenic mice (5.3 ± 0.5% and 5.8 ± 0.7%, respectively) (Fig. 6Bd and e). Furthermore, immunostaining of lymphoid organs of TRAF2DN/Bcl-2 mice with Ab to proliferating cell nuclear antigen (PCNA) revealed only occasional (<6%) PCNA-positive cells before and after progression to overt disease (data not shown).
Fig. 6.
(A) Analysis of lymphocyte proliferation. Lymphocytes were isolated from spleens of wild-type (▪), Bcl-2 (♦), TRAF2DN (○), and TRAF2DN/Bcl-2 (Δ) transgenic mice. Cells (5 × 104) were seeded in triplicate in 96-well plates and pulsed with 1 μCi (1 Ci = 37 GBq) of [3H]-methylthymidine for 12 h at days 0 (immediately after purification), 3, and 6. Proliferation was measured as the mean of [3H]-methylthymidine incorporation (cpm) ± SEM (n = 3) from three mice of each genotype. (B) DNA content analysis; 106 lymphocytes from spleen (a–d), ascitic fluid (e), and lung (f) from wild-type (a; n = 4), Bcl-2 (b; n = 3), TRAF2DN (c; n = 4), and symptomatic TRAF2DN/Bcl-2 (d–f; n = 4) mice were lysed in hypotonic buffer and stained with PI. Cells with DNA content ≥ of 2n were gated, and the percentage of G1 cells (DNA content = 2n) and cells in S, G2, or M phases (>2n) was quantified. Shown are representative DNA content profiles of the various genotypes. The statistical analysis in the text corresponds to mean ± SEM. (C) Bcl-2 protects cells from spontaneous apoptosis. Splenocytes isolated from wild-type, Bcl-2, TRAF2DN, and TRAF2DN/Bcl-2 transgenic mice were cultured for 24 h and then incubated with APC-anti-B220 mAb for 1 h, washed, and incubated with annexinV-FITC/PI. B lymphocytes (B220+ cells) were gated, and the average percentage of annexin V-FITC-positive (apoptotic) B lymphocytes and the SD (n = 3) is shown (P = 0.01) comparing apoptosis of wild-type vs. Bcl-2 (*) or TRAF2DN/Bcl-2 (**). B cells are compared by unpaired t test. (D) Effects of chemotherapeutic drugs on apoptosis. Splenocytes isolated from wild-type (▪), Bcl-2 (♦), TRAF2DN (○), and TRAF2DN/Bcl-2 (Δ) transgenic mice were incubated with or without dexamethasone (0.01, 0.1, and 0.5 μM) or F-ara-A (1.25, 2.5, and 5 μM). Cells were harvested after 24 (Dex) or 48 (F-ara-A) h and incubated with APC-anti-B220 mAb for 1 h, then washed and incubated with annexin-V-FITC and PI. B lymphocytes (B220+ cells) were gated, and the percentage of apoptotic cells (annexin V+) was determined by flow cytometry. Data were corrected for differences in spontaneous apoptosis (mean ± SEM) (n = 6).
We next tested whether B cells from the transgenic mouse lines displayed resistance to apoptosis. Splenocytes from the TRAF2DN single-transgenic mice and wild-type littermates had similar spontaneous apoptosis rates after 24 h in culture (36.2 ± 10.8% and 34.6 ± 6.2%, respectively). In contrast, the apoptosis rates were significantly reduced in Bcl-2 single- and TRAF2DN/Bcl-2 double-transgenic mice (14.7 ± 7.5 and 18.7 ± 9.4%, respectively) (Fig. 6C).
Lymphocytes from the Bcl-2, TRAF2DN, and TRAF2DN/Bcl-2 double-positive mice were also tested for apoptosis induced by chemotherapeutic drugs commonly used in the treatment of CLL, including the nucleoside analog fludarabine and the glucocorticoid dexamethasone (Fig. 6D). B cells from the Bcl-2 and TRAF2DN/Bcl-2 double-transgenic mice displayed marked resistance to apoptosis induced by these drugs, particularly to dexamethasone (Fig. 6D). Interestingly, B cells from the TRAF2DN single-transgenic mice also displayed significant resistance to both dexamethasone- and fludarabine-induced apoptosis (Fig. 6D), thus suggesting that both TRAF2DN and Bcl-2 protect B cells against apoptosis.
Altogether, these results strongly suggest that resistance to apoptosis rather than deregulation of proliferation accounts for the malignant transformation of lymphocytes in TRAF2DN/Bcl-2 double-transgenic mice.
Discussion
The results described here show that the combined deregulated expression of Bcl-2 and TRAF2 resulted in development of small B cell lymphoma/CLL in mice. Our criteria for defining this disease as a CLL-like disorder are as follows: (i) the mice develop leukemia; (ii) the neoplastic B cells express CD5; (iii) they do not express Bcl-6; (iv) they progress to clonal malignancies that are lethal; (v) the cells are not rapidly proliferating; and (vi) the B cells display an apoptosis-resistant phenotype.
The data presented above suggest that a blockage to apoptosis with subsequent additional secondary genetic lesions resulting in further growth advantage is the cause of the malignant B cell expansion that occurs during disease progression in the TRAF2DN/Bcl-2 mice. In this regard, some lines of transgenic mice overexpressing Bcl-2 in B cells develop plasmacytoma and pre-B cell lymphoma (13), although the frequency of lethal malignancy is generally low (only 3–15% of the mice). However, the Bcl-2 transgenic line we used (12) did not develop tumors and had a normal life span, despite polyclonal expansion of B cells. These results indicate that Bcl-2 alone is not sufficient for malignant transformation, and that additional factors are required for development of B cell malignancy. An example of a cooperating gene is c-MYC, which collaborates with Bcl-2 to induce aggressive lymphomas (27). However, those lymphomas are comprised of large cells (diffuse histocytic lymphocytes) different from the small B cell lymphoma that arises in TRAF2DN/Bcl-2 mice.
Our data revealed a role for TRAF-family proteins in tumorigenesis. TRAFs integrate signals from many members of the TNFR family and regulate multiple cellular processes, including proliferation, gene expression, and apoptosis. However, until now, no evidence has been reported supporting direct involvement of TRAF-family proteins in cancer.
TRAF2 is a prototypical member of the TRAF family, comprised of a RING finger domain within its N terminus, followed by five zinc finger domains and the TRAF domain (5). In contrast, TRAF1 lacks the RING and zinc finger domains found in TRAF2 and other members of the TRAF family (5, 6). TRAF2DN is similar to TRAF1, and both proteins are known to bind many of the same proteins via their TRAF domains (10, 14). The RING and zinc finger domains are essential for many TRAF2 activities, such as Jun N-terminal kinase activation and association with lipid rafts and the cytoskeleton (10). Consequently, TRAF1 and TRAF2DN are considered to be dominant inhibitors of TRAF2 through their ability to compete with endogenous TRAF2 for binding to various TNFRs and downstream signaling molecules. Interestingly, we observed that TRAF2DN expression in B cells also resulted in a marked decrease in endogenous TRAF2 protein, further supporting a role for TRAF2DN as a TRAF2 antagonist in our system.
In humans, TRAF1 is overexpressed in many CLL and FLs. In CLL, TRAF1 expression is associated with more aggressive chemorefractory disease (7). Whether TRAF1 confers a survival advantage to CLL is unclear at present. However, our evidence of an antiapoptotic role for TRAF2DN in B cells suggests that TRAF1 may have a similar function. The mechanisms underlying the antiapoptotic effects of TRAF1 and TRAF2DN in B lymphocytes could involve (i) effects on Jun N-terminal kinase, which plays an essential role in TNF-induced apoptosis (28); (ii) effects on NFκB activation, which is required for resistance to apoptosis in some circumstances (29); and (iii) inhibition of signaling by TNF-family death receptors, thus complementing Bcl-2-mediated suppression of the mitochondrial pathway for apoptosis.
The B cells that accumulate in the spleen and nodes of TRAF2DN single- and TRAF2DN/Bcl-2 double-transgenic mice express elevated surface levels of adhesion proteins. Thus, TRAF2DN may modulate cell invasiveness through upregulation of cell adhesion proteins such as CD54. However, despite increased expression of adhesion proteins, B cells of TRAF2DN single-transgenic mice did not accumulate in extranodal tissues to nearly the extent observed in TRAF2DN/Bcl-2 mice. We speculate, therefore, that once leaving the nodal compartment, TRAF2DN B cells die unless apoptosis is blocked by Bcl-2. In this scenario, Bcl-2 overexpression would allow the survival of those B cells that have migrated into extranodal environments, as has been suggested for other members of the Bcl-2 family (30). We also suggest that the ability of TRAF2DN/Bcl-2 B cells to accumulate in extranodal tissues is a manifestation of the complementary abilities of Bcl-2 to promote survival in ectopic sites and TRAF2DN to promote invasiveness. It remains to be determined which genetic events contribute to progression of TRAF2DN/Bcl-2 B cells to monoclonal malignancies followed by induction of a leukemic phase and death.
Supplementary Material
Acknowledgments
We thank Dr. J. Ward (Veterinary and Tumor Pathology Section, National Cancer Institute, Frederick, MD) for expert evaluation of tissue sections; Dr. W. Plunkett (MD Anderson Cancer Center, Houston) for F-ara-A; Dr. S. Janz (Center for Cancer Research, National Cancer Institute, Bethesda) for the pJ11 IgH probe; Dr. L. Glimcher (Harvard Medical School, Boston) for the antilung Krüppel-like factor polyclonal Ab; and C. L. Kress, M. Thomas, I. M. Pedersen, M. Mcalonis, X. Xiao, C. Yost, L. Fiorentino, and the personnel of the animal facility at the Burnham Institute for excellent technical assistance. We also thank Dr. S. Krajewski, members of the CLL Research Consortium and Guava Technologies (Hayward, CA) for helpful discussions and advice. This study was supported by National Cancer Institute Grants CA69381 and CA81534.
Author contributions: J.M.Z. and J.C.R. designed research; J.M.Z. and M.K. performed research; J.M.Z., M.K., H.C.M., and Y.C. analyzed data; and J.M.Z. wrote the paper.
Abbreviations: TNFR, TNF family receptor; TRAF, TNF-receptor-associated factor; CLL, chronic lymphocytic leukemia; PI, propidium iodide; TRAF2DN, TRAF2 mutant lacking the RING and zinc finger domains located at the N terminus of the molecule; APC, allophycocyanin; PE, phycoerythrin; IgH, Ig heavy chain; FL, follicular lymphoma.
References
- 1.Kipps, T. (1997) Curr. Opin. Hematol. 4, 268–276. [DOI] [PubMed] [Google Scholar]
- 2.Reed, J. (1998) Clin. Immunol. Newslett. 17, 125–140. [Google Scholar]
- 3.Kitada, S., Pedersen, I. M., Schimmer, A. & Reed, J. C. (2002) Oncogene 21, 3459–3474. [DOI] [PubMed] [Google Scholar]
- 4.Bradley, J. R. & Pober, J. S. (2001) Oncogene 20, 6482–6491. [DOI] [PubMed] [Google Scholar]
- 5.Zapata, J. M. (2003) Exp. Opin. Ther. Targets 7, 411–425. [DOI] [PubMed] [Google Scholar]
- 6.Zapata, J. M. & Reed, J. C. (2002) Science STKE, www.stke.org/cgi/content/full/OC_sigtrans;2002/133/pe27n. [DOI] [PubMed]
- 7.Zapata, J. M., Krajewska, M., Krajewski, S., Kitada, S., Welsh, K., Monks, A., McCloskey, N., Gordon, J., Kipps, T., Gascoyne, R. D., et al. (2000) J. Immunol. 165, 5084–5096. [DOI] [PubMed] [Google Scholar]
- 8.Munzert, G., Kirchner, D., Stobbe, H., Bergmann, L., Schmid, R. M., Dohner, H. & Heimpel, H. (2002) Blood 100, 3749–3756. [DOI] [PubMed] [Google Scholar]
- 9.Wang, C., Mayo, M., Korneluk, R., Goeddel, D. & Baldwin, A. (1998) Science 281, 1680–1683. [DOI] [PubMed] [Google Scholar]
- 10.Arron, J. R., Pewzner-Jung, Y., Walsh, M. C., Kobayashi, T. & Choi, Y. (2002) J. Exp. Med. 196, 923–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin, Y., Ryan, J., Lewis, J., Wani, M. A., Lingrel, J. B. & Liu, Z. G. (2003) Mol. Cell. Biol. 23, 5849–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsumata, M., Siegel, R. M., Louie, D. C., Miyashita, T., Tsujimoto, Y., Nowell, P. C., Greene, M. I. & Reed, J. C. (1992) Proc. Natl. Acad. Sci. USA 89, 11376–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasser, A., Harris, A. W. & Cory, S. (1993) Oncogene 8, 1–9. [PubMed] [Google Scholar]
- 14.Lee, S. Y., Reichlin, A., Santana, A., Sokol, K. A., Nussenzweig, M. C. & Choi, Y. (1997) Immunity 7, 703–713. [DOI] [PubMed] [Google Scholar]
- 15.Fotin-Mleczek, M., Henkler, F., Hausser, A., Glauner, H., Samel, D., Graness, A., Scheurich, P., Mauri, D. & Wajant, H. (2003) J. Biol. Chem. [DOI] [PubMed]
- 16.Krajewski, S., Bodrug, S., Gascoyne, R., Berean, K., Krajewska, M. & Reed, J. C. (1994) Am. J. Pathol. 145, 515–525. [PMC free article] [PubMed] [Google Scholar]
- 17.Zapata, J. M., Takahashi, R., Salvesen, G. S. & Reed, J. C. (1998) J. Biol. Chem. 273, 6916–6920. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita, T., Krajewski, S., Krajewska, M., Wang, H. G., Lin, H. K., Hoffman, B., Lieberman, D. & Reed, J. C. (1994) Oncogene 9, 1799–1805. [PubMed] [Google Scholar]
- 19.Kuo, C. T., Veselits, M. L. & Leiden, J. M. (1997) Science 277, 1986–1990. [DOI] [PubMed] [Google Scholar]
- 20.Kovalchuk, A. L., Kim, J. S., Park, S. S., Coleman, A. E., Ward, J. M., Morse, H. C., III, Kishimoto, T., Potter, M. & Janz, S. (2002) Proc. Natl. Acad. Sci. USA 99, 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bichi, R., Shinton, S. A., Martin, E. S., Koval, A., Calin, G. A., Cesari, R., Russo, G., Hardy, R. R. & Croce, C. M. (2002) Proc. Natl. Acad. Sci. USA 99, 6955–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyer, K. K., French, S. W., Turner, D. E., Nguyen, M. T., Renard, M., Malone, C. S., Knoetig, S., Qi, C. F., Su, T. T., Cheroutre, H., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 14392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berland, R. & Wortis, H. H. (2002) Annu. Rev. Immunol. 20, 253–300. [DOI] [PubMed] [Google Scholar]
- 24.Niu, H. (2002) Hematol. Oncol. 20, 155–166. [DOI] [PubMed] [Google Scholar]
- 25.Morse, H. C. I., Anver, M. R., Fredrickson, T. N., Haines, D. C., Harris, A. W., Harris, N. L., Jaffe, E. S., Kogan, S. C., MacLennan, I. C. M., et al. (2002) Blood 100, 246–258. [DOI] [PubMed] [Google Scholar]
- 26.Drillenburg, P. & Pals, S. T. (2000) Blood 95, 1900–1910. [PubMed] [Google Scholar]
- 27.Harris, A., Strasser, A., Bath, M., Elefanty, A. & Cory, S. (1997) Curr. Top. Microbiol. Immunol. 224, 221–230. [DOI] [PubMed] [Google Scholar]
- 28.Deng, Y., Ren, X., Yang, L., Lin, Y. & Wu, X. (2003) Cell 115, 61–70. [DOI] [PubMed] [Google Scholar]
- 29.Karin, M. & Lin, A. (2002) Nat. Immunol. 3, 221–227. [DOI] [PubMed] [Google Scholar]
- 30.Pelengaris, S., Khan, M. & Evan, G. I. (2002) Cell 109, 321–334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.