Abstract
Estrogen (E) deficiency leads to an expansion of the pool of tumor necrosis factor (TNF)-producing T cells through an IFN-γ-dependent pathway that results in increased levels of the osteoclastogenic cytokine TNF in the bone marrow. Disregulated IFN-γ production is instrumental for the bone loss induced by ovariectomy (ovx), but the responsible mechanism is unknown. We now show that mice with T cell-specific blockade of type β transforming growth factor (TGFβ) signaling are completely insensitive to the bone-sparing effect of E. This phenotype results from a failure of E to repress IFN-γ production, which, in turn, leads to increased T cell activation and T cell TNF production. Furthermore, ovx blunts TGFβ levels in the bone marrow, and overexpression of TGFβ in vivo prevents ovx-induced bone loss. These findings demonstrate that E prevents bone loss through a TGFβ-dependent mechanism, and that TGFβ signaling in T cells preserves bone homeostasis by blunting T cell activation. Thus, stimulation of TGFβ production in the bone marrow is a critical “upstream” mechanism by which E prevents bone loss, and enhancement of TGFβ levels in vivo may constitute a previously undescribed therapeutic approach for preventing bone loss.
Although multiple genotropic and nongenotropic effects contribute to explain how estrogen (E) controls bone remodeling (1, 2), most of the bone-sparing activity exerted by E occurs through modulation of bone cell life span and decreased cytokine-driven osteoclastogenesis (3, 4). Among the factors that up-regulate osteoclast (OC) formation and lead to bone loss in estroprevic humans and rodents is tumor necrosis factor (TNF) α (5). This E-regulated cytokine promotes osteoclastogenesis by augmenting the production of receptor activator of nuclear factor-κB ligand (RANKL) (1), the nonredundant cytokine responsible for OC development (6) and by increasing the responsiveness of maturing OCs to this factor (7–9). Furthermore, TNF stimulates the production of other cytokines known to be implicated in the pathogenesis of ovariectomy (ovx)-induced bone loss, such as IL-1, IL-6, IL-7, and macrophage colony-stimulated factor (1, 10).
ovx increases TNF levels in the bone marrow (BM) via an expansion of the pool of TNF-producing T cells (7, 11) induced by a complex mechanism driven by IFN-γ (12). This cytokine augments antigen (Ag) presentation by enhancing MHCII expression on BM macrophages (BMMs), through induction of class II transactivator (CIITA) expression (13). Up-regulation of Ag presentation results, in turn, in increased T cell activation. Thus, up-regulation of IFN-γ production induced by ovx leads to increased T cell proliferation and life span, a phenomenon that results in an increase in both the total number of T cells and the pool of TNF-producing T cells (12). T cell-produced TNF plays a pivotal role in the mechanism of ovx-induced bone loss, as demonstrated by the failure of ovx to induce bone loss in T cell-deficient nude mice and by the ability to reconstitute with WT T cells, but not TNF–/– T cells, to restore a normal response to ovx (7, 11).
The mechanism by which ovx up-regulates the production of IFN-γ remains undetermined, but type β transforming growth factor (TGFβ) could be involved. TGFβ has been reported to repress the production of IFN-γ by directly targeting T cells and inhibiting their proliferation (14) and differentiation into effector cells (15). In addition to its effects on IFN-γ production, TGFβ is recognized for its ability to repress the production of and responsiveness to numerous cytokines relevant for bone homeostasis. For example, TGFβ signaling in BMMs decreases the responsiveness of the CIITA gene to IFN-γ (16, 17) via a mechanism involving Smad3 (18), whereas targeting of stromal cells and osteoblasts by TGFβ blunts BM production of IL-7 (19).
Because of its capacity to repress Ag presentation through modulation of CIITA expression, its direct effect on T cell proliferation and differentiation, and its ability to blunt the production of IL-7, TGFβ blocks T cell activation and T cell TNF production. Because the levels of TGFβ in bone and serum are increased by E and blunted by ovx (20–22), TGFβ may be a pivotal upstream target of E in bone.
To test this hypothesis, we investigated the contribution of the regulatory effect of TGFβ on T cells to ovx-induced bone loss. The data show that E fails to prevent bone loss in mice with a T cell-specific blockade of TGFβ signaling. In contrast, the overexpression of TGFβ in vivo completely prevents ovx-induced bone loss, thus confirming that E prevents bone loss through a TGFβ-dependent mechanism.
Materials and Methods
Animal Protocol. All animal procedures were approved by the Institutional Animal Care and Use Committee of Emory University. C57BL/6 homozygous (nu/nu) nude mice and WT C57BL/6 mice were purchased from The Jackson Laboratory. Transgenic mice overexpressing dominant-negative TGFβ type II receptor in T cells exclusively (CD4dnTGFβIIR), also on a C57BL/6 background, have been described (23). Mice were either sham-operated or ovariectomized at 16 weeks of age and killed at 20 weeks of age. At death, one tibia was excised for histology and one femur for microcomputerized tomography (μCT) analysis, and BM was harvested from remaining bones for ex vivo analysis. Uterine weight was determined at death to verify successful ovx.
BMM and T Cell Purification. BMM and T cells were purified from the BM and spleen by positive immunoselection using MACS Microbeads (Miltenyi Biotec, Auburn, CA) coupled to anti-CD11b and anti-CD90 (Thy1.2) antibodies, as described (12). Cell purity was verified to be >90% by fluorescence-activated cell sorting.
T Cell Adoptive Transfer. T cells were purified from spleens of WT or CD4dnTGFβIIR mice and injected (2 × 107 cells per mouse) i.p. into sham or ovariectomized nude mouse recipients, as described (24). T cell engraftment was assessed by fluorescence-activated cell sorting analysis of the spleens of recipient mice 4 weeks after the adoptive transfer.
TGFβ1 Expression Vector Construction. The mouse TGFβ1 full-length cDNA cloned into the mammalian expression vector pCI-Neo (Promega), to generate pCMV-TGFβ1, was kindly provided by Matthias Lohr (University of Rostock, Rostock, Germany) (25, 26). Large-scale plasmid preparation was accomplished by using Qiagen Endo-free Giga kit (Qiagen, Santa Clarita, CA). Plasmid DNA for i.m. injections was resuspended in sterile saline, and DNA purity was confirmed by 1% agarose gel electrophoresis and spectrophotometric analysis (OD at 260/280 nm ≥ 1.80).
i.m. Injection of Plasmid DNA. i.m. injections of plasmid DNA were performed as described (26). Briefly, mice were anesthetized, and 50 μl of sterile saline containing 100 μg of pCMV-TGFβ1 plasmid or empty vector control (pCMV-Null) was injected into each rectus femoris and tibialis anterior muscle for a total of 400 μg per mouse, by using a 1-ml sterile 27-gauge insulin syringe.
pCMV-TGFβ1 RT-PCR. To verify plasmid transfection of the muscles after injection, total RNA was isolated from tibialis anterior muscles, and RT-PCR was performed by using Taq Ready-To-Go PCR Beads (Amersham Pharmacia Biosciences). TGFβ1 primers were designed to specifically amplify the TGFβ1 cDNA derived from the pCMV-TGFβ1 vector, without cross reactivity with the endogenous TGFβ1 gene. The forward primer used was 5′-TTA ATA CGA CTC ACT ATA GGG A, and the reverse primer used was 5′-GTC ATC GGC GTC GGG G. β-Tubulin was used as normal control; the forward primer used for β-tubulin was 5′-TTC TGG GAG GTC ATA AGC GAT-3′, and the reverse primer used was 5′-GGG AAA CGG AGG CAG GTG-3′.
Real-Time RT-PCR. TGFβ1 and CIITA mRNA was quantitated by real-time PCR after reverse transcription of mRNA, as described, by using a GeneAmp 5700 system (PE Biosystems) (27). The primers used for TGFβ1 were 5′-AGG ACC TGG GTT GGA AGT GG-3′ (forward) and 5′-AGT TGG CAT GGT AGC CCT TG-3′(reverse). For CIITA, we used 5′-CCT ATG CCA ACA TTG CGG A-3′ (forward) and 5′-GCT GGG TAT CCT GGA ACA CG-3′ (reverse). mRNA was normalized against β-tubulin by using the primers 5′-GGA GAG CTG TGA TTG CCT GC (forward) and 5′-CCA CCC AGT GAG TGG GTC AG-3′ (reverse).
Flow Cytometry. Flow cytometry was performed as described (11), by using whole spleen or BM cells labeled with anti-CD3-conjugated phycoerythrin (PE) and either anti-CD25-conjugated FITC or anti-CD69-conjugated FITC antibodies (Pharmingen). Nonspecific staining was assessed by using FITC or PE-conjugated isotype-matched normal IgG antibodies. Cells were analyzed on a FACScan flow cytometer (Becton Dickinson).
BrdUrd Labeling. In vivo BrdUrd labeling was performed by using a BrdUrd Flow Kit from Pharmingen, as described (12). Briefly, 100 μl of 10 mg/ml BrdUrd in PBS was injected i.p. 3 days before mice were killed. Spleen or BM cells were labeled with phycoerythrin-conjugated CD3 and FITC-conjugated anti-BrdUrd antibodies. Total DNA was stained with 7-amino-actinomycin D, and the cells were analyzed by three-color flow cytometry.
Determination of Bone Mineral Density (BMD). Femoral BMD was determined in vivo at baseline and 2 and 4 weeks after surgery by using PIXImus2 bone densitometry (GE Medical Systems, Madison, WI), as described (12). The short-term reproducibility of this technique is 1.7% (27). Data points represent the average of both femurs for each mouse. Data were expressed as percent change from baseline.
μCT. Femurs were fixed for 24 h in 10% formalin in PBS (pH 7.2), washed twice in PBS, and stored in 70% ethanol at 4°C until analyzed. The distal end of the femur was imaged by a Scanco μCT-40 (Scanco Medical, Bassersdorf, Switzerland) by using a voxel resolution of 16 μm and analyzed as described (28, 29). For details, see Supporting Text, which is published as supporting information on the PNAS web site.
Histology and Histomorphometry. Histological analyses and histomorphometry were performed as described (27, 30). Sections were cut and stained with hematoxylin/eosin and tartrate-resistant acid phosphatase to quantitate OCs. OC surface [osteoclast surface/bone surface (Os/Bs)], an index of bone resorption, and mineral apposition rate, a dynamic index of bone formation, were measured and analyzed by using Osteomeasure (OsteoMetrics, Atlanta) by an operator blinded to the nature of the samples.
Ag Presentation Activity Assay. Ag presentation was measured as described (12). For details, see Supporting Text.
Cytokine Assays. BM supernatant and blood were collected at killing. To minimize platelet activation and release of endogenous TGFβ, platelet-poor plasma was obtained as described (26) and stored at –80°C until analysis for TGFβ1 by ELISA (R & D Systems). T cells were cultured for 3 days in the presence of 100 ng/ml phorbol 12-myristate 13-acetate and 100 ng/ml ionomycin. The levels of TNF, IFN-γ, and RANKL in the culture media were determined by ELISA (R & D Systems).
C-Terminal Telopeptide Measurements. The C-terminal telopeptide levels, a specific marker of bone resorption, were determined by using a mouse-specific ELISA assay according to protocols from the manufacturer (Nordic, Herlev, Denmark).
Statistical Analysis. All cross-sectional data were analyzed by ANOVA and Fisher-protected lysergic acid diethylamide (LSD) tests as well as the Kruskal–Wallis test for data not normally distributed. Prospective data were analyzed by ANOVA for repeated measures. Multiple comparison tests were performed by the Fisher-protected LSD test. Simple comparisons were made by using the unpaired Student t test. Nondetectable vs. detectable cytokine levels were compared by Fisher's exact test.
Results
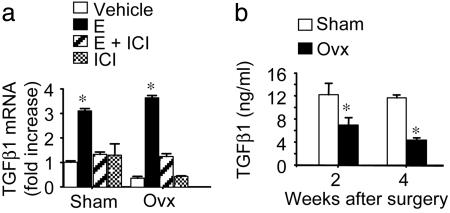
E Represses the Production of TGFβ by BMMs. E is known to repress the production of TGFβ in the bone microenvironment, but its effect on TGFβ1 production by BMMs has not been described. Because BMMs are a major source of TGFβ in the BM and play a central role in the mechanism of ovx-induced bone loss, we measured the levels of TGFβ1 mRNA by real-time RT-PCR in BMMs harvested from sham-operated and ovariectomized WT mice 4 weeks after surgery. Ovariectomized BMMs exhibited TGFβ1 mRNA levels ≈50% lower than those from sham-operated mice (Fig. 1a). Furthermore, in vitro E treatment caused an ≈3-fold increase of TGFβ1 mRNA in BMMs from sham-operated mice and an ≈8-fold increase in ovariectomized BMMs. Attesting to specificity, the stimulatory effect of E was blunted by concomitant treatment with the complete E antagonist ICI 182720. Thus, E increases TGFβ levels in BMMs and does so through direct targeting of these cells. A time course in vivo experiment further revealed that serum levels of TGFβ are significantly lower in ovariectomized than in sham-operated mice at 2 and 4 weeks after surgery (Fig. 1b), a time period when E deficiency is associated with rapid bone loss.
Fig. 1.
ovx decreases BMM TGFβ1 mRNA and plasma TGFβ1 levels (mean ± SE). (a) BMMs from sham-operated and ovariectomized mice were treated with vehicle, 17β estradiol (10–8 M), or 17β estradiol plus ICI 182720 (10–8 M) for 3 days. *, P < 0.05, as compared with vehicle-treated sham-operated BMMs. (b) BM plasma TGFβ1 levels. *, P < 0.05 as compared with sham-operated mice (n = 6).
Silencing of TGFβ Signaling in T Cell Leads to Impaired Skeletal Maturation and Bone Loss in E Replete Mice. One relevant target of TGFβ in the BM is the T lymphocyte. TGFβ signaling in T cells is known to repress T cell proliferation and differentiation as well as production of cytokines relevant for osteoclastogenesis and bone homeostasis (15). To examine the contribution of TGFβ signaling in T cells to skeletal homeostasis and the mechanism of ovx-induced bone loss, we made use of a conditional transgenic mouse that overexpresses a dominant-negative TGFβ type II receptor (CD4dnTGFβIIR) under the control of the CD4 promoter (31). TGFβ signaling in all T cells is blocked in this transgenic mouse, because the CD8 silencer has been removed from the CD4 promoter. Because all isoforms of TGFβ signal through the same receptor complex, CD4dnTGFβIIR mice are insensitive to TGFβ1, -2, and -3.
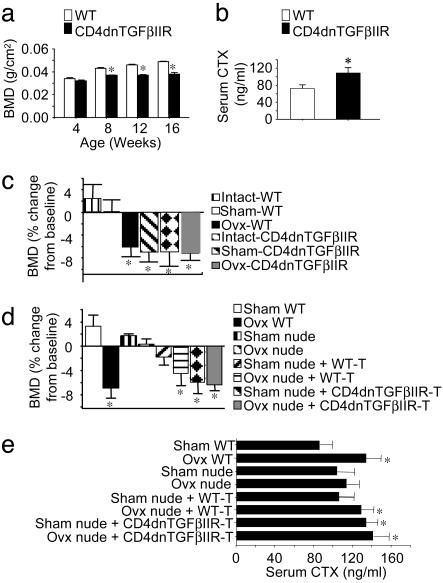
In a first study, we measured BMD in vivo in CD4dnTGFβIIR mice and WT littermates during physiological bone modeling. At birth, there was no difference in the skeletal phenotype between CD4dnTGFβIIR mice and WT littermates, as determined by alizarin red and alcian blue staining (data not shown). Prospective measurements of BMD revealed a lower rate of bone density accumulation in CD4dnTGFβIIR mice as compared with WT controls. As a result, CD4dnTGFβIIR mice exhibited significantly lower (P < 0.05) BMD values than WT mice as early as 8 weeks after birth. The difference in mean BMD values continued to increase until the end of the study at 16 weeks of age (Fig. 2a). Furthermore, CD4dnTGFβIIR mice displayed higher serum levels of C-terminal telopeptides compared with WT controls (Fig. 2b). These findings suggest that T cells insensitive to TGFβ signaling stimulate bone resorption, leading to alterations in physiological bone modeling and impaired skeletal development.
Fig. 2.
Silencing of TGFβ signaling in T cells decreases BMD in growing mice. (a) BMD (mean ± SE) was measured in intact WT and CD4dnTGFβIIR mice (n = 6) between the ages of 4 and 16 weeks. *, P < 0.05 as compared with age-matched WT mice. (b) Silencing of TGFβ signaling in T cells increases bone resorption in adult mice. Serum C-terminal telopeptide (CTX) (mean ± SE), a marker of bone resorption, was measured in WT and CD4dnTGFβIIR mice (n = 6) at 16 weeks of age. *, P < 0.05 as compared with age-matched WT mice. (c) Silencing of TGFβ signaling in T cells induces bone loss in E-replete mice (n = 6). BMD was measured in vivo at baseline and 4 weeks after surgery. Data (mean ± SE) are expressed as percentage change from baseline. *, P < 0.05 as compared with sham-operated WT controls. (d and e) Transfer of T cells insensitive to TGFβ into E-replete nude mice leads to bone loss (d) and increases bone resorption (e). T cells from WT or CD4dnTGFβIIR mice were transferred into nude recipients (n = 6), which were then subjected to ovx or sham operation. BMD was measured in vivo at baseline and at 4 weeks. Data (mean ± SE) are expressed as percentage change from baseline. Serum C-terminal telopeptide levels (mean ± SE) were measured at 4 weeks after surgery. *, P < 0.05 as compared with sham-operated WT mice.
To investigate the contribution of TGFβ signaling in T cells to the bone waste induced by E deficiency, WT and CD4dnTGFβIIR mice were either sham-operated or subjected to ovx at 16 weeks of age, and BMD was measured by bone densitometry at baseline and at 4 weeks after surgery. In WT mice, a significant bone loss was observed (Fig. 2c) in ovariectomized but not in intact and sham-operated controls. In contrast, intact, sham-operated, and ovariectomized CD4dnTGFβIIR mice exhibited a significant loss of bone that was equal to that observed in WT ovariectomized mice. These findings demonstrate that CD4dnTGFβIIR mice lose bone despite the presence of physiologic E levels, thus establishing that silencing of TGFβ signaling in T cells renders these mice insensitive to the bone-sparing effect of E. Furthermore, the lack of a more severe bone loss in ovariectomized CD4dnTGFβIIR mice as compared with E-replete CD4dnTGFβIIR mice attests to the pivotal role of TGFβ signaling in T cells, because T lymphocytes are the only cells that do not sense the decreased levels of TGFβ induced by ovx.
To confirm that the specific role of TGFβ signaling in T cells for ovariectomized mice induced bone loss, T cells from CD4dnTGFβIIR mice and WT littermates were harvested and adoptively transferred into nude mice. On the same day, reconstituted recipient mice were either sham-operated or ovariectomized. BMD was measured by bone densitometry at baseline and at 4 weeks after T cell reconstitution. Confirming earlier reports, ovx caused no bone loss in T cell-deficient nude mice (Fig. 2d). Likewise, the transfer of WT T cells into sham-operated nude mice failed to induce bone loss, because the E of the recipient mice maintained donor T cells in a quiescent state. In contrast, the transference of T cells from CD4dnTGFβIIR mice into sham-operated nude recipients was followed by a bone loss equal to that observed in both WT ovariectomized mice and ovariectomized nude mice reconstituted with WT T cells.
These findings were confirmed by μCT measurements of morphometric indices in femurs harvested at 4 weeks after surgery (Table 1). This analysis showed that sham-operated nude mice reconstituted with CD4dnTGFβIIR T cells have lower values of bone volume (relative bone volume, BV/TV) and trabecular thickness (Tb.Th) and higher values of structure model index (SMI) than both sham-operated WT animals and sham-operated nude mice reconstituted with WT T cells. In nude mice reconstituted with CD4dnTGFβIIR T cells, the values of BV/TV, Tb.Th, and SMI were similar to those of both WT ovariectomized mice and ovariectomized nude mice reconstituted with WT T cells.
Table 1. μCT analysis of femur morphometric indices (mean ± SD) 4 weeks after sham operation or ovx.
| BV/TV, % | Tb.Th, μm | SMI* | |
|---|---|---|---|
| Sham WT | 21.81 ± 1.91 | 58.12 ± 2.89 | 0.85 ± 0.25 |
| Ovx WT | 18.19 ± 1.51† | 51.38 ± 4.11† | 1.19 ± 0.19† |
| Sham nude | 18.63 ± 0.7† | 45.50 ± 1.98† | 1.34 ± 0.25† |
| Ovx nude | 17.95 ± 1.37† | 43.13 ± 1.06† | 1.32 ± 0.34† |
| Sham nude + WT T cells | 18.83 ± 1.35† | 46.23 ± 3.49† | 1.41 ± 0.13† |
| Ovx nude + WT T cells | 14.22 ± 2.32†‡ | 40.50 ± 2.75†‡ | 1.88 ± 0.33†‡ |
| Sham nude + CD4dnTGFβIIR-T | 14.11 ± 2.52†‡ | 40.00 ± 3.09†‡ | 1.71 ± 0.29†‡ |
| Ovx nude + CD4dnTGFβIIR-T | 14.99 ± 2.59†‡ | 40.7 ± 1.28†‡ | 1.70 ± 0.48†‡ |
BV/TV, relative bone volume; Tb.Th, trabecular thickness; SMI, structure model index; sham, sham-operated.
0 (plate) to 3 (rod).
P < 0.05 from sham-operated WT group.
P < 0.05 from sham-operated nude group.
Transfer of CD4dnTGFβIIR T cells into sham-operated nude recipients also led to an increase in the serum levels of C-terminal telopeptide, an index of bone resorption, similar to those observed in both ovariectomized WT controls and ovariectomized nude mice reconstituted with WT T cells (Fig. 2e).
Together, the data demonstrate that T cells rendered insensitive to TGFβ are also insensitive to the bone-sparing effect of E. Thus, TGFβ signaling in T cells plays a pivotal role in the mechanism by which E prevents bone loss.
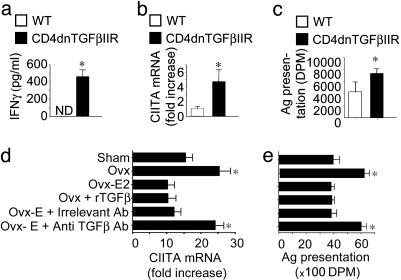
Silencing of TGFβ Signaling in T Cells Stimulates IFN-γ Production, CIITA Expression, and Ag Presentation. Because TGFβ is known to repress IFN-γ production and signaling, we investigated whether silencing of TGFβ signaling in T cells abolishes the bone-sparing activity of E by interfering with IFN-γ induction of CIITA and the resulting stimulation of Ag presentation. T cells were purified from CD4dnTGFβIIR and WT controls and cultured in vitro in the presence of phorbol 12-myristate 13-acetate and ionomycin for 72 h and the culture media assayed for IFN-γ by ELISA. These experiments revealed that TGFβ signaling in T cells represses IFN-γ production. In fact, T cells from CD4dnTGFβIIR mice released into the culture media ≈400 pg/ml IFN-γ, whereas this cytokine was undetectable in the conditioned media of WT T cells (Fig. 3a). Because IFN-γ is the physiologic inducer of CIITA expression in BMM, CIITA mRNA levels were measured by real-time RT-PCR in freshly isolated BMMs. This analysis revealed CIITA mRNA levels to be ≈4-fold higher in BMMs from CD4dnTGFβIIR mice than in those from WT controls (Fig. 3b). Because CIITA is a key regulator of Ag presentation by BMM, we examined how blockade of TGFβ signaling in T cells affects BMM Ag presentation. We found (Fig. 3c) Ag presentation to be ≈2-fold higher in BMMs from CD4dnTGFβIIR mice than in those from WT controls. Together, these findings demonstrate that TGFβ signaling in T cells represses the production of IFN-γ leading to blunted CIITA induction and decreased Ag presentation by macrophages.
Fig. 3.
Silencing of TGFβ signaling in T cells increases secretion of IFN-γ by phorbol 12-myristate 13-acetate and ionomycin-stimulated T cells (a), CIITA expression in BMMs (b), and BMM Ag presentation (c). Data are expressed as mean ± SE. *, P < 0.05 as compared with WT mice. (d and e) TGFβ1 neutralization blocks the repressive effect of E on BMM CIITA mRNA expression (d) and Ag presentation activity (e). BMMs from WT-operated sham and ov mice were treated for 3 days in vitro with either 17β estradiol or control vehicle and anti-TGFβ1 antibody or irrelevant antibody. *, P < 0.05 as compared with sham-operated controls. DPM, disintegrations per minute.
Because TGFβ is known to decrease the responsiveness of the CIITA gene to IFN-γ, the blunted production of TGFβ induced by ovx could also explain how E deficiency increases the responsiveness of the CIITA gene to IFN-γ. To investigate this matter, sham and ovariectomized BMMs were stimulated with IFN-γ and CIITA mRNA levels measured by real-time RT-PCR. IFN-γ stimulated CIITA expression in both sham and ovariectomized BMMs. However, when BMMs were stimulated with a saturating concentration of IFN-γ (100 units/ml), CIITA mRNA levels were almost 2-fold higher in ovariectomized BMMs compared with sham-operated BMMs (Fig. 3d). In vitro treatment with either E or recombinant TGFβ decreased CIITA mRNA levels by ≈50% in BMMs from ovariectomized mice, whereas no significant changes were observed in BMMs from sham-operated mice. Importantly, the repression of CIITA levels induced by E treatment in ovariectomized BMMs was completely abolished by a neutralizing antibody directed against TGFβ, whereas an irrelevant antibody had no effect. These findings demonstrate that ovx increases the responsiveness of the CIITA gene to IFN-γ via a TGFβ-dependent mechanism.
Because our data suggest that ovx up-regulates the production of IFN-γ, the enhanced responsiveness of the CIITA gene to IFN-γ, and IFN-γ-induced CIITA expression through TGFβ, we further investigated whether E represses BMM Ag presentation through a TGFβ-dependent mechanism. Experiments showed Ag presentation to be ≈2-fold higher in BMMs from WT ovariectomized mice than those from WT sham-operated mice (Fig. 3e). Furthermore, in vitro treatment of BMMs from ovariectomized mice with either E or recombinant TGFβ decreased their Ag presentation activity to the level of sham-operated BMMs. As in the case of CIITA expression, treatment with anti-TGFβ antibody completely abolished the increased Ag presentation characteristic of BMMs from ovariectomized mice, whereas an irrelevant antibody had no effect. Thus, E represses Ag presentation by BMMs through a mechanism hinging on the ability of TGFβ to blunt IFN-γ-mediated induction of CIITA.
TGFβ Represses T Cell Activation and Proliferation in E-Replete Mice. To determine whether TGFβ plays a role in the increase in T cell activation induced by E deficiency, spleen cells were collected to measure indices of T cell activation by fluorescence-activated cell sorting and T cell proliferation by in vivo BrdUrd incorporation. CD4dnTGFβIIR mice exhibited a higher percentage of CD69+ (Fig. 6a, which is published as supporting information on the PNAS web site) and CD25+ T cells (Fig. 6b) as compared with WT littermates. Consistent with the presence of increased T cell activation, in vivo BrdUrd incorporation studies revealed that CD4dnTGFβIIR mice display an ≈2-fold higher percentage of T cells in S phase, an index of proliferation, than WT controls (Fig. 6c). The finding of increased T cell activation and proliferation in mice with T cell-specific blockade of TGFβ signaling is consistent with both the augmented Ag presentation observed in these transgenic mice and the direct repressive action of TGFβ on T cell proliferation and differentiation.
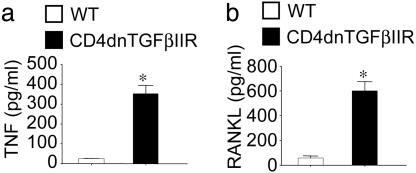
An expected consequence of increased T cell activation is increased production of osteoclastogenic cytokines (12, 32, 33). Measurements by ELISA of cytokine levels in 72-h culture media revealed that T cells derived from CD4dnTGFβIIR mice produce significantly higher levels of TNF (Fig. 4a) and RANKL (Fig. 4b) than those from WT controls.
Fig. 4.
Silencing of TGFβ signaling in T cells increases T cell production of osteoclastogenic cytokines. Levels (mean ± SE) of TNF (a) and RANKL (b) were measured in the culture media of phorbol 12-myristate 13-acetate and ionomycin-stimulated T cells harvested from WT and CD4dnTGFβIIR mice. *, P < 0.05 compared with WT controls.
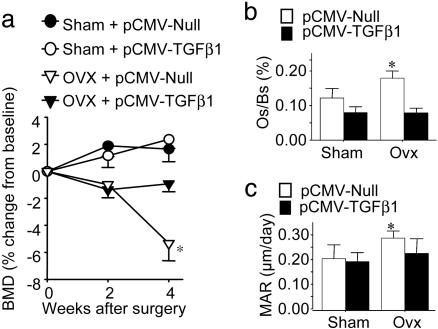
Overexpression of TGFβ1 Prevents ovx-Induced Bone Loss by Blunting T Cell Activation. The development of a TGFβ1 expression vector that, when injected i.m., causes long-term local and systemic elevation of TGFβ1 levels (26) provided an opportunity to use a somatic gene therapy approach to demonstrate a proof of principle that overexpression of TGFβ may represent a viable means of preventing ovx-induced bone loss. Thus, 16-week-old mice were injected i.m. with a TGFβ1-expressing plasmid (pCMV-TGFβ1) under control of a constitutively active cytomegalovirus promoter and then subjected to sham operation or ovx. Analysis by RT-PCR 4 weeks later revealed that mRNA expression of the TGFβ1 transgene was still detectable at the site of injection (Fig. 7a, which is published as supporting information on the PNAS web site). A significant increase in the serum levels of TGFβ was detected (Fig. 7b) as early as 3 days after pCMV-TGFβ1 plasmid injection. Serum TGFβ1 levels decreased slowly over time but were still significantly elevated 4 weeks after injection relative to baseline.
In vivo BMD measurements obtained at baseline and at 2 and 4 weeks after surgery revealed that mice treated with the pCMV-TGFβ1 plasmid were completely protected against ovx-induced bone loss (Fig. 5a). Although ovx caused a significant bone loss as compared with baseline in mice injected with control empty plasmid (pCMV-Null), mice injected with pCMV-TGFβ1 plasmid failed to lose bone. Sham operation did not cause significant bone loss in mice treated with either pCMV-TGFβ1 or pCMV-Null. Furthermore, analysis of tibiae harvested at 4 weeks by quantitative histomorphometry revealed that treatment with pCMV-TGFβ1 plasmid completely prevented the ovx-induced increase in osteoclast surface/bone surface (Os/Bs), a marker for bone resorption, and dynamic mineral apposition rate, a marker of bone formation (Fig. 5 b and c).
Fig. 5.
(a) Treatment with pCMV-TGFβ1 prevents ovx-induced bone loss. BMD was measured at baseline and 2 and 4 weeks after surgery in mice treated with pCMV-TGFβ1 plasmid or pCMV-Null (n = 10). Data (mean ± SE) are expressed as percent change from baseline. *, P < 0.05 compared with sham-operated mice treated with pCMV-Null. (b and c) Histomorphometric analysis (mean ± SE) of proximal tibia. Osteoclast surface/bone surface (b) and mineral apposition rate (MAR) (c) from sham-operated and ovariectomized mice were measured at 4 weeks. *, P < 0.05 as compared with sham-operated mice treated with pCMV-Null.
Fluorescence-activated cell sorting analysis of CD3+ cells for the activation markers CD25 and CD69 (Fig. 7 c and d) revealed that treatment with pCMV-TGFβ1 plasmid completely prevented the increase in T cell activation induced by ovx, whereas treatment with control plasmid did not. Together, these data indicate that overexpression of TGFβ1 prevents the increase in bone turnover and the decrease in bone density induced by ovx by preventing T cell activation.
Discussion
TGFβ has long been implicated in the pathogenesis of ovx-induced bone loss, although its effects on bone remodeling remain controversial (1, 34). In support of a role for TGFβ in the bone-sparing effect of E, we have found ovx to blunt both the BM serum levels of TGFβ1 and TGFβ1 mRNA levels in BMMs. These findings extend earlier animal and human studies indicating that E up-regulates serum concentrations of TGFβ and TGFβ expression in osteoblasts, bone extracts, and BM cells (20, 21, 35). The stimulatory effect of E on TGFβ gene expression is the result of the direct association of ligand-bound E receptor to an E-responsive element in the TGFβ promoter (36). Further attesting to the relevance of TGFβ as mediator of the bone-sparing effect of E, we have found that overexpression of TGFβ1 prevents bone loss and the increase in bone turnover induced by ovx.
TGFβ is recognized as a powerful repressor of T cell activation, differentiation, proliferation, and cytokine secretion (15). Accordingly, we have found disruption of TGFβ signaling in T cells to lead to bone loss despite the presence of physiological E levels, through induction of T cell activation and proliferation, events that result in increased T cell production of TNF and RANKL, two factors that, in concert, potently stimulate OC formation and activity (8). The capacity of TGFβ to dampen T cell activation has herein been found to also play a relevant role in the regulation of physiological bone modeling during skeletal growth. Disruption of TGFβ signaling, in fact, not only abolishes the capacity of E to prevent bone loss but also retards the increase in bone density induced by aging, through stimulation of bone resorption.
The finding that E blunts T cell production of TNF and RANKL through TGFβ provides insights on the mechanism of ovx-induced bone loss. We have shown that ovx increases T cell production of TNF through an expansion of the pool of TNF-producing T cells in the BM (11). T cell-produced TNF, in turn, augments RANKL-induced osteoclastogenesis (7, 11). The T cell expansion that follows E withdrawal results from increased activation-induced T cell proliferation and life span. This, in turn, is caused by an increase in BMM Ag presentation activity resulting from up-regulated CIITA-dependent MHCII expression (12). The responsible mechanism hinges on the ability of E to indirectly repress the production of IFN-γ by effector T lymphocytes in vivo (12). Such indirect stimulatory influence prevails over a direct stimulatory effect of E on IFN-γ gene expression (37). IFN-γ plays a pivotal role in ovx-induced bone loss, not only enhancing Ag presentation but also increasing the responsiveness of T cells to IL-7, a cytokine known to induce the peripheral expansion of T cells (38) and the T cell production of RANKL and TNF (39, 40). The BM levels of IL-7 are indeed elevated in ovariectomized mice, and IL-7 neutralization in vivo prevents ovx-induced bone loss (41).
Although the relevance of IFN-γ as an inducer of bone loss in ovariectomized mice is demonstrated by the failure of ovx to induce bone loss in IFN-γR–/– mice (12), the mechanism by which ovx stimulates IFN-γ in vivo remains undetermined. The finding that silencing of TGFβ signaling in T cells up-regulates T cell production of IFN-γ indicates that E deficiency up-regulates IFN-γ production by repressing TGFβ levels. It is also likely that direct repressive effects of TGFβ on T cell activation may further contribute to block the production of osteoclastogenic cytokines and thus to the bone-sparing activity of E. Therefore, the data identify TGFβ targeting of T cells as the “upstream” mechanism by which E prevents bone loss.
The identification of IFN-γ as a pro-bone wasting factor is in apparent contrast with studies showing IFN-γ to be a potent inhibitor of osteoclastogenesis in vitro (42). However, the net effect of IFN-γ in bone in vivo remains controversial, because studies have identified several conditions associated with IFN-γ stimulation of OC formation and bone resorption. For example, IFN-γ has been reported to increase bone resorption and to be efficacious in the treatment of osteopetrosis in humans (43) and rodents (44). IFN-γ is also known to stimulate bone resorption and bone loss in rats (45). Conversely, randomized controlled trials have shown that IFN-γ does not prevent bone loss in patients with rheumatoid arthritis (46, 47). It is likely that IFN-γ indirectly stimulates OC formation in vivo, whereas it directly blocks OC formation in vitro through targeting of maturing OCs. The inability of IFN-γ to blunt the differentiation of maturing OCs arising in a microenvironment such as bone, where RANKL is abundant (48), contributes to explaining why the in vivo proresorptive effects of IFN-γ are more potent than the suppression of osteoclastogenesis induced by IFN-γ in vitro.
Conclusion
Our findings may contribute to explaining why the mechanism of ovx-induced bone loss results from increased T cell activation and T cell TNF production. Should these findings be confirmed in humans, postmenopausal osteoporosis could be shown to result from an increased immune response elicited as a consequence of the elimination of the suppressive effect of TGFβ on T cells. Thus, inhibition of T cell activation or stimulation of TGFβ signaling may represent a strategy to prevent bone loss.
Supplementary Material
Acknowledgments
This study was supported in part by National Institutes of Health Grants AR 49659, DK 055746, and AR 41412. This work made use of Engineering Research Center Shared Facilities supported by the National Science Foundation under Award Number EEC-9731643.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: E, estrogen; ovx, ovariectomy; OC, osteoclast; TNF, tumor necrosis factor; BM, bone marrow; Ag, antigen; BMM, BM macrophage; CIITA, class II transactivator; TGFβ, type β transforming growth factor; BMD, bone mineral density; μCT, microcomputerized tomography; RANKL, receptor activator of nuclear factor-κB ligand.
References
- 1.Pfeilschifter, J., Koditz, R., Pfohl, M. & Schatz, H. (2002) Endocr. Rev. 23, 90–119. [DOI] [PubMed] [Google Scholar]
- 2.Manolagas, S. C., Kousteni, S. & Jilka, R. L. (2002) Recent Prog. Horm. Res. 57, 385–409. [DOI] [PubMed] [Google Scholar]
- 3.Kousteni, S., Bellido, T., Plotkin, L. I., O'Brien, C. A., Bodenner, D. L., Han, L., Han, K., DiGregorio, G. B., Katzenellenbogen, J. A., Katzenellenbogen, B. S., et al. (2001) Cell 104, 719–730. [PubMed] [Google Scholar]
- 4.Riggs, B. L., Khosla, S. & Melton, L. J., III (2002) Endocr. Rev. 23, 279–302. [DOI] [PubMed] [Google Scholar]
- 5.Nanes, M. S. (2003) Gene 321, 1–15. [DOI] [PubMed] [Google Scholar]
- 6.Hofbauer, L. C., Khosla, S., Dunstan, C. R., Lacey, D. L., Boyle, W. J. & Riggs, B. L. (2000) J. Bone Miner. Res. 15, 2–12. [DOI] [PubMed] [Google Scholar]
- 7.Cenci, S., Weitzmann, M. N., Roggia, C., Namba, N., Novack, D., Woodring, J. & Pacifici, R. (2000) J. Clin. Invest. 106, 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam, J., Takeshita, S., Barker, J. E., Kanagawa, O., Ross, F. P. & Teitelbaum, S. L. (2000) J. Clin. Invest. 106, 1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, Y. H., Heulsmann, A., Tondravi, M. M., Mukherjee, A. & Abu-Amer, Y. (2001) J. Biol. Chem. 276, 563–568. [DOI] [PubMed] [Google Scholar]
- 10.Ross, F. P. (2003) Trends Endocrinol. Metab. 14, 147–149. [DOI] [PubMed] [Google Scholar]
- 11.Roggia, C., Gao, Y., Cenci, S., Weitzmann, M. N., Toraldo, G., Isaia, G. & Pacifici, R. (2001) Proc. Natl. Acad. Sci. USA 98, 13960–13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cenci, S., Toraldo, G., Weitzmann, M. N., Roggia, C., Gao, Y., Qian, W. P., Sierra, O. & Pacifici, R. (2003) Proc. Natl. Acad. Sci. USA 100, 10405–10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boss, J. M. & Jensen, P. E. (2003) Curr. Opin. Immunol. 15, 105–111. [DOI] [PubMed] [Google Scholar]
- 14.Kehrl, J. H., Wakefield, L. M., Roberts, A. B., Jakowlew, S., Alvarez-Mon, M., Derynck, R., Sporn, M. B. & Fauci, A. S. (1986) J. Exp. Med. 163, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorelik, L. & Flavell, R. A. (2002) Nat. Rev. Immunol. 2, 46–53. [DOI] [PubMed] [Google Scholar]
- 16.Lee, Y. J., Han, Y., Lu, H. T., Nguyen, V., Qin, H., Howe, P. H., Hocevar, B. A., Boss, J. M., Ransohoff, R. M. & Benveniste, E. N. (1997) J. Immunol. 158, 2065–2075. [PubMed] [Google Scholar]
- 17.Nandan, D. & Reiner, N. E. (1997) J. Immunol. 158, 1095–1101. [PubMed] [Google Scholar]
- 18.Dong, Y., Tang, L., Letterio, J. J. & Benveniste, E. N. (2001) J. Immunol. 167, 311–319. [DOI] [PubMed] [Google Scholar]
- 19.Tang, J., Nuccie, B. L., Ritterman, I., Liesveld, J. L., Abboud, C. N. & Ryan, D. H. (1997) J. Immunol. 159, 117–125. [PubMed] [Google Scholar]
- 20.Gray, T. K., Lipes, B., Linkhart, T., Mohan, S. & Baylink, D. (1989) Connect Tissue Res. 20, 23–32. [DOI] [PubMed] [Google Scholar]
- 21.Finkelman, R. D., Bell, N. H., Strong, D. D., Demers, L. M. & Baylink, D. J. (1992) Proc. Natl. Acad. Sci. USA 89, 12190–12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bord, S., Beavan, S., Ireland, D., Horner, A. & Compston, J. E. (2001) Bone 29, 216–222. [DOI] [PubMed] [Google Scholar]
- 23.Gorelik, L., Constant, S. & Flavell, R. A. (2002) J. Exp. Med. 195, 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers, A. & Eastell, R. (1998) J. Bone Miner. Res. 13, 1577–1586. [DOI] [PubMed] [Google Scholar]
- 25.Lohr, M., Schmidt, C., Ringel, J., Kluth, M., Muller, P., Nizze, H. & Jesnowski, R. (2001) Cancer Res. 61, 550–555. [PubMed] [Google Scholar]
- 26.Piccirillo, C. A., Chang, Y. & Prud'homme, G. J. (1998) J. Immunol. 161, 3950–3956. [PubMed] [Google Scholar]
- 27.Cenci, S., Weitzmann, M. N., Gentile, M. A., Aisa, M. C. & Pacifici, R. (2000) J. Clin. Invest. 105, 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hildebrand, T., Laib, A., Muller, R., Dequeker, J. & Ruegsegger, P. (1999) J. Bone Miner. Res. 14, 1167–1174. [DOI] [PubMed] [Google Scholar]
- 29.Hildebrand, T. & Ruegsegger, P. (1997) Comput. Methods Biomech. Biomed. Eng. 1, 15–23. [DOI] [PubMed] [Google Scholar]
- 30.Kimble, R., Bain, S. & Pacifici, R. (1997) J. Bone Miner. Res. 12, 935–941. [DOI] [PubMed] [Google Scholar]
- 31.Gorelik, L. & Flavell, R. A. (2000) Immunity 12, 171–181. [DOI] [PubMed] [Google Scholar]
- 32.Kong, Y. Y., Feige, U., Sarosi, I., Bolon, B., Tafuri, A., Morony, S., Capparelli, C., Li, J., Elliott, R., McCabe, S., et al. (1999) Nature 402, 304–309. [DOI] [PubMed] [Google Scholar]
- 33.Weitzmann, M. N., Cenci, S., Rifas, L., Haug, J., Dipersio, J. & Pacifici, R. (2001) J. Bone Miner. Res. 16, 328–337. [DOI] [PubMed] [Google Scholar]
- 34.Centrella, M., Horowitz, M. C., Wozney, J. M. & McCarthy, T. L. (1994) Endocr. Rev. 15, 27–39. [DOI] [PubMed] [Google Scholar]
- 35.Grainger, D. J., Percival, J., Chiano, M. & Spector, T. D. (1999) Osteoporos Int. 9, 398–404. [DOI] [PubMed] [Google Scholar]
- 36.Yang, N. N., Venugopalan, M., Hardikar, S. & Glasebrook, A. (1996) Science 273, 1222–1225. [DOI] [PubMed] [Google Scholar]
- 37.Fox, H. S., Bond, B. L. & Parslow, T. G. (1991) J. Immunol. 146, 4362–4367. [PubMed] [Google Scholar]
- 38.Fry, T. J. & Mackall, C. L. (2001) Trends Immunol. 22, 564–571. [DOI] [PubMed] [Google Scholar]
- 39.Weitzmann, M. N., Cenci, S., Rifas, L., Brown, C. & Pacifici, R. (2000) Blood 96, 1873–1878. [PubMed] [Google Scholar]
- 40.Toraldo, G., Roggia, C., Qian, W. P., Pacifici, R. & Weitzmann, M. N. (2003) Proc. Natl. Acad. Sci. USA 100, 125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weitzmann, M. N., Roggia, C., Toraldo, G., Weitzmann, L. & Pacifici, R. (2002) J. Clin. Invest. 110, 1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takayanagi, H., Ogasawara, K., Hida, S., Chiba, T., Murata, S., Sato, K., Takaoka, A., Yokochi, T., Oda, H., Tanaka, K., Nakamura, K. & Taniguchi, T. (2000) Nature 408, 600–605. [DOI] [PubMed] [Google Scholar]
- 43.Key, L. L., Jr., Rodriguiz, R. M., Willi, S. M., Wright, N. M., Hatcher, H. C., Eyre, D. R., Cure, J. K., Griffin, P. P. & Ries, W. L. (1995) N. Engl. J. Med. 332, 1594–1599. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguiz, R. M., Key, L. L., Jr., & Ries, W. L. (1993) Pediatr. Res. 33, 384–389. [DOI] [PubMed] [Google Scholar]
- 45.Mann, G. N., Jacobs, T. W., Buchinsky, F. J., Armstrong, E. C., Li, M., Ke, H. Z., Ma, Y. F., Jee, W. S. & Epstein, S. (1994) Endocrinology 135, 1077–1083. [DOI] [PubMed] [Google Scholar]
- 46.Cannon, G. W., Pincus, S. H., Emkey, R. D., Denes, A., Cohen, S. A., Wolfe, F., Saway, P. A., Jaffer, A. M., Weaver, A. L., Cogen, L., et al. (1989) Arthritis Rheum. 32, 964–973. [DOI] [PubMed] [Google Scholar]
- 47.Veys, E. M., Menkes, C. J. & Emery, P. (1997) Arthritis Rheum. 40, 62–68. [DOI] [PubMed] [Google Scholar]
- 48.Huang, W., O'Keefe, R. J. & Schwarz, E. M. (2003) Arthritis Res. Ther. 5, R49–R59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.