Abstract
Realistic studies of plumage color need to consider that many birds can see near-UV light, which normal humans cannot perceive. Although previous investigations have revealed that UV-based plumage reflectance is an important component of various intraspecific social signals, the contribution of UV signals to inter-specific divergence and speciation in birds remains largely unexplored. I describe an avian example of an interspecific phenomenon in which related sympatric species that appear similar to humans (sibling species) differ dramatically in the UV. Both UV video images and physical reflectance spectra indicate that the dorsal plumage of the tanager Anisognathus notabilis has a strong UV-limited reflectance band that readily distinguishes this species from its sibling congener Anisognathus flavinuchus. The main human-visible distinction between A. notabilis (olive back) and coexisting A. flavinuchus (black back) also occurs among different geographic populations of A. flavinuchus. Notably, however, olive- and black-backed taxa interbreed (differentiated populations of A. flavinuchus) unless the additional UV distinction is present (A. notabilis vs. A. flavinuchus). Thus, UV-based reflectance can be an essential component of plumage divergence that relates to reproductive isolation, a key attribute of biological species.
Keywords: Andes, communication, reproductive isolation, speciation, tanager
Since Darwin, the description of plumage variation has figured prominently in the development of theories about speciation (1, 2). Virtually all studies of avian speciation have assumed that human spectral sensitivities (400–700 nm) are adequate guides to plumage colors as they relate to divergence and the development of reproductive isolation. However, mounting evidence indicates that many plumages reflect appreciable near-UV light, a portion of the spectrum (320–400 nm) invisible to normal humans but encompassed by the sensitivity ranges (320–700 nm) of many birds (3, 4). Although studies of UV plumage reflectance have focused on intraspecific communication, a role for UV in avian speciation is suggested by observations that UV plumage reflectance is important for individual recognition and mate choice (3, 5–7) and that such reflectance is taxonomically widespread (8–11).
The classic demonstration of UV's role in speciation was developed during the study of UV-sensitive insects such as lepidopterans and odonatans. In these organisms, sympatric (coexisting) relatives that looked similar from the human perspective (so-called sibling species) often could be readily distinguished by UV-based signals (12). Such UV differences indicated the existence of hidden morphological divergence; in addition, female insects themselves were shown to use species-specific UV reflectance patterns to identify mates (13). Unlike insect UV reflectance, however, most UV plumage reflectance appears to be strongly correlated with human-visible reflectance (11, 14, 15). Such patterns suggest only that UV may augment species distinctions encoded over non-UV wavelengths. Two avian genera are known to express a principal reflectance band limited to the UV. However, the respective congeners are not sibling species, and all members of both genera have the UV features (14, 15). Thus, no evidence currently exists to suggest that UV plumage reflectance is ever essential for avian speciation.
Here I describe an example of sibling bird species whose plumages differ dramatically in the UV. As with UV distinctions between insect species (12, 13), analysis of interactions within and between the sibling bird species indicates that UV signals can promote reproductive isolation, a key attribute of biological species (2).
Materials and Methods
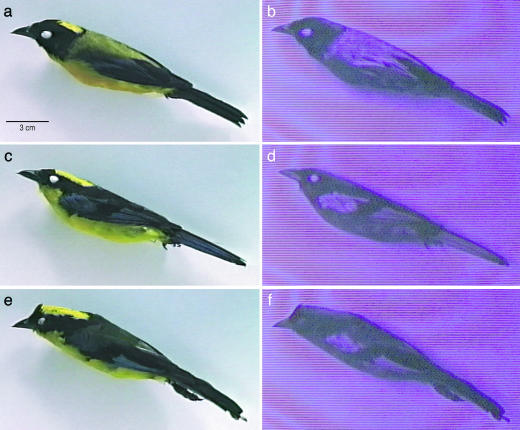
Background Information. Most species of tanager (Emberizinae, Passeriformes) live at tropical latitudes, where they often form feeding flocks composed of many distinctive and brightly colored species. In light of this general pattern, the colorful plumages of the black-chinned (Anisognathus notabilis) and blue-winged (Anisognathus flavinuchus) mountain-tanagers show surprising similarity. These two species coexist without interbreeding along the Pacific slope of the northern Andes in Colombia and Ecuador, with various subspecies of A. flavinuchus also occurring in other Andean regions (16). To humans, both of these Anisognathus species appear to share rich yellow crowns and underparts, black heads, electric blue trim on dark wings and tail, olive rumps, and dull-colored backs (Fig. 1 a, c, and e). In the region of sympatry, the species differ mainly in back plumage, which appears olive in A. notabilis and blackish in the local subspecies of A. flavinuchus, A. flavinuchus cyanopterus (Fig. 1 a and c). This distinction is comparable to ones observed among other brightly colored sibling species such as Pachycephala (2). Indeed, field guides specifically caution that one can easily confuse the two Anisognathus species (17), which may occur in the same feeding flocks (18).
Fig. 1.
Human-visible (a, c, and e) and UV (b, d, and f) still video images of males of A. notabilis [a and b; Academy of Natural Sciences of Philadelphia (ANSP) catalog no. 177661], A. flavinuchus cyanopterus (c and d; ANSP catalog no. 181175), and A. flavinuchus victorini (e and f; ANSP catalog no. 155701). Note the strong UV reflectance by the dorsal plumage in A. notabilis and by blue wing trim in both species.
Specimens Examined. I examined a total of 25 museum study skins collected from throughout the geographic ranges of both A. notabilis (n = 7, Colombia to Ecuador) and A. flavinuchus (n = 18, Venezuela to Bolivia), including exemplars of both sexes from six of the most distinctive subspecies of the polytypic A. flavinuchus (dubious subspecies were not examined). I included only adult birds and pooled the sexes (based on preliminary data indicating only minute sexual, as compared to taxonomic, differences). Nomenclature here follows standard references, although the name somptuosus may have priority over flavinuchus (18).
Visible and UV Imaging. The wavelength range to which both birds and humans are sensitive (400–700 nm) is here referred to as the “human-visible” range. Even these wavelengths probably are perceived differently by birds because various fundamental differences in avian and human visual physiology exist (3). Images based on the human-visible range, however, do provide a useful context for judging the extent to which UV differences bias human interpretations of plumage distinctions.
Still video images of museum study skins in the human-visible and UV ranges were made with a JVC model GX-S700U color video camera fitted with a Pentax Takumar f/1.8 lens. For UV images, the lens was capped with a Kodak 18A Wratten filter, which selectively passes UV between 310 and 400 nm and has a transmission maximum of 70% at 365 nm. This set-up (19) approximates for the UV waveband, both the range (320–400 nm) and maximum (≈370 nm) of spectral sensitivity for passerines with the ability to detect UV (3–6). UV plumage reflectance in Anisognathus also is greatest over these wavelengths. GretagMacbeth (New Windsor, NY) SpectraLight II and BLAK-RAY (San Gabriel, CA) UVL-56 lamps were used to provide human-visible vs. UV illumination.
Spectral Analyses. Reflectance spectra (n = 441) over the wavelength range visible to UV-sensitive passerines (320–700 nm) and to normal humans (400–700 nm) were generated with a PerkinElmer Lambda-9 UV-VIS spectrophotometer equipped with a 60-mm integrating sphere. Percent reflectance, estimated relative to a BaSO4 white standard, was measured in 1-nm intervals with a band pass of 2 nm and a slit width of 4 mm. Specimens were positioned in the (9 × 17 or 5 × 5 mm) sample acceptance port so that the approximate center of a visibly uniform patch filled the port. Both the human-visible and UV lamps were activated before each recording session to allow sufficient time for them to warm up. The background standard was measured before data acquisition for each specimen.
Spectra were generated from standard plumage patches (crown, back, rump, throat, breast, belly, face, shoulder, and flight feathers), excluding some small or narrow markings that could not be measured in the apparatus. To minimize any systematic measurement bias that might arise with respect to taxon or patch location, I randomized both the order in which specimens were measured and then the order in which each patch was measured for each specimen. To improve the accuracy of reflectance measurements, two or three successive scans were made for each plumage patch, and the specimen was repositioned after each scan. Successive scans were averaged for use in subsequent analyses because of the high repeatability of spectra (<2%, usually <1%, different). All data were analyzed in sas 8.0 (20).
Results and Discussion
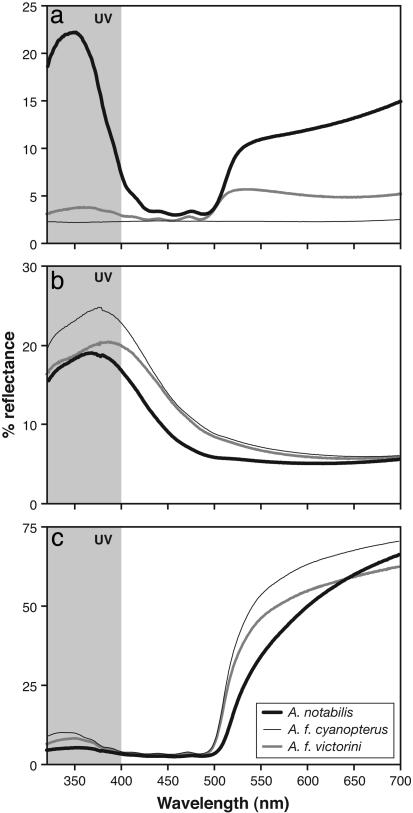
Remarkably, striking differences in UV reflectance by the seemingly dull-colored dorsal plumages comprise the major distinction between A. notabilis and A. flavinuchus cyanopterus (Figs. 1 and 2). For both sexes, UV reflectance by dorsal plumage is high in A. notabilis but very low in A. flavinuchus cyanopterus. The general intensity differences in UV reflectance can be visualized easily with the UV video-viewing system (Fig. 1 a–d). More precise characterizations with physical reflectance spectra indicate that reflectance by dorsal plumage in A. notabilis is maximal in the UV, expressed as a discrete UV-limited reflectance band that is absent in A. flavinuchus cyanopterus (Fig. 2a).
Fig. 2.
Reflectance spectra for Anisognathus plumage patches across the passerine range of spectral sensitivity. Shown are mean (replicates × patch × subspecies) reflectance spectra for back (a), shoulder (b), and breast (c) plumages of A. notabilis, A. flavinuchus cyanopterus (black-backed subspecies), and A. flavinuchus victorini (olive-backed subspecies). Shaded region indicates UV waveband (320–400 nm). See ref. 11 for additional details on recording equipment and methods. For back plumage (a), note variation in reflectance amplitude and shape between subspecies of A. flavinuchus, which parallels the distinction between sympatric A. notabilis and A. flavinuchus cyanopterus.
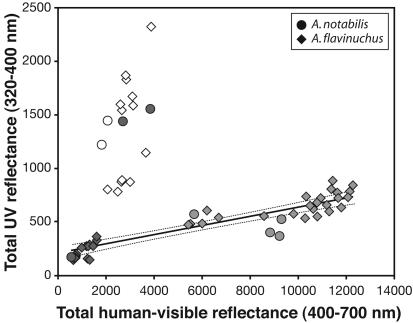
UV-based reflectance is likely to be relevant to plumage differences perceived by Anisognathus because UV spectral sensitivity is expressed in all related emberizid passerines that have been examined (3, 4). Moreover, UV-sensitive birds demonstrate an ability to discriminate UV-based plumage differences that are much smaller than those between A. notabilis and A. flavinuchus (3, 5–7). Given the cone-based opponent color system of vertebrates (3), the strong UV peak expressed by the dorsum of A. notabilis should enhance plumage colorfulness, a psychological quality that depends on the perception of deviations from uniform physical reflectance (3). UV reflectance by A. notabilis is the most remarkable example of hidden avian colors because it is truly invisible to humans. In black or brown feathers with a UV-reflectance band, the tail of the UV peak extends into the human-visible range so that a violet sheen is evident; the dorsal plumage of A. notabilis lacks any such violet sheen (Fig. 1a). Other plumage patches also contribute to UV distinctions between the two Anisognathus species. However, these differences are less dramatic (Fig. 2) and follow the more usual avian pattern of a strong correlation between UV and human-visible reflectance (Fig. 3).
Fig. 3.
UV (320–400 nm) vs. human-visible (400–700 nm) plumage reflectance among Anisognathus taxa. Each data point is the average of replicate spectra per plumage patch per individual for taxa examined (A. notabilis, A. flavinuchus venezuelanus, A. flavinuchus victorini, A. flavinuchus baezae, A. flavinuchus cyanopterus, A. flavinuchus sumptuosus, and A. flavinuchus flavinuchus). Human-visible color coded on gray-scale (white = blue; light gray = yellow, dark gray = blackish to olive). Note linear regression (solid line) and 95% confidence interval (dotted lines) of UV vs. human-visible reflectance among pigmented (black, olive, and yellow) colors, exclusive of dorsal plumage of A. notabilis (y = 0.043x + 205; adjusted r2 = 0.7150, t = 11.02, P < 0.0001). Olive plumages (points for back, rump) of A. notabilis express UV reflectance levels comparable to those of blue plumages (accompanied by similar modifications to feather architecture; R.B., unpublished work) rather than to other pigmented feathers that look similar to humans.
Despite frequent plumage differences between sympatric relatives, one cannot assume that these distinctions necessarily enhance reproductive isolation, because some bird populations that differ dramatically in visible plumage colors may nevertheless interbreed (1, 2). However, the best natural experiment that one could expect for demonstrating a role for UV in reproductive isolation between Anisognathus taxa is provided by geographic variation in A. flavinuchus. Human-visible differences similar to those between A. notabilis and A. flavinuchus cyanopterus also occur among populations of A. flavinuchus, expressed as (darker) olive-backed and black-backed (Figs. 1 c and e and 2a) subspecies (16–18). However, all subspecies of A. flavinuchus have low UV reflectance on the dorsum (Figs. 1 d and f and 2a), and olive-backed (A. flavinuchus victorini and A. flavinuchus baezae) and black-backed (A. flavinuchus cyanopterus and A. flavinuchus sumptuosus) forms intergrade in back color and in several minor differences (size and human-visible shades of blue and yellow) across their many putative geographic contact zones (21, 22). Such graded (clinal) geographic variation implies that the differentiated populations of A. flavinuchus readily interbreed (2). Therefore, no reproductive isolation is achieved between olive and black-backed populations (subspecies of A. flavinuchus) without the UV distinction (A. notabilis vs. A. flavinuchus cyanopterus).
Comparisons with all other Anisognathus taxa also suggest that the major UV-based distinctions between A. notabilis and A. flavinuchus are essential components of species-level divergence. Although to UV-blind humans A. notabilis and A. flavinuchus look very similar, their differences are more in line with those between other sympatric (and nonsibling) congeneric species pairs when colorfulness includes consideration of the UV (Table 1). Without considering the UV, A. notabilis and A. flavinuchus are no more distinct than either geographically isolated forms (recognized as different species) or interbreeding subspecies within recognized species (Table 1). These patterns are consistent with the hypothesis that species status (reproductive isolation in sympatry) for A. notabilis vis vis á A. flavinuchus depends in part on divergence in UV-based colors.
Table 1. Difference in plumage colorfulness among Anisognathus taxa.
| No. of patches that differ in color*
|
||
|---|---|---|
| Taxon comparison | Without UV (human) | With UV (avian) |
| Sympatric species | ||
| igniventris vs. lacrymosus | 8 | 8 |
| flavinuchus vs. notabilis | 3 | 6 |
| Allopatric species | ||
| lacrymosus vs. melanogenys | 3 | 3 |
| Subspecies† | ||
| igniventris, lacrymosus, and flavinuchus | 0-2 | 0-2 |
Plumage colorfulness was based on number of different colorful plumage patches among 14 standard regions (see below). Color vision in both birds and humans is based on differential stimulation of populations of different cone classes by nonuniform physical reflectance. Therefore, plumage reflectance spectra with well defined local (peaks and troughs) and/or absolute (step-functions) maxima or minima (see Fig. 2) should be deemed colorful to both birds and humans (23). By the avian standard, the UV-reflecting dorsal plumage of A. notabilis qualifies as colorful, even though this plumage appears dull-colored to humans.
Excludes comparisons among noncolorful black, brown, olive, or white patches. Patches scored: forecrown, hindcrown, back, rump, upper-tail coverts, throat, breast, belly, under-tail coverts, face, auriculars, thigh, wing, and tail.
Subspecies not enumerated.
Reproductive isolation can be established either directly through species-specific communication (prezygotic isolation) or indirectly as a by-product of genetic divergence that prevents viable offspring (postzygotic isolation). Visual signals appear to play a major role in the interspecific social interactions of tanagers, as evidenced by behavioral observations (24) and the unparalleled diversity of plumages among sympatric species (16–18). More generally, adaptive evolution by dorsal plumage reflectance in A. notabilis is implied by the marked deviation of the UV component from the usual covariation between UV and human-visible reflectance among pigment-based (black, olive, and yellow) colors (Figs. 2 and 3). Conversely, the existence of UV reflectance in the black plumage of other birds (14, 15) implies that no global constraint prevents the development of UV reflectance by dorsal blackish plumage in sympatric A. flavinuchus cyanopterus. Taken together, these additional considerations suggest that the UV distinction between A. notabilis and A. flavinuchus cyanopterus arose via selection on signaling in this waveband, rather than as a by-product of other factors.
Therefore, Anisognathus provides prima facie evidence that application of a UV-based standard of perception is important for studies of avian speciation. This does not mean that UV has a more special significance than wavelengths in the humanvisible range (25–27), but only that some birds may rely mainly on the UV for species discrimination. This evidence does imply that UV reflectance may have a biological significance that extends beyond those intraspecific interactions that have provided the principal focus for studies of UV-based plumage reflectance (3, 28).
Inclusion of the UV also provides a more realistic assessment of the connection between reproductive isolation and morphological change (Table 1). In this regard, ignorance of humans to UV-based plumage differences between A. notabilis and A. flavinuchus highlights that sibling species really are no different from other biological species except that their distinctions are more obvious to the organisms involved than to humans. Indeed, one could mistakenly conclude based on their similar appearance to humans that A. notabilis and A. flavinuchus mimic each other to facilitate their social interactions in feeding flocks (24). Evidence for UV's role in avian speciation also informs studies of speciation in other vertebrates, many of which have only recently been determined to see UV (29).
Acknowledgments
I thank D. Geary and two anonymous reviewers for helpful comments on the manuscript; William Feeny for drafting the figures; Russ Attoe and Dave Hoffman for supplies; the curators of the Academy of Natural Sciences of Philadelphia, American Museum of Natural History (New York), and Museum of Comparative Zoology (Cambridge, MA) for loans of specimens; Ponnampalam Mathiaparanam (Appleton) and Frank Padera (PerkinElmer) for equipment; and the Biology New Media Center (University of Wisconsin, Madison) for video-to-digital image transfer.
Author contributions: R.B. designed research, performed research, analyzed data, and wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Darwin, C. (1859) On the Origin of Species by Means of Natural Selection: Or the Preservation of Favoured Races in the Struggle for Life (Murray, London). [PMC free article] [PubMed]
- 2.Mayr, E. (1963) Animal Species and Evolution (Harvard Univ. Press, Cambridge, MA).
- 3.Cuthill, I. C., Partridge J., Bennett, A. T. D., Church, S. C., Hart, N. S. & Hunt, S. (2000) Adv. Study Behav. 29, 159–214. [Google Scholar]
- 4.Ödeen, A. & Håstad, O. (2003) Mol. Biol. Evol. 20, 855–861. [DOI] [PubMed] [Google Scholar]
- 5.Andersson, S, Örnborg, J. & Andersson, M. (1998) Proc. R. Soc. London Ser. B 265, 445–450. [Google Scholar]
- 6.Bennett, A. T. D., Cuthill, I. C., Partridge, J. C. & Lunau, K. (1997) Proc. Natl. Acad. Sci. USA 94, 8618–8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keyser, A. J. & Hill, G. E. (2000) Behav. Ecol. 11, 202–209. [Google Scholar]
- 8.Burkhardt, D. (1989) J. Comp. Physiol. A 164, 787–796. [Google Scholar]
- 9.Eaton, M. D. & Lanyon, S. M. (2003) Proc. R. Soc. London Ser. B 270, 1721–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prum, R. O., Andersson, S. & Torres, R. H. (2003) Auk 120, 163–170. [Google Scholar]
- 11.Bleiweiss, R. (2005) Biol. J. Linn. Soc., in press.
- 12.Silberglied, R. E. (1979) Annu. Rev. Ecol. Syst. 10, 373–398. [Google Scholar]
- 13.Silberglied, R. E. & Taylor, O. R. (1973) Nature 241, 406–408. [DOI] [PubMed] [Google Scholar]
- 14.Burkhardt, D. & Finger, E. (1991) Naturwissenschaften 78, 279–280. [Google Scholar]
- 15.Andersson, A. (1996) Proc. R. Soc. London Ser. B 263, 843–848. [Google Scholar]
- 16.Isler, M. L. & Isler, P. R. (1999) The Tanagers: Natural History, Distribution, and Identification (Smithsonian Institution Press, Washington, DC).
- 17.Hilty, S. L. & Brown, W. L. (1986) A Guide to the Birds of Colombia (Princeton Univ. Press, Princeton).
- 18.Ridgely, R. S. & Greenfield, P. J. (2001) The Birds of Ecuador Field Guide (Cornell Univ. Press, Ithaca, NY), Vol. II.
- 19.Bleiweiss, R. (1994) Anim. Behav. 48, 978–981. [Google Scholar]
- 20.SAS Institute Inc. (2003) SAS User's Guide, Version 8 (SAS Inst., Cary, NC).
- 21.Chapman, F. M. (1926) Bull. Am. Mus. Nat. Hist. 55, 1–790. [Google Scholar]
- 22.Zimmer, J. T. (1944) Am. Mus. Nov. 1262, 1–21. [Google Scholar]
- 23.Endler, J. (1990) Biol. J. Linn. Soc. 41, 315–352. [Google Scholar]
- 24.Moynihan, M. (1968) Evolution 22, 315–331. [DOI] [PubMed] [Google Scholar]
- 25.Hunt, S., Cuthill, I. C., Bennett, A. T. D., Church, S. C. & Partridge, J. C. (2001) J. Exp. Biol. 204, 2499–2507. [DOI] [PubMed] [Google Scholar]
- 26.Maddocks, S. A., Church, S. C. & Cuthill, I. C. (2001) J. Exp. Biol. 204, 2509–2515. [DOI] [PubMed] [Google Scholar]
- 27.Hausmann, F., Arnold, K. E., Marshall, N. J. & Owens, I. P. F. (2003) Proc. R. Soc. London Ser. B 270, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheldon, B. C., Andersson, S., Griffith, S. C., Örnborg, J. & Sendecka, J. (1999) Nature 402, 874–877. [Google Scholar]
- 29.Shi, Y. & Yokoyama, S. (2003) Proc. Natl. Acad. Sci. USA 100, 8308–8313. [DOI] [PMC free article] [PubMed] [Google Scholar]