Abstract
Background
CD117 is expressed on double-negative (DN; CD4–CD8–) cells (Nat Rev Immunol 14:529–545; 2014), but whether it is expressed in other stages and its subsequent functions are unclear. We used an improved method of flow cytometry to analyze different populations of thymocytes (Sci Rep 4:5781; 2014). The expression of CD117 and CTLA-4 were directly assayed in the early stage of thymocytes.
Methods
Flow cytometry was used to analyze different populations of thymocytes, and T-cell proliferation assays, RT-PCR, and real-time RT-PCR were used to characterize the stem cells and examine the function of CD44+CD117+ cells.
Results
In DN cells, CD117 expression was greatest on CD44+CD25+ cells (DN2), followed by CD44+CD25– (DN1), CD44–CD25+ (DN3), and CD44–CD25– (DN4) cells. In thymocytes, CD117 expression was highest in DN cells, followed by single-positive (SP; CD4 or CD8) and double-positive (DP; CD4+CD8+) cells. Especially, CD117 expression was positively associated with CD44 and CTLA-4 expression. CTLA-4 expression was highest in DN cells, followed by SP and DP cells. CTLA-4 expression was positively associated with CD25, CD44, and Foxp3 expression. CD44+CD117+ T cells expressed more CTLA-4, which suppressed T-cell proliferation and blocked CTLA-4 to cause antibody-induced T-cell proliferation.
Conclusion
These results suggest that CD44+CD117+ T cells are stem cells and a specific T-cell phenotype that initially develops in the thymus, but they do not progress through DN3 and DN4 stages, lack a DP stage, and potently suppress T-cell proliferation and modulate the CTLA-4 pathway.
Keywords: CD117, CTLA-4, CD25, CD44, Stem cell, Flow cytometry
Background
T-cell development in mice begins with hematopoietic progenitor cells that migrate from the bone marrow to the thymus [1, 2]. The earliest precursors in the T-cell lineage are found within thymocyte populations and lack CD4 and CD8 expression, so they are referred to as CD4−CD8− or double-negative (DN) thymocytes [3]. DN thymocytes consist of four subpopulations based on CD44 and CD25 expression [4]. DN cells that express CD4 and CD8 are CD4+CD8+ or double-positive (DP) thymocytes before differentiating into single-positive (SP; CD4+ or CD8+) thymocytes [5].
Stems cells include embryonic stem (ES) cells, induced pluripotent stem (iPS) cells, adult tissue stem/progenitor cells, and cancer stem cells. Mesenchymal stem cells (MSCs) have been shown to inhibit abnormally activated immune functions in dendritic cells, T cells, and B cells; however, preclinical efficacy studies to evaluate MSCs in autoimmune animal models have been contradictory [6–8].
Mammalian CD117 (c-kit) gene protein is a stem cell marker and a type III tyrosine kinase receptor [9, 10], widely expressed in hematopoietic stem cells (HSC), myeloid progenitor cells, as well as pro-B cells and pro-T cells [11–13]. In the hematopoietic system this protein contributes to cell growth, metabolism, proliferation, and survival [14, 15] during gametocyte development, pigmentation, and intestinal motility [16], and in the immune system contributes to inflammation [17]. The four DN subsets are defined by surface expression of c-kit, CD44, and CD25. Cells at the DN3 stage are positive for c-kit loCD44−CD25+. CD25 expression is downregulated as cells transition to the DN4 cell stage [14, 18].
Nanog is an important transcription factor for maintaining self-renewal and pluripotency of ES cells and regulates the fate of inner cell mass of blastocysts in the embryo during early development. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells [19].
Cytotoxic T lymphocyte antigen-4 (CTLA-4) is an inhibitory relative of the T-cell costimulatory molecule, CD28. Whereas CD28 signaling promotes T-cell activation, CTLA-4 serves an immunoregulatory function by suppressing the T-cell response [20–22]. CTLA-4 also has intrinsic functions for T-conv cells and regulates trafficking of self-reactive T cells [23, 24].
Flow cytometry can be used to characterize single cells in an efficient and expedient manner [25], and can be used to describe DN cells which are present in limited amounts in the mouse thymus. Previously, a highly reproducible multi-color flow cytometry method was developed to increase thymocytes collected from 104 to more than 106 [26]. Using this method, we identified genes with low expression in DN cells and this permitted characterization of DN cell subsets.
In the present study, CD117, CTLA-4, CD4, CD8, CD25, and CD44 expression was measured in thymocytes and expression of CD117 and CTLA-4 was assayed directly in thymocytes and CD117+ stem T cells in the thymus and spleen. These data provide evidence of a relationship among CD117, CTLA-4, CD25, and CD44 cell surface markers on DN cells and other thymocytes.
Methods
Animals
Female C57BL/6 mice (Taconic Farms, Germantown, NY, USA) were maintained under specific pathogen-free conditions and were studied at 4–6 weeks of age, according to protocols approved by the Institutional Animal Care and Use Committee at Xi’an Jiaotong University, China.
Flow cytometric analysis and cell sorting
Thymocytes and splenocytes were obtained from naive mice and suspended in PBS plus 1% fetal calf serum (FCS). To avoid nonspecific binding to mouse Fc-γ receptors, cells were blocked with mouse CD16/CD32 mAb (0.25 μg/100 μl; BD Biosciences, Franklin Lakes, NJ, USA) for 15 min at room temperature. Cells were washed and then stained with FITC anti-CD4 (clone RM4-5), PercP5.5-anti-CD8 (clone 53-6.7), APC-CY7-anti-CD44 (clone IM7), PE-CY7-anti-CD25 (clone PC61), PE-anti-CD117 (clone 2B8), FITC-CD117 (Clone 2B8), and APC-anti-CD3e (Clone 145-2C11) antibodies for 60 min at 4 °C. For some samples, surface-stained cells were fixed/permeabilized with a Cytofix/Cytoperm Kit (BD Biosciences) and stained with PE-anti-Foxp3 (clone FJK-16 s; eBioscience) and APC-anti-CTLA-4 (clone UC10-4B9; eBioscience) antibody for 45 min at 4 °C. The corresponding isotype controls (Armenian Hamster IgG, and Rat IgG2b,k) were purchased from eBioscience. Cells were sorted and analyzed using a FACSAria II, and data were analyzed using BD FACSDiva software (BD Biosciences).
Culture medium and antibodies
RPMI 1640 medium containing 10% FCS was used for CD4 naive T-cell culture. Antibodies used for cell culture were anti-mouse CD3 (145-2C11) and anti-mouse IL2 (300 u/ml) (BD BioSciences, San Jose, CA, USA).
Coculture system development
A coculture system was developed using a six-well Transwell plate (0.4 μm pore size insert; Corning Incorporated, Corning, NY, USA).
Naive T cell and CD44+CD117+ cell sorting and stimulation
Lymphocytes from the spleen and thymus were stained with fluorochrome-conjugated antibodies, CD4 (FITC), CD8 (PerCp5.5), CD44 (APC-CY7), and CD117 (PE) for 45 min at 4 °C. Cells were then washed twice and resuspended at a density of 1 × 108 cells/ml. Naive CD4, CD4+CD44+CD117+, and CD8+CD44+CD117+ cells were sorted using BD FACS Aria II. Anti-CD3 antibody was coated onto 24-well flat-bottom plates at 1 μg/ml in PBS overnight and then washed once with PBS. Naive T cells and CD4+CD44+CD117+ (CD8+CD44+CD117+) were then placed into the wells at a density of 1 × 106 cells/ml in culture. Cells were incubated at 37 °C containing 5% CO2 for 7 days.
T-cell proliferation assays
Cell proliferation was quantified using a Cell Trace CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA, USA). Splenic T cells were prepared by passing splenocytes through nylon-wool columns (108 splenocytes were incubated in the column for 1 h at 37 °C in 5% CO2 before eluting with RPMI medium), in which CD4+CD117+ cells (from flow cytometric cell sorting) were added to effecter cells (splenic T cells) at ratios of 1:20, 1:10, and 1:5. Cells were incubated with 2.5 μM CFSE (Invitrogen, USA) in PBS for 20 min at 37 °C. An excess of ice-cold RPMI 1640 with 10% FBS was added to the cells to quench the reaction. CFSE-labeled cells (1 × 106/well) were washed twice with RPMI 1640 culture medium and then cultured in complete RPMI 1640 medium with 2 mM l-glutamine (Gibco), supplemented with 10 mM HEPES, 1% penicillin–streptomycin solution (Gibco), and 10% FBS (Gibco). A total 3 μg/ml of ConA or IL-2 and anti-CD3 antibody was added for polyclonal stimuli. Samples not stimulated with ConA or IL-2 and anti-CD3 were used as negative controls. Cells were then incubated for 7 days and stained with CD4 and CD8. T-cell proliferation was measured by quantifying CFSE fluorescence intensity via flow cytometry.
CTLA-4 block assay
To block CTLA-4, 8 μl anti-mouse CD152 (CTLA-4) or isotype Ctrl (Armenian Hamster IgG) antibody was added in medium before incubation during the T-cell proliferation assay.
RT-PCR and real-time RT-PCR
Total RNA was extracted from 106 sorted thymocytes from C57BL/6 mice, using TRIzol (Invitrogen). Chloroform (0.2 ml; Sigma-Aldrich, USA) was added for every 1 ml of TRIzol used. Extracted RNA was shaken vigorously for 15 sec and incubated at room temperature for 2 min. After transferring the aqueous phase to a clean tube, isopropanol (Sigma-Aldrich) was added and the RNA was incubated at room temperature for 5 min. The RNA pellet was then washed with 1 ml of 75% ethanol, air-dried for 10 min, dissolved in 20 μl of water, and incubated at 55 °C for 10 min. The OD ratio (λ 260 nm/280 nm) was between 1.9 and 2.0, and RNA samples were assessed with electrophoresis on 1.5% agarose gels and visualized under UV light after ethidium bromide staining. RNA preparations were treated with DNase I to remove genomic DNA. cDNA was synthesized by incubating 20 μl of mRNA in a sprint C1000 terminal cycler (Bio-Rad, USA). Negative controls contained the reaction mixture except for template DNA. For quantification, relative mRNA expression of specific genes was obtained by the 2−ΔΔCt method. The gene-specific primers (5′ → 3′) used are presented in Table 1. Data were normalized using transcripts for β-actin as controls, which were amplified with primers as presented in Table 1. Diluted cDNA (10 μl) was mixed with 2 μl of primer and 10 μl IQ SYBR Green SuperMix and was assayed in triplicate using a Stepone real-time system (ABI) under the following conditions: denaturation at 95 °C for 3 min, 40 cycles of 95 °C for 15 sec, and 60 °C for 1 min, followed by 30 sec of extension at 72 °C. Each sample was analyzed in triplicate.
Table 1.
Primers used for RT-PCR to detect CTLA-4, Nanog mRNAs
| β-actin sense | GAA ATC GTG CGT GAC ATC AAA G |
| Antisense | TGT AGT TTC ATG GAT GCC ACA G |
| CTLA-4 sense | TCTGCAAGGTGGAACTCA |
| Antisense | GCTAACTG CGACAAGGAT |
| Nanog sense | ATCCCGAGAACTATTCTTG |
| Antisense | GGTACTTCTGCTTCTGAAAC |
Statistical analysis
Mean and SD values were calculated with Microsoft Excel. At least three independent experiments were performed. A Tukey–Kramer post test was used to compare three or more means and a two-tailed unpaired Student’s t test was used to compare two groups (p ≤ 0.05 was considered statistically significant).
Results
CD117 and CD44 expression in different thymocyte subpopulations
CD117 plays an important role in the differentiation of T cells [9, 10]. T cells develop from CD3–CD4–CD8–c-kit+ cells through the modulation of cell surface molecules [27]. To confirm CD117 expression in the thymus during T-cell development, thymocytes were harvested from C57BL/6 mice, stained with CD4 (FITC), CD8 (PercP5.5), CD44 (APC-CY7), CD25 (PE-CY7), and CD117 (PE), and analyzed by flow cytometry. This group of antibodies is now termed the “antibody cocktail.” Isotype Rat IgG2b,k was used as a negative control (CD117). DN2 (CD44+CD25+) cells had the highest frequency CD117 expression, followed by DN1, DN3, and DN4 cells (Fig. 1a). Pooled data from three independent experiments are shown in Fig. 1c. DN cells had more CD117-expressing cells in C57BL/6 mice followed by CD8+ SP cells, CD4+ SP cells, and DP cells (Fig. 1b); the pooled data from three independent experiments (Fig. 1d) indicate that CD117 expression was lowest in DP cells.
Fig. 1.
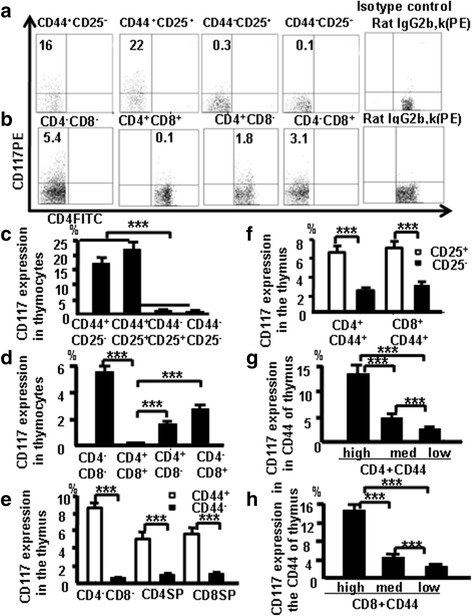
CD117 and CD44 expression in different thymocyte subpopulations. a CD117 expression in DN1, DN2, DN3, and DN4 thymocytes. b CD117 expression in DN, DP, and SP thymocytes. DN thymocyte data (c) and thymocyte data (d) pooled from three independent experiments. e CD44 expression analyzed in the different subpopulations of mouse thymocytes. f CD117 expression evaluated on CD4+CD44+CD25+ and CD8+CD44+CD25+ cells. g CD117 expression in CD44+CD4 SP cells, according to varying CD44 expression. h CD117 in CD44+CD8 SP cells, according to varying CD44 expression. Data are mean percent of total cells ± SD from three independent experiments. ***p < 0.001
Thymocytes from naive mice were stained with antibody cocktail to confirm whether CD117 expression is associated with CD44 expression in the other thymocyte subpopulations. In three experiments performed independently, more DN, CD4, and CD8 CD44+ cells expressed CD117 compared with DN, CD4 and CD8 CD44– cells (Fig. 1e). There was also a positive correlation between CD25 and CD117 expression among CD4+CD44+ cells and CD8+CD44+ cells (Fig. 1f). Data (Fig. 1g, h) indicate that CD117 expressed in early stage T cells in the thymus positively correlates with CD44 in thymocytes.
CTLA-4 expression correlates with CD25 and CD44 expression in different thymocyte populations
CTLA-4 expression was examined in different subsets of thymocytes at various stages of T-cell development to confirm CTLA-4 expression in T-cell development. More DN cells expressed CTLA-4 compared with CD4 SP, CD8 SP, and DP cells (Fig. 2a). The pooled data from three independent experiments are shown in Fig. 2b, and the data show that CTLA-4 expression is greater in early stages of T-cell development. More CD4+CD25+CD44+ cells produced CTLA-4 compared with CD4+CD25+CD44– and CD4+CD25– cells (Fig. 2c, d). Data show that surface expression of CD25 and CD44 appears to be positively correlated with CTLA-4 expression.
Fig. 2.
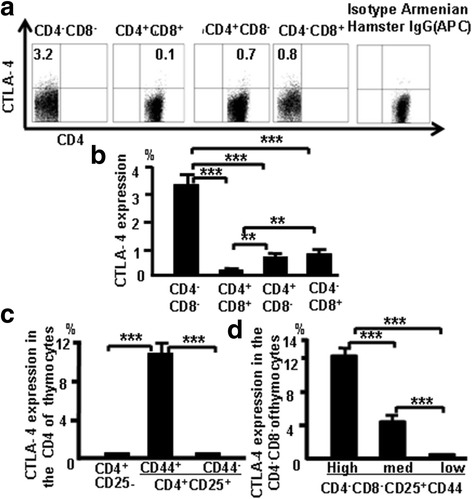
CTLA-4 expression correlates with CD25 and CD44 expression in different populations of thymocytes. a CTLA-4 expression in thymocytes of C57BL/6 mice. b CTLA-4 expression data pooled from three independent experiments. c CTLA-4 expression evaluated in CD4+CD25– and CD4+CD25+ cells. d CTLA-4 expression in CD25+ DN cells, according to varying CD44 expression. Data are mean percent of total cells ± SD from three independent experiments. **p < 0.01, ***p < 0.001
CTLA-4 correlates with Foxp3 expression in thymocytes
Thymocytes from naive mice were stained with CD4, CD8, CD44, CD25, CTLA-4 (APC), and Foxp3 (PE), and were analyzed by flow cytometry to study the relationship between CTLA-4 and Foxp3. More CTLA4 expression was observed in Foxp3-positive CD4 SP cells compared with Foxp3-positive DN cells (Fig. 3). These data indicate that there is overlap of Foxp3 and CTLA-4 expression in some thymocytes.
Fig. 3.
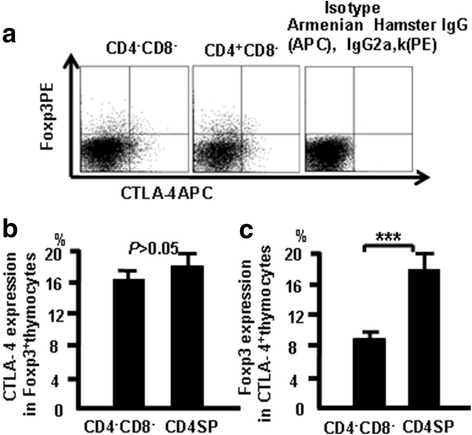
CTLA-4 expression correlates with Foxp3 expression in thymocytes. Cells were stained as in Fig. 1 with Foxp3 (PE) and analyzed by flow cytometry. a CTLA-4 and Foxp3 expression in different thymocyte populations. b CTLA-4 expression in Foxp3-positive thymocytes. c Foxp3 expression in CTLA-4-positive thymocytes. Data are mean percent of total and live thymocytes in each cell subset ± SD from three independent experiments. ***p < 0.001
CTLA-4 expression in CD44+CD117+cells in the thymus
Thymocytes from naive mice were stained with the antibody cocktail and CTLA-4 (APC), we studied whether CD117 and CD44 expression correlates with CTLA-4 expression in the thymus (Fig. 4a). Data showed 0.5% of thymocytes were CD117+ cells, 0.7% of thymocytes were CTLA-4+ cells, and 0.2% of thymocytes were CTLA-4+CD117+ cells. Furthermore, 2.9% of the CD44+ thymocytes were CD117+ cells, 3.2% of the CD44+ thymocytes were CTLA-4+ cells, and 0.6% of the CD44+ thymocytes were CTLA-4+CD117+ cells (Fig. 4b). CTLA-4 expression was found in 19% of DN CD117+ cells, 18% of CD4+CD117+ cells, and 16% of CD8+CD117+ cells. Interestingly, CTLA-4 expression was also found in 34% of DN CD44+CD117+ cells, 36% of CD4+CD44+CD117+ cells, and 32% of CD8+CD44+CD117+ cells (Fig. 4c), demonstrating that CD44+ and CD117+ expression is closely associated with CTLA-4 in thymocyte stem T cells.
Fig. 4.
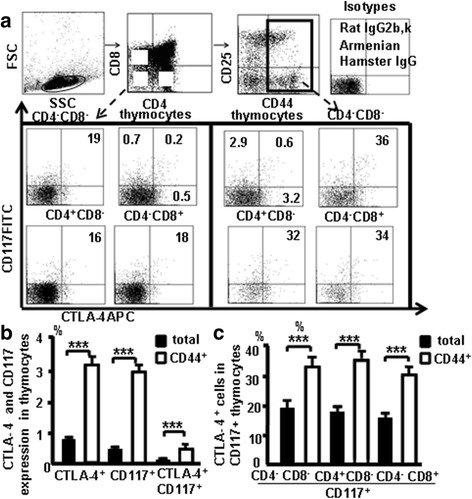
CTLA-4 expression in CD44+CD117+cells in the thymus. a CD117 and CTLA-4 expression in the different population in the thymocytes. b CD117 and CTLA-4 in thymocytes and in the CD44+ thymocytes. c CTLA-4 in CD117+ thymocytes and in CD117+CD44+ thymocytes. Data are mean percent of total ± SD from three independent experiments. ***p < 0.001
CD4+ SP cells and CD4+CD44+CD117+ cells were sorted from the thymocytes via FACS (Fig. 5a) to further verify whether CD44+CD117+ cells express higher levels of CTLA-4. The expression of CTLA-4 mRNA in CD44+CD117+ cells and CD4+ SP cells was compared by RT-PCR. Results showed that the level of CTLA-4 was higher in CD4+CD44+CD117+ cells, as determined by densitometry measurements of the gels (Fig. 5b). Data from three independent experiments were pooled (Fig. 5c, d). Furthermore, CD4+ SP and CD4+CD44+CD117+ cells were sorted from the thymocytes and the levels of CTLA-4 mRNA were measured by real-time RT-PCR to further confirm the aforementioned findings. As shown in Fig. 5e, CD4+CD44+CD117+ cells expressed higher levels of CTLA-4 than CD4+ SP cells (~14× higher). These results corroborate the finding that CTLA-4 expression was much higher in CD4+CD44+CD117+ cells.
Fig. 5.
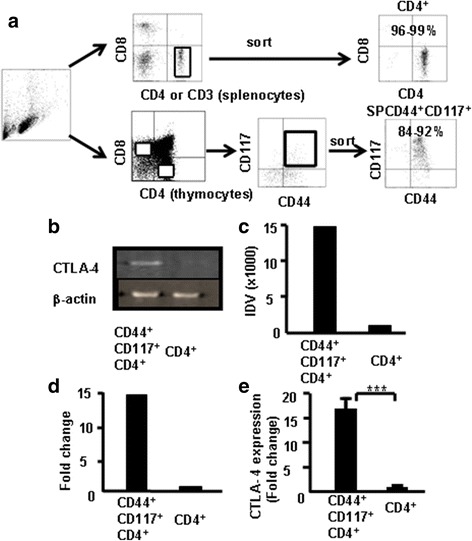
CTLA-4 expression in CD4+CD117+CD44+ thymocytes by RT-PCR and real-time RT-PCR. CD4+CD117+CD44+ and CD4+ SP cells sorted from the thymocytes of C57BL/6 mice (a). Total RNA extracted for RT-PCR and real-time RT-PCR analysis of CTLA-4 expression. b CTLA-4 expression in thymocytes by RT-PCR. c Integrated density value (IDV) for CTLA-4 transcripts quantified and normalized to β-actin. Representative results from one of three independent experiments. d Data pooled from three independent experiments. e Total RNA isolated for measuring CTLA-4 mRNA by real-time RT-PCR. Data are fold-induction relative to β-actin and representative results from one of three independent experiments. ***p < 0.001
CTLA-4 expression correlates with CD44 expression in different populations of CD117-positive thymocytes
Thymocytes were stained with the combination of antibodies to CD4, CD8, CD44, CD25, CTLA-4 (APC), and CD117 (PE) and analyzed by flow cytometry, and data show that CTLA-4 expression correlates with thymic CD44 expression. Fig. 6a–c show these data which suggest that CD44 expression positively correlates with CTLA-4 expression in CD117-positive thymocytes.
Fig. 6.
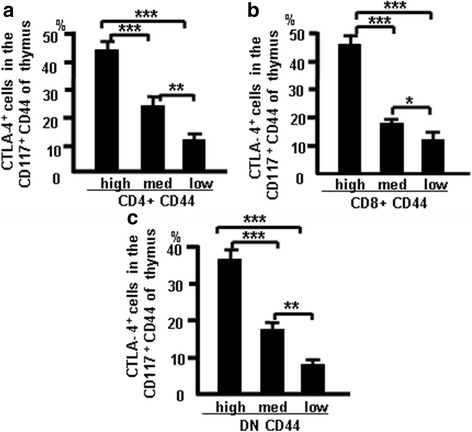
CTLA-4 correlates with CD44 expression in different populations of CD117-positive thymocytes. Thymocytes were treated as indicated in Methods and analyzed by flow cytometry. a CTLA-4 expression in CD4+CD117+CD44+ cells. b CTLA-4 expression in CD8+CD117+CD44+ cells. c CTLA-4 expression in DNCD117+CD44+ cells. Data show mean percent of total cells ± SD from three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001. DN double-negative
CD44+CD117+ cells expressed more Nanog in thymocytes. Nanog is an important transcription factor for maintaining the self-renewal and pluripotency of ESCs. It regulates the fate of the inner cell mass of blastocysts in the embryo during early development. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells [19]. To characterize the CD44+CD117+ cells in the thymus, CD4+ SP cells and CD4+CD44+CD117+ cells were sorted from the thymocytes via FACS to verify whether CD44+CD117+ cells express more Nanog. The Nanog mRNA expression in CD44+CD117+ cells and CD4+ SP cells was compared using RT-PCR. Results showed that Nanog were greater in CD4+CD44+CD117+ cells than in CD4+ SP cells as determined by densitometric analysis of gels (Fig. 7a). Pooled data from three independent experiments are shown in Fig. 7b. CD4 SP and CD4+CD44+CD117+ cells were sorted from thymocytes and Nanog mRNA expression was measured using real-time RT-PCR to confirm previous findings. CD4+CD44+CD117+ cells expressed more Nanog than CD4+ SP cells as shown in Fig. 7c. Data suggest that CD44+CD117+ cells are stem cells.
Fig. 7.
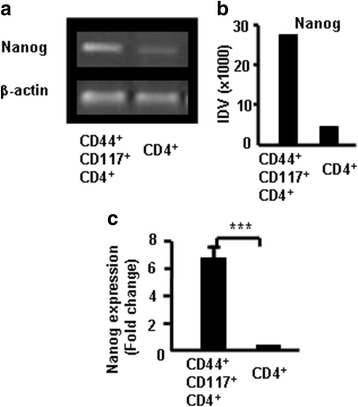
Nanog expression in CD117+CD44+ cells measured with RT-PCR and real-time RT-PCR. a Nanog in the thymocytes by RT-PCR. b Integrated density value (IDV) for the Nanog transcripts quantified and normalized to those of β-actin. c Total RNA isolated for measuring Nanog mRNA using real-time RT-PCR. Data presented as fold-induction relative to β-actin. Data represent one of three independent experiments. ***p < 0.001
CD44+CD117+ stem T cells from the thymus and spleen suppressed T-cell proliferation
CD117+ cells develop in the thymus and do not downregulate CD44 and CD117 expression, which is measurable in SP cells. We hypothesized that CD44+CD117+ T cells are stem T cells, which are key to the development and function of the immune system, especially of T cells. To confirm whether CD44+CD117+ T cells affect T-cell proliferation, stem T-cell function was examined in vitro using a CFSE assay. CD4+CD117+CD44+ T cells from the thymus were sorted by FACSAria II and were cocultured with T cells (CD4+CD117+CD44+ T cells:CD4+ T cells) in the presence of Con A (3 μg/ml). T-cell proliferation was measured by flow cytometry to determine the CFSE (5 μM) fluorescence intensity, as shown in Fig. 8. Stem T cells from the thymus and T cells from the spleen were sorted by FACSAria and were incubated with cocultured T cells (stem T cells:T cells) at ratios of 1:20, 1:10, and 1:5 in the presence of Con A. T-cell proliferation was measured by flow cytometry to determine the CFSE fluorescence intensity (Fig. 8a, b). The percentage of T-cell inhibition was 80%, 21%, and 5.1%, respectively. Further, both stem T cells and T cells from the spleen were sorted by FACSAria and were incubated with cocultured T cells (stem T cells:T cells) at ratios of 1:20, 1:10, and 1:5 in the presence of Con A. The percentage of cell inhibition was 84%, 26%, and 11%, respectively. The data from three independent experiments were pooled and are shown in Fig. 8c (stem cells from the thymus) and Fig. 8d (stem cells from the spleen). These results suggest that CD44+CD117+ T cells significantly suppressed T-cell proliferation.
Fig. 8.
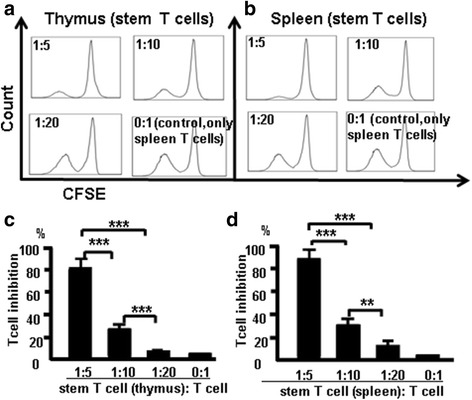
CD4+CD117+ cells from the thymus and spleen suppress T cells in vitro. T-cell proliferation was measured by flow cytometry to measure fluorescence intensity. a CD4+CD117+ cells sorted from the thymus. b CD4+CD117+ cells sorted from the spleen. Thymocyte data (c) and spleen data (d) pooled from three independent experiments. Data are mean percent of the total ± SD from three independent experiments. **p < 0.01, ***p < 0.001
CTLA-4 suppressed T-cell proliferation and blocking CTLA-4 led to antibody-induced T-cell proliferation
CD44+CD117+ stem T cells from the thymus were sorted by FACSAria and incubated alone or cocultured (stem T cell:T cell) at a 1:2 ratio in the presence of anti-CD3 and IL-2 (300 u/ml) or blocking antibodies (1 μg/100 μl) to CTLA-4. Isotype Armenian Hamster IgG was used as a control. T-cell proliferation was measured by flow cytometry to determine the CFSE (2 μM) fluorescence intensity after 7-day incubation. The percentage of T-cell inhibition was 2.5% in T cells only (unstimulated) (Fig. 9Aa), 33% in T cells containing anti-CD3 + IL-2 (T cells only) (Fig. 9Ab), 5.3% in Isotype Armenian Hamster IgG (control, T cells were cultured with CD44+CD117+ T cells and Isotype Armenian Hamster IgG together) (Fig. 9Ac), 39% in T cells + CD44+CD117– T cells containing anti-CD3 + IL-2 (control, T cells were cultured with CD44+CD117– T cells) (Fig. 9Ad), 54% in the medium containing CTLA-4 broke antibody (CD44+CD117+ T cells were cultured with CTLA-4 broke, after 1 h the cells were washed and cultured with T cells in the medium) (Fig. 9Ae), and 25% in CD44+CD117+ T cells and T cells in Transwell plate coculture system (CD44+CD117+ T cells and T cells were cultured in the Transwell system) (Fig. 9Af). The data from three independent experiments were pooled and are shown in Fig. 9B. Data show that CD44+CD117+ stem T cells suppressed T-cell proliferation by the interaction between the cells, and suppressive activity of stem T cells was reduced in the presence of anti-CTLA-4 antibody.
Fig. 9.
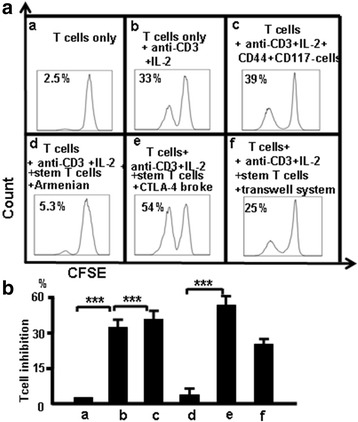
Blocking CTLA-4 reduces the ability of CD4+CD117+CD44+ cells to suppress T-cell proliferation. a a T cells only; b T cells + anti-CD3 + IL-2; c CD44+CD117– T cells + anti-CD3 + IL-2; d CD44+CD117+ T cells + anti-CD3 + IL-2 + Isotype Armenian Hamster IgG; e (CD44+CD117+ T cells + CTLA-4 broke) + anti-CD3 + IL-2 + T cells; f CD44+CD117+ T cells cultured with T cells in a Transwell plate. B Data pooled from three independent experiments representing mean percent of totals ± SD. ***p < 0.001
CD44+CD117+ stem T cells can self-renew
To confirm that CD44+CD117+ T cells can self-renew, thymic CD44+CD117+ stem T cells were sorted with FACSAria and incubated alone or cocultured (stem T cell:T cell) at a 1:2 ratio in the presence of anti-CD3 and IL-2 (300 u/ml). T-cell proliferation was measured by flow cytometry to measure CFSE (2 μM) fluorescence intensity after 7-day incubation. T-cell proliferation was low in CD44+CD117+ stem T cells only (unstimulated), and this was greater in CD44+CD117+ T cells containing anti-CD3 + IL-2 (Fig. 10). Thus, CD44+CD117+ T cells can self-renew.
Fig. 10.
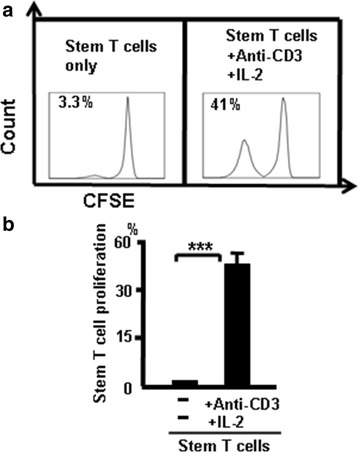
CD44+CD117+ T cells have the ability to self renew. a CD4+CD44+CD117+ T cells only and CD4+CD44+CD117+ T cells + anti-CD3 + IL-2. b Data pooled from three independent experiments representing mean percent ± SD. ***p < 0.001
CD44+CD117+ cells in blood and lymph nodes
To confirm CD44+CD117+ cells are exist in blood and lymph nodes, cells from naive mice were stained with the combination of antibodies CD4 (FITC), CD8 (PerCp), CD25(PE-CY7), CD44 (APC-CY7), and CD117 (FITC) and analyzed by flow cytometry. The results showed 0.5% of CD4+CD117+ cells and 1.2% of CD4+CD117+ cells in blood and lymph nodes, respectively (Fig. 11). Furthermore, 6.9% of CD4+ CD117+ cells and 14.6% of CD4+ CD117+ cells were present in CD44+ cells of blood and lymph nodes, respectively. These results suggest CD44+CD117+ T cells are exist in blood and lymph nodes.
Fig. 11.
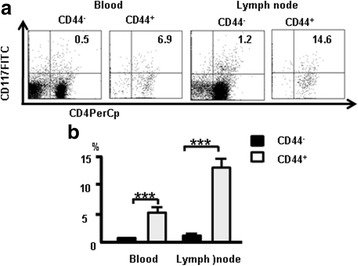
CD44+CD117+ cells in blood and lymph nodes. Cells from naive mice were stained with the combination of antibodies CD4 (FITC), CD8 (PerCp), CD25 (PE-CY7), CD44 (APC-CY7), and CD117 (FITC) and analyzed by flow cytometry. a CD117 expression and CD44+CD117+ cells in blood and lymph nodes. b Data pooled from three independent experiments and represent mean percent ± SD. ***p < 0.001
CD44+CD117+ cells expressed Foxp3 in blood and lymph nodes. The cells from naive mice were stained with the following combination of antibodies: the antibody cocktail with Foxp3 (PE) and used to confirm whether CD117 and CD44 expression correlates with Foxp3 expression in the blood and lymph nodes. Data show that 4.5% of CD4+CD117+ cells expressed Foxp3, 10.3% of CD4+CD44CD117+ cells expressed Foxp3+ in blood cells, 11% of CD4+Foxp3+ cells expressed CD117, and 9.5% of CD4+CD44+ cells expressed Foxp3 in lymph nodes (Fig. 12a). The pooled data from three independent experiments are shown in Fig. 12b, c. These results suggested that CD44+CD117+ cells and nTreg cells are two different populations of cells, although part of them overlap.
Fig. 12.
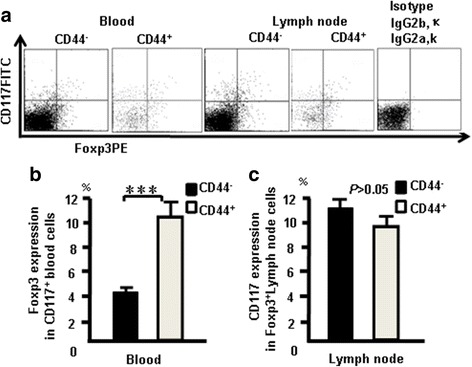
CD44+CD117+ cells expressed Foxp3 in blood and lymph nodes. Cells from naive mice were stained with the following combination of antibodies: the antibody cocktail with CD117 (FITC) and Foxp3 (PE). a CD117 and Foxp3 expression in blood and lymph nodes. b Foxp3 expression in CD117+ blood cells. c Foxp3 expression in CD117+ lymph node cells. Data pooled from three independent experiments and represent mean percent ± SD. ***p < 0.001
Discussion
Using an advanced flow cytometry method, we researched the characteristic of T-cell development in the thymus by measuring expression of CD117 and CTLA-4 in thymocytes. Surprisingly, CD117+ stem T cells were found in mature thymocytes and CD117 and CTLA-4 expression occurred at each stage of T-cell development. Changes in CD25, CD44, CD117, and CTLA-4 expression were examined in DN, DP, and SP cells using multi-color flow cytometry. CD117 was expressed in the early stages (DN1 and DN2) of T cells (Fig. 1a, c), and DN cells expressed more CD117 than did DP and SP cells (Fig. 1b, d). CD117 expression was positively correlated with CD44 in thymocytes (Fig. 1e–h). CD44+CD117+ T cells could be also found in blood and lymph nodes (Fig. 11). CD4SP and CD8SP cells contain many CD117+ cells, most of which are CD44+CD117+ cells. CD44+CD117+ cells have been used to label tumor stem cells [8, 10, 23, 28]. In the current study, CD117 and CD44 were used to label stem T cells and development stages were identified in the thymus.
Next, CTLA-4 expression was measured at different stages of T-cell development. CTLA-4 expression was higher in DN cells compared with SP and DP cells (Fig. 2a, b) and was positively correlated with CD25 and CD44 (Fig. 2c, d). CTLA-4 and Foxp3 overlapped in DN and CD4+cells (Fig. 3). CD44+CD117+ cells and nTreg cells are two different populations of cells, although parts of them overlap (Fig. 12). CD44+CD117+ cells had more CTLA-4 expression (Figs. 4 and 5). CD44 expression was positively correlated with CTLA-4 in CD117+ thymocytes (Fig. 6). CD117+CD44+ T cells are stem T cells that expressed more Nanog than other T cells in thymocytes (Fig. 7) and have the ability to self-renew (Fig. 10), confirming that CD44+CD117+ stem T cells suppressed T-cell proliferation and CD4+CD117+CD44+ thymus cells had a potent suppressive function (Fig. 8). Because there is more CTLA-4 expression in stem T cells, we studied the effect of CTLA-4 on stem T cells. CD117+ stem T cells were incubated with CTLA-4 blocking antibodies to confirm whether blocking CTLA-4 abrogated the suppressive mechanism of CD117+ stem T cells. CD44+CD117+ stem T cells and T cells proliferated, and when cultured separately this proliferation ceased. Blocking CTLA-4 abrogated the suppressive function of CD44+CD117+ stem T cells, and induced T-cell proliferation (Fig. 9). The suppressive activity of stem T cells was partly aborted by anti-CTLA-4 antibodies and the ability of CD44+CD117+ cells to inhibit T cells was diminished in a Transwell plate, suggesting that this inhibition depends on cell-to-cell contact.
Stem T cells in the immune system differ from other stem cells with respect to development and why this is true is unclear. Research for study the effects of MSCs on lymphocyte proliferation and their immune modulation has provided invaluable data [29]. Stem T cells are analogous to MSCs; however, they highly express abundant CTLA-4 and have a greater ability to modulate the immune system. How stem T cells interact with, and regulate, T cells, B cells, and natural killer cells, DCs, or neutrophils is unclear, as is whether stem T cells alter lymphocyte phenotype or induce development of T regulatory cells in vitro and/or in vivo, or whether stem T cells are anti-inflammatory in vivo. Tumor cells are associated with tumor stem cells [30], but CD44+CD117+ stem T cells regulate T-cell proliferation. The difference between tumor stem cells and CD44+CD117+ stem T cells is worthy of exploration.
CD44+CD117+ stem T-cell development in the thymus and spleen. We sought to identify various gene/protein expression changes at different stages of T-cell development. Many changes were identified, which are contradictory to the current theory of T-cell development. In this study, peak CD117 expression occurred in DN and CD117 cells and could still be detected in SP T cells in the thymus and spleen (Fig. 13). Thus, studies are needed to explain this phenomenon.
Fig. 13.
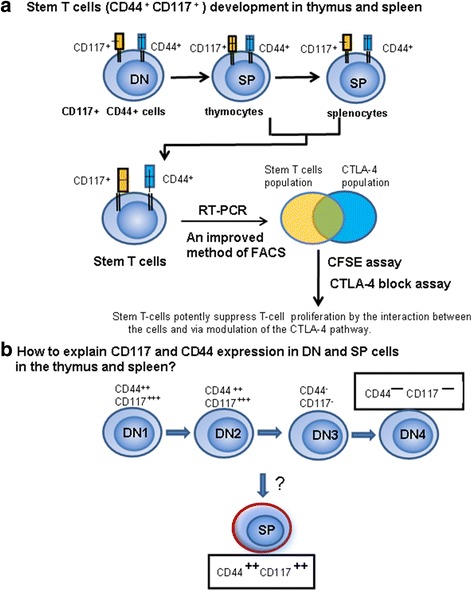
Stem T-cell development in the thymus and spleen. a Methods of stem T-cell development. b Important question of whether there is a contradictory problem with traditional DN T-cell development theory. DN double-negative, SP single-positive
Previous work indicates that CD117 cells are present in DN1 and DN2, but not in SP cells [31]. We observed that CD117 cells such as NK T cells and Tregs develop in the thymus, and do not follow the theory of T-cell or DN T-cell development. CD117 cell development may begin from CD44+ DN cells and proceed directly to CD44+ SP cells. It is unclear why NK T cells, Tregs and CD44+CD117+ cells are T cells in phenotype but do not share the T-cell development pathway. We propose that CD44+CD117+ T cells may not have been derived from a common precursor as T cells, but rather pass through a different pathway.
Conclusions
In summary, CD44+CD117+ T-cell development occurred in the thymus and spleen. CD44+CD117+ T cells expressed abundant CTLA-4 and suppressed T-cell proliferation. Blocking CTLA-4 reduced the suppression of T-cell proliferation. CD44+CD117+ T cells are stem cells that expressed more Nanog and CTLA-4 and potently suppressed T-cell proliferation via modulating the CTLA-4 pathway. Finally, CD44+CD117+ T cells may have developed from CD4–CD8–CD44+CD25– T progenitor cells, without the involvement of DN3, DN4, and CD4+CD8+ (DP) stages in the thymus.
Acknowledgements
The authors gratefully acknowledge The Xi'an Jiaotong University Cardiovascular Research Center for providing their flow cytometry services.
Funding
This study was supported by The First Affiliated Hospital, Xi'an Jiaotong University and Natural Science Foundation of Shaanxi Province of China (No. 2016JM8039).
Availability of data and materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Authors’ contributions
TL contributed to the design of the study and writing the manuscript, performed experiments, and analyzed data. YW, ZSH, WG, GL, BYS, and EQL participated in the experimental design and manuscript preparation, performed some experiments, and analyzed data. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All protocols were approved by the Institutional Animal Care and Use Committee at Xi’an Jiaotong University (Permit Number: 2016-278). All surgeries and sacrifices were performed under chloral hydrate anesthesia. Every effort was made to minimize animal suffering.
Abbreviations
- CTLA-4
Cytotoxic T lymphocyte antigen-4
- DN
Double-negative CD4–CD8–
- DN1
Double-negative CD44+CD25–
- DN2
Double-negative CD44+CD25+
- DN3
Double-negative CD44–CD25+
- DN4
Double-negative CD44–CD25–
- DP
Double-positive CD4+CD8+
- SP
Single-positive CD4 or CD8
Contributor Information
Yang Wei, Email: weizxche@163.com.
Zhansheng Hu, Email: icuhzs@163.com.
Wen Gu, Email: desaix@stu.xjtu.edu.cn.
Gang Liu, Email: gangliu_guangyi@163.com.
Bingyin Shi, Email: shibingy@126.com.
Enqi Liu, Email: Liuenqi@mail.xjtu.edu.cn.
Tie Liu, Phone: +86-29-85459925, Email: xiantieliu@mail.xjtu.edu.cn.
References
- 1.Ritter MA, Boyd RL. Development in the thymus. it takes two to tango. Immunol Today. 1993;14:462–9. doi: 10.1016/0167-5699(93)90250-O. [DOI] [PubMed] [Google Scholar]
- 2.Anderson G, Pongracz J, Parnell S, Jenkinson EJ. Notch ligand-bearing thymic epithelial cells initiate and sustain Notch signaling in thymocytes independently of T cell receptor signaling. Eur J Immunol. 2001;31:3349–54. doi: 10.1002/1521-4141(200111)31:11<3349::AID-IMMU3349>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Shortman K. Cellular aspects of early T-cell development. Curr Opin Immunol. 1992;4:140–6. doi: 10.1016/0952-7915(92)90003-W. [DOI] [PubMed] [Google Scholar]
- 4.Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nat Rev Immunol. 2002;2:888–97. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3–CD4–CD8– triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–52. [PubMed] [Google Scholar]
- 6.Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J, Ruzek M, Tubo R, Kaplan J, Lodie T. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clin Exp Immunol. 2010;161:176–86. doi: 10.1111/j.1365-2249.2010.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, et al. Interferon-gamma-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776–86. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Balch C, Chan MW, Lai HC, Matei D, Schilder JM, Yan PS, Huang TH, Nephew KP. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–20. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hombach-Klonisch S, Panigrahi S, Rashedi I, Seifert A, Alberti E, Pocar P, Kurpisz M, Schulze-Osthoff K, Mackiewicz A, Los M. Adult stem cells and their trans-differentiation potential—perspectives and therapeutic applications. J Mol Med (Berl) 2008;86:1301–14. doi: 10.1007/s00109-008-0383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 11.Qiu FH, Ray P, Brown K, Barker PE, Jhanwar S, Ruddle FH, Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family—oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003–11. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-kit. a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–51. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray P, Krishnamoorthy N, Ray A. Emerging functions of c-kit and its ligand stem cell factor in dendritic cells: regulators of T cell differentiation. Cell Cycle. 2008;7:2826–32. doi: 10.4161/cc.7.18.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plas DR, Thompson CB. Akt-dependent transformation. there is more to growth than just surviving. Oncogene. 2005;24:7435–42. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 15.Keshet E, Lyman SD, Williams DE, Anderson DM, Jenkins NA, Copeland NG, Parada LF. Embryonic RNA expression patterns of the c-kit receptor and its cognate ligand suggest multiple functional roles in mouse development. EMBO J. 1991;10:2425–35. doi: 10.1002/j.1460-2075.1991.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heissig B, Werb Z, Rafii S, Hattori K. Role of c-kit/Kit ligand signaling in regulating vasculogenesis. Thromb Haemost. 2003;90:570–6. doi: 10.1160/TH03-03-0188. [DOI] [PubMed] [Google Scholar]
- 17.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112(4):946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aifantis I, Mandal M, Sawai K, Ferrando A, Vilimas T. Regulation of T-cell progenitor survival and cell-cycle entry by the pre-T-cell receptor. Immunol Rev. 2006;209:159–69. doi: 10.1111/j.0105-2896.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 19.Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–42. doi: 10.1016/S0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- 20.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 21.Gough SC, Walker LS, Sansom DM. CTLA4 gene polymorphism and autoimmunity. Immunol Rev. 2005;204:102–15. doi: 10.1111/j.0105-2896.2005.00249.x. [DOI] [PubMed] [Google Scholar]
- 22.Ise W, Kohyama M, Nutsch KM, Lee HM, Suri A, Unanue ER, Murphy TL, Murphy KM. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat Immunol. 2010;11:129–35. doi: 10.1038/ni.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–63. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A. 2010;107:1524–8. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tung JW, Heydari K, Tirouvanziam R, Sahaf B, Parks DR, Herzenberg LA. Modern flow cytometry: a practical approach. Clin Lab Med. 2007;27:453–68. v. [DOI] [PMC free article] [PubMed]
- 26.Liu G, Li Z, Wei Y, Lin Y, Yang C, Liu T. Direct detection of FoxP3 expression in thymic double-negative CD4–CD8- cells by flow cytometry. Sci Rep. 2014;4:5781. doi: 10.1038/srep05781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Santo JP, Radtke F, Rodewald HR. To be or not to be a pro-T? Curr Opin Immunol. 2000;12:159–65. doi: 10.1016/S0952-7915(99)00066-7. [DOI] [PubMed] [Google Scholar]
- 28.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328–37. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 29.Carrade DD, Borjesson DL. Immunomodulation by mesenchymal stem cells in veterinary species. Comp Med. 2013;63:207–17. [PMC free article] [PubMed] [Google Scholar]
- 30.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Bhandoola A, von Boehmer H, Petrie HT, Zúñiga-Pflücker JC. Commitment and developmental potential of extrathymic and intrathymic T cell precursors: plenty to choose from. Immunity. 2007;26:678–89. doi: 10.1016/j.immuni.2007.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.


