The composition of the plasma membrane is highly complex and so are the cellular functions it conveys. Among those, the plasma membrane plays a key role in isolating the intracellular and extracellular environments. Nevertheless, communication between these 2 compartments has still to be maintained. If transmembrane receptors have long been known to integrate communicative functions by transducing various signals from outside, how these tasks are embodied with the plasma membrane dynamics and nanoscale organization remains an open question. Twenty years ago, the concept of “lipid rafts” was proposed1 as an explanation for the observed heterogeneous organization of plasma membrane constituents. For long, the only available methods to study lipid domains were limited to detergent-based biochemical isolation, which certainly brought a wealth of novel information but was also prone to artifacts. The situation dramatically changed with newly developed light microscopy techniques that break diffraction limit and follow single molecules. These technical improvements led to a recent refinement of the initial "raft" concept with the description of rafts as highly dynamic nanoscale lipid-protein assemblies that are endowed with the possibility to regulate the spatio-temporal sorting and/or activation of membrane receptors.
N-glycosylation is one of the most common co-translational modifications associated with several physiological and pathological processes, including membrane trafficking, signal transduction, cell growth and cancer. The synthesis of N-linked glycan starts in the endoplasmic reticulum, and continues during export to the plasma membrane where glycoproteins are either secreted or embedded as transmembrane proteins. Whereas most glycosylation genetic disorders are associated with a loss of glycosylation, recent studies show that the frequency of gain-of- N-glycosylation has been underestimated and that it may cause a more deleterious effect on protein function. Several patients with Mendelian susceptibility to mycobacterial diseases (MSMD) were shown to present a common mutation resulting in the amino acid substitution T168N which create an extra N-glycosylation site in the interferon γ receptor subunit IFN-γR2.2 This inherited modification of the IFN-γR2 subunit caused a complete lack of gene response to IFN-γ, a key cytokine for host defense against infection and disease.
To decipher the molecular basis underlying the defects of IFN-γ responsiveness in these patients, we investigated the dynamics of IFN-γR diffusion at the surface of living cells.3 Individual IFN-γR complexes were followed by spot variation fluorescence correlation spectroscopy (svFCS). svFCS is a powerful microscopy technique with high spatiotemporal resolution which allows not only to monitor the long-range mobility of receptors at the plasma membrane but also to determine the type of lateral diffusion whether it is free diffusion, dynamic partitioning into specific membrane nanodomains, or trapping into a cytoskeleton meshwork.4 Thus, we could monitor the dynamic diffusion of the IFN-γR2 subunit within sphingolipid and cholesterol nanodomains. In contrast, the diffusion of the T168N mutated form of the IFN-γR2 was confined into actin-based nanodomains (Fig. 1). IFN-γ stimulation triggered IFN-γR2 trapping in the actin cytoskeleton meshwork whereas the mutated receptor lateral diffusion remained unchanged. The next question was why the T168N IFN-γR2 mis-partitioned in actin nanodomains? Proteomics revealed that the additional N-glycosylation renders the IFN-γR2 mutant more affine for galectin-1 and -3 (Gal1 & Gal3), extracellular lectins that specifically bind to N-acetyllactosamine glycans. Indeed, when Gal1 and Gal3 were removed, the lateral diffusion of IFN-γR2 T168N at steady state in lipid nanodomains was restored together with the confinement in the cytoskeleton meshwork after IFN-γ stimulation. Galectins removal restored also the efficient activation of JAK/STAT signaling and the biological activity of IFN-γ in patient cells. Conversely, incubating the wild type IFN-γR with excess of galectins resulted also in receptor mis-partitioning and reproduced the IFN-γR T168N mutant phenotype.
Figure 1.
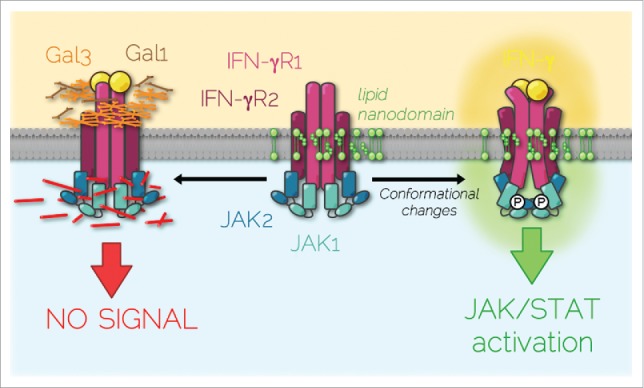
IFN-γR partitioned diffusion at the plasma membrane. At steady-state, IFN-γR is dynamically diffusing within plasma membrane sphingolipid/cholesterol nanodomains that are necessary for receptor subunits and associated JAK kinase conformational changes induced by IFN-γ binding. These molecular rearrangements lead to signal transduction. On the contrary, excess galectins (Gal1 & Gal3) binding on IFN-γR2 subunit caused by the gain of glycosylation mutation (T168N) or by increased extracellular galectin concentration, segregates IFN-γR complex in actin nanodomains, which prevents JAK/STAT signaling pathway activation.
It is believed that ligand-induced receptor complex rearrangements condition JAK/STAT signaling initiation and therefore the cell response to the cytokine. As recently shown for the growth hormone receptor, JAK kinases that are associated to the receptor can activate in trans by sliding the pseudo-kinase domain away from the kinase domain of the corresponding JAK partner leading to trans-activation.5 Our study further established that the lipid environment was necessary for the IFN-γR complex conformational changes required for JAK activation (Fig. 1). These results raise a pivotal question. What drives the partitioning of the IFN-γR complex in lipid nanodomains? In this context, it is interesting that a direct protein-lipid interaction between the IFN-γR1 subunit and SM18 sphingomyelin species was recently reported.6 Whether this is also true for IFN-γR2, and whether both subunits can bind cholesterol has not been unexplored. It is likely that these potential receptor-lipid interactions may condition the conformational changes observed after ligand binding.
Our results demonstrate the crucial role that lipid nanodomain dynamics play in the partitioning of IFN-γR complex and the initiation of JAK/STAT signaling at the plasma membrane. They reveal also the unsuspected key role of receptor glycosylation and galectin binding in these processes. The number of identified protein N-glycosylation congenital disorders has largely increased during the last 10 years. Galectins can be involved in many pathologies including, infection and cancer.7 In this context, a better understanding of galectin/N-glycosylated protein interaction consequences may provide new tools for potential therapeutical treatments.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by institutional grants from the Institut Curie, INSERM, CNRS, and by specific grants from Agence Nationale de la Recherche (ANR NANOSTAT-15-CE11-0025-01 to C.L.), Institut National du Cancer (INCa PLBIO12-203 to C.L.), and Marie Curie Actions-Networks for Initial Training (FP7-PEOPLE-2010-ITN to C.L.). C.M.B. was supported by a postdoctoral fellowship from Ligue Nationale contre le Cancer. The Lamaze team received support under the program “Investissements d'Avenir” launched by the French Government and implemented by ANR with the references Labex Cel- TisPhyBio ANR-10-LBX-0038 part of the IDEX Idex PSL ANR-10-IDEX-0001-02 PSL.
References
- [1].Simons K, Ikonen E. Functional rafts in cell membranes. Nature 1997; 387:569-72; PMID:9177342; http://dx.doi.org/ 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- [2].Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, Boisson-Dupuis S, Alcais A, Filipe-Santos O, Bustamante J, et al.. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat Genet 2005; 37:692-700; PMID:15924140; http://dx.doi.org/ 10.1038/ng1581 [DOI] [PubMed] [Google Scholar]
- [3].Blouin CM, Hamon Y, Gonnord P, Boularan C, Kagan J, Viaris de Lesegno C, Ruez R, Mailfert S, Bertaux N, Loew D, et al.. Glycosylation-Dependent IFN-γR partitioning in lipid and actin nanodomains is Critical for JAK Activation. Cell 2016; 166:920-34; PMID:27499022; http://dx.doi.org/ 10.1016/j.cell.2016.07.003 [DOI] [PubMed] [Google Scholar]
- [4].Lasserre R, Guo XJ, Conchonaud F, Hamon Y, Hawchar O, Bernard AM, Soudja SM, Lenne PF, Rigneault H, Olive D, et al.. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nat Chem Biol 2008; 4:538-47; PMID:18641634; http://dx.doi.org/ 10.1038/nchembio.103 [DOI] [PubMed] [Google Scholar]
- [5].Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, et al.. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science 2014; 344:1249783; PMID:24833397; http://dx.doi.org/ 10.1126/science.1249783 [DOI] [PubMed] [Google Scholar]
- [6].Contreras FX, Ernst AM, Haberkant P, Björkholm P, Lindahl E, Gönen B, Tischer C, Elofsson A, von Heijne G, Thiele C, et al.. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012; 481:525-9; PMID:22230960; http://dx.doi.org/ 10.1038/nature10742 [DOI] [PubMed] [Google Scholar]
- [7].Rabinovich GA, Conejo-García JR. Shaping the immune landscape in cancer by galectin-driven regulatory pathways. J Mol Biol 2016; 428:3266-81; PMID:27038510; http://dx.doi.org/ 10.1016/j.jmb.2016.03.021 [DOI] [PubMed] [Google Scholar]


