Primary microcephaly is a genetically heterogeneous neurodevelopmental disorder characterized by a severe reduction in brain growth, accompanied by variable degrees of intellectual, language and motor-skill disability.1
Mutations in 16 loci (MCPH1–MCPH16) have been described as associated with MCPH.1 MCPH gene products are shown to be highly expressed in neuroepithelial or neuroprogenitor cells during early brain development and found to be implicated in the biogenesis and function of the centrosome, an organelle intimately connected with cell division, suggesting that proper cell cycle control could play an important role in neurogenesis.1
Initial studies focused on the roles of MCPH-associated proteins at the spindle pole in determining mitotic-spindle orientation. This process may play an important role to regulate the delicate balance between proliferation and differentiation that underlies normal brain development. Conceivably, any perturbation that upsets the balance between symmetric and asymmetric division can drastically reduce the number of neuroprogenitor and neuronal cells, leading to reduced brain size. Although such a mechanism is appealing, additional mechanisms, including cell proliferation defects and enhanced cell death/apoptosis can also impair brain development and contribute to the development of MCPH.2
Very recent studies described families affected by recessive MCPH caused by novel pathogenic variants in CIT (MIM: 605629), encoding citron rho-interacting kinase (CITK).3,4 CITK is a conserved protein that localizes at the cleavage furrow and at the midbody of mitotic cells where it functions in cytokinesis, the final step of mitosis.5
We reported previously that midbody microtubules are a primary target of CITK in abscission control and that dividing neuronal precursors become progressively more sensitive to CITK loss as they commit to terminal differentiation. Indeed, the sensitivity of cytokinesis to CITK loss is increased by the expression of TUBB3, a β-tubulin isoform expressed in the CITK-dependent tissues and CITK stabilizes midbody microtubules by increasing the phosphorylation of TUBB3.6
Our recent work provides new insight on the role of CITK in microcephaly.7 Indeed, we have shown that, in addition to its well documented role in cytokinesis, CITK is functionally implicated in the control of spindle orientation. This conclusion is based on the phenotypes observed in CITK-depleted HeLa cells, in the developing neocortex of CITK-knockout mice and also in 2 different Drosophila mutant lines, underscoring the physiological and phylogenetic relevance of this function.
CITK physically interacts with ASPM (abnormal spindle-like microcephaly-associated, MCPH5), that is the most frequently mutated gene in MCPH. ASPM localizes at the spindle poles and midbody and controls spindle orientation and cytokinesis from insects to mammals.
We found that the 2 proteins are tightly co-localized not only at the midbody during cytokinesis, but also at earlier mitotic stages. A pool of CITK is indeed stably associated with the spindle and the spindle poles during metaphase. Remarkably, this localization is dependent on ASPM, while the localization of ASPM is not dependent on CITK. Even more importantly, both CITK and ASPM knockdown do not disturb the localization of the proteins required to anchor astral microtubules (MT) to the membrane, but significantly alter the organization of astral MT. We also demonstrated that MT stability is crucial for the spindle orientation phenotypes elicited by depletion of both proteins, as they are rescued by low doses of MT-stabilizing agent. Our observations strongly suggest that ASPM and CITK regulate spindle orientation primarily by affecting the dynamics of astral MT. In this function, CITK is most likely a downstream mediator of ASPM, because its recruitment to the spindle is ASPM-dependent, while CITK knockdown does not affect ASPM localization, and because the overexpression of CITK can rescue the effects of ASPM knockdown. Therefore, we propose that ASPM promotes the correct organization of astral MT by recruiting CITK to them, thus allowing the anchorage of the spindle to the cell cortex required for a proper orientation (Fig. 1).7
Figure 1.
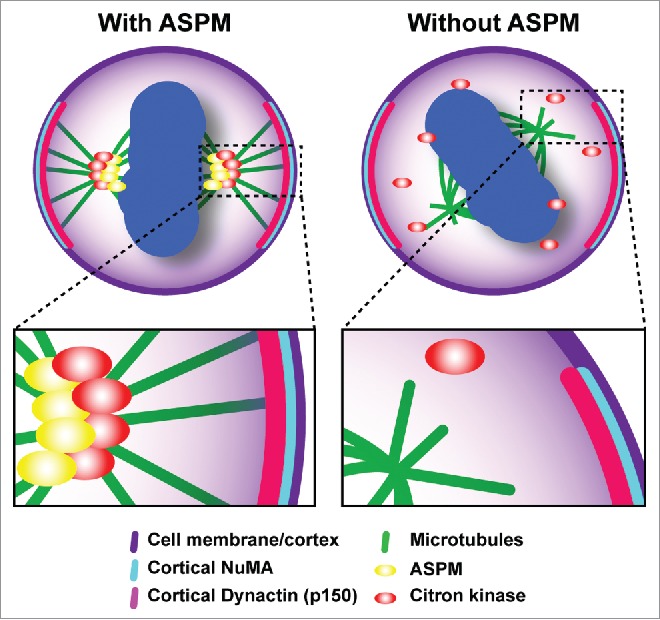
CITK regulates mitotic spindle orientation. The cytokinesis regulator CITK is recruited by the microcephaly gene ASPM to the mitotic spindle of neural progenitor cells, where it promotes the correct organization of astral MT, thus allowing the anchorage of the spindle to the cell cortex required for a proper orientation.7
Moreover, the kinase activity of CITK is essential for this function, since the expression of mutants completely lacking the kinase domain or mutated in the ATP-binding pocket does not rescue the spindle orientation phenotype.7
The catalytic activity of CITK is required also for cytokinesis. Indeed, Li and colleagues described families affected by recessive MCPH caused by biallelic missense mutations of CITK leading to undetectable kinase activity. These mutations were remarkable in that they did not alter mRNA expression or CITK localization.3
These findings highlight the evolutionarily conserved function of CITK in neurodevelopment and the disproportionate sensitivity of the neuraxis to pathogenic variants in this gene. Moreover, these data support the hypothesis that both cytokinesis failure and subsequent apoptosis and mitotic spindle orientation defects in neuronal precursors could be underlying mechanisms for the genetic forms of MCPH. Deeper exploration of the role of CITK in neurogenesis could help to better understand to what extent does each of these phenotypes (spindle orientation/cytokinesis failure) contribute to brain development and possible functional links between these processes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Faheem M, Naseer MI, Rasool M, Chaudhary AG, Kumosani TA, Ilyas AM, Pushparaj PN, Ahmed F, Algahtani HA, Al-Qahtani MH, et al.. Molecular genetics of human primary microcephaly: an overview. BMC Med Genomics [Internet] 2015; 8(Suppl 1):S4; PMID:25951892; http://dx.doi.org/ 10.1186/1755-8794-8-S1-S4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gilmore EC, Walsh CA. Genetic causes of microcephaly and lessons for neuronal development. Wiley Interdiscip Rev Dev Biol 2013; 2:461-78; PMID:24014418; http://dx.doi.org/ 10.1002/wdev.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li H, Bielas SL, Zaki MS, Ismail S, Farfara D, Um K, Rosti RO, Scott EC, Tu S, Chi NC, et al.. Biallelic Mutations in Citron Kinase Link Mitotic Cytokinesis to Human Primary Microcephaly. Am J Hum Genet [Internet] 2016; 99(2):501-10; PMID:27453578; http://dx.doi.org/ 10.1016/j.ajhg.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Harding BN, Moccia A, Drunat S, Soukarieh O, Tubeuf H, Chitty LS, Verloes A, Gressens P, El Ghouzzi V, Joriot S, et al.. Mutations in Citron Kinase Cause Recessive Microlissencephaly with Multinucleated Neurons. Am J Hum Genet [Internet] 2016; 99:511-20; PMID:27453579; http://dx.doi.org/ 10.1016/j.ajhg.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gai M, Camera P, Dema A, Bianchi F, Berto G, Scarpa E, Germena G, Di Cunto F. Citron kinase controls abscission through RhoA and anillin. Mol Biol Cell [Internet] 2011; 22:3768-78; PMID:21849473; http://dx.doi.org/ 10.1091/mbc.E10-12-0952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sgr∫ F, Bianchi FT, Falcone M, Pallavicini G, Gai M, Chiotto AMA, Berto GE, Turco E, Chang YJ, Huttner WB, et al.. Tissue-specific control of midbody microtubule stability by Citron kinase through modulation of TUBB3 phosphorylation. Cell Death Differ [Internet] 2015; 23(5):801-13; PMID:26586574; http://dx.doi.org/ 10.1038/cdd.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gai M, Bianchi FT, Vagnoni C, Vernì F, Bonaccorsi S, Pasquero S, Berto GE, Sgr∫ F, Chiotto AM, Annaratone L, et al.. ASPM and CITK regulate spindle orientation by affecting the dynamics of astral microtubules. EMBO Rep [Internet] 2016; 17(10):1396-409. PMID:27562601; http://dx.doi.org/ 10.15252/embr.201541823 [DOI] [PMC free article] [PubMed] [Google Scholar]


