Abstract
Background
Diagnosis of chronic Hepatitis C Virus (HCV) infection requires both a positive HCV antibody screen and confirmatory nucleic acid test (NAT). HCV core antigen (HCVcAg) is a potential alternative to NAT.
Purpose
This systematic review evaluated the accuracy of diagnosis of active HCV infection among adults and children for five HCVcAg tests compared to NAT.
Data Sources
EMBASE, PubMed, Web of Science, Scopus, and Cochrane from 1990 through March 31, 2016.
Study Selection
Cohort, cross-sectional, and randomized controlled trials were included without language restriction
Data Extraction
Two independent reviewers extracted data and assessed quality using an adapted Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Data Synthesis
44 studies evaluated 5 index tests. Studies for the ARCHITECT had the highest quality, while those for Ortho ELISA were the lowest. From bivariate analyses, the sensitivity and specificity with 95% CI were: ARCHITECT 93.4% (90.1, 96.4) and 98.8% (97.4, 99.5), Ortho ELISA 93.2% (81.6, 97.7) and 99.2% (87.9, 100), and Hunan Jynda 59.5% (46.0, 71.7) and 82.9% (58.6, 94.3). Insufficient data were available for a meta-analysis for Lumipulse and Lumispot. In three quantitative studies using ARCHITECT, HCVcAg correlated closely with HCV RNA above 3000 IU/mL.
Limitations
There was insufficient data on covariates such as HIV or HBV status for sub-group analyses. Few studies reported genotypes of isolates and there were scant data for genotypes 4, 5, and 6. Most studies were conducted in high resource settings within reference laboratories.
Conclusions
HCVcAg assays with signal amplification have high sensitivity, high specificity, and good correlation with HCV RNA above 3000 IU/mL. HCVcAg assays have the potential to replace NAT in high HCV prevalence settings.
INTRODUCTION
There are 130–150 million people worldwide infected with chronic Hepatitis C Virus (HCV) and approximately 75% of all cases occur in low to middle income countries (LMICs)(1, 2). The development of direct acting antiviral therapy (DAAs) now allows for safe and effective curative treatment, but treatment is the final step in a long cascade of care that requires screening, confirmation, notification of results, and linkage to care (3, 4). Currently, HCV diagnosis is a two-step process that starts with screening for exposure with an HCV antibody (HCVAb) assay, followed by nucleic acid testing (NAT) for those with reactive HCVAb to confirm active viremia. Among those who acquire a primary infection, 15–50% will spontaneously clear the virus within the first 2–6 months and remain HCVAb positive though they are not actively infected and do not require treatment (5). The diagnostic process is designed to be cost-efficient with a low cost screening test followed by targeted testing with the more expensive NAT. In LMICs where implementation of a complex algorithm is often not feasible and diagnostic capacity is low, less than 1% of patients are aware of their infection (6). Additionally, a significant proportion of HCVAb positive patients fail to have a diagnostic NAT and are lost to follow-up (7). The two-step diagnostic process represents a major bottleneck to the HCV cascade of care that needs to be addressed in order to achieve the ambitious elimination strategy proposed by the World Health Organization (WHO)(8).
HCV core antigen (HCVcAg) testing is a potential replacement for NAT. The HCVcAg forms the internal capsid, which is highly conserved and antigenic (9, 10). During viral assembly, nucleocapsid peptides 22 (p22) are released into plasma(11) and can be detected earlier than antibodies and throughout the course of infection (12). Currently, five tests for HCVcAg detection are commercially available: 1) the Abbott ARCHITECT HCV Ag assay, an automated chemiluminescent microparticle immunoassay (CMIA) 2) the Fujirebio Lumipulse Ortho HCV Ag test, and 3) the EIKEN Lumispot HCV Ag, which are similar automated chemiluminescent enzyme immunoassays (CLEIA) available in Japan and China, 4) the Hunan Jynda Bioengineering Group HCVcAg ELISA and 5) the Ortho ELISA-Ag, which are both enzyme-linked immunosorbent assays (ELISA).
While all current HCVcAg tests require laboratory capacity, the development of a highly sensitive point of care (POC) platform is feasible and likely possible at a lower cost than NAT POC tests. Such a test has been defined as the highest priority target product profile in a global stakeholder consultation process(13). As such, tests targeting HCVcAg could be attractive as a single step diagnosis for chronic HCV infection in high prevalence settings, streamlining the HCV cascade of care and reducing loss to follow-up. This WHO-commissioned systematic review to inform forthcoming WHO guidelines on hepatitis testing evaluated the accuracy of diagnosis of active HCV infection among adults and children for five commercially available HCVcAg tests compared to NAT.
METHODS
We performed a systematic review of HCV diagnostics literature, extracted data from selected studies, and conducted a bivariate meta-analysis of the test characteristics of HCVcAg as a diagnostic test for HCV infection. We employed standard methods for systematic reviews and meta-analyses of diagnostic tests (14–18), including preparation of an a priori protocol (see Data Supplement) for the literature search, article selection, data extraction, quality assessment and analysis.
Data Sources and Searches
We searched EMBASE, PubMed, Scopus, Web of Science, and Cochrane, for citations related to HCVcAg screening and diagnosis published up until March 31, 2016. We did not restrict the search by language, and terms were selected under guidance of medical librarians. The search strategy included terms related to HCV, antigen, and nucleic acid amplification. Please refer to the Data Supplement Section I for specific search strategies and number of studies retrieved from each database.
Two authors (JMF and TMT) independently assessed titles and abstracts identified by the literature search to select eligible studies (Screen 1). Citations identified by either review author during screen 1 were selected for full text-review. The same two authors then independently assessed the full text articles for inclusion using the predefined inclusion and exclusion criteria (screen 2). Discrepancies were resolved by discussion between the review authors, and when needed, by the decision of a third author (CMD).
Study Selection
Study inclusion criteria were the following: 1) case-control, cross-sectional, cohort or randomized trial designs, 2) commercially available NAT as a reference test, 3) whole blood, plasma, or serum specimens, 4) at least 10 independent clinically collected samples. Studies performed using commercially prepared reference panel specimens were excluded. Studies published in abstract form only or presented as slides or posters were excluded.
We included papers that reported results from populations with any distribution of patient age, from any country, and in any screening setting (e.g. hospital based or community based). Although, primarily interested in test performance among those at risk for HCV and with known infection, we also included studies using healthy blood donor specimens. As the performance characteristics of NATs are very similar above 50 IU/mL, we accepted any of the following NAT techniques as the reference standard: polymerase chain reaction, branched-chain DNA, or transcription mediated amplification. Tests were classified as either qualitative or quantitative.
Data Extraction and Quality Assessment
Two authors (JMF and TMT) independently assessed all studies for inclusion in the systematic review, and extracted data on study methodology, characteristics and test accuracy using a standardized extraction form (available in Data Supplement Section II). Foreign language studies were translated and extracted by native speakers using the same extraction form. We crosschecked data points for 25% of the included studies. Disagreements between review authors were resolved by discussion or by a third reviewer (CMD). When elements for extraction were missing, we contacted the authors to request further data. Additionally, we requested individual specimen data to allow for a quantitative assessment of HCVcAg against HCV RNA. Studies without extractable sensitivity and specificity data were excluded if no further information was acquired after three attempts to contact the study authors.
Methodological quality of the included studies was assessed using a validated tool for Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (19). Details of the QUADAS-2 questions and interpretation are reported in the Data Supplement Section III.
Data Synthesis and Analysis
We defined HCVcAg sensitivity as the proportion of samples with a positive NAT that were also positive on HCVcAg testing. We defined specificity as the proportion of samples with negative NAT that were also negative on HCVcAg testing. Sensitivity and specificity were the primary outcome measures. Positive and negative likelihood ratios were calculated when pooled sensitivity and specificity data were available from meta-analysis. Indeterminate test results accounted for less than 1% for all index tests and were excluded from further analyses.
We constructed forest plots for each HCVcAg index test to visually assess heterogeneity by examining the confidence intervals of individual studies. We then used summary plots to examine the width of the prediction region with a wider prediction region suggesting more heterogeneity. When at least 4 studies with limited heterogeneity were available, we used a bivariate random-effects model and carried out meta-analyses using the ‘metandi’ command in STATA (20, 21).
When at least 4 studies provided sensitivity data only, a univariate random effects meta-analysis was performed on the sensitivities in order to use all the available data. Results from the univariate analyses (including all studies) were compared with the pooled estimates from the bivariate analyses where possible. Descriptive analyses were done for index tests with less than four studies and when substantial heterogeneity was evident from the inspection of the forest and summary plots.
Where quantitative data were available, a locally weighted regression smoother was used to visually assess the linearity of quantitative HCVcAg measured in fmol/L to HCV RNA measured in IU/mL(22). We identified outliers and performed descriptive statistics of these points. There was only sufficient quantitative data to assess the ARCHITECT assay.
We assessed for publication bias where there were more than 10 studies available for an index test. We generated funnel plots displaying the Youden Index (YI = Sensitivity + Specificity −1) versus the Standard Error (SE) for each study (18, 23). We additionally performed the trim and fill statistical assessment in STATA using the ‘metatrim’ command (24). Unpublished data were not included in this review.
Statistical analyses were performed using STATA (version 14; STATA Corporation, College Station, TX) and RStudio (version 0.99.467).
This systematic review was supported by grants from the National Institutes of Health: 5T32AI052074-10, 5R01DA031059-04, 1P30DA040500-01, and 5P30AI042853-18.
RESULTS
Study selection and characteristics
The systematic review identified 8508 citations, of which we reviewed 299 full text articles and identified 44 that met the a priori defined inclusion criteria (Data Supplement Figure 1).
Forty-four included studies utilized the five different HCVcAg assays, with one performing direct comparisons between three antigen tests. Four studies were translated from Mandarin(25–28), one from German(29), and two from Japanese (30, 31). Characteristics for each study are presented in Table 1.
Table 1.
Characteristics of included studies grouped alphabetically by index test type.
| Author, year | Country and income category | Study design | Study Pop | Age group | Number of subjects | Proportion with HIV infection | Proportion with HBV infection | Proportion female | Sample type | Sample condition |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbott ARCHITECT HCV Ag | ||||||||||
| Buket, 2014 | Kazakhstan (B) | Cohort | Broad | Adults | 115 | Unknown | Unknown | 56.5% | Serum | Unknown |
| Chevaliez, 2014 | France (A) | Cross-sectional | Broad | Adults | 514 | Unknown | Unknown | 36.6% | Serum | Unknown |
| Cresswell, 2015 | UK (A) | Cohort | Broad | Adults | 111 | 100% | Unknown | 5.4% | Serum | Unknown |
| Descamps, 2012 | France (A) | Cross-sectional | Broad | Adults | 22 | Unknown | Unknown | 40.1% | Serum | Frozen |
| Durante-Mangoni, 2013 | Italy (A) | Cohort | Broad | Adults | 114 | 0 % | 0% | 43% | Serum | Frozen |
| Duy Thong, 2015 | Thailand (B) | Cohort | Broad | Adults | 189 | 44.9% | 0% | 28.6% | Serum | Frozen |
| Ergünay, 2011 | Turkey (A) | Cohort | Broad | Mixed | 272 | Unknown | Unknown | Unknown | Serum | Frozen |
| Florea, 2014 | Romania (B) | Cross-sectional | Broad | Adults | 76 | 0% | 0% | 75% | Serum | Frozen |
| Garbuglia, 2014 | Italy (A) | Cohort | Broad | Adults | 292 | 100% | 3.8% | 25.9% | Serum | Frozen |
| Gu, 2014 | China (B) | Cross-sectional | Broad | Unknown | 304 | Unknown | Unknown | Unknown | Whole | Unknown |
| Hadziyannis, 2013 | Greece (A) | Cross-sectional | Broad | Unknown | 105 | Unknown | Unknown | Unknown | Serum | Frozen |
| Heidrich, 2014 | Germany (A) | Cohort | Broad | Adults | 596 | Unknown | Unknown | 43% | Serum | Unknown |
| Kadkhoda, 2014 | Canada (A) | Cross-sectional | Broad | Adults | 154 | 1.3% | 0% | 50% | Serum | Unknown |
| Kesli, 2011 | Turkey (A) | Cohort | Healthy | Adults | 212 | Unknown | Unknown | 57.5% | Serum | Unknown |
| Köroglu, 2012 | Turkey (A) | Cohort | Broad | Unknown | 32 | Unknown | Unknown | 45.5% | Serum | Unknown |
| Kuo, 2012 | Taiwan (A) | Cohort | Broad | Adults | 405 | Unknown | Unknown | 52.6% | Serum | Unknown |
| Li Cavoli, 2012 | Italy (A) | Cohort | Broad | Adults | 92 | 1.1% | 2.2% | 41.3% | Serum | Unknown |
| Mederacke. 2009 | Germany (A) | Cohort | Broad | Unknown | 118 | 0% | 0% | Unknown | Serum | Unknown |
| Mederacke, 2012 | Germany (A) | Cross-sectional | Broad | Unknown | 237 | 49.50% | 50.50% | Unknown | Serum | Unknown |
| Medici, 2011 | Italy, Spain (A) | Cross-sectional | Broad | Unknown | 1480 | Unknown | Unknown | 52.6% | Serum | Frozen |
| Medici, 2016 | Italy (A) | Cross-sectional | Broad | Adults | 188 | Unknown | Unknown | 44.25% | Serum | Unknown |
| Miedouge, 2010 | France (A) | Cohort | Broad | Unknown | 2850 | Unknown | Unknown | Unknown | Serum | Frozen |
| Mixson-Hayden | USA (A) | Cohort | Broad | Unknown | 551 | Unknown | Unknown | Unknown | Serum | Frozen |
| Murayama, 2012 | Japan (A) | Cross-sectional | Broad | Unknown | 80 | Unknown | Unknown | Unknown | Plasma | Frozen |
| Ottiger, 2013 | Switzerland (A) | Cross-sectional | Broad | Adults | 97 | 6% | 0% | 38.1% | Plasma | Frozen |
| Park, 2010 | South Korea (A) | Cohort | Broad | Adults | 282 | Unknown | Unknown | 49.3% | Serum | Unknown |
| Reyes-Méndez, 2014 | Mexico (B) | Cross-sectional | Broad | Unknown | 211 | Unknown | Unknown | Unknown | Serum | Unknown |
| Rouet, 2015 | Gabon (B) | Cross-sectional | Broad | Adults | 54 | 100.00% | Unknown | 70.1% | Plasma | Frozen |
| Russi, 2014 | Italy (A) | Cohort | Broad | Adults | 102 | 0% | 0% | 78.4% | Serum | Frozen |
| Tedder, 2013 | UK (A) | Cohort | Broad | Unknown | 54 | 0% | 0% | Unknown | Plasma | Frozen |
| van Helden, 2014 | Germany (A) | Cross-sectional | Broad | Unknown | 3558 | 4.40% | 6.60% | Unknown | Serum | Unknown |
| Vanhommerig. 2015 | Netherlands (A) | Cohort | Broad | Unknown | 93 | 100.00% | Unknown | 0% | Serum | Unknown |
| Vermehren, 2012 | Germany (A) | Cohort | Broad | Adults | 160 | 0% | 0% | 54% | Serum | Frozen |
| EIKEN Lumispot HCV Ag | ||||||||||
| Saito, 2003 | Japan (A) | Cross-sectional | Broad | Unknown | 155 | Unknown | Unknown | Unknown | Serum | Frozen |
| Murayama, 2012 | Japan (A) | Cross-sectional | Broad | Unknown | 80 | Unknown | Unknown | Unknown | Plasma | Frozen |
| Fujirebio Lumipulse Ortho HCV Ag | ||||||||||
| Murayama, 2012 | Japan (A) | Cross-sectional | Broad | Unknown | 80 | Unknown | Unknown | Unknown | Plasma | Frozen |
| Hunan Jynda Bioengineering Group HCV Core Ag ELISA | ||||||||||
| Lu, 2007 | China (B) | Cohort | Broad | Unknown | 191 | Unknown | Unknown | Unknown | Serum | Unknown |
| Ouyang, 2006 | China (B) | Cross-sectional | Broad | Unknown | 149 | Unknown | Unknown | Unknown | Serum | Unknown |
| Zhang, 2007 | China (B) | Cohort | Healthy | Unknown | 11 | Unknown | Unknown | Unknown | Serum | Frozen |
| Zhu, 2010 | China (B) | Cross-sectional | Broad | Mixed | 173 | Unknown | Unknown | Unknown | Serum | Unknown |
| Ortho ELISA-Ag | ||||||||||
| Agha, 2004 | Egypt, Japan, Uzbekistan (AB) | Cohort | Broad | Unknown | 246 | Unknown | Unknown | Unknown | Serum | Unknown |
| El-Sayed, 2004 | Egypt (B) | Cross-sectional | Broad | Unknown | 50 | Unknown | Unknown | Unknown | Serum | Frozen |
| Letowska, 2004 | Poland (A) | Cohort | Healthy | Unknown | 124 | Unknown | Unknown | Unknown | Serum | Unknown |
| Nübling, 2002 | USA (A) | Cohort | Broad | Unknown | 52 | Unknown | Unknown | Unknown | Plasma | Frozen |
| Ohta, 2004 | Japan (A) | Cross-sectional | Broad | Unknown | 225 | Unknown | Unknown | Unknown | Serum | Unknown |
| Okazaki, 2008 | Japan (A) | Cohort | Broad | Unknown | 300 | Unknown | Unknown | 50.3% | Serum | Unknown |
HCV = Hepatitis C Virus, Cat = category, Pop = population, HIV = Human Immunodeficiency Virus, HBV = Hepatitis B Virus, Ag = Antigen, Ab = Antibody, ELISA = enzyme linked immunosorbent assay, EIA = enzyme immunoassay, UK = United Kingdom, USA = United States of America, A = High-income countries, B = Middle-income countries, C = Low-income countries by World Bank List of Economies (July 2015).
Risk of Bias Assessment (QUADAS-2)
The overall risk of bias assessment for all included studies across each QUADAS domain is summarized in the Data Supplement Figure 2 and presented for each individual study by index test in the Data Supplement Figure 3. The quality of studies utilizing the ARCHITECT test was the highest. However, 15 of the 33 studies did not report on whether patients were recruited consecutively, and one study only included healthy blood donors (32). Quality among the six studies using the Ortho ELISA-Ag test was the lowest; two did not report patient selection methods (33, 34) and only one included healthy blood donors (35). Additionally, two studies performed convenience sampling (30, 31) and it was unclear in both studies whether the index test and reference tests were performed on the same sample or within 30 days from the same participant and whether the index test was performed in accordance with manufacturer recommendations. For the Hunan Jynda test, patient selection was unclear in one study (26), timing of index test and reference test was unclear in one study (27), and one study enrolled only healthy blood donors (36). For the Lumispot test, both studies had unclear participant selection (37, 38). The study that assessed Lumipulse also had unclear participant selection (38).
HCV Core Ag for Diagnosis of Active HCV Infection
Abbott ARCHITECT
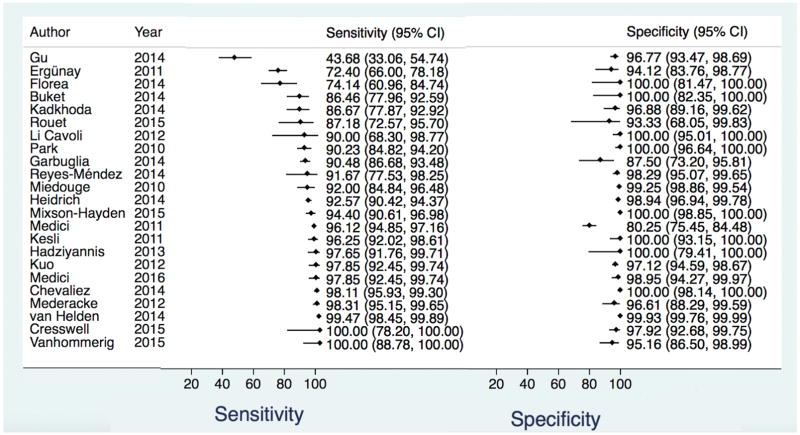
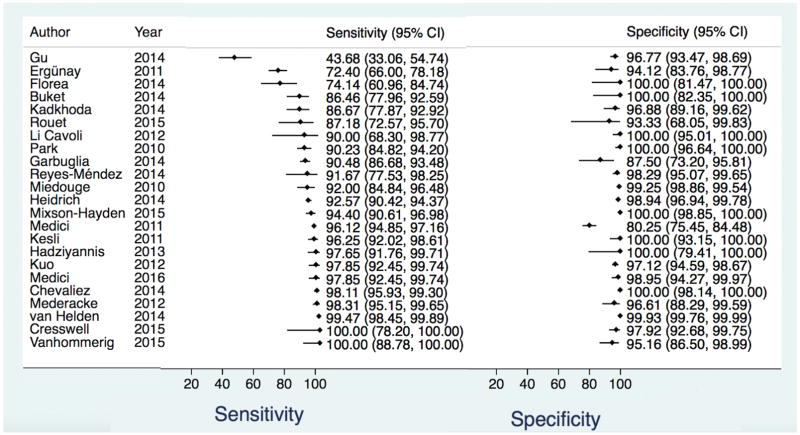
There were 33 studies assessing the Abbott ARCHITECT HCV Ag assay (11, 25, 29, 32, 38–66). All study designs were either cross-sectional or cohort, with a broad study population (included patients with HCV disease, and those susceptible to HCV disease) with the exception of one study that evaluated only healthy blood donors (32). Demographic data were available in 21 studies, the remainder utilized anonymous specimens and authors were unable to provide further information. HIV status was known in 16 of the studies with three including only HIV co-infected subjects (60, 62, 65). HBV status was known in 13 studies and all but four excluded patients with HBV co-infection. The highest prevalence of HBV co-infection (defined as hepatitis B surface Ag positivity) was 50.5% (54). Only one study included children (44).
There were 23 studies included in the bivariate analysis. The ten remaining studies did not have data to calculate specificity (11, 38, 41–43, 50, 53, 57, 61, 63) and were only included in the univariate pooled sensitivity estimate. For the bivariate analysis there were 12,670 total samples. The pooled sensitivity and specificity with 95% CI regardless of HCV Ab status were 93.4% (90.1, 96.4) and 98.8% (97.4, 99.5), respectively. The positive and negative likelihood ratios were 80.6 (36.4, 178.8), and 0.06 (0.04, 0.1), respectively (Table 2, Figure 1, Data Supplement Figure 4). The pooled sensitivity estimate from univariate analysis including the ten additional studies (total of 13,638 samples) was similar to that of the bivariate analysis: 94.3% (92.8, 95.9) (Table 2, Data Supplement Figure 5) though higher among the 10 studies when evaluated alone: 99% (97.8, 100). Among 16 studies with known HCV Ab positive samples, the sensitivity was 92.5% (86.9, 95.8) and specificity 97.8% (94.7, 99.1) (Table 2, Data Supplement Figure 6). From five studies that analyzed HCV Ab negative samples in the acute/pre-seroconversion phase, the pooled sensitivity was lower 92.3% (3.7, 99.9) with a wide confidence interval. The specificity among antibody-negatives remained high: 98.8% (97.3, 99.4) (Table 2, Data Supplement Figure 7).
Table 2.
Diagnostic accuracy by HCV core antigen (HCVcAg) index test type for diagnosis of active HCV infection compared to nucleic acid testing as the reference standard. Results from bivariate, univariate, range of studies, and single studies are all reported.
| Index Test | HCV Ab Status | # Studies (# Samples) | Sensitivity 95% CI | Specificity 95% CI | Positive LR 95% CI | Negative LR 95% CI |
|---|---|---|---|---|---|---|
| Abbott ARCHITECT1 | All | 23 (12,670) | 93.4%1 (90.1, 96.5) | 98.8%1 (97.4, 99.5) | 80.6 (36.4, 178.8) | 0.06 (0.04, 0.1) |
| Abbott ARCHITECT2 | All | 33 (13,638) | 94.3%2 (92.8, 95.9) | ND | NA | NA |
| Abbott ARCHITECT1 | Known Ab Positive | 16 (5,246) | 92.5%1 (86.9, 95.8) | 97.8%1 (94.7, 99.1) | 42 (16.4, 106.4) | 0.05 (0.03, 0.08) |
| Abbott ARCHITECT2 | Known Ab positive | 27 (6,189) | 93.4%2 (91.4, 95.4) | ND | NA | NA |
| Abbott ARCHITECT1 | Known Ab Negative | 5 (3,415) | 92.3%1 (3.7, 99.9) | 98.8%1 (97.3, 99.4) | 73.9 (32.6, 167.9) | 0.08 (0.004, 15.7)) |
| Ortho ELISA-Ag1 | All | 5 (1,177) | 93.2%1 (81.6, 97.7) | 99.2%1 (87.9, 100) | 116.5 (6.7, 977) | 0.06 (0.02, 0.07) |
| Ortho ELISA-Ag2 | All | 6 (1,423) | 90.8%2 (83.5, 98.2) | ND | NA | NA |
| EIKEN Lumispot HCV Ag3 | All | 2 (235) | 97.5–98.1%3 | ND | NA | NA |
| Fujirebio Lumipulse Ortho HCV Ag4 | All | 1 (80) | 95.0%4 (90.2, 99.8) | ND | NA | NA |
| Hunan Jynda Bioengineering Group HCV Core Ag ELISA1 | All | 4 (562) | 59.5%1 (46.0, 71.7) | 82.9%1 (58.6, 94.3) | 3.5 (1.1, 12.6) | 0.28 (0.2, 0.3) |
HCV = Hepatitis C Virus, cAg = Core Antigen, Ab = Antibody, CI = Confidence Interval, LR = likelihood ratio, ELISA = enzyme linked immunosorbent assay, ND = no data, NA = Not applicable—if sensitivity and specificity results were not available from meta-analysis, likelihood ratios were not calculated.
Determined by bivariate meta-analysis – “metandi” command in STATA
Determined by univariate meta-analysis – “metan” command in STATA
Meta-analysis not possible, range of results seen across studies reported
Results from one study only
Figure 1.
Forest plot of Abbott ARCHITECT HCV Ag Assay sensitivity and specificity for the diagnosis of active HCV infection compared to NAT reference test for all samples regardless of HCV Ab status.
HCV = Hepatitis C Virus, Ag = antigen, NAT = nucleic acid testing, Ab = Antibody, CI = Confidence Interval
Heterogeneity was visually assessed in Figure 1 and Data Supplement Figures 4–7. The studies appear to be homogeneous in the overall bivariate analysis with the exception of one outlier study (25), which had no demographic information to perform further analysis. Overall, genotype distribution was reported for 18 studies (Data Supplement Table 1) with genotype 1b being the most prevalent and genotypes 5 and 6 only minimally studied. In the univariate analysis, there were three outlier studies (25, 44, 45). In the Ergünay study from Turkey, HIV and HBV co-infection status were unknown and the genotype distribution was overall similar to other studies that reported data: 60.2% of participants had HCV genotype 1b infection, 2.2% genotype 1a, 0.8% genotypes 3 and 4, and 35.8% were unknown (Data Supplement Table 1). In the Florea study from Romania, there were no HIV or HBV infected patients, and genotype of HCV was unknown. For specificity, the results were even more homogeneous with only one outlier, the Medici 2011 study from Italy and Spain (55) that reported 63 false positive tests. There were no demographic data for this study as it was performed on anonymous samples.
There was a symmetric peak in the Youden Index funnel plot (Data Supplement Figure 8) with a rightward tail suggesting little publication bias. This was further supported by the trim and fill statistical test, which found no change between the random effects model for sensitivity compared to a filled model (data not shown).
Ortho ELISA-Ag
Six studies utilized the Ortho ELISA-Ag test (30, 31, 33–35, 67). All were either cross-sectional or cohort designs in general study populations except for one study performed in healthy blood donors (35). All had unknown demographic information.
Five studies were included in the bivariate analysis with 1,177 total samples. The pooled sensitivity and specificity regardless of HCVAb status with 95% CI were 93.2% (81.6, 97.7) and 99.2% (87.9, 100), respectively. The positive and negative likelihood ratios were 116.5 (6.7, 977) and likelihood ratio 0.06 (0.02, 0.07) respectively (Table 2). The summary plot showed the summary point approaching the upper left corner suggesting good accuracy of the Ortho ELISA-Ag test for diagnosis of active HCV infection though these data exhibited some heterogeneity given the wide confidence intervals (Figure 2, Data Supplement Figure 9). The only outlier study (34) found 91 false negative HCVcAg tests but did not report antibody status, HIV or HBV co-infection information, though the genotype distribution was similar to other studies where it was reported (11.5% genotype 1 not subtyped, 42.3% genotype 1a, 19.2% genotype 1b, 11.5% genotype 2, and 15.4% genotype 3; Data Supplement Table 1). Furthermore, this study was performed in 494 plasma samples collected from only 52 donors at various time points during HCV infection and thus these samples did not provide independent data points. Raw quantitative data was not available.
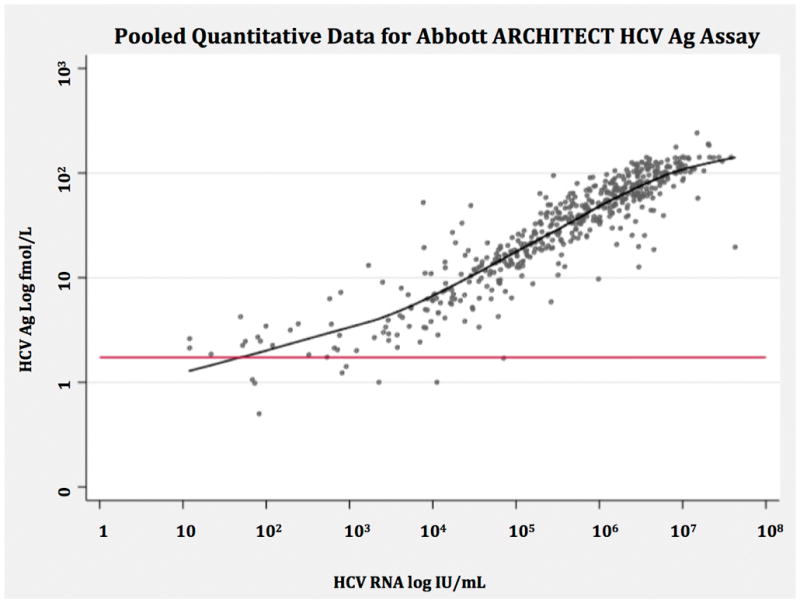
Figure 2.
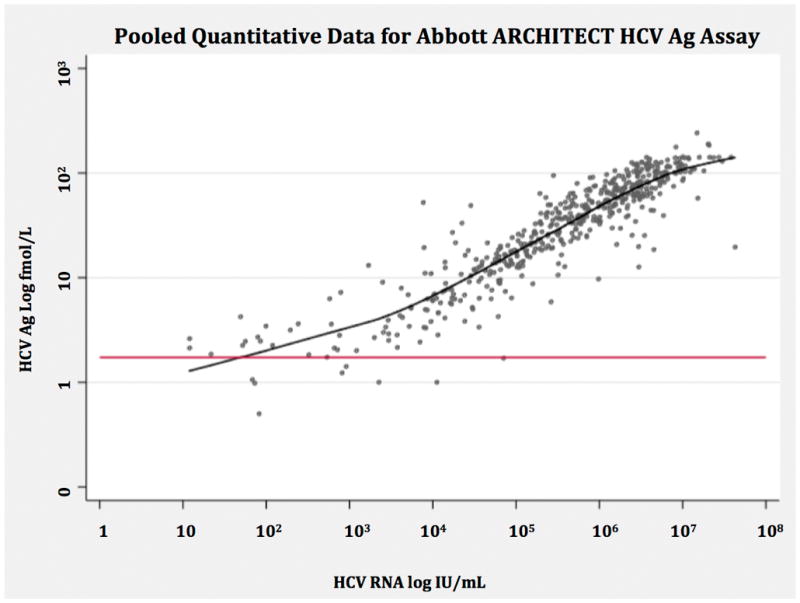
Non-parametric regression smoother of pooled quantitative data assessing correlation between Abbott ARCHITECT HCV Core Ag measured in log fmol/L and HCV RNA measured in log IU/mL. The red line indicates the positivity threshold of the core antigen index test corresponding to 3 fmol/L.
HCV = hepatitis C virus, Ag = antigen, fmol/L = femtomoles per liter, IU = international units, RNA = ribonucleic acid
EIKEN Lumispot HCV Ag
The EIKEN Lumispot HCV Ag was performed in one cross-sectional study of a general study population (37). Further demographic information was unavailable. The EIKEN Lumispot HCV Ag, Fujirebio Lumipulse Ortho HCV Ag, and Abbott ARCHITECT HCV Ag were compared in one cross-sectional study (38) with unknown demographic information.
The first study included 155 samples and the sensitivity reported was 98.1% (95% CI 95.9, 100)(Table 2)(37). The majority of samples were genotype 1 (65.2%) with the remaining genotype 2. The second study comparing three assays (38) included 80 participants, and reported a sensitivity of 97.5% (95% CI 94.1, 100) for the Lumispot. There were insufficient data reported to determine specificity in either study.
Fujirebio Lumipulse Ortho HCV Ag
One study was performed using the Lumipulse test with 80 participants (38). Sensitivity for the Lumipulse was reported as 95.0% (95% CI 90.2, 99.8) (Table 2). There were insufficient data reported to determine specificity.
Hunan Jynda Bioengineering Group HCV Core Ag ELISA
Four studies assessed the Hunan Jynda Bioengineering Group HCV Core Ag ELISA (26–28, 36). Two studies each had a cohort or cross-sectional design. One assessed a healthy blood donor population(36) while the others included broad study populations. HIV and HBV co-infection status were unknown in all studies. One included children (28), and for the remaining the age groups were unknown.
All four studies were included in the bivariate analysis with 562 total samples. The pooled sensitivity, specificity and 95% CI were 59.5% (46.0, 71.7) and 82.9% (58.6, 94.3), respectively. The positive and negative likelihood ratios were 3.5 (1.1, 12.6) and 0.28 (0.2, 0.3) (Table 2). Both the forest plot (Figure 3) and bivariate analysis (Data Supplement Figure 10) showed heterogeneity among the four studies, which limited confidence in the pooled estimate. No covariate assessment was performed, as HIV status, HBV status, and genotype distribution were unknown for all studies.
Quantitative Data
Three studies provided quantitative data for analysis (49, 55, 58). All used the ARCHITECT assay in comparison with NAT. There were 90 HCVAb positive specimens analyzed in the Kadkhoda study (49), 205 HCVAb positive and 77 HCVAb negative specimens in the Park study (58), and 1152 HCVAb positive specimens in the Medici study (55). The HCVcAg was shown to correlate well with RNA except for RNA values below 3000 IU/mL, where negative HCVcAg were observed (Figure 4). In the Kadkhoda study among the 8 specimens with HCV RNA > 3000 IU/mL and negative HCVcAg, the genotype distribution was similar to the cohort as a whole (12.5% unspecified, 37.5% genotype 1, 25% genotype 2, 25% genotype 3). No genotype or co-infection information was available for the specimens in the Park and Medici studies to further characterize outlier points.
DISCUSSION
This systematic review concludes that a well-performing HCVcAg test can achieve similar diagnostic accuracy to NAT for identification of active HCV infection when the viral load exceeds 3000 IU/ml.
Both the Abbott ARCHITECT HCV Ag test and Ortho ELISA-Ag perform similarly in regards to sensitivity—93.4% (90.1, 96.4) vs 93.2% (81.6, 97.7)—and specificity—98.8% (97.4, 99.5) vs 99.2% (87.9, 100). However, the large amount of consistent, homogenous data on the ARCHITECT (33 studies vs. six on Ortho) allows for greater precision and more confidence in these estimates. The likelihood ratios for both tests are also very favorable and allow for clinical decision-making based on test results. The EIKEN Lumispot and Fujirebio Lumipulse were designed with the same principle technology as the ARCHITECT and have similar sensitivity and specificity, though assessment was limited to one and two studies respectively despite the fact that our systematic review included Chinese and Japanese literature. Tests such as the Hunan Jynda assay have the lowest sensitivity (59.5%, 95% CI 46.0, 71.7), which supports the notion that an ELISA is insufficient for detection and signal amplification (as with chemiluminescence) is necessary to achieve adequate detection limits.
Quantitative analysis of data available from three studies using the ARCHITECT supported close correlation between HCVcAg and RNA, though the linearity declined around an HCV RNA level of 3,000 IU/mL, which is consistent with the analytical limit of detection reported by Abbott.
We expanded on the literature search described in a systematic review of HCVcAg performed in 2012 (68) to identify additional relevant studies reported since its publication and update the analysis to include only tests that remain commercially available. Further, this study builds on that prior work by evaluating performance of each commercial test separately, rather than pooling all HCVcAg tests into one multivariate random effects model. Our approach is useful because test characteristics and thresholds differ between various detection technologies. Our results allow indirect between test comparisons and avoid the problematic heterogeneity introduced by pooling together performance characteristics of different detection technologies.
Strengths of this review include the development of an a priori protocol and analysis plan. The search was performed without language restriction, though ultimately three articles were excluded for inability to find appropriate translation for Russian, Korean, and Polish. Nevertheless, studies may have been missed in the comprehensive search, and subsequent studies published after the search date was performed could not be included. Article selection and standardized data extraction in accordance with the predefined protocol was ensured by independent reviewers. Authors were contacted for missing data and clarifications, though some studies were excluded due to lack of author response. In the analysis, bivariate random effects modeling was used when appropriate to derive pooled estimates and univariate analyses were performed in an effort to utilize all available data.
There were limitations with the data summarized in this review. We planned to examine the effects of HBV co-infection, HIV co-infection, and HCV genotype in a meta-regression but this was not possible due to limited available data on these covariates. Data on HCVcAg test performance in genotypes 4, 5 and 6 is largely lacking, which limits the conclusions. Additionally, a sensitivity analysis to examine the impact of the specimen condition (fresh vs. frozen) could not be performed as all studies used frozen samples or did not specify the specimen condition. There were not enough studies with Lumispot and Lumipulse to derive pooled estimates and only descriptive analyses could be completed. Most of the studies were performed in high-resource settings and within reference laboratories. This might not reflect the population that would be tested if HCVcAg tests were implemented in LMICs, particularly given limited data for genotypes 4, 5, and 6, which are more prevalent in these countries (69).
The data limitations in this review highlight a need for better surveillance data that will inform an understanding of how many patients are missed (false negatives) by assays that have higher limits of detection (e.g 3000 IU/ml for ARCHITECT), and whether covariates like HIV or HBV co-infection and HCV genotype impact assay results. More information is necessary on the fluctuation in RNA and HCVcAg during the pre-seroconversion phase, as well as for the rare patients with high viral loads and negative HCVcAg to inform the optimization of antigen detection. This study focused only on the use of HCVcAg as a screening and diagnostic test though NAT is also used in treatment monitoring and to assess sustained viral response (SVR) after therapy is completed. The performance of HCVcAg to confirm SVR at completion of therapy should be further investigated. However, viral load measurements while on DAA therapy may no longer be necessary as suggested by recent publications (70, 71).
For both HCVcAg tests and NATs to reach a larger population at risk in LMICs, tests with better POC suitability need to be developed or alternatively transport mechanisms with dried-blood spots need to be improved to enable better centralized testing depending on local settings. For any HCVcAg POC test, careful sample processing is necessary to lyse viral particles, expose antigen and dissociate antibody from antigen to optimize detection. Signal amplification will be necessary to achieve sufficient sensitivity (as suggested by this review); therefore, an instrument-free assay (e.g. lateral-flow assay) is unlikely to be feasible in the near future. Cost of testing to the patient or health care provider is also a key factor for implementation in LMICs. The cost estimates from LMICs are highly variable and often country specific, though generally cost estimates for HCVcAg tests are lower (from $10–50) than for HCV RNA tests ($13–100) (65, 70, 72, 73) (unpublished survey of high burden countries by Foundation for Innovative New Diagnostics and WHO).
In summary, this systematic review showed that there are several HCVcAg assays with high sensitivity (>90%) and specificity (>98%). While even those with the highest performance do not reach the sensitivity of NAT, well-performing HCVcAg tests with an analytical sensitivity reaching into the femtomolar range (equivalent of 3000 IU/ml) could serve as a replacement for NAT for HCV detection, particularly if a lower cost per test allows reaching more patients. HCVcAg should be explored for POC testing to increase the number of patients diagnosed and streamline the HCV cascade of care.
Supplementary Material
Appendix Figure 1.
Funnel plot of published studies that used the Abbott ARCHITECT HCV Ag assay.
Appendix Figure 2.
Forest plot Ortho ELISA-Ag sensitivity and specificity for diagnosis of active HCV infection compared to NAT reference test for all samples regardless of HCV Ab status.
ELISA = enzyme linked immunosorbent assay, Ag = antigen, HCV = Hepatitis C Virus, NAT = nucleic acid testing, Ab = Antibody, CI = Confidence Interval
Acknowledgments
Funding Source: This systematic review was supported by grant money from the NIH: 5T32AI052074-10, 5R01DA031059-04, 1P30DA040500-01, and 5P30AI042853-18.
We would like to thank all of the study authors who provided additional data necessary to complete this review. We also wish to thank the following individuals: Jeanne Chauffor at Médecins Sans Frontières and Audrey Albertini at FIND for translation of studies in French, Kuniaki Arai at the World Health Organization for translation of studies in Japanese, Joseph Tucker at University of North Carolina for translation of author communication in Mandarin, David Flynn from Boston University School of Medicine Alumni Medical Library and Genevieve Gore from McGill University for their help with search terms, and Ranald Sutherland and Martin Brusdeilins from FIND for their input on the technical aspects and commercial availability of HCVcAg tests.
References
- 1.Gower EEC, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. Journal of Hepatology, Supplement. 2014;61(1 Suppl):S45–57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C Fact Sheet. 2015. [Google Scholar]
- 3.Ward JW, Mermin JH. Simple, Effective, but Out of Reach? Public Health Implications of HCV Drugs. N Engl J Med. 2015 doi: 10.1056/NEJMe1513245. [DOI] [PubMed] [Google Scholar]
- 4.Linas BP, Barter DM, Leff JA, Assoumou SA, Salomon JA, Weinstein MC, et al. The hepatitis C cascade of care: identifying priorities to improve clinical outcomes. PLoS One. 2014;9(5):e97317. doi: 10.1371/journal.pone.0097317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamal SM. Acute hepatitis C: a systematic review. Am J Gastroenterol. 2008;103(5):1283–97. doi: 10.1111/j.1572-0241.2008.01825.x. quiz 98. [DOI] [PubMed] [Google Scholar]
- 6.Global regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rongey CA, Kanwal F, Hoang T, Gifford AL, Asch SM. Viral RNA testing in hepatitis C antibody-positive veterans. Am J Prev Med. 2009;36(3):235–8. doi: 10.1016/j.amepre.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Glasgow Declaration on Hepatitis. 2015. [Google Scholar]
- 9.Reed KE, Rice CM. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr Top Microbiol Immunol. 2000;242:55–84. doi: 10.1007/978-3-642-59605-6_4. [DOI] [PubMed] [Google Scholar]
- 10.Dubuisson J. Hepatitis C virus proteins. World J Gastroenterol. 2007;13(17):2406–15. doi: 10.3748/wjg.v13.i17.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tedder RS, Tuke P, Wallis N, Wright M, Nicholson L, Grant PR. Therapy-induced clearance of HCV core antigen from plasma predicts an end of treatment viral response. Journal of Viral Hepatitis. 2013;20(1):65–71. doi: 10.1111/j.1365-2893.2012.01630.x. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka E, Kiyosawa K, Matsumoto A, Kashiwakuma T, Hasegawa A, Mori H, et al. Serum levels of hepatitis C virus core protein in patients with chronic hepatitis C treated with interferon alfa. Hepatology. 1996;23(6):1330–3. doi: 10.1053/jhep.1996.v23.pm0008675147. [DOI] [PubMed] [Google Scholar]
- 13.Foundation for Innovative New Diagnostics. High Priority Target Product Profile for Hepatitis C Diagnosis in Decentralized Settings. 2015. [Google Scholar]
- 14.Deville WL, Buntinx F, Bouter LM, Montori VM, de Vet HC, van der Windt DA, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai M, McCulloch M, Gorman JD, Pai N, Enanoria W, Kennedy G, et al. Systematic reviews and meta-analyses: an illustrated, step-by-step guide. Natl Med J India. 2004;17(2):86–95. [PubMed] [Google Scholar]
- 16.Eden J, Levit L, Berg A, Morton S, editors. Institute of Medicine Committee on Standards for Systematic Reviews of Comparative Effectiveness R. Finding What Works in Health Care: Standards for Systematic Reviews. Washington (DC): National Academies Press (US); 2011. Copyright 2011 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 18.Burkner PC, Doebler P. Testing for publication bias in diagnostic meta-analysis: a simulation study. Stat Med. 2014;33(18):3061–77. doi: 10.1002/sim.6177. [DOI] [PubMed] [Google Scholar]
- 19.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 20.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 21.Harbord R. metandi: Meta-analysis of diagnostic accuracy using hierarchical logistic regression. STATA J. 2009;9:211–29. [Google Scholar]
- 22.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–36. [Google Scholar]
- 23.Wickham H. Elegant Graphics for Data Analysis. New York: Springer-Verlag; 2009. [Google Scholar]
- 24.Palmer T, Peters J, Sutton A, Moreno S. Contour-enhanced funnel plots for meta-analysis. The STATA Journal. 2008;8(2):242–54. [Google Scholar]
- 25.Gu JL, Yu Y, Liang ZL. Performances of HCV Ag or HCV RNA kits for screening of HCV-infected samples. Chinese Journal of Biologicals. 2014;27(9):1181–4. [Google Scholar]
- 26.Lu YC, Jiang ZY, Kuang YL, Chen WS, Tan YZ, Li DR. Clinical value of the detection of hepatitis C virus core antigen. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2007;23(7):635–7. [PubMed] [Google Scholar]
- 27.Ouyang Y, Tan DM, Li TG, Zhou H, Tan C. Qualitative detection of hepatitis C virus core antigen in the serum in patients with chronic hepatitis C. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31(6):894–6. 905. [PubMed] [Google Scholar]
- 28.Zhu H, Zhang T, Fan CL, Xiao XG, Zhang FH. Clinical significance of testing hepatitis C virus core antigen. Journal of Dalian Medical University. 2010;32(2) 211-2-+8. [Google Scholar]
- 29.van Helden J, Weiskirchen R. Hepatitis C Diagnostics: Clinical Evaluation of the HCV-Core Antigen Determination. Zeitschrift Fur Gastroenterologie. 2014;52(10):1164–70. doi: 10.1055/s-0034-1366618. [DOI] [PubMed] [Google Scholar]
- 30.Ohta H, Takemura M, Furuta N, Akiyama M, Katagiri Y, Ohashi H, et al. Clinical significance and problems in HCV measurement--comparison of CLEIA method with PCR method. Rinsho Byori. 2004;52(10):813–8. [PubMed] [Google Scholar]
- 31.Okazaki K, Nishiyama Y, Saitou T, Shibata N, Yamamoto C, Oosaga J, et al. Fundamental evaluation of HCV core antigen method comparison with Cobas Amplicor HCV monitor v2.0 (high range method) Rinsho Byori. 2008;56(2):95–100. [PubMed] [Google Scholar]
- 32.Kesli R, Polat H, Terzi Y, Kurtoglu MG, Uyar Y. Comparison of a Newly Developed Automated and Quantitative Hepatitis C Virus (HCV) Core Antigen Test with the HCV RNA Assay for Clinical Usefulness in Confirming Anti-HCV Results. Journal of Clinical Microbiology. 2011;49(12):4089–93. doi: 10.1128/JCM.05292-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.el-Sayed Zaki M, el-Adrosy H. Recent approach for diagnosis of early HCV infection. Egypt J Immunol. 2004;11(1):123–9. [PubMed] [Google Scholar]
- 34.Nübling CM, Unger G, Chudy M, Raia S, Löwer J. Sensitivity of HCV core antigen and HCV RNA detection in the early infection phase. Transfusion. 2002;42(8):1037–45. doi: 10.1046/j.1537-2995.2002.00166.x. [DOI] [PubMed] [Google Scholar]
- 35.Letowska M, Brojer E, Mikulska M, Gronowska A, Rosiek A. Hepatitis C core antigen in Polish blood donors. Transfusion. 2004;44(7):1067–71. doi: 10.1111/j.1537-2995.2004.03340.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhang HQ, Li SB, Wang GH, Chen K, Song XG, Feng XY. Detection of hepatitis C virus core antigen for early diagnosis of hepatitis C virus infection in plasma donor in China. World Journal of Gastroenterology. 2007;13(19):2738–42. doi: 10.3748/wjg.v13.i19.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saito R, Yokota H, Takahashi E, Mashige F, Yoneyama A, Nakahara K, et al. Performance of an automated system for quantitation of hepatitis C virus core antigen. Journal of Virological Methods. 2003;112(1–2):93–7. doi: 10.1016/s0166-0934(03)00195-2. [DOI] [PubMed] [Google Scholar]
- 38.Murayama A, Sugiyama N, Watashi K, Masaki T, Suzuki R, Aizaki H, et al. Japanese Reference Panel of Blood Specimens for Evaluation of Hepatitis C Virus RNA and Core Antigen Quantitative Assays. Journal of Clinical Microbiology. 2012;50(6):1943–9. doi: 10.1128/JCM.00487-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buket CA, Ayse A, Selcuk K, Suleyman T, Emel SC. Comparison of HCV core antigen and anti-HCV with HCV RNA results. African Health Sciences. 2014;14(4) doi: 10.4314/ahs.v14i4.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chevaliez S, Soulier A, Poiteau L, Bouvier-Alias M, Pawlotsky JM. Clinical utility of hepatitis C virus core antigen quantification in patients with chronic hepatitis C. Journal of Clinical Virology. 2014;61(1):145–8. doi: 10.1016/j.jcv.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Descamps V, de Beeck AO, Plassart C, Brochot E, Francois C, Helle F, et al. Strong Correlation between Liver and Serum Levels of Hepatitis C Virus Core Antigen and RNA in Chronically Infected Patients. Journal of Clinical Microbiology. 2012;50(2):465–8. doi: 10.1128/JCM.06503-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durante-Mangoni E, Vallefuoco L, Sorrentino R, Iossa D, Perna E, Molaro R, et al. Clinico-Pathological Significance of Hepatitis C Virus Core Antigen Levels in Chronic Infection. Journal of Medical Virology. 2013;85(11):1913–8. doi: 10.1002/jmv.23672. [DOI] [PubMed] [Google Scholar]
- 43.Duy Thong V, Akkarathamrongsin S, Avihingsanon A, Theamboonlers A, Poovorawan Y, Tangkijvanich P. The Correlation between Hepatitis C Core Antigen and Hepatitis C Virus RNA Levels with Respect to Human Immunodeficiency Virus Status, Hepatitis C Virus Genotype and Interferon-Lambda-4 Polymorphism. Intervirology. 2015;58(2):73–9. doi: 10.1159/000370070. [DOI] [PubMed] [Google Scholar]
- 44.Ergunay K, Sener B, Alp A, Karakaya J, Hascelik G. Utility of a commercial quantitative hepatitis C virus core antigen assay in a diagnostic laboratory setting. Diagnostic Microbiology and Infectious Disease. 2011;70(4):486–91. doi: 10.1016/j.diagmicrobio.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Florea D, Neaga E, Nicolae I, Maxim D, Popa M, Otelea D. Clinical Usefulness of HCV Core Antigen Assay for the Management of Patients with Chronic Hepatitis C. Journal of Gastrointestinal and Liver Diseases. 2014;23(4):393–6. doi: 10.15403/jgld.2014.1121.234.chcv. [DOI] [PubMed] [Google Scholar]
- 46.Garbuglia AR, Monachetti A, Galli C, Sabatini R, Ferreri ML, Capobianchi MR, et al. HCV core antigen and HCV-RNA in HIV/HCV co-infected patients with different HCV genotypes. Bmc Infectious Diseases. 2014:14. doi: 10.1186/1471-2334-14-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadziyannis E, Minopetrou M, Georgiou A, Spanou F, Koskinas J. Is HCV core antigen a reliable marker of viral load? An evaluation of HCV core antigen automated immunoassay. Ann Gastroenterol. 2013;26(2):146–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Heidrich B, Pischke S, Helfritz FA, Mederacke I, Kirschner J, Schneider J, et al. Hepatitis C virus core antigen testing in liver and kidney transplant recipients. Journal of Viral Hepatitis. 2014;21(11):769–79. doi: 10.1111/jvh.12204. [DOI] [PubMed] [Google Scholar]
- 49.Kadkhoda K, Smart G. HCV Antigen Testing for the Diagnosis of Hepatitis C Infection: A Cost-Efficient Algorithm. Clinical Laboratory. 2014;60(4):677–80. doi: 10.7754/clin.lab.2013.130634. [DOI] [PubMed] [Google Scholar]
- 50.Kooglu M, Ak S, Ak M, Yakupogullari Y, Ozer A. Evaluation of diagnostic performance of new antigen-based enzyme immune assay for diagnosis of Hepatitis C virus (HCV) infections. African Journal of Microbiology Research. 2012;6(4):809–12. [Google Scholar]
- 51.Kuo YH, Chang KC, Wang JH, Tsai PS, Hung SF, Hung C, et al. Is hepatitis C virus core antigen an adequate marker for community screening? Journal of Clinical Microbiology. 2012;50(6):1989–93. doi: 10.1128/JCM.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Cavoli G, Zagarrigo C, Schillaci O, Servillo F, Tralongo A, Coglitore M, et al. Hepatitis C virus core antigen test in monitoring of dialysis patients. Hepat Res Treat. 2012;2012:832021. doi: 10.1155/2012/832021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mederacke I, Ciesek S, Raupach R, Wursthorn K, Deterding K, Steinmann E, et al. Kinetics of HCV core antigen during antiviral treatment of acute and chronic hepatitis C as determined by a novel chemiluminescent microparticle immunoassay. Journal of Hepatology. 2009;50:S129. [Google Scholar]
- 54.Mederacke I, Potthoff A, Meyer-Olson D, Meier M, Raupach R, Manns MP, et al. HCV core antigen testing in HIV- and HBV-coinfected patients, and in HCV-infected patients on hemodialysis. Journal of Clinical Virology. 2012;53(2):110–5. doi: 10.1016/j.jcv.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 55.Medici MC, Furlini G, Rodella A, Fuertes A, Monachetti A, Calderaro A, et al. Hepatitis C virus core antigen: Analytical performances, correlation with viremia and potential applications of a quantitative, automated immunoassay. Journal of Clinical Virology. 2011;51(4):260–5. doi: 10.1016/j.jcv.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Miedouge M, Saune K, Kamar N, Rieu M, Rostaing L, Izopet J. Analytical evaluation of HCV core antigen and interest for HCV screening in haemodialysis patients. Journal of Clinical Virology. 2010;48(1):18–21. doi: 10.1016/j.jcv.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 57.Ottiger C, Gygli N, Huber AR. Detection limit of architect hepatitis C core antigen assay in correlation with HCV RNA, and renewed confirmation algorithm for reactive anti-HCV samples. Journal of Clinical Virology. 2013;58(3):535–40. doi: 10.1016/j.jcv.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 58.Park Y, Lee JH, Kim BS, Kim DY, Han KH, Kim HS. New Automated Hepatitis C Virus (HCV) Core Antigen Assay as an Alternative to Real-Time PCR for HCV RNA Quantification. Journal of Clinical Microbiology. 2010;48(6):2253–6. doi: 10.1128/JCM.01856-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reyes-Mendez MA, Juarez-Figueroa L, Iracheta-Hernandez P, Medina-Islas Y, Ruiz-Gonzalez V. Comparison of two diagnostic algorithms for the identification of patients with HCV viremia using a new HCV antigen test. Ann Hepatol. 2014;13(3):337–42. [PubMed] [Google Scholar]
- 60.Rouet F, Deleplancque L, Mboumba BB, Sica J, Mouinga-Ondémé A, Liégeois F, et al. Usefulness of a fourth generation ELISA assay for the reliable identification of HCV infection in HIV-positive adults from Gabon (Central Africa) PLoS ONE. 2015;10(1) doi: 10.1371/journal.pone.0116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Russi S, Sansonno D, Mariggio MA, Vinella A, Pavone F, Lauletta G, et al. Assessment of total hepatitis C virus (HCV) core protein in HCV-related mixed cryoglobulinemia. Arthritis Research & Therapy. 2014;16(2) doi: 10.1186/ar4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vanhommerig JW, van de Laar TJW, Koot M, van Rooijen MS, Schinkel J, Speksnijder AGCL, et al. Evaluation of a hepatitis C virus (HCV) antigen assay for routine HCV screening among men who have sex with men infected with HIV. Journal of Virological Methods. 2015;213:147–50. doi: 10.1016/j.jviromet.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 63.Vermehren J, Susser S, Berger A, Perner D, Peiffer KH, Zeuzem S, et al. Clinical utility of the architect HCV AG assay for early treatment response monitoring in patients with chronic Hepatitis C genotype 1 infection. Journal of Hepatology. 2012;56:S461. doi: 10.1016/j.jcv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Medici MC, Chezzi C, Conto FD, Ferraglia F, Pinardi F, Arcangeletti MC, et al. Evolving strategy for HCV testing in an Italian tertiary care hospital. Journal of Clinical Virology. 2016;77:92–8. doi: 10.1016/j.jcv.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 65.Cresswell FV, Fisher M, Hughes DJ, Shaw SG, Homer G, Hassan-Ibrahim MO. Hepatitis C core antigen testing: A reliable, quick, and potentially cost-effective alternative to hepatitis C polymerase chain reaction in diagnosing acute hepatitis C virus infection. Clinical Infectious Diseases. 2015;60(2):263–6. doi: 10.1093/cid/ciu782. [DOI] [PubMed] [Google Scholar]
- 66.Mixson-Hayden T, Dawson GJ, Teshale E, Le T, Cheng K, Drobeniuc J, et al. Performance of ARCHITECT HCV core antigen test with specimens from US plasma donors and injecting drug users. Journal of Clinical Virology. 2015;66:15–8. doi: 10.1016/j.jcv.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 67.Agha S, Tanaka Y, Saudy N, Kurbanov F, Abo-Zeid M, El-Malky M, et al. Reliability of hepatitis C virus core antigen assay for detection of viremia in HCV genotypes 1, 2, 3, and 4 infected blood donors: A collaborative study between Japan, Egypt, and Uzbekistan. Journal of Medical Virology. 2004;73(2):216–22. doi: 10.1002/jmv.20078. [DOI] [PubMed] [Google Scholar]
- 68.Gu S, Liu J, Zhang H, Gu B, Lai H, Zhou H, et al. Core antigen tests for hepatitis C virus: a meta-analysis. Mol Biol Rep. 2012;39(8):8197–208. doi: 10.1007/s11033-012-1667-z. [DOI] [PubMed] [Google Scholar]
- 69.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cohn J, Roberts T, Amorosa V, Lemoine M, Hill A. Simplified diagnostic monitoring for hepatitis C, in the new era of direct-acting antiviral treatment. Curr Opin HIV AIDS. 2015;10(5):369–73. doi: 10.1097/COH.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 71.Sidharthan S, Kohli A, Sims Z, Nelson A, Osinusi A, Masur H, et al. Utility of hepatitis C viral load monitoring on direct-acting antiviral therapy. Clin Infect Dis. 2015;60(12):1743–51. doi: 10.1093/cid/civ170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamal SM, Kassim S, El Gohary E, Fouad A, Nabegh L, Hafez T, et al. The accuracy and cost-effectiveness of hepatitis C core antigen assay in the monitoring of anti-viral therapy in patients with chronic hepatitis C genotype 4. Alimentary Pharmacology and Therapeutics. 2015;42(3):307–18. doi: 10.1111/apt.13261. [DOI] [PubMed] [Google Scholar]
- 73.Frontières Médecins Sans. Putting HIV and HCV to the test: a product guide for point-of-care CD4 and laboratory-based and point-of-care virological HIV and HCV tests. 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.