Abstract
BACKGROUND
Enoxaparin (ENX) has been shown to reduce cerebral edema and improve neurologic recovery after traumatic brain injury (TBI), through blunting of cerebral leukocyte (LEU) recruitment. High mobility group box 1 (HMGB1) protein may induce inflammation through LEU activation. We hypothesized that ENX after TBI reduces LEU-mediated edema through blockade of HMGB1 signaling.
METHODS
Twenty-three CD1 mice underwent severe TBI by controlled cortical impact and were randomized to one of four groups receiving either monoclonal antibody against HMGB1 (MAb) or isotype (Iso) and either ENX (1 mg/kg) or normal saline (NS): NS + Iso (n = 5), NS + MAb (n = 6), ENX + Iso (n = 6), ENX + MAb (n = 6). ENX or NS was administered 2, 8, 14, 23 and 32 hours after TBI. MAb or Iso (25 μg) was administered 2 hours after TBI. At 48 hours, cerebral intravital microscopy served to visualize live LEU interacting with endothelium and microvascular fluorescein isothiocyanate–albumin leakage. The Neurological Severity Score (NSS) graded neurologic recovery; wet-to-dry ratios determined cerebral/lung edema. Analysis of variance with Bonferroni correction was used for statistical analyses.
RESULTS
ENX and MAb similarly reduced in vivo pial LEU rolling without demonstrating additive effect. In vivo albumin leakage was greatest in vehicle-treated animals but decreased by 25% with either MAb or ENX but by 50% when both were combined. Controlled cortical impact–induced cerebral wet-to-dry ratios were reduced by MAb or ENX without additive effect. Postinjury lung water was reduced by ENX but not by MAb. Neurologic recovery at 24 hours and 48 hours was similarly improved with ENX, MAb, or both treatments combined.
CONCLUSION
Mirroring ENX, HMGB1 signaling blockade reduces LEU recruitment, cerebrovascular permeability, and cerebral edema following TBI. ENX further reduced lung edema indicating a multifaceted effect beyond HMGB1 blockade. Further study is needed to determine how ENX may play a role in blunting HMGB1 signaling in brain injury patients.
Keywords: Enoxaparin, HMGB1, TBI, Leukocyte, mice
Persistence of cerebral inflammation following brain trauma is believed to be a key component of the progression to secondary brain injury and contributes significantly to death in traumatic brain injury (TBI) patients. Circulating leukocytes (LEUs) and in particular activated polymorphonuclear neutrophils (PMNs) are recruited to the penumbral region surrounding injured tissue and have been observed to migrate out of circulation through interactions with cerebrovascular endothelium.1 Once in the interstitial milieu, degranulation and free radical release by these tissue-phase LEU occurs and may continue unhindered, promoting persistent local microvascular leakage, edema, and tissue destruction.2
High mobility group box 1 (HMGB1) is a structural DNA-binding protein secreted by various cell lines including those of the central nervous system (CNS).3–5 In addition to serving in transcription regulation within the nucleus, HMGB1 released by activated or necrotic cells may also function as an intercellular messenger promoting the host inflammatory response.6,7 As a member of the danger-associated molecular patterns (DAMPs),8 HMGB1 has been implicated in the pathogenesis of various CNS diseases including TBI,9 subarachnoid hemorrhage,10 autoimmune encephalomyelitis,11 transient ischemia,12 and seizures.13
Although a recent report has raised doubts,14 TBI is considered an independent risk factor for developing venous thromboembolic events (VTEs) following injury. Enoxaparin (ENX), a low-molecular-weight heparin, is the preferred agent for VTE prophylaxis in trauma but is often withheld in TBI patients fearing progression of intracranial hemorrhage from its anti-coagulant properties. In the last two decades, unexpected but potent anti-inflammatory properties of heparinoids have been reported. In particular, heparinization before hemorrhage and trauma resulted in blunted endothelial activation and restoration of endothelial cells' (ECs) ability to release nitric oxide.15–17 In these rodent models, this resulted in the maintenance of microvascular patency with decreased capillary sludging and, ultimately, improved organ function. In the CNS, heparins were shown to blunt cerebrovascular LEU and EC activation following injury and reduce cerebral edema while accelerating neurologic recovery.18–21 The mechanism by which heparinoids exert these effects on inflammation remains unclear but has been investigated in different injury models. In a stretch-induced lung injury model, ENX administration was found to inhibit HMGB1 production, pulmonary PMN infiltration, oxygen free radical release, and microvascular permeability in injured lung tissue.22 Others demonstrated similar findings relating heparinoids to LEU recruitment through HMGB1 signaling in sepsis23 and ischemia reperfusion24 models. To date, it remains unclear if ENX alters HMGB1 signaling following cerebral injury.
In the current study, we sought to determine if ENX-related reductions of LEU-mediated cerebral inflammation were mirrored by HMGB1 monoclonal antibody blockade in the same TBI model. We hypothesized that HMGB1 blockade would replicate effects of ENX in reducing live LEU recruitment to pericontusional cerebral tissue, decreasing microvascular permeability and cerebral edema while accelerating neurologic recovery following experimental TBI in mice. We further postulated that both ENX and HMGB1 blockade would independently reduce pulmonary edema in this setting.
MATERIALS AND METHODS
Reagents and Monoclonal Antibodies
Normal saline (NS) was purchased from Baxter Healthcare Corporation (Deerfield, IL), ENX (100 mg/mL) from SANOFI (Bridgewater, NJ), ketamine from Hospira (Lake Forest, IL), xylazine from Akorn (Decatur, IL), and acepromazine from Boehringer Ingelheim (St. Joseph, MO). Bovine fluorescein isothiocyanate (FITC)–labeled albumin and 0.3% rhodamine 6G were purchased from Sigma-Aldrich (St. Louis, MO).
Blocking monoclonal antibodies were purified IgG (mouse antihuman HMGB1 monoclonal antibody [MAb], clone 3B1, 1 mg/mL) from Oncolmmube, Inc. (Rockville, MD) and its purified protein A/G (mouse) IgG2A negative isotype antibody from R&D Systems (Minneapolis, MN).
Animals and Surgical Procedures
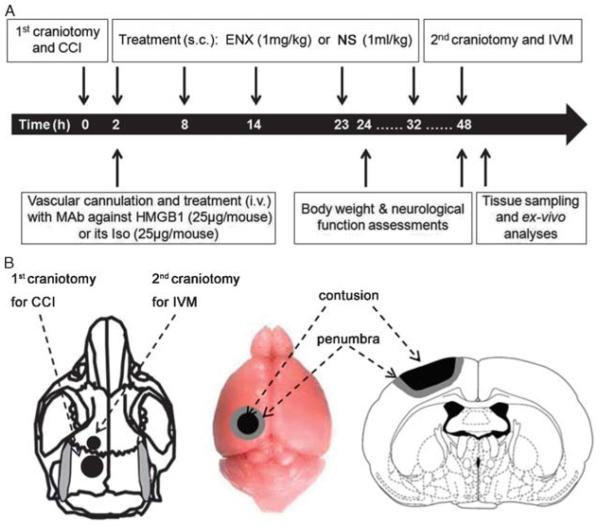
All experimental procedures were approved and performed according to the Institutional Animal Care and Use Committee guidelines of the University of Pennsylvania. Twenty-three 25-g to 30-g male CD1 mice (Charles River Laboratories, Wilmington, MA) underwent severe TBI by controlled cortical impact (CCI) and were randomly assigned to receive either anti-HMGB1 MAb or its isotype (Iso) and either ENX (1 mg/kg) or 0.9% NS. Animals were randomized to four groups as follows: NS + Iso (n = 5), NS + MAb (n = 6), ENX + Iso (n = 6), ENX + MAb (n = 6). ENX (1 mg/kg, subcutaneous) or an equal volume of NS (1 mL/kg, subcutaneous) was administered 2, 8, 14, 23, and 32 hours after TBI in respective animals (Fig. 1A). The right jugular vein was cannulated, and MAb (25 μg/mouse, intravenous) or Iso (25 μg/mouse, intravenous) was administered 2 hours after TBI. This dose of MAb was chosen based on previous studies in different validated brain injury models evaluating HMGB1.5,12 CCI was used to induce severe TBI as previously described.25,26 Briefly, after anesthesia with ketamine/xylazine/acepromazine (100/10/1 mg/kg, intraperitoneal) and placement of the animal in a stereotactic device, mice underwent a midline sagittal incision to expose the surface of the skull. A left-sided, 4-mm circular craniotomy was prepared between the bregma and the lambda using a dental drill (Henry Schein, Melville, NY) (Fig. 1B). Anesthetized mice were then mounted on a CCI device (AMS 201, AmScien Instruments, Richmond, VA) to undergo severe TBI using the following validated CCI parameters: 6-m/s striking velocity, 1.0-mm depth of penetration, using a 3-mm impactor tip.
Figure 1.
Experimental design and procedures. A, Timeline of experimental procedures. B, Schematic diagram showing the location of craniotomies and CCI-induced cortical contusion surrounded by penumbral area.
Observation with Intravital Microscopy
To investigate the effect of MAb blockade and ENX on LEU/EC interactions and blood-brain barrier (BBB) permeability, direct live observation of the pial microcirculation was performed using intravital microscopy (IVM) 48 hours after CCI.26 Briefly, a second 2.5-mm craniotomy was made just anterior to the first craniotomy and covered with a 5-mm coverslip (Fisher Scientific, Waltham, MA) (Fig. 1B). Live mice were then transferred under an IVM microscope (ECLIPSE FN1, Nikon Instruments, Melville, NY), and their brain was observed under a 40× water immersion objective. After receiving 50-μL IV of 0.3% rhodamine 6G (to label LEUs), unbranched venules with a diameter of 25 μm to 50 μm were randomly selected and video-recorded for 1 minute using an attached digital camera (QuantEM, Photometrics, Tucson, AR). Thereafter, to visualize macromolecular leakage from the BBB, 100-mg/kg FITC-labeled albumin was intravenously administrated, and a second 10-second-long video was recorded of the same pial location. All videos were subsequently analyzed offline by a blinded observer.
Microcirculatory Analysis
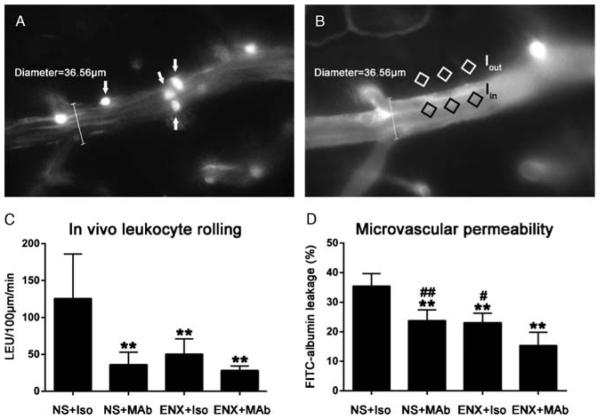
Offline video analysis of microcirculatory LEU/EC interactions and permeability in a 100-μm venular segment was performed using NIS-Elements software (Nikon Instruments, Melville, NY). LEUs were identified as spherical cells with a diameter of 7 μm to 12 μm (Fig. 2A). Rolling LEUs were defined as labeled cells traveling at a velocity significantly slower than central blood flow velocity, and adherent LEUs were defined as those immobile in the vessel for at least 30 seconds, and both are expressed as cells per 100 micrometer per minute. Quantification of microvascular permeability in the same venules was conducted by measuring the mean ratio of fluorescence intensity in three separate locations outside the vessel wall (Iout) to that of three separate locations within the venule (Iin) (Fig. 2B), whereby Iin / Iout = permeability index.
Figure 2.
Effect of ENX and HMGB1 MAb blockade on in vivo LEU/EC interactions and microvascular permeability. A, Representative image showing leukocytes interacting with endothelium. White arrows indicate live circulating leukocytes labeled with Rhodamine 6G. B, Representative image showing FITC-albumin leakage in the cerebral microcirculation. The microvascular permeability index is expressed as the ratio of mean fluorescence of three separate locations outside the vessel wall (Iout) to mean fluorescence of three separate locations within the venule (Iin). C, Quantification of the number of rolling leukocytes in the pial penumbral microcirculation 48 hours after CCI. **p < 0.01 versus NS + Iso. D, Quantification of FITC-albumin leakage in the same pial venule 48 hours after CCI. **p < 0.01 versus NS + Iso; #p < 0.05, ##p < 0.01 versus ENX + MAb.
Brain and Lung Water Content
Brain and lung water content was determined by wet-to-dry ratios. Following IVM and euthanasia, brain tissue was rapidly extracted from the skull and divided into contralateral and ipsilateral hemispheres (in relation to CCI injury). Lungs were then extracted following sternotomy. All samples were immediately weighed to obtain wet weight (WW) and then dried at 70°C for 72 hours to obtain dry weight (DW). Percent brain and lung water content was calculated with the formula (WW − DW) / WW × 100%.
Body Weight Loss and Neurologic Recovery
Animal weights were measured at 0, 24, and 48 hours after CCI, and body weight loss ratio was calculated with the formula (W0h − W24h or 48h)/W0h.
Twenty-four hours and 48 hours after CCI, evaluation of neurologic function was performed using the 18-point modified Neurological Severity Score (NSS) specifically scoring animal motor, sensory, reflex, and balance as previously described.27
Statistical Analysis
All values are presented as mean (SD). SPSS software (SPSS, Chicago, IL) was used for all statistical analyses. For all outcomes measured, 6 post hoc pairwise comparisons were conducted using analysis of variance with Bonferroni correction to determine significance between group means (corrected α = 0.008). A p < 0.05 was considered statistically significant.
RESULTS
In Vivo LEU/EC Interactions and Microvascular Permeability
Compared with positive control (NS + Iso) animals (125.6 [60.4] LEU/100 μm/min), rolling LEUs were significantly reduced by both MAb (36.0 [16.9] LEU/100 μm/min, p = 0.001) or ENX (50.3 [20.6] LEU/100 μm/min, p = 0.005) alone, 48 hours after CCI (Fig. 2C). Combining both ENX and MAb (28.2 [5.9] LEU/100 μm/min) also resulted in less LEUs rolling than in NS + Iso animals (p = 0.001) but no less than with either treatment alone (p = 1.0). No differences were found in LEU adhesion among NS + Iso (0.2 [0.4] LEU/100 μm/min), NS + MAb (0.3 [0.5] LEU/100 μm/min), ENX + Iso (0.2 [0.4] LEU/100 μm/min), and ENX + MAb (0.2 [0.4] LEU/100 μm/min) groups, but numbers of adherent cells were low. Concurrently, FITC-labeled albumin leakage was notably reduced in the NS + MAb (23.8% [3.6%]; p = 0.001), ENX + Iso (23.1% [3.1%]; p = 0.001), and ENX + MAb (15.3% [4.5%], p < 0.001) groups as compared with that measured in the NS + Iso group (35.4% [4.3%], Fig. 2D). However, combining ENX and MAb treatment further reduced venular leakage as compared with either treatment alone (p = 0.02).
Brain and Lung Edema
Compared with positive controls (NS + Iso) (ipsilateral, 81.7% [1.4%]; contralateral, 77.6% [0.4%]; Fig. 3A), brain water content was similarly reduced by MAb (NS + MAb: ipsilateral, 78.2% [0.3%], p < 0.001; contralateral, 76.3% [0.5%], p < 0.001), ENX (ENX + Iso: ipsilateral, 77.9% [0.5%], p < 0.001; contralateral, 76.4% [0.4%], p < 0.001), or combined treatments (ENX + MAb: ipsilateral, 77.2% [0.3%]; contralateral, 76.2% [0.2%], p < 0.001) 48 hours after CCI. At 48 hours after injury, lung wet-to-dry ratios in NS + Iso animals (77.2% [0.7%]) were significantly reduced in either ENX groups irrespective of MAb (75.2% [1.2%], p = 0.03) or Iso administration (75.2% [1.1%], p = 0.04) (Fig. 3B).
Figure 3.
Animal outcomes. A, CCI-induced ipsilateral and contralateral cerebral edema was markedly reduced by NS + MAb, ENX + Iso, and ENX + MAb. Ipsilateral, **p < 0.01 versus NS + Iso; contralateral, ##p < 0.01 versus NS + Iso. B, Postinjury lung water content was reduced by ENX + Iso and ENX + MAb but not NS + MAb 48 hours after CCI. *p < 0.05 versus NS + Iso. C, Body weight loss ratios were decreased by NS + MAb and ENX + MAb but not ENX + Iso 24 hours after CCI. 24 hours, *p < 0.05 versus NS + Iso. D, Neurologic recovery 24 hours and 48 hours after injury was lowest in the vehicle-treated group as compared with any of the treated groups without an additive effects of ENX and MAb. 24 hours, **p < 0.01 versus NS + Iso; 48 hours, ##p < 0.01 versus NS + Iso.
Body Weight Loss and Neurologic Function
Persistent animal weight loss, a surrogate of poor neurologic recovery, was greatest in NS + Iso animals (12.5% [3.9%]) 1 day after injury. Twenty-four hours after CCI, antibody against HMGB1 showed a similar reduction in body weight loss regardless of ENX therapy (ENX + MAb, −7.2% [2.6%], p = 0.03; NS + MAb, −7.1% [1.2%]; p = 0.03) (Fig. 3C). All animals regained similar body weights by 48 hours after injury.
NSSs were lowest in the NS + Iso group both at 24 hours [0.5]) and 48 hours (15.2 [0.4]), and treatment with monoclonal blockade (24 hours, 16.3 [0.8], p = 0.001; 48 hours, 16.7 [0.5], p < 0.001), ENX (24 hours, 16.3 [0.5], p = 0.001; 48 hours, 16.7 [0.5], p < 0.001), or both (24 hours, 16.5 [0.5], p < 0.001; 48 hours, 16.8 [0.4], p < 0.001) resulted in similar improvements across treatment groups at both time frames.
DISCUSSION
In the present study, we report in vivo data demonstrating ENX-related reductions in cerebral LEU recruitment and brain edema that are correlated to greater concomitant neurologic recovery following TBI. We further demonstrate how HMGB1 blockade in this model closely mirrored these effects of ENX in an identical setting.
Following the initial mechanical cerebral tissue damage imparted by TBI, persistent local and regional neuroinflammation may contribute to progressive tissue injury promoting secondary brain injury.28,29 In this setting, progressive microvascular recruitment of activated LEUs to the area of tissue injury may play a critical role in promoting and sustaining this pervasive inflammation.30 Focal cerebrovascular sequestration of activated LEUs and in particular PMN may activate local ECs and increase microvascular disruption of the BBB leading to LEU extravasation into brain parenchyma. This tissue migration of circulating LEUs has been characterized to begin with the margination and rolling of LEU on endothelium instigated by surface up-regulation of carbohydrate-binding adhesion molecules or selectins including L-selectin, E-selectin, and P-selectin on both cell surfaces.31 LEUs then slow out of circulatory flow along the luminal endothelial surface and finally firmly adhere to the activated EC via covalent interactions between surface PMN CD11b receptors and intercellular adhesion molecule 1 or vascular adhesion molecule 1 expressed on endothelium.32 Schwarzmaier et al.33 demonstrated how LEU rolling can be observed as early as 1 hour and LEU adhesion to EC 4 hours following brain trauma. In a time-response study, our group found that LEU recruitment continues in the subsequent hours with maximal sequestration at 48 hours to 60 hours after injury.1 Tissue infiltrated, blood-borne LEUs facilitate activation of resident microglia resulting in their release of proinflammatory cytokines and oxidative metabolites.28,29 The resultant neurotoxic effects on the cerebral microcirculation are thought to exacerbate BBB disruption and cerebral edema and may result in persistent neurologic impairment.34
HMGB1 has been identified as a member of the family of DAMPs and may be secreted by activated CNS cells including astrocytes, microglia, and neurons.3–5 Once released after CNS injury, HMGB1 acts as an inflammatory modulator with cytokine- and chemokine-like activity,35 promoting LEU recruitment to the injured tissue by forming a chemotactic HMGB1/CXCL12 heterocomplex.36 HMGB1 may additionally modulate EC/PMN interactions by acting as a potent proinflammatory cytokine, readily activating ECs.37 Robinson et al.38 demonstrated that monoclonal blockade against HMGB1 reduced LEU infiltration into CNS tissues and blocked neuro-inflammatory responses in experimental autoimmune encephalomyelitis. Similar to other DAMPs, HMGB1 has also been shown to promote neuronal apoptosis39 and secondary lesion expansion.3 Using anti-HMGB1 MAb blockade, Liu et al.12 further reported attenuation of BBB permeability and brain infarction through reductions in the production of proinflammatory cytokines and microglia activation. Similarly, but using a middle cerebral artery occlusion model, instead, Zhang et al.5 reported that neutralization of neuron-derived HMGB1 suppressed BBB damage and subsequent brain edema. Specifically in a TBI rat model, others found that anti-HMGB1 blockade also inhibited overexpression of proinflammatory molecules and BBB disruption, reducing brain edema.40 In line with these data, our IVM findings also demonstrated that anti-HMGB1 MAb administration after TBI greatly reduced live recruitment of LEU to injured brain 48 hours after injury. This was further associated with both a reduction in concurrent microvascular permeability and an overall reduction in tissue water, suggesting that HMGB1 blockade directly prevented BBB injury, limiting expansion of the cerebral lesion. However, in vivo microvascular leakage of albumin captured 48 hours after injury decreased more by administration of ENX than anti-HMGB1 blockade, suggesting a broader effect of ENX on leakage at that moment in time.
TBI patients are among the trauma populations most at risk of developing VTEs. Heparin and low-molecular-weight heparins such as ENX have been shown to be highly effective in safely preventing VTE complications in these patients.41,42 Furthermore, some evidence suggests that if ENX prophylaxis is initiated late (>11 [1]days after admission), this may no longer protect TBI patients from VTEs.43 Unfortunately, routine earlyclinical administration of heparinoid VTE prophylaxis in TBI populations is often restricted at the bedside because of fears of worsening intracranial bleeding through their anticoagulant properties.
Unexpectedly, intriguing reports in the last decade have suggested that in addition to their anticoagulant effect, heparinoids may also possess powerful anti-inflammatory properties limiting progression of tissue injury in different organs. More than decade ago, in a series of elegant trauma and hemorrhagic shock rodent studies, Chaudry et al.15–17 demonstrated how heparin administration before shock/resuscitation prevented endothelial dysfunction, improving rheology and microvascular flow and ultimately improving end-organ (hepatic) function. In a study using a rat TBI model, early initiation of ENX (2 hours after trauma) reduced brain edema, lesion volume, and neurologic impairment.44 Our group investigated the role of cerebral LEU infiltration in this setting and showed how ENX administration after TBI diminished live LEUs rolling on pial endothelium, and that this was associated with concurrent reductions in microvascular leakage, also attenuating brain edema and accelerating neurologic recovery.21 While the modulation of the HMGB1 signaling pathway by heparins has been demonstrated in different animal models replicating sepsis,23,45 ischemia reperfusion,24 and lung injury,22,46 little is known of their interrelation following TBI. In the current study, we investigated the role of the HMGB1 signaling pathway in this setting and showed how HMGB1 blockade alone almost identically mirrored ENX effects, reducing penumbral LEU recruitment, cerebral edema (post mortem), and neurologic recovery. Of note, in vivo microvascular permeability was further reduced by combined treatment with ENX and HMGB1 blockade suggesting an additional effect by ENX. This may have occurred because ENX effects in brain tissue may more broadly protect the BBB beyond HMGB1 signaling, resulting a greater effect at the microscopic level but not when grossly evaluating wet-to-dry ratios of the entire cerebral hemisphere histologically. This greater multifaceted effect of ENX beyond attenuation of the HMGB1 pathway was also supported by our findings of greatly reduced lung tissue water with ENX but not by MAb blockade against HMGB1 alone.
Our finding of reduced lung edema with ENX treatment is in line with those of other studies which also demonstrated how heparinoids reduce PMN influx and pulmonary edema following stretch induced lung injury.46 Interestingly, in other lung injury and sepsis models, treatment with systemic ENX significantly reduced pulmonary HMGB1 levels.22,23 Nonetheless, we did not find a reduction in lung edema following monoclonal blockade of HMGB1 when ENX was absent. These authors did not evaluate pulmonary edema in a TBI model, and it remains unknown if it would have been reduced in groups subjected to HMGB1 monoclonal blockade. Clearly, additional investigation is needed to better characterize the relationship between HMGB1 and ENX on pulmonary edema following TBI.
The current study is small and has important limitations. First, we did not conduct a dose-response evaluation for optimal HMGB1 MAb concentration. Instead, we opted for a 25-μg MAb dose selected to mirror reports using this clone in other studies evaluating CNS injury models.5,12 Second, it would have been useful to include an uninjured, untreated negative (sham) control group to further characterize normalization of observed parameters. Yet, we included this group in a previous study,21 and to keep the study simpler to interpret, we restricted it to four experimental groups and chose not to add this group in this report. Third, it would have been beneficial to also measure circulating and tissue HMGB1 levels before and after MAb blockade or ENX treatment but because of the already lengthy time frame of each animal experiment and insufficient tissue available for this analysis, we were unable to do so. Irrespective, other models have confirmed heparinoid-related reductions in tissue HMGB1 levels.22,23 Fourth, we did not find differences among groups in visualized adherent LEU. While LEU rolling is an important step in LEU/EC interactions leading to transmigration into the tissue phase, in the current study, the number of sticking or adherent LEUs was extremely low as we could only safely record 30 seconds of fluorescent exposure at a time to prevent fluorescent light effects on the pial surface.21,26,47 We can only postulate that differences could not be ascertained with such low frequency of adhesion. It is important to note that other plausible explanations may also exist linking heparinoids to reduced LEU rolling. In particular, heparinoids could have reduced small vessel microthrombi formation, improving rheology and circulatory flow and increasing shear stress, thereby reducing interactions between LEU and EC.15,16,48,49 Alternatively, the circulating LEU count may have differed among groups, giving rise to parallel differences in neutrophils that were rolling, but systemic white blood cell count was not measured. Finally, ENX doses used were greater than usual human doses. This was done to reflect previously published models of CNS rodent studies and was not associated with increased bleeding complications.21,44,50
Taken together, the current study confirms previous findings of reduced live LEU recruitment to injured brain by ENX, diminishing cerebrovascular permeability and brain edema and accelerating neurologic recovery. HMGB1 blockade seems to confer near-identical effects but, unlike ENX, did not significantly reduce lung edema, suggesting that ENX has anti-inflammatory properties distinct from the HMGB1 pathway. Furthermore, ENX reductions in lung edema after TBI in this model were not replicated by HMGB1 blockade. Whether ENX definitively targets the HMGB1 pathway will need further study measuring circulating and tissue levels or using genetic knockout models.
ACKNOWLEDGMENT
We thank Ms. Robin Armstrong for her technical and organizational assistance. We also thank Isabel Xu for her assistance in the analysis of the in vivo video footage.
Discussion
Dr. Ronald V. Maier (Seattle, Washington): As we were discussing earlier regarding science in trauma surgery, this study is a definite example of a well-performed series of experiments that is well-considered and may have significant contributions in the treatment of our patients. It is what we hope for going forward.
The authors’ hypothesis is based on, as stated, that traumatic brain injury releases HMBG1, which is an inflammatory mediator or DAMP that is recognized to increase leukocyte activation. The leukocytes stick in the brain microcirculation and they contribute to secondary injury, which we worry about in brain injury that causes a worse outcome, increased edema, and more difficulties. They also base their study on a series of studies in the literature that actually show that heparinoids are very effective at blocking many of these deleterious effects in brain injury and there may be a relation to HMBG1.
Since it is based on a series of studies in the literature, the fact that they were able to reconfirm many of the studies in the literature and find that the heparinoid actually did improve neurologic recovery after brain injury seems to me to further confirm that what they have observed and demonstrated is real and a worthwhile proposal to seek and chase. What is less clear is they have this commitment to their hypothesis that the benefit is due to blocking HMGB1; whereas the data, while supporting it, has no means of proving causality.
There are many alternative explanations. The most common would be that heparin provides microcirculatory sledging in the brain injury, therefore there is less ischemia and there is less brain injury, the brain does better, it has nothing to do with HMGB1.
And, as they mentioned, the results are variable and they did not have an impact of blocking HMGB1 on lung edema, which further supports that these may be different mechanisms. They say knock-out models to attest this but I would ask them if they have some more simplistic ways to try and unravel this complex question.
Secondly, many factors are not controlled in this study. Does the TBI model cause differential intracranial pressures? They had access to the brain. Did they measure the pressures? Was there a differential impact on the number of leukocytes circulating? This would affect their analyses were they the same number. Was the blood pressure affected? Did heparin produce a normal blood pressure and better perfusion?
And, lastly, as they alluded to, the dose of enoxaparin they gave was enormous. And this, again, proves that mice are different than men. It was probably necessary to get a positive outcome. But does it mean that it is not reasonable to attempt clinical trials? Can the enoxaparin be given without causing increased bleeding and increased death? And can it actually be a therapeutic?
Thank you.
Dr. Carl Hauser (Boston, Massachusetts): As usual it’s hard to follow Ron. I would say things a little differently. HMGB1 is a very interesting molecule with many roles, and it is probably released in two waves at different times with completely different effects.
First of all, it is a true ‘DAMP’ in that it is released passively from destroyed cells where it resides normally in the nucleus. But in inflammation it can also be actively released, perhaps from endothelium as a second wave of inflammation. Here it acts as a cytokine, signaling through TLR-4. That is an inflammatory pathway so anti-HMGB1 will be an anti-inflammatory molecule.
Now heparin is also well known to be an anti-inflammatory molecule. So I have to wonder why the authors think those two events are linked? I can’t figure out after listening to this paper how anyone could claim there’s causation here. And how can you distinguish heparin effect here from HMGB1-mediated anti-inflammation?
The other question I would ask is what chance do you think you have of getting a neurosurgeon to treat a brain injury with Lovenox?
Dr. Mitchell J. Cohen (San Francisco, California): A really nice study, nicely presented. I do have a couple of questions.
The first is the relative levels of HMGB1 not measured either in the brain injury or after the antibody. You alluded to you didn’t have those measurements. You know there is an alternative explanation that you have enoxaparin preventing thrombin signaling which is known to be a pro-inflammatory and to break down blood-brain barrier and cause endothelial permeability through PAR 1. I am wondering if you know the thrombin levels and the relative thrombin HMGB1 levels?
The second question is: what is the antibody to HMGB1C? Are you sure that you are really knocking out all HMGB1 signaling with whatever the antibody you had was?
And then the third question is: do you know what your endothelium in the brain looks like histologically in these, in this model? You know, apropos of Carl’s question, is this coming from the blood-brain barrier just breaking apart or is this happening via some other mechanism?
Dr. Paula Ferrada (Richmond, Virginia): Virginia, a great paper. A couple of questions. Did you have adverse events related to bleeding? And the second, did you measure Factor XA levels?
Thanks.
Dr. Vishal Bansal (San Diego, California): Very interesting paper. I love TBI work. Two questions. Does your antibody actually cross the blood-brain barrier or is it dependent solely on blood barrier breakdown secondary to severe TBI?
And, secondly, you know, your cognitive recovery is not a very good measure, frankly, for neurocognitive outcome. You need to extend that to seven days to fourteen days, including other balance beam assay—a Morris water maze or a rotarod test. These are the types of assays that will allow you to determine whether there is true cognitive differentiation with your therapy.
Dr. James Hoth (Wake Forest, North Carolina): My question is: have you considered alternative pathways, in particular complement? Enoxaparin has been shown to inhibit complement activation.
Dr. Joshua Marks (Pittsburgh, Pennsylvania): Thank you Dr. Maier for your great discussion and questions, and thank you others for your insightful questions as well. I will try to address as many of them as possible.
First, many of the questions asked focus on how we attribute our findings to causality and precise mechanism. I think that to jump that far would really be the next series of experiments. In the literature there is a lot discussed about HMGB1 and its associations in sepsis and in lung injury. We wanted to start looking at these interactions in the brain. This series of traumatic brain injury experiments ask whether HMGB1 blockade mirrors the effects we have observed with enoxaparin. Certainly further studies are necessary to establish a true causation and mechanism. HMGB1 blockage is one possible mechanistic pathway. There is room for other interpretations and other possibilities.
Measuring HMGB1 levels pre- and post-blockade and after enoxaparin, as we are now doing in our next wave of experiments, would help in further elucidating the relationship and possibly mechanism as it has been seen in sepsis and in lung injury models.
We specifically did not measure ICP, leukocyte count or blood pressure, as asked. All animals were treated the same. This work builds on many other protocols that we have in our lab and other things previously presented at this meeting and at others where we have looked specifically at some of those markers to assure that our model holds validity and that the animals are all treated the same. Obviously with a small animal and a small amount of blood there are only so many assays that can be run on each animal at each moment. With each series of experiments we have been examining different aspects while keeping other factors constant.
Our prior work looked specifically at bleeding and the Lovenox dosing. As I said this is an animal mouse model standard, rather than reinvent something that is not published in the literature, we have used this standard here and in our prior work. We have not seen an increase in contusion size with this dosing. It is certainly much higher dosing than we are clinically used to and one that would certainly make our neurosurgical colleagues very nervous.
These are preliminary animal data that we are looking forward to continuing and that hopefully one day will translate into our care of human patients.
Thank you very much again for the opportunity and privilege of the podium.
Footnotes
This study was presented at the 74th annual meeting of the American Association for the Surgery of Trauma, September 9–12, 2015, in Las Vegas, Nevada.
AUTHORSHIP
Each author has contributed significantly to and is willing to take public responsibility for one or more aspects of the study including its design, data acquisition, as well as analysis and interpretation of data.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Li SM, Marks J, Sanati P, Eisenstadt R, Gong W, Browne K, Smith D, Becker L, Pascual J. In vivo evolution of microvascular inflammation after traumatic brain injury: an intravital microscopy study. Crit Care Med. 2011;39(12 Suppl 1):1. [Google Scholar]
- 2.Schoettle RJ, Kochanek PM, Magargee MJ, Uhl MW, Nemoto EM. Early polymorphonuclear leukocyte accumulation correlates with the development of posttraumatic cerebral edema in rats. J Neurotrauma. 1990;7(4):207–217. doi: 10.1089/neu.1990.7.207. [DOI] [PubMed] [Google Scholar]
- 3.Fang P, Schachner M, Shen YQ. HMGB1 in development and diseases of the central nervous system. Mol Neurobiol. 2012;45(3):499–506. doi: 10.1007/s12035-012-8264-y. [DOI] [PubMed] [Google Scholar]
- 4.Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci. 2011;31(3):1081–1092. doi: 10.1523/JNEUROSCI.3732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, et al. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke. 2011;42(5):1420–1428. doi: 10.1161/STROKEAHA.110.598334. [DOI] [PubMed] [Google Scholar]
- 6.Magna M, Pisetsky DS. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol Med. 2014;20:138–146. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang H, Antoine DJ, Andersson U, Tracey KJ. The many faces of HMGB1: molecular structure-functional activity in inflammation, apoptosis, and chemotaxis. J Leukoc Biol. 2013;93(6):865–873. doi: 10.1189/jlb.1212662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao TL, Yuan XT, Yang D, Dai HL, Wang WJ, Peng X, Shao HJ, Jin ZF, Fu ZJ. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J Trauma Acute Care Surg. 2012;72(3):643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- 10.Sun Q, Wu W, Hu YC, Li H, Zhang D, Li S, Li W, Li WD, Ma B, Zhu JH, et al. Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage invivo and invitro. J Neuroinflammation. 2014;11:106. doi: 10.1186/1742-2094-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uzawa A, Mori M, Taniguchi J, Masuda S, Muto M, Kuwabara S. Anti–high mobility group box 1 monoclonal antibody ameliorates experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2013;172(1):37–43. doi: 10.1111/cei.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, et al. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J. 2007;21(14):3904–3916. doi: 10.1096/fj.07-8770com. [DOI] [PubMed] [Google Scholar]
- 13.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, et al. HMGB1–Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 14.Valle EJ, Van Haren RM, Allen CJ, Jouria JM, Bullock MR, Schulman CI, Namias N, Livingstone AS, Proctor KG. Does traumatic brain injury increase the risk for venous thromboembolism in polytrauma patients? J Trauma Acute Care Surg. 2014;77(2):243–250. doi: 10.1097/TA.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 15.Rana MW, Singh G, Wang P, Ayala A, Zhou M, Chaudry IH. Protective effects of preheparinization on the microvasculature during and after hemorrhagic shock. J Trauma. 1992;32(4):420–426. doi: 10.1097/00005373-199204000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs after hemorrhage in nonheparinized but not in preheparinized models. J Surg Res. 1993;54(5):499–506. doi: 10.1006/jsre.1993.1077. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Singh G, Rana MW, Ba ZF, Chaudry IH. Preheparinization improves organ function after hemorrhage and resuscitation. Am J Physiol. 1990;259(3):R645–R650. doi: 10.1152/ajpregu.1990.259.3.R645. Pt 2. [DOI] [PubMed] [Google Scholar]
- 18.Maugeri N, Di Fabio G, Barbanti M, de Gaetano G, Donati MB, Cerletti C. Parnaparin, a low-molecular-weight heparin, prevents P-selectin–dependent formation of platelet-leukocyte aggregates in human whole blood. Thromb Haemost. 2007;97(6):965–973. doi: 10.1160/th06-12-0680. [DOI] [PubMed] [Google Scholar]
- 19.Miller SJ, Hoggat AM, Faulk WP. Heparin regulates ICAM-1 expression in human endothelial cells: an example of non–cytokine-mediated endothelial activation. Thromb Haemost. 1998;80(3):481–487. [PubMed] [Google Scholar]
- 20.Peter K, Schwarz M, Conradt C, Nordt T, Moser M, Kubler W, Bode C. Heparin inhibits ligand binding to the leukocyte integrin Mac-1 (CD11b/CD18) Circulation. 1999;100(14):1533–1539. doi: 10.1161/01.cir.100.14.1533. [DOI] [PubMed] [Google Scholar]
- 21.Li S, Marks JA, Eisenstadt R, Kumasaka K, Samadi D, Johnson VE, Holena DN, Allen SR, Browne KD, Smith DH, et al. Enoxaparin ameliorates post-traumatic brain injury edema and neurologic recovery, reducing cerebral leukocyte endothelial interactions and vessel permeability in vivo. J Trauma Acute Care Surg. 2015;79(1):78–84. doi: 10.1097/TA.0000000000000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li LF, Yang CT, Huang CC, Liu YY, Kao KC, Lin HC. Low-molecular-weight heparin reduces hyperoxia-augmented ventilator-induced lung injury via serine/threonine kinase-protein kinase B. Respir Res. 2011;12:90. doi: 10.1186/1465-9921-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning F, Wang X, Shang L, Wang T, Lv C, Qi Z, Wu D. Low molecular weight heparin may prevent acute lung injury induced by sepsis in rats. Gene. 2015;557(1):88–91. doi: 10.1016/j.gene.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, He Y, Wu X, Zhang S, Chong Z, Jiang Y, Zhou C, Jin X. Heparin attenuates HMGB1 expression in arterial tissue subjected to limb ischemia/reperfusion. Int J Cardiol. 2014;176(2):543–546. doi: 10.1016/j.ijcard.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Schwarzmaier SM, Kim SW, Trabold R, Plesnila N. Temporal profile of thrombogenesis in the cerebral microcirculation after traumatic brain injury in mice. J Neurotrauma. 2010;27(1):121–130. doi: 10.1089/neu.2009.1114. [DOI] [PubMed] [Google Scholar]
- 26.Pascual JL, Murcy MA, Li S, Gong W, Eisenstadt R, Kumasaka K, Sims C, Smith DH, Browne K, Allen S, et al. Neuroprotective effects of progesterone in traumatic brain injury: blunted in vivo neutrophil activation at the blood-brain barrier. Am J Surg. 2013;206:840–845. doi: 10.1016/j.amjsurg.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaar KL, Brenneman MM, Savitz SI. Functional assessments in the rodent stroke model. Exp Transl Stroke Med. 2010;2(1):13. doi: 10.1186/2040-7378-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Loane DJ. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26(8):1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Ziebell JM, Morganti-Kossmann MC. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7(1):22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzmaier SM, Plesnila N. Contributions of the immune system to the pathophysiology of traumatic brain injury—evidence by intravital microscopy. Front Cell Neurosci. 2014;8:358. doi: 10.3389/fncel.2014.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vestweber D. Novel insights into leukocyte extravasation. Curr Opin Hematol. 2012;19(3):212–217. doi: 10.1097/MOH.0b013e3283523e78. [DOI] [PubMed] [Google Scholar]
- 32.Reglero-Real N, Marcos-Ramiro B, Millan J. Endothelial membrane reorganization during leukocyte extravasation. Cell Mol Life Sci. 2012;69(18):3079–3099. doi: 10.1007/s00018-012-0987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzmaier SM, Zimmermann R, McGarry NB, Trabold R, Kim SW, Plesnila N. In vivo temporal and spatial profile of leukocyte adhesion and migration after experimental traumatic brain injury in mice. J Neuroinflammation. 2013;10:32. doi: 10.1186/1742-2094-10-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vestweber D. Relevance of endothelial junctions in leukocyte extravasation and vascular permeability. Ann N Y Acad Sci. 2012;1257:184–192. doi: 10.1111/j.1749-6632.2012.06558.x. [DOI] [PubMed] [Google Scholar]
- 35.Yanai H, Ban T, Taniguchi T. High-mobility group box family of proteins: ligand and sensor for innate immunity. Trends Immunol. 2012;33(12):633–640. doi: 10.1016/j.it.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Venereau E, Schiraldi M, Uguccioni M, Bianchi ME. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol Immunol. 2013;55(1):76–82. doi: 10.1016/j.molimm.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 37.Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, et al. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. J Exp Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson AP, Caldis MW, Harp CT, Goings GE, Miller SD. High-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitis. J Autoimmun. 2013;43:32–43. doi: 10.1016/j.jaut.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine (Phila Pa 1976) 2010;35(11):1109–1115. doi: 10.1097/BRS.0b013e3181bd14b6. [DOI] [PubMed] [Google Scholar]
- 40.Okuma Y, Liu K, Wake H, Zhang J, Maruo T, Date I, Yoshino T, Ohtsuka A, Otani N, Tomura S, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72(3):373–384. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- 41.Dudley RR, Aziz I, Bonnici A, Saluja RS, Lamoureux J, Kalmovitch B, Gursahaney A, Razek T, Maleki M, Marcoux J. Early venous thromboembolic event prophylaxis in traumatic brain injury with low-molecular-weight heparin: risks and benefits. J Neurotrauma. 2010;27(12):2165–2172. doi: 10.1089/neu.2010.1366. [DOI] [PubMed] [Google Scholar]
- 42.Norwood SH, Berne JD, Rowe SA, Villarreal DH, Ledlie JT. Early venous thromboembolism prophylaxis with enoxaparin in patients with blunt traumatic brain injury. J Trauma. 2008;65(5):1021–1026. doi: 10.1097/TA.0b013e31818a0e74. [DOI] [PubMed] [Google Scholar]
- 43.Daley MJ, Ali S, Brown CV. Late venous thromboembolism prophylaxis after craniotomy in acute traumatic brain injury. Am Surg. 2015;81(2):207–211. [PubMed] [Google Scholar]
- 44.Wahl F, Grosjean-Piot O, Bareyre F, Uzan A, Stutzmann JM. Enoxaparin reduces brain edema, cerebral lesions, and improves motor and cognitive impairments induced by a traumatic brain injury in rats. J Neurotrauma. 2000;17(11):1055–1065. doi: 10.1089/neu.2000.17.1055. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Ling Y, Huang M, Yin T, Gou SM, Zhan NY, Xiong JX, Wu HS, Yang ZY, Wang CY. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72(1):36–42. doi: 10.1016/j.cyto.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Li LF, Huang CC, Lin HC, Tsai YH, Quinn DA, Liao SK. Unfractionated heparin and enoxaparin reduce high-stretch ventilation augmented lung injury: a prospective, controlled animal experiment. Crit Care. 2009;13(4):R108. doi: 10.1186/cc7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumasaka K, Marks JA, Eisenstadt R, Murcy MA, Samadi D, Li S, Johnson V, Browne KD, Smith DH, Schwab CW, et al. In vivo leukocyte-mediated brain microcirculatory inflammation: a comparison of osmotherapies and progesterone in severe traumatic brain injury. Am J Surg. 2014;208(6):961–968. doi: 10.1016/j.amjsurg.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dong C, Lei XX. Biomechanics of cell rolling: shear flow, cell-surface adhesion, and cell deformability. J Biomech. 2000;33(1):35–43. doi: 10.1016/s0021-9290(99)00174-8. [DOI] [PubMed] [Google Scholar]
- 49.Sundd P, Pospieszalska MK, Cheung LS, Konstantopoulos K, Ley K. Biomechanics of leukocyte rolling. Biorheology. 2011;48(1):1–35. doi: 10.3233/BIR-2011-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zupan Z, Pilipovic K, Dangubic B, Frkovic V, Sustic A, Zupan G. Effects of enoxaparin in the rat hippocampus following traumatic brain injury. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(8):1846–1856. doi: 10.1016/j.pnpbp.2011.08.005. [DOI] [PubMed] [Google Scholar]