Abstract
BACKGROUND AND OBJECTIVES:
The occurrence of hemolysis due to transfusion of ABO plasma-incompatible platelets (PLTs) is challenging. There has been no consensus for critical antibody titers in the transfusion community. This study was conducted to understand the trends of anti-A and anti-B antibody titer levels in O group donors and to identify any specific patterns of distribution in relation to age and gender.
MATERIALS AND METHODS:
A total of 1635 Group O PLT donors were randomly selected for this prospective study. Serial 2-fold doubling dilutions were prepared for each sample to calculate the titer of anti-A and anti-B in a standard 96 well micro-plate. Tube technique was used for comparison with the microplate method for 100 samples.
RESULTS:
Out of 1635 donors, 1430 (87.46%) were males and 205 (12.54%) were females. The median titer for anti-A and anti-B was 128 with range from 4 to 2048. Spearman's correlation coefficient for microplate versus tube technique was estimated to be 0.803 (P < 0.01, two-tailed). 57.12% and 51.19% of all donors had titers ≥128 for anti-A and anti-B, respectively. The geometric mean of anti-A and anti-B was 155.7 and 137.28, respectively. The titers were significantly higher (P < 0.001) in female donors. An inverse relation between titer levels and age was seen.
CONCLUSION:
Microplate can be used to perform titers in resource-constrained settings. Screening for critical titers in O group donors is essential as they are more implicated in hemolytic transfusion reactions. In the absence of a global consensus on this topic, institutes may need to formulate their own guidelines on handling ABO plasma-incompatible PLT transfusions.
Keywords: Antibody titers, hemolytic transfusion reaction, O group, platelet donor
Understanding issues related to ABO compatibility for the purpose of platelet (PLT) transfusion is necessary.[1] Maintaining platelet concentrate (PC) inventory is a challenging task due to a high demand and a short shelf life of the product. Although guidelines suggest the transfusion of ABO-compatible PC, it is an acceptable practice to transfuse other blood group PCs.[2] Intravascular hemolysis due to passively transferred antibodies in a minor ABO-incompatible PC transfusion can cause severe morbidity and mortality. These antibodies are naturally occurring anti-A and anti-B of IgM type and can readily activate complement leading to hemolysis.[3] Many recent studies have focused on the role of antibody titers in the causation of hemolytic transfusion reactions (HTRs). Case reports from different workers have tried to report the titer levels which may be considered dangerous.[4,5] There has been a growing interest in defining safe or critical titer levels for the purpose of issuing a minor incompatible PC. However, there is a lack of consensus over what constitutes a safe titer level because of differences in methodologies adopted to quantify titers, type, and class of antibody detected, and the lack of studies necessary to correlate these methods.[6,7,8]
A review of literature indicates that titers between 16 and 600 may be clinically relevant.[4] The United Kingdom have addressed the issue by implementing a policy to screen all O group donors for IgM titers and label those with titers ≥100 as “high-titer” and allowed to be transfused only to O group patients.[9] However, there is no recommended standard in the blood bank community.[3] In India, there is a dearth of national standards of practice to guide ABO-incompatible PLT transfusions. This is coupled with a paucity of data on titer levels in Indian population with only a few reports about them.[10,11,12] Our institute follows a policy of performing titer levels and doing a plasma volume reduction before issuing a plasma ABO-incompatible PC. However, volume reduction needs time, technical expertise, and causes PLT loss.[13] This study screens Group O PLT donors for anti-A and anti-B titers. Our O group donor population is 40%, and many can be high titer. The aim was to identify the isoagglutinin levels in them. We did a manual microplate method to evaluate titers as it is known to be faster and cost-effective.[14] We compared the manual microplate method with conventional tube technique.
Materials and Methods
Study setting
The study was conducted in a tertiary care oncology center over a period of 4 months from June to September 2014. The study protocol was approved by the Institutional Ethics Committee of the hospital.
Study population
The study population comprised Group O donors presenting for blood or PLT donation after being declared fit as per national guidelines for donor selection.
Samples and reagents
Plasma from blood samples collected at the time of donation in 3 ml ethylenediaminetetraacetic acid vacutainers was used to study titers. The vacutainers were centrifuged at 2500 rpm for 3 min. All samples were subjected to titration for anti-A and anti-B antibodies by microplate method. In-house freshly prepared pooled cells of Group A1, and B were used for titer studies. The cells were prepared daily using departmental standard operating procedures and subjected to quality control check before use. There was no variation in antigen expression of pooled cells as ascertained by the strength of agglutination with commercial antisera.
Methods
1635 O group donors were randomly selected for the purpose of the study. A standard 96 well, “U” bottom polystyrene microplate (Greiner™) was used to study the titers. The methodology was adapted from principles of using microplate technique for ABO typing.[15] Two rows of serial 2-fold doubling dilutions were prepared for each sample to calculate titer of anti-A and anti-B. Testing was performed for dilutions 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512 and 1:1024. If necessary, higher dilutions were assessed. Each microplate sufficed testing for 4 donors. Multichannel pipettes were calibrated periodically and used to load the wells due to their accuracy and ease of performance of test and lesser time required to load the wells. A 3% suspension of pooled cells prepared as described above was used for titer study. The plates were incubated for 15 min and centrifuged in a microplate centrifuge (Labofuge 400, Heraeus, Inc.,), for 30 s at 400 rpm. Microplates were later kept on a mechanical agitator (Titramax 1000, Heidolph, Inc.,) at 900 rpm for 1 min. The agglutination was read manually by placing the microplate at 45° angle to the horizontal for 15 s and observing a streaming pattern of cells. The plates were read by observing the under-surface for better visualization of the agglutinates which was read as a positive result and turbid smooth suspension as a negative result. One hundred samples were run in parallel by the tube and microplate technique to compare between the two methods. All the samples were run using the same batch of pooled cells on a given day. The reporting was done by one observer and these results were verified by another person. Both the observers were blind to the microplate results while reporting results on tube technique. Antibody titer was reported as the reciprocal of the highest dilution of plasma that produced a visible macroscopic agglutination.
Statistical analysis
Data related to donor demographic characteristics such as age and gender was reported using descriptive statistics. The sample was corrected for gender, and statistical analysis was performed using Mann–Whitney U-test. Two-tailed Spearman's correlation coefficient (r vs. r2) was calculated for tube technique and microplate method. Concordance for microplate with tube technique was calculated using parameters such as single tube difference, dual tube difference, and percentage concordance.
Results
O group donors were randomly selected for the study (n = 1635). Of these, 1430 (87.46%) were males, and 205 (12.54%) were females.
To compare the efficacy of microplate method with a standard reference method (tube), we tested 100 samples out of these 1635. Spearman's correlation coefficient for tube technique versus microplate method (r vs. r2) was estimated to be 0.803 (P < 0.01, two-tailed). 40% of samples matched accurately in both techniques. In the remainder 60% samples, microplate showed one tube higher reading in 40/60 samples.
The mean age of our study population was 30.96 years. Distribution of anti-A and anti-B titers in donor population is represented in Table 1. The antibody titer frequency in donors is represented in Figures 1 and 2. The anti-A and anti-B titer were ≥128 in 57.12% and 51.19% of all donors, respectively. The Anti-A and anti-B titer were ≥64 in 79.9% and 76.33% of all donors, respectively. Across the entire donor population, the anti-A titers were significantly higher (P < 0.001) as compared to anti-B titers. Among the female donors anti-A and anti-B titers were significantly higher (P < 0.001) when compared to males. There is an inverse relation between titer levels and age (males: anti-A and anti-B, females: anti-B) with titer levels reducing as age progressed [Figures 3, 4 and 6].
Table 1.
Description of overall distribution of anti-A and anti-B titers in donor population (n=1635)
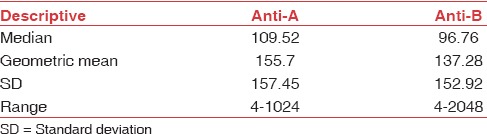
Figure 1.
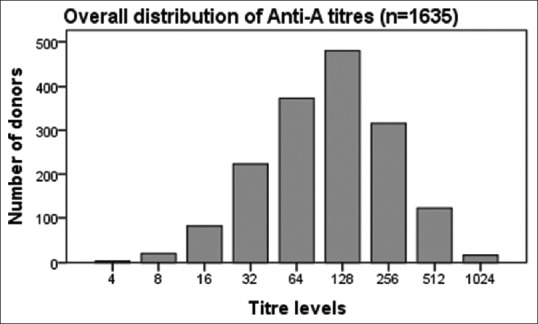
Frequency distribution of anti-A in all donors.
Figure 2.

Frequency distribution of anti-B in all donors.
Figure 3.
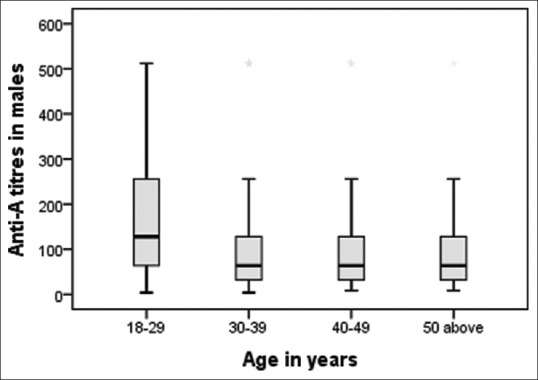
Age-wise distribution of anti-A in males.
Figure 4.
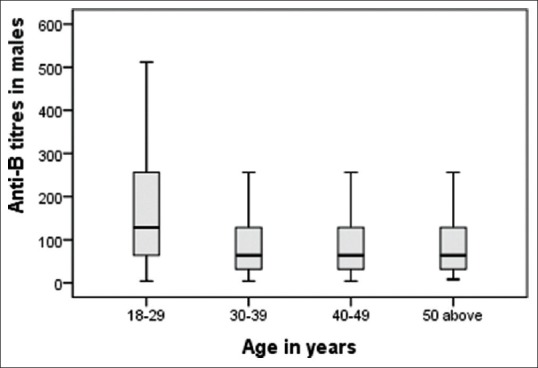
Age-wise distribution of anti-B in males.
Figure 6.
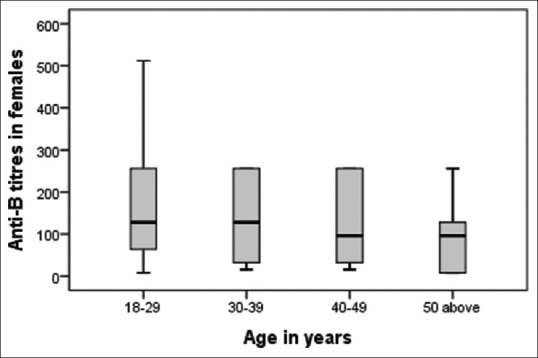
Age-wise distribution of anti-B in females.
Titer levels as per age are shown in Tables 2 and 3. We found the titers were significantly higher (P < 0.01) in donors of age 18–29 years. Fifty two percent of our donor population belongs to this age group. The inverse relation of titer and age was not seen in females for anti-A [Figure 5].
Table 2.
Age-related distribution of titers in O group male donors
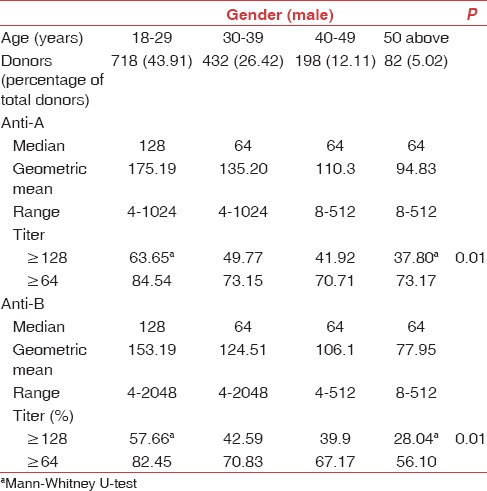
Table 3.
Age-related distribution of titers in O group female donors
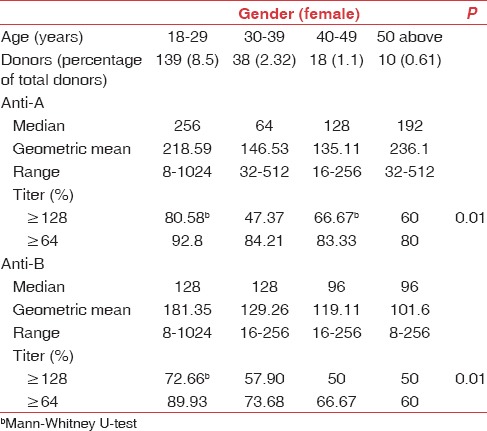
Figure 5.
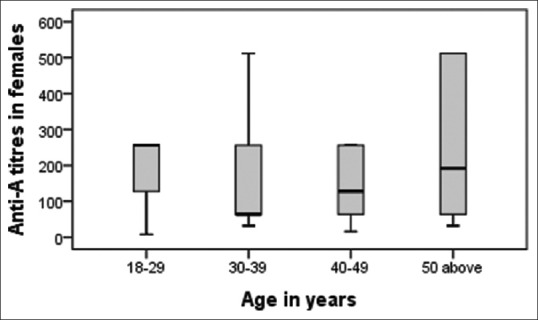
Age-wise distribution of anti-A in females.
Discussion
This study examines the prevalence of high titers in Group O donors in our setting. Products from such donors can lead to a fatal intravascular HTR if minor ABO-incompatible PLT transfusions are attempted.[13,16] We explored the advantages of using a microplate method for performing titers. Literature cites column agglutination technology (CAT) and tube techniques for the same.[4,6,7,8] To the best of our knowledge, this is the first study in our region assessing the microplate method for performing antibody titers.
This study showed a correlation of 0.803 (Spearman's correlation coefficient) and a concordance of 40% between manual microplate and tube method. The studies comparing CAT-based methods with tube technique have reported similar concordance between the two methods.[4] We found that with proper standardization and validation, the microplate can be an acceptable method. We hope that these findings would help resource-constrained setups to consider microplate as against CAT-based techniques which need expertise and higher investments.[17] The implementation of microplate-based technique requires minimal training of existing staff and can be performed using in-house prepared reagents.[14] The technique has an advantage of high throughput, less time, and high sensitivity. Compared to tube method, it requires lesser reagents and working space. From our findings, we can conclude that among other benefits, microplate testing for antibody titers does not give any false negative results.
The median titers for anti-A and anti-B were 128 in our study. Out of 1635, 57.12% and 51.19% donors had titers ≥128 for anti-A and anti-B, respectively. Literature reports on titers in white population are available however methods of titration vary.[4,18] Our results are similar to those reported in Asians and Africans as compared to Caucasians.[19,20] A high isoagglutinin titer in the Asian and Black populations has been attributed to increased the incidences of mosquito bites and intestinal parasitic infections.[21] Among O group individuals, high titers of ABO antibodies (IgM) and hemolysins (IgG) can be due to vaccination and other antigen exposures such as pregnancy, and transfusions.[22] The levels of naturally occurring antibodies depend on the ethnic background of the donors and also on environmental factors.[19,23] These titers levels can change over a period as cited in a study on Japanese population. The reasons cited for titer reduction were betterment of environmental hygiene leading to lesser parasitic and enteric infections along with higher consumption of processed food by the Japanese donors as compared to other Asiatic population.[24]
Josephson et al.[4] found a high prevalence of Group O apheresis donors having titers of ≥64 for IgM and ≥256 for IgG using gel CAT. The critical titer value of 64 and 256 was selected based on cited literature.[25,26,27] The authors agreed that there was no consensus on a definitive value for critical titers. If our study considers a critical titer value of 64 for IgM, then 79.9% and 76.33% of our donors have anti-A and anti-B titers, respectively. Thus, this percentage is more than that reported by other studies.[4,28]
In the United States, regulatory agencies including the American Association of Blood Banks and College of American Pathologists mandates the blood banks to have the policy to prevent HTR from plasma-incompatible products.[3] In the absence of regulation, coupled with a lack of consensus over a critical titer level and a large proportion of O group donors showing antibody titers ≥64 in our country, there are high chances of an HTR. In our institute, the percentage of out of group PLT transfusions is about 10.5% of all PLT transfusions. Hence, it would be prudent to consider methods to reduce hazards caused by such ABO-incompatible PLT transfusions.
We studied the age-wise distribution of titers in O group donors. The mean age of the donors was 30.96 years, and 52.41% of donors belong to age group of 18–29 years. 85.88% and 83.66% of donations from this age group have titers ≥64 for anti-A and anti-B respectively. Hence, the chances of finding Group “O” PLTs with titers <64 is miniscule. Thus, we find it beneficial to screen all O group donors for isoagglutinin titers. Similar findings are also reflected in a report from Brazil,[18] wherein the authors have found a higher mean titer level in younger population.
We studied the gender-wise distribution of titers and found the titers to be higher in females as compared to males across all age groups. The study highlighted a rising trend for titers of anti-A in females after 40 years of age. This can be attributed to the immune exposures such as pregnancy and vaccinations.[22] An opposite trend is revealed in males, with titer level progressively reducing in men across all age groups. Similar findings have been reported from Brazilian population.[18]
We studied the antibody titer levels and their distribution in our donors to help us in understanding the trends and ameliorating the problems associated with ABO-incompatible PLT transfusions. The last published data on this topic was studied by Indian authors three decades ago.[11,12,29] The range of titers implicated in HTR is between 32 and 16,384 in saline phase.[3] Hence, there is a need to develop a screening program for O group donors, especially in areas having higher density of such donors. In our study, we found titers in O group donors ranging from 4 to 1024 for anti-A and 4 to 2048 for anti-B. Issues to be considered while developing such a program include serologic technique (manual tube versus microplate versus gel CAT on automated platform), the antibody to be studied (IgM, IgG), determining a threshold for critical antibody titer, performance of serial titration versus single predetermined dilution.[4,6]
We estimated IgM antibody titers in O group donors. The IgG and hemolysin test also help in predicting the possibility of HTR. However, it has been reported that hemolysins are found in higher percentage of donors with IgM titers ≥64.[10]
It has been reported that lifestyle changes are associated with changes in titer levels.[30] Hence, it would be prudent to screen the donors for titers at each visit to look for any changes in titer levels.
Conclusion
To summarize, our study shows that manual microplate technique can be used as a reliable method to perform titers in resource-constrained settings. There is a need to define critical titer values in O group donors as they are more implicated in HTRs. In the absence of a global consensus on this topic, institutes may need to formulate their own guidelines on handling ABO plasma-incompatible PLT transfusions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
All authors fulfill the following three criteria: substantial contributions to research design or the acquisition, analysis or interpretation of data, drafting the paper or revising it critically, and approval of the submitted and final versions.
References
- 1.Lee EJ, Schiffer CA. ABO compatibility can influence the results of platelet transfusion. Results of a randomized trial. Transfusion. 1989;29:384–9. doi: 10.1046/j.1537-2995.1989.29589284135.x. [DOI] [PubMed] [Google Scholar]
- 2.British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 3.Fung MK, Downes KA, Shulman IA. Transfusion of platelets containing ABO-incompatible plasma: A survey of 3156 North American laboratories. Arch Pathol Lab Med. 2007;131:909–16. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 4.Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high-titer” anti-A/A, B: Implications for transfusion policy. Transfusion. 2004;44:805–8. doi: 10.1111/j.1537-2995.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 5.Quillen K, Sheldon SL, Daniel-Johnson JA, Lee-Stroka AH, Flegel WA. A practical strategy to reduce the risk of passive hemolysis by screening plateletpheresis donors for high-titer ABO antibodies. Transfusion. 2011;51:92–6. doi: 10.1111/j.1537-2995.2010.02759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AuBuchon JP, de Wildt-Eggen J, Dumont LJ. Biomedical Excellence for Safer Transfusion Collaborative; Transfusion Medicine Resource Committee of the College of American Pathologists. Reducing the variation in performance of antibody titrations. Vox Sang. 2008;95:57–65. doi: 10.1111/j.1423-0410.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 7.Kumlien G, Wilpert J, Säfwenberg J, Tydén G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007;84(12 Suppl):S17–9. doi: 10.1097/01.tp.0000296019.85986.af. [DOI] [PubMed] [Google Scholar]
- 8.Park ES, Jo KI, Shin JW, Park R, Choi TY, Bang HI, et al. Comparison of total and IgG ABO antibody titers in healthy individuals by using tube and column agglutination techniques. Ann Lab Med. 2014;34:223–9. doi: 10.3343/alm.2014.34.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadani DT, Urbaniak SJ, Bruce M, Tighe JE. Repeat ABO-incompatible platelet transfusions leading to haemolytic transfusion reaction. Transfus Med. 2006;16:375–9. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 10.Mathai J, Sindhu PN, Sulochana PV, Sathyabhama S. Haemolysin test for characterization of immune ABO antibodies. Indian J Med Res. 2003;118:125–8. [PubMed] [Google Scholar]
- 11.Jolly JG, Roy S, Kulkarni AG. Study of naturally occurring blood group antibodies in North Indians. Indian J Med Res. 1974;62:1202–7. [PubMed] [Google Scholar]
- 12.Jain PC, Singh SN, Rajvanshi VS, Jain R. Naturally occurring ABO antibodies in Kanpur (U.P.) J Indian Med Assoc. 1981;76:53–5. [PubMed] [Google Scholar]
- 13.Fontaine MJ, Mills AM, Weiss S, Hong WJ, Viele M, Goodnough LT. How we treat: Risk mitigation for ABO-incompatible plasma in plateletpheresis products. Transfusion. 2012;52:2081–5. doi: 10.1111/j.1537-2995.2012.03596.x. [DOI] [PubMed] [Google Scholar]
- 14.Crawford MN, Gottman FE, Gottman CA. Microplate system for routine use in blood bank laboratories. Transfusion. 1970;10:258–63. doi: 10.1111/j.1537-2995.1970.tb00740.x. [DOI] [PubMed] [Google Scholar]
- 15.Fung M, Hillyer CD, Westhoff CM, editors. Determining ABO Group of Red Cells and Serum: Microplate Test. AABB Technical Manual. 18th ed. Bethesda, MD: AABB; 2014. pp. 873–5. [Google Scholar]
- 16.Lozano M, Cid J. The clinical implications of platelet transfusions associated with ABO or Rh (D) incompatibility. Transfus Med Rev. 2003;17:57–68. doi: 10.1053/tmrv.2003.50003. [DOI] [PubMed] [Google Scholar]
- 17.Bajpai M, Kaur R, Gupta E. Automation in immunohematology. Asian J Transfus Sci. 2012;6:140–4. doi: 10.4103/0973-6247.98914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de França ND, Poli MC, Ramos PG, Borsoi CS, Colella R. Titers of ABO antibodies in group O blood donors. Rev Bras Hematol Hemoter. 2011;33:259–62. doi: 10.5581/1516-8484.20110073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redman M, Malde R, Contreras M. Comparison of IgM and IgG anti-A and anti-B levels in Asian, Caucasian and Negro donors in the North West Thames region. Vox Sang. 1990;59:89–91. doi: 10.1111/j.1423-0410.1990.tb05016.x. [DOI] [PubMed] [Google Scholar]
- 20.Adewuyi JO, Gwanzura C, Mvere D. Characteristics of anti-A and anti-B in black Zimbabweans. Vox Sang. 1994;67:307–9. doi: 10.1111/j.1423-0410.1994.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 21.Issitt PD, Anstee DJ, editors. Applied Blood Group Serology. 4th ed. Durham, NC: Montgomery Scientific Publications; 1998. The ABO and H blood group system; pp. 175–246. [Google Scholar]
- 22.Gupte SC, Bhatia HM. Anti-A and anti-B titre response after tetanus toxoid injections in normal adults and pregnant women. Indian J Med Res. 1979;70:221–8. [PubMed] [Google Scholar]
- 23.Toy PT, Reid ME, Papenfus L, Yeap HH, Black D. Prevalence of ABO maternal-infant incompatibility in Asians, Blacks, Hispanics and Caucasians. Vox Sang. 1988;54:181–3. doi: 10.1111/j.1423-0410.1988.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 24.Mazda T, Yabe R, NaThalang O, Thammavong T, Tadokoro K. Differences in ABO antibody levels among blood donors: A comparison between past and present Japanese, Laotian, and Thai populations. Immunohematology. 2007;23:38–41. [PubMed] [Google Scholar]
- 25.Garratty G. Problems associated with passively transfused blood group alloantibodies. Am J Clin Pathol. 1998;109:769–77. doi: 10.1093/ajcp/109.6.769. [DOI] [PubMed] [Google Scholar]
- 26.Grove-Rasmussen M. Selection of “safe” group O blood. Transfusion. 1966;6:331–5. doi: 10.1111/j.1537-2995.1966.tb04751.x. [DOI] [PubMed] [Google Scholar]
- 27.Tisdall LH, Garland DM. The effects of the transfusion of group O blood of high iso-agglùtinin titer into recipients of other blood groups. Am J Clin Pathol. 1946;16:193–206. doi: 10.1093/ajcp/16.3.193. [DOI] [PubMed] [Google Scholar]
- 28.Cooling LL, Downs TA, Butch SH, Davenport RD. Anti-A and anti-B titers in pooled group O platelets are comparable to apheresis platelets. Transfusion. 2008;48:2106–13. doi: 10.1111/j.1537-2995.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 29.Dutta SB, Roy MN. Observation on the titres of anti-A and anti-B isoagglutinins among normal Bengali males. J Indian Med Assoc. 1972;58:460–1. [PubMed] [Google Scholar]
- 30.Daniel-Johnson J, Leitman S, Klein H, Alter H, Lee-Stroka A, Scheinberg P, et al. Probiotic-associated high-titer anti-B in a group A platelet donor as a cause of severe hemolytic transfusion reactions. Transfusion. 2009;49:1845–9. doi: 10.1111/j.1537-2995.2009.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


